Abstract
Background
Healthcare-associated infections (HAIs) are preventable. Globally, laws aimed at reducing HAIs have been implemented. In the USA, these laws are at the federal and state levels. It is not known whether the state interventions are more effective than the federal incentives alone.
Objective
The aims of this study were to explore the impact federal and state HAI laws have on state departments of health and hospital stakeholders in the USA and to explore similarities and differences in perceptions across states.
Methods
A qualitative study was conducted. In 2012, we conducted semistructured interviews with key stakeholders from states with and without state-level laws to gain multiple perspectives. Interviews were transcribed and open coding was conducted. Data were analysed using content analysis and collected until theoretical saturation was achieved.
Results
Ninety interviews were conducted with stakeholders from 12 states (6 states with laws and 6 states without laws). We found an increase in state-level collaboration. The publicly reported data helped hospitals benchmark and focus leaders on HAI prevention. There were concerns about the publicly reported data (eg, lack of validation and timeliness). Resource needs were also identified. No major differences were expressed by interviewees from states with and without laws.
Conclusions
While we could not tease out the impact of specific interventions, increased collaboration between departments of health and their partners is occurring. Harmonisation of HAI definitions and reporting between state and federal laws would minimise reporting burden. Continued monitoring of the progress of HAI prevention is needed.
BACKGROUND
For over 15 years, public disclosure of hospital outcome data has been thought to improve patient safety.1 One of the most serious issues for ensuring patient safety is preventing healthcare associated infections (HAIs); at any given time, an estimated 1 in 25 hospitalised patients in the USA has a HAI, leading to significant morbidity and mortality.2 The Centers for Disease Control and Prevention (CDC) estimated the annual national hospital costs of HAI to be US$25–31 billion.3 These data, coupled with growing demand for transparency and accountability by policy makers, have led several countries to legally mandate the public reporting of HAI indicators including England, France and the USA.4
In the USA, HAI reporting laws have been enacted at the federal and state levels.5,6 In 2008, the federal Department of Health and Human Services implemented a national Action Plan for reducing HAIs across healthcare and identifying measureable goals.7 In support of the Action Plan, further federal initiatives were legislated. For example, as part of the American Recovery and Reinvestment Act of 2009 (Public Law 111-5, 42 U.S.C 241(a)), US$40 million were appropriated through the CDC. These funds were available to all states to support state departments of health (DOH) for HAI prevention planning and infrastructure including supporting a state-specific dedicated programme coordinator (HAI coordinator). As part of the Patient Protection and Affordable Care Act of 2010 (Public Law 111–148), the Hospital Value Based Purchasing Program built on earlier legislation that allowed Medicare to pay hospitals for reporting quality measures, rather than on the quantity of care (eg, service or patient counts). This programme included the Inpatient Quality Reporting Program requiring hospitals to report specific HAIs to the CDC’s National Healthcare Safety Network to receive full Medicare payment.
The federal laws apply across all 50 states and provide incentives only; these interventions do not mandate action. As of 2013, 37 states (including the District of Columbia and Puerto Rico) have introduced laws that require facilities to report HAI indicators to each state’s DOH, which then may report HAI data publicly.8 While there is support of the general concept of HAI prevention, the evidence on the benefits of public reporting has been inconclusive and opinions are mixed.9,10 Furthermore, it is not known whether the state interventions are more effective than the federal legislation alone. Therefore, our aims were to explore the impact federal and state HAI laws have on state DOH and hospital stakeholders in the USA and to explore the similarities and differences in perceptions from those in states with and without state-level laws.
STUDY DESIGN AND METHODS
Our research team conducted a qualitative public health law study.11–13 This design was chosen because of its inductive nature, which may provide invaluable insights from multiple perspectives.
Selection of states
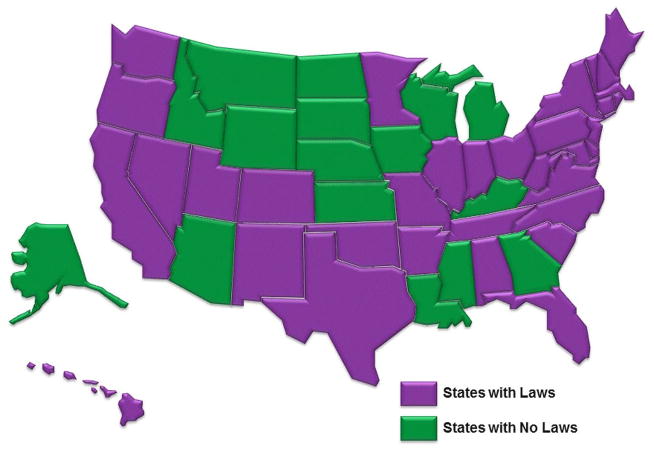
First, a legal review was conducted to determine the states that had HAI reporting laws. LexisNexis research system databases were used along with other relevant administrative law resources to capture state statutes and administrative regulations. To ensure accuracy, HAI coordinators in states and territories with legal reporting mandates (n=36 at the time of our study) were contacted and verification was obtained. Figure 1 displays all states with and without mandatory reporting laws as of 1 May 2012. States were purposefully sampled to include equal numbers of those with and without state-level mandatory reporting laws and to ensure geographical variety until theoretical saturation was achieved.
Figure 1.
Map of states with healthcare-associated infection laws.
Selection of participants
From each of the states, stakeholders with key roles (ie, state DOH HAI personnel, regulatory or legal officials, clinicians and community partners) were purposefully recruited to elicit multiple perspectives. Using a snowballing technique, each state’s HAI coordinator was asked to recommend knowledgeable individuals from each role. At least 5 and up to 10 participants were recruited from each state based on availability, willingness to participate and theoretical saturation.
Interviews
Semistructured interview guides (available upon request) were tailored to the key roles and state type (ie, state-level law or no law). These guides were based on: (1) Donabedian’s quality framework that proposes evaluation of healthcare delivery should be based on the quality of system structure, process and outcomes, (2) our legal review and (3) knowledge gained from previous research.5,14–16 All interview guides included demographic and open-ended questions about the impact state and federal laws have on structure of the state HAI programme; changes in the programme; working relationships in HAI prevention; community and non-governmental partnerships; HAI data uses; funding; and lessons learned.
A two-person interview team was trained by the investigators (PWS and JAM) so there would be consistent interviewing techniques used.17 Prior to beginning, the interview team piloted all procedures. Interviews were audiotaped, professionally transcribed and reviewed for accuracy.
Analysis
Transcripts were entered into NVivo 10 (QSR International) software and identified by state and role only. All data were combined. Transcripts were read several times by multiple team members to gain an understanding of the data. An inductive open coding process was conducted by two research assistants who were supervised by the investigators (PWS and JAM). Coding began as soon as transcripts were received and throughout the coding process, two transcripts were double coded on a bi-weekly basis and per cent agreement was computed and adequate (ie, >90%). Discrepancies were discussed until a consensus was reached. Throughout the interviewing and coding process, weekly debriefings occurred with all research team members to ensure a shared understanding of the data. Using a qualitative content analysis approach,18 coded data were reviewed to develop themes, looking for similarities and differences between respondents from states with and without state-level laws. This comprehensive, conscious and collaborative analysis contributes to the credibility of the findings.
RESULTS
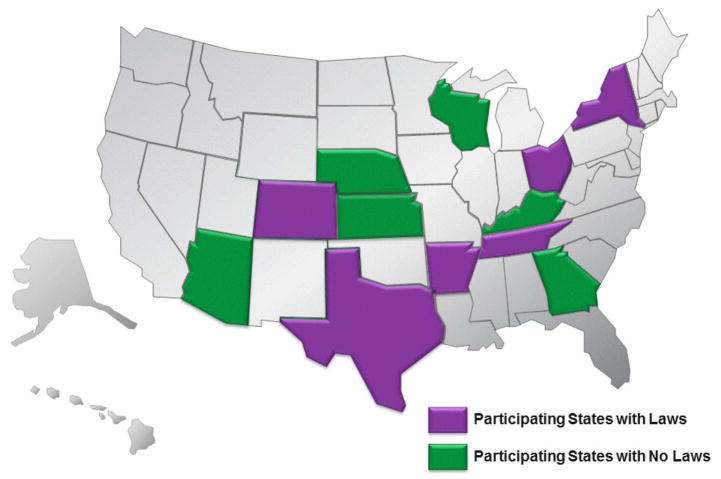
Theoretical saturation was achieved after recruiting 12 states (see figure 2). Of the states with HAI mandatory reporting laws, Arkansas, Colorado, New York, Ohio, Tennessee and Texas were selected (randomly assigned numbers from 1 to 6). Arizona, Georgia, Kansas, Kentucky, Nebraska and Wisconsin were states selected without laws (randomly assigned numbers from 7 to 12).
Figure 2.
States participating in the study.
Ninety interviews were conducted and the respondent roles included: (1) state DOH officials and HAI coordinators; (2) DOH regulatory officials and legal counsel; (3) clinicians involved in HAI prevention including infection preventionists (IPs), hospital epidemiologists, and department managers from acute care and specialty care settings (eg, dialysis); and (4) community partners including state hospital association representatives, quality improvement organisation representatives, and if applicable, consumer advocates. Table 1 describes the key stakeholders by state type. A majority (59%) came from states with HAI laws.
Table 1.
Key respondents from each type of state
| Role | State categories
|
||
|---|---|---|---|
| With laws* (n=6) | No laws† (n=6) | Total | |
| State DOH HAI personnel | |||
| DOH official | 3 | 5 | 8 |
| HAI coordinator | 6 | 5 | 11 |
| Regulatory or legal | |||
| State DOH regulatory official | 4 | 3 | 7 |
| State DOH legal counsel | 6 | 1 | 7 |
| Clinicians | |||
| Acute care facility IP/HE | 14 | 9 | 23 |
| Specialty care facility IP/HE | 4 | 3 | 7 |
| Community partners | |||
| Hospital association | 6 | 5 | 11 |
| QIO | 6 | 4 | 10 |
| Consumer advocate | 4 | 2 | 6 |
| Total personnel interviewed | 53 | 37 | 90 |
Arkansas, Colorado, New York, Ohio, Tennessee, Texas.
Arizona, Georgia, Kansas, Kentucky, Nebraska, Wisconsin.
DOH, Department of Health; HAI, healthcare-associated infection; HE, hospital epidemiologist; IP, infection preventionist; QIO, quality improvement organisation.
Our findings are organised around four themes: increased collaboration, using public reported data for benchmarking and prioritising, concerns related to public reported data and resource needs. Narrative exemplar quotes supporting each theme from the various stakeholders from states with and without laws are provided below. Table 2 displays the narratives consistent with the themes by role and state type. There is some dissimilarity in the narrative responses by role (ie, only 50% of regulatory or legal stakeholders discussed resource needs compared with 70–80% of the other stakeholders). The narratives are similar from stakeholders from states with and without state-level laws.
Table 2.
Percentage of respondents in states with and without laws that contributed data to the themes
| State law status | DOH HAI personnel
|
Regulatory or legal
|
Clinicians
|
Community partners
|
||||
|---|---|---|---|---|---|---|---|---|
| Yes* N=9 (%) | No† N=10 (%) | Yes* N=10 (%) | No† N=4 (%) | Yes* N=18 (%) | No† N=12 (%) | Yes* N=16 (%) | No† N=11 (%) | |
| Increased collaboration | 9 (100) | 10 (100) | 8 (80) | 3 (75) | 13 (72) | 12 (100) | 14 (88) | 11 (100) |
| Using publicly reported data for benchmarking and prioritising | 7 (78) | 8 (80) | 8 (80) | 3 (75) | 17 (94) | 12 (100) | 14 (88) | 10 (91) |
| Concerns related to publicly reported data | 9 (100) | 10 (100) | 7 (70) | 3 (75) | 15 (83) | 9 (75) | 9 (56) | 9 (82) |
| Resource needs | 9 (100) | 8 (80) | 5 (50) | 2 (50) | 17 (94) | 11 (92) | 14 (88) | 10 (91) |
States with laws
States without laws; N=number of interviewees.
DOH, Department of Health; HAI, healthcare-associated infections.
Increased collaboration
Many respondents commented on the increase in state-level partnerships and collaboration stemming from the HAI laws. For example, one respondent stated:
Before [the federal HAI prevention actions] we were … working alone in our own little spheres and not necessarily discovering what other places are doing. (Participant 56, IP, state 7)
Indeed, collaboration came up repeatedly in discussions about Quality Improvement Organizations, Hospital Engagement Networks (ie, learning collaboratives funded through the federal government), hospital associations and DOHs. As one respondent discussed:
…we try to coordinate the work between state [DOH], and state hospital association, and the HEN [hospital engagement network] work, and then what I’m doing, so we’re not reinventing the wheel and duplicating efforts. (Participant 72, quality improvement organization representative, state 11)
Furthermore, many recognised the state DOH as leading these collaborations as elucidated below:
The state is leading …getting the right people in the room, distribution across the state, different types of providers, stakeholders, infection preventionists, other disciplines. There’s somebody knowledgeable at the state level very involved. (Participant 39, hospital association representative, state 4)
This respondent also discussed the collaboration between the hospital association and the hospitals as follows:
We’re very hands-on. We’ve become an extension of each hospital’s team and use regional expertise to coach and mentor hospitals. (Participant 39, hospital association representative, state 4)
Using publicly reported data for benchmarking and prioritising
Publicly reported data were useful in obtaining the focus of the hospital administrators and gaining support for local prevention efforts. It was perceived that state and federal level HAI reporting laws influenced the prioritisation of infection prevention efforts. Due to institutional accountability and reputation, the publicly reported data helped hospitals benchmark and focus leaders on HAI prevention. This benchmarking often translated into an increased recognition of the importance of infection prevention in the facility as explained below:
When you take data…put names along with it…put it on a website or in the newspaper…you see monumental change in the interest and funding that the CEO [Chief Executive Officer] provides. (Participant 14, community representative, state 2)
The data resulting from the state reporting mandate were useful for prioritising state prevention efforts as described in the following:
A few years ago we added C. difficile infections…based on what we’re seeing in the state [publically reported data]. (Participant 21, HAI coordinator, state 4)
Benchmarking and prioritising was also present in states without state-level mandatory reporting laws as described:
We provide benchmarking for both individual hospitals and then compare it [to] state and national data when [they are] available. And we provide targeted interventions and resources to hospitals that are having challenges in bringing those rates down. (Participant 51, hospital association representative, state 9)
Concerns related to publicly reported data
There were concerns about the data reported as a result of the HAI laws. These concerns stemmed from a need for validation and standardisation of definitions as well as the specificity and timeliness of the aggregated publicly reported data to inform practice. As discussed below, inconsistently defined data for benchmarking between institutions was seen as problematic:
The worry would be whether everybody was collecting data the same way and if we were capturing really what we thought we were capturing. (Participant 46, IP, state 7)
Many stakeholders expressed doubts about using unvalidated data as described:
Data validation is also important. You know there are quite a few states that publish reports, but the data aren’t validated. (Participant 27, DOH official, state 4)
The concern about validation was echoed in the following statement:
We’re collecting all of this information, but we haven’t validated the data, and while I trust that we have a very savvy and robust network of IPs, I think folks would feel a lot more comfortable with the data knowing that some validation has occurred. (Participant 65, DOH official, state 10)
Others were concerned about the timeliness and specificity of the data; state-specific data were perceived as timelier than federal data as described in the following:
When it comes to CMS [Centers for Medicare and Medicaid Services] reporting, by the time they get that data out…it doesn’t have as much relevance because it’s…24 months old or 18 months old…versus the state [DOH] HAI data, which is very specific. (Participant 28, community representative, state 5)
Resource needs
Sustainable and sufficient funding for the state level programmes was an issue. In all states, respondents discussed resource constraints and stressed the need for sustainable and sufficient funding for the state HAI programme. Many respondents expressed the belief that their state’s HAI programme would not exist without a federal investment and expressed concern that this funding was unreliable as explained below:
ARRA [American Recovery and Reinvestment Act] was huge for us. We wouldn’t have the [state HAI] program…the state [HAI program] has to have funding. I don’t know how the government can come up with the [future] funding, but you know somehow, [in] some way that needs to happen. (Participant 33, regulatory stakeholder, state 5)
These sentiments were echoed in the following:
Our biggest issue is ongoing funding. So that’s worrisome when [the state HAI program is] 100% federally funded. (Participant 59, HAI coordinator, state 5)
Another important aspect of resources was trained personnel. The need for sufficient numbers of personnel with adequate, relevant expertise was discussed by respondents from all states. For example, one respondent stated:
It’s really hard to build expertise. It’s hard enough at the state level to get expertise, to help these IPs out. At the hospital level there often aren’t enough trained IPs.(Participant 88, DOH official, state 12)
Many stakeholders expressed concern that meeting reporting requirements (ie, federal and state) overburdened the IPs leaving little time for development and execution of interventions aimed at prevention. As one respondent explained:
Many of our IPs complain that they’re spending all of their time doing data entry, collection and management and have very little time for education and infection prevention efforts. When you’re spending all of your time collecting all this different data, there is not enough time to actually do the infection prevention effort, so those are unintended consequences. (Participant 28, community representative, state 5)
DISCUSSION
We explored perceptions of the impact federal and state HAI laws have on state DOHs and hospital stakeholders in the USA and similarities and differences in perceptions from those working in states with and without state-level laws. While some findings were expected others were surprising.
We found there was a perception that state HAI laws fostered a climate of collaboration. There is a growing evidence base establishing the efficacy of HAI prevention collaboratives that emphasise health system change through surveillance, data feedback, systematic implementation of prevention measures, and intrafacility and interfacility collaboration.19–21 Other researchers have surveyed DOH about their HAI prevention activities and found that between 2009 and 2011, just prior to our study period, federal funding had resulted in 60 prevention collaboratives.22 Therefore, it is not surprising that we found increased partnerships described by interviewees in all states. What was not known was the partnerships were frequently attributed to the role the state DOH played as a convenor and leader.
Stakeholders from both types of states (ie, with state-level laws and without) discussed how the publicly reported data were used to benchmark and prioritise HAI prevention efforts. The federal financial incentives were perceived to be an important motivator for healthcare facility prevention efforts. This is despite a lack of evidence on the efficacy of the federal incentives; in 2008, researchers found that federal non-payment policy did not affect rates of targeted HAIs.23 However, that research only focused on the non-payment policy, which was found to have negligible impact on hospital finances.24 The more recent value-based purchasing programme may act as a stronger financial incentive. Even without clear evidence of the efficacy of financial incentives, publicly reported data were considered useful for engaging institutional leadership around issues of harm reduction and reputation.
It is not surprising that validation of publicly reported data and use of standard definitions were seen as paramount by stakeholders. The importance of data validation has been recognised by the CDC.25 Without validation, there may be incentives to under-report due to the data being tied to payment and hospital reputation. In Europe, due to concerns about under-reporting as well as potential to negatively impact prevention efforts, experts are in favour of reporting process indicators over HAI rates.10
The timeliness and specificity of the data were persistent concerns among the respondents, a problem also identified in prior CDC HAI stakeholder engagement activities.5,14 The benefit of rapid-cycle feedback to clinicians and hospital administration is well known26 and given sufficient staffing, facilities would be expected to provide feedback to clinicians directly while providing their state DOH with data. Indeed, the CDC’s National Healthcare Safety Network provides two mechanisms for facilities to voluntarily share HAI data with their state DOH: the National Healthcare Safety Network Group Function and data use agreements between the CDC and individual state DOHs. These resources afford states and the individual hospital immediate access to data to focus prevention efforts.
Sustainable resources and funding for HAI programmes were identified as important themes. State DOHs that are facing budget cuts depend on vulnerable programmatic funding streams and must balance competing priorities against scarce resources.27 Targeted funding can play a critical role in reducing HAIs.21 While increased resources for infectious disease epidemiology and laboratory capacity have recently been awarded through the CDC, future funding is not guaranteed.28
Respondents repeatedly expressed a need for experienced personnel, especially IPs. Over the past few decades, the role of IPs has changed.29 IPs have shifted their focus from merely monitoring infections to proactively intervening to prevent them and have expanded their scope to lead performance improvement teams.30 However, with mandatory reporting, some IPs are forced to spend more time on surveillance and less time on preventing infections.31
While the focus of responses varied slightly by role, we were surprised we did not uncover major differences in those from states with or without state reporting laws. We attribute this to the impact of federal HAI prevention initiatives on stakeholders in all states. It is possible that the state laws had an impact on healthcare delivery processes and may have laid the groundwork for the federal legislation. However, we cannot definitively determine that impact with our current data.
Limitations
This study has limitations; findings need to be interpreted with caution. Efforts were made to have a diverse sample of states and respondents to gain multiple perspectives. However, the respondents did not represent all states and the findings may have limited transferability. The data are narrative self-report and bias due to social desirability cannot be ruled out.
CONCLUSIONS
Legal interventions such as state-level reporting mandates and value based purchasing federal incentives should be, in theory, associated with decreasing HAIs. While we could not tease out the impact of specific interventions, progress has been made in the effort to eliminate HAIs; however, more work is needed to improve patient safety and reduce HAIs. Continued monitoring of the progress made towards goals to decrease HAIs is needed. To prevent HAIs, collaboration between public health agencies and their partners in the healthcare sector is essential. Harmonisation of HAI definitions and reporting between state and federal laws would minimise reporting burden.
Acknowledgments
Funding National Institute of Nursing Research (R01NR010107), Robert Wood Johnson Foundation (270282), Centers for Disease Control and Prevention (400648).
Footnotes
Contributors All authors contributed to the planning of the research design and reporting of the results. PWS, MP-M, JR, JAM and BS contributed to acquisition and analysis of the data.
Competing interests None declared.
Ethics approval Columbia University Medical Center’s Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Davies HT, Marshall MN. Public disclosure of performance data: does the public get what the public wants? Lancet. 1999;353:1639–40. doi: 10.1016/s0140-6736(99)90047-8. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott RD., II . The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention; 2009. http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. [Google Scholar]
- 4.Haustein T, Gastmeier P, Holmes A, et al. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis. 2011;11:471–81. doi: 10.1016/S1473-3099(10)70315-7. [DOI] [PubMed] [Google Scholar]
- 5.Eliminating healthcare associated infections: state policy options. Arlington, VA: Association of State and Territorial Health Officials; 2011. http://www.cdc.gov/hai/pdfs/toolkits/toolkit-HAI-POLICY-FINAL_01-2012.pdf. [Google Scholar]
- 6.Reagan J, Hacker C. Laws pertaining to healthcare-associated infections: a review of 3 legal requirements. Infect Control Hosp Epidemiol. 2012;33:75–80. doi: 10.1086/663204. [DOI] [PubMed] [Google Scholar]
- 7.National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination. Office of Disease Prevention and Health Promotion, Office of the Assistant Secretary for Health, Office of the Secretary, U.S. Department of Health and Human Services; Apr 2, 2015. http://www.health.gov/hcq/prevent_hai.asp. [Google Scholar]
- 8.Herzig CT, Reagan J, Pogorzelska-Maziarz M, et al. State-mandated reporting of health care-associated infections in the United States: trends over time. Am J Med Qual. 2014 Jun 20; doi: 10.1177/1062860614540200. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–96. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 10.Martin M, Zingg W, Hansen S, et al. Public reporting of healthcare-associated infection data in Europe. What are the views of infection prevention opinion leaders? J Hosp Infect. 2013;83:94–8. doi: 10.1016/j.jhin.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Burris S, Mays GP, Douglas Scutchfield F, et al. Moving from intersection to integration: public health law research and public health systems and services research. Milbank Q. 2012;90:375–408. doi: 10.1111/j.1468-0009.2012.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope C, van Royen P, Baker R. Qualitative methods in research on healthcare quality. Qual Saf Health Care. 2002;11:148–52. doi: 10.1136/qhc.11.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood J. Using qualitative research strategies for public health law evaluation: a methods monograph for the public health law research program. Public Health Law Research; 2012. Forthcoming. SSRN: http://ssrn.com/abstract=2017750. [Google Scholar]
- 14.Policies for eliminating healthcare-associated infections: lessons learned from state stakeholder engagement. Arlington, VA: Association of State and Territorial Health Officials Centers for Disease Control and Prevention; 2012. http://www.cdc.gov/hai/pdfs/toolkits/HAI-policy-case-studies-lesssons-learned.PDF. [Google Scholar]
- 15.Uchida M, Stone PW, Conway LJ, et al. Exploring infection prevention: policy implications from a qualitative study. Policy Polit Nurs Pract. 2011;12:82–9. doi: 10.1177/1527154411417721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 17.Merton R, Fiske M, Kendall P. The focused interview: a manual of problems and procedures. New York, NY: The Free Press; 1990. [Google Scholar]
- 18.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 19.DePalo VA, McNicoll L, Cornell M, et al. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19:555–61. doi: 10.1136/qshc.2009.038265. [DOI] [PubMed] [Google Scholar]
- 20.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–30. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 21.Lipitz-Snyderman A, Steinwachs D, Needham DM, et al. Impact of a statewide intensive care unit quality improvement initiative on hospital mortality and length of stay: retrospective comparative analysis. BMJ. 2011;342:d219. doi: 10.1136/bmj.d219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellingson K, McCormick K, Sinkowitz-Cochran R, et al. Enhancement of health department capacity for health care-associated infection prevention through Recovery Act-funded programs. Am J Public Health. 2014;104:e27–33. doi: 10.2105/AJPH.2013.301809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in U.S. hospitals. N Engl J Med. 2012;367:1428–37. doi: 10.1056/NEJMsa1202419. [DOI] [PubMed] [Google Scholar]
- 24.McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood) 2009;28:1485–93. doi: 10.1377/hlthaff.28.5.1485. [DOI] [PubMed] [Google Scholar]
- 25.Menu of selected provisions in healthcare-associated infection laws. Atlanta, GA: Centers for Disease Control and Prevention; 2012. http://www.cdc.gov/phlp/docs/HAI%20Menu-508.pdf. [Google Scholar]
- 26.Bradley EH, Holmboe ES, Mattera JA, et al. Data feedback efforts in quality improvement: lessons learned from US hospitals. Qual Saf Health Care. 2004;13:26–31. doi: 10.1136/qhc.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). . The epidemiology workforce in state and local health departments-United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:205–8. [PubMed] [Google Scholar]
- 28.Committee on Public Health Strategies to Improve Health Board on Population Health and Public Health Practice. For the public’s health: investing in a healthier future. Washington DC: Institute of Medicine of the National Academies; 2012. http://www.iom.edu/Reports/2012/For-the-Publics-Health-Investing-in-a-Healthier-Future.aspx. [PubMed] [Google Scholar]
- 29.Goldrick BA. The practice of infection control and applied epidemiology: a historical perspective. Am J Infect Control. 2005;33:493–500. doi: 10.1016/j.ajic.2005.04.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy DM, Hanchett M, Olmsted RN, et al. Competency in infection prevention: a conceptual approach to guide current and future practice. Am J Infect Control. 2012;40:296–303. doi: 10.1016/j.ajic.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Conway LJ, Raveis VH, Pogorzelska-Maziarz M, et al. Tensions inherent in the evolving role of the infection preventionist. Am J Infect Control. 2013;41:959–64. doi: 10.1016/j.ajic.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]