Abstract
The discovery of new vaccines against infectious diseases and cancer requires the development of novel adjuvants with well-defined activities. The TLR4 agonist adjuvant GLA-SE elicits robust TH1 responses to a variety of vaccine antigens and is in clinical development for both infectious diseases and cancer. We demonstrate that immunization with a recombinant protein antigen and GLA-SE also induces granzyme A expression in CD4 T cells and produces cytolytic cells that can be detected in vivo. Surprisingly these in vivo CTLs were CD4 T cells, not CD8 T cells and this cytolytic activity was not dependent on granzyme A/B or perforin. Unlike previously reported CD4 CTLs the transcription factors Tbet and Eomes were not necessary for their development. CTL activity was also independent of the FasL-Fas, TRAIL-DR5, and canonical death pathways, indicating a novel mechanism of CTL activity. Rather, the in vivo CD4 CTL activity induced by vaccination required T cell expression of CD154 (CD40 ligand) and target cell expression of CD40. Thus, vaccination with a TLR4 agonist adjuvant induces CD4 CTLs which kill through a previously unknown CD154-dependent mechanism.
Introduction
MHC class II restricted CD4 T cells have traditionally been characterized as helper T cells (TH) based on their ability to modify or enhance the immune response mediated by CD8 T cells, B cells and innate immune cells. Help is mediated by both cell-cell interactions such as CD154-CD40 cross talk with B cells and secretion of cytokines including TNF and IFN-γ which cause maturation of phagocytic cells such as macrophages. CD8 T cells also produce some of these same cytokines but can also directly kill target cells presenting a cognate MHC class I:peptide complex. CD8 cytolytic T lymphocytes (CTLs) use two primary mechanisms of cytolysis: exocytosis of lytic granules containing perforin and granzymes and cell surface receptors including FasL that bind receptors on the target cell that initiate a cell death pathway. Death of the target cell can proceed via several different signaling pathways including a caspase 3- or caspase 7-dependent pathway and Bad/Bax pathway of mitochondria cytochrome c release (1).
CD4 T cells with lytic activity have also been described, however early work was based on long-term cultured CD4 T clones, suggesting this may be an in vitro artifact resulting from chronic antigen stimulation and IL-2 signaling (2). More recent in vivo and directly ex vivo work has described CD4 CTLs that express perforin and the most well characterized cytolytic granzyme, granzyme B (reviewed in (3, 4)). These CD4 CTL have been implicated in the control of a number of viral infections including LCMV, influenza, mousepox, and West Nile virus in mice (5–8). Human CD4 CTLs expressing lytic granules have also been described for HIV, HCMV, and Epstein-Barr virus as well as mycobacteria including BCG and Mycobacterium tuberculosis (M.tb.) infections (9–16). Human and mouse CD4 CTL can also kill via cell-cell contact by expressing FasL or the related surface protein TRAIL which bind Fas or death receptor 5 (DR5), respectively, on target cells to induce death (9, 17, 18). Of note Woodworth et al. found that M.tb.-specific CD4 CTLs were induced in mice infected with M.tb., but unlike those produced by viral infection, these CD4 CTL killed via an undefined mechanism that was independent of perforin, Fas-FasL, and TNFR1 (19).
The major lineages of CD4 T cell differentiation including TH1, TH2, TH17, Treg and TFH have been linked to expression of a fate determining transcription factor, Tbet, GATA3, RORγt, FoxP3, or Bcl-6, respectively. CTL activity was originally ascribed to a subset of TH1 cells, although other groups found that non-polarized CD4 T cells could also mediate CTL activity. More recently the T-box transcription factor Eomes was found to be necessary for the expression of granzyme B in mouse CD4 T cells stimulated via CD134 and CD137, a regimen sufficient to produce CD4 CTL (20). Similarly ectopic expression of Eomes drove perforin and FasL expression in mouse TH2 cells, converting them to CD4 CTL (21). The exact conditions necessary to induce CD4 CTL in vitro and in vivo are still being established but it seems clear that both antigen concentration and IL-2 availability can affect CD4 CTL programming (22).
Given the contribution of CD4 CTL to the immune response to a number of bacterial and viral infections it would be useful to develop a vaccination scheme that can intentionally elicit these cells. We have developed a number of adjuvants that preferentially augment TH1 or TH2 responses or boost antibody responses to protein antigens indicating the induction of TFHs (23–26). Using the recombinant M.tb. protein antigen ID93 we have found that the synthetic TLR4 agonist GLA augments IFN-γ and TNF CD4 T cell responses when formulated in an oil-in-water stable emulsion (SE) (24, 26). We now report that this vaccination scheme also elicits CD4 T cells that express granzyme A and are lytic in vivo.
Materials and Methods
Mice and immunizations
Wild type C57Bl/6, B6.SJL-PtprcaPepcb/BoyJ (CD45.1), 129X1/SvJ-Gzmatm1Ley Gzmbtm2.1Ley/J (Gzm A/B−/−, B6.Cg-Tg(Cd4-cre)1Cwi/BfluJ (CD4-Cre+), B6.129S1(cg)-Eomestm1.1Bflu/J (Eomes fl/fl), Tbet−/−, B6Smn.C3-Faslgld/J (FasL−/−), B6.MRL-Faslpr/J (Fas−/−), C57BL/6-Pfr1tm1Sdz/J (Pfr−/−), B6N.129S1-Casp3tm1Flv/J (Casp3−/−), B6.129S6-Casp7tm1Flv/J (Casp7−/−), B6.129X1-Baxtm1Sjk/J (Bax−/−), B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J (TNFR1/2−/−), B6.129P2-Cd40tm1Kik/J (CD40−/−), and B6.129S2-Cd40lgtm1Imx/J (CD154−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Splenocytes from DR5−/− mice on the C57Bl/6 background (27) were a kind gift from Stephen Schoenberger (La Jolla Institute for Allergy and Immunology). CD4-Cre+ and Eomesfl/fl mice were interbred to establish CD4-Cre+ Eomesfl/fl (Eomes0/0) and CD4-Cre− Eomesfl/fl (Eomesfl/fl) lines. Mice were immunized by intramuscular injection with the recombinant protein ID93 unadjuvanted or formulated with the adjuvant GLA-SE to provide a final vaccine dose of 0.5 µg antigen and 5 µg GLA-SE (28). All mice were maintained in specific pathogen-free conditions. All procedures were approved by the IDRI institutional animal care and use committee.
Intracellular cytokine and tetramer staining
One week after immunization splenocytes were plated at 2×106 cells/well and stimulated for 2 hours at 37°C with ID93 (10 µg/mL) or unstimulated. GolgiPlug (BD Biosciences) was added and the cells were incubated for an additional 8 hours at 37°C. Cells were washed and surface stained with fluorochrome-labeled antibodies to CD4 (clone RM4-5) and CD8 (clone 53-6.7) (BioLegend and eBioscience) in the presence of anti-CD16/32 (clone 2.4G2) for 20 minutes. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 minutes. Cells were washed with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome-labeled antibodies to CD154 (clone MR1), IFN-γ (clone XMG-1.2), IL-2 (clone JES6-5H4) and TNF (clone MP6-XT22) (BioLegend and eBioscience) for 20 minutes. Cells were washed and resuspended in PBS. Up to 106 events were collected on an LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo v9. Cells were gated as singlets > lymphocytes > CD4+ CD8− or CD4− CD8+ > cytokine positive. ID93 specific response frequencies were determined by subtracting the frequency of response positives of unstimulated cells from ID93 stimulated cells in matched samples.
Alternatively splenocytes were stained for 90 minutes at 37°C with an I-Ab tetramer presenting the dominant epitope of Rv3619 (VIYEQANAHGQ), one of the components of ID93 (24). APC labeled tetramers were provided by the NIH Tetramer Core Facility. Cells were then surface stained with fluorochrome-labeled antibodies to CD4, CD8, CD44 (clone IM7), and lineage (CD19 (clone 1D3), CD11b (clone M1/70), and I-Ab (clone AF6-120.1)) in the presence of anti-CD16/32 for 20 minutes. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 minutes. Cells were washed with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome-labeled antibodies to granzyme A (clone GzA-3G8.5), granzyme B (clone GB11), perforin (clone eBioOMAK-D), and Eomes (clone Dan11mag) (BioLegend, eBioscience, and BD Biosciences). Cells were washed and resuspended in PBS. Up to 106 events were collected on an LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo v9. Cells were gated as singlets > lymphocytes > lin− > CD4+ CD8−> CD44hi tetramer+ > intracellular marker.
In vivo killing
Target CD45.1 splenocytes were isolated and red blood cells were lysed. Cells were split equally and labeled with 4 µM or 0.4 µM CFSE for 10 minutes in PBS at 37C. Cells were washed twice with RPMI + 20% FCS. CFSEhi cells were then incubated for 1 hr at 37°C with ID93 (10 µg/mL) and washed. CFSEhi and CFSElo cells were combined 1:1 in PBS. In some experiments CD45.1 and CD40−/−, Fas−/−, or DR5−/− (all CD45.2) target cells were mixed 1:1 prior to CFSE and ID93 labeling and analyzed in the same recipients. Up to 107 target cells were intravenously injected into immunized animals and matched unimmunized controls one week after immunization. One day later splenocytes were harvested from recipients and red blood cells were lysed. Depending on the experiment cells were stained for CD45.1 (clone A20), CD45.2 (clone 104), and I-Ab (eBioscience and BioLegend). Cells were gated as singlets > lymphocytes > CFSE+ donors > CD45.1+ CD45.2− or CD45.1− CD45.2+ > I-Ab+ or I-Ab− > CFSEhi or CFSElo. Specific lysis was calculated as (1 – (CFSEhi immunized/ CFSElo immunized)/average (CFSEhi unimmunized/ CFSElo unimmunized))*100 as described previously (5). In some experiments immunized mice were depleted of CD4+ cells (clone GK1.5) or CD8+ cells and NK1.1+ cells (clones 53-6.7 and PK136, respectively) two days prior to transfer of target cells by intraperitoneal injection of 0.25 mg of each depleting antibody.
Statistics
The Student’s t test was used to analyze data sets consisting of only two groups. ANOVA analysis with the Bonferroni correction for multiple comparisons was used when more than two groups were analyzed. Statistical analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
Expression of cytolytic markers in CD4 T cells after immunization
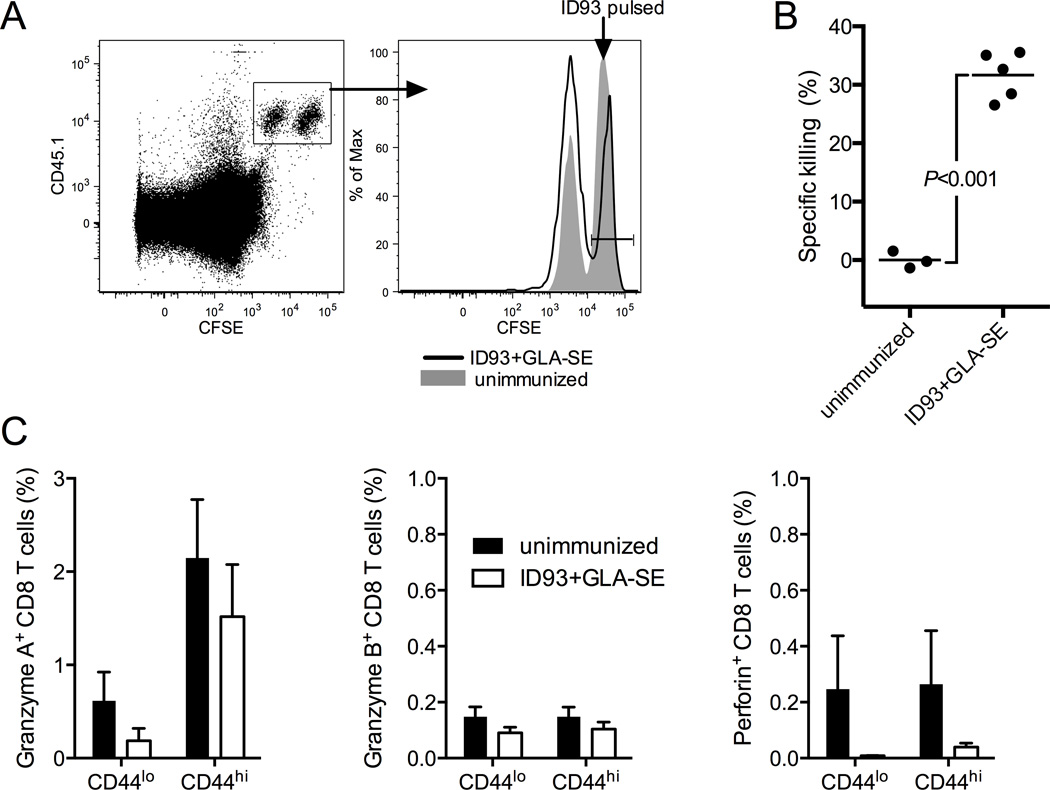
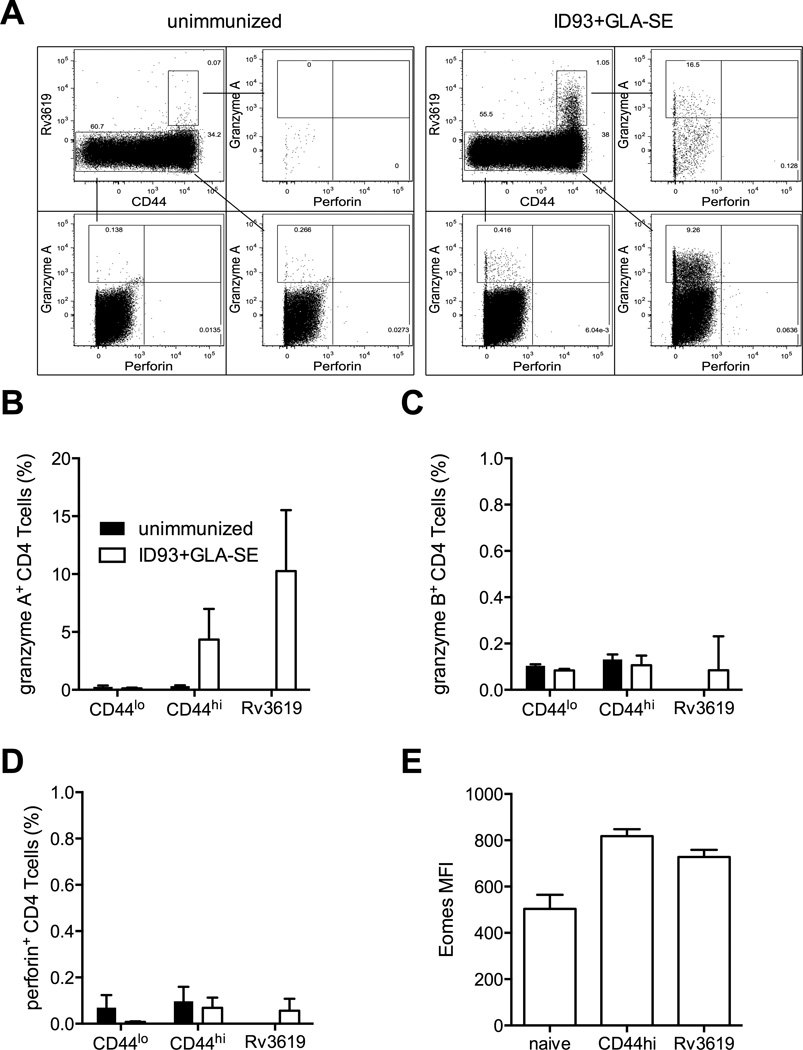
Parenteral immunization with ID93+GLA-SE produces ID93-specific CD4 T cells that produce a number of TH1-associated cytokines upon stimulation, including IFN-γ, TNF, IL-2, and GM-CSF, as well as expression of CD154 (CD40 ligand) (24). However cytokine-producing CD8 T cells are not evident (29). To determine whether immunization produced cytolytic CD8 T cells that were not detectable by cytokine secretion we employed an in vivo killing assay. CD45.1 congenic splenocytes were differentially labeled with high or low concentrations of CFSE ex vivo and pulsed with ID93 or left unpulsed. Labeled cells were mixed 1:1 and intravenously injected into immunized or unimmunized animals. 18 hours later there was a substantial reduction in the frequency of ID93-pulsed target cells in the immunized recipients compared to the unimmunized controls, indicating the presence of ID93-specific CTLs (Figure 1A and B). Surprisingly CD8 T cells from these same animals did not degranulate upon in vitro stimulation with ID93, as measured by surface capture of CD107a during stimulation (data not shown). Further immunization did not induce the expression of granzyme A, granzyme B or perforin in CD44hi CD8 T cells (Figure 1C). Unexpectedly, we found that a significant number of CD44hi CD4 T cells contained granzyme A, but not granzyme B or perforin (Figure 2 A–D). Granzyme A was further enriched in ID93-specific CD4 T cells identified with an I-Ab tetramer loaded with the dominant epitope from Rv3619, one of the component proteins of ID93. Additionally these cells had an increased level of expression of the transcription factor Eomes, which has been linked to the development of cytolytic CD4 T cells (Figure 2E) (20, 21).
Figure 1. ID93+GLA-SE immunization produces cytolytic cells.
(A) ID93+GLA-SE (black line) and unimmunized (gray fill) mice received a 1:1 mix of ID93 pulsed (CFSEhi) and unpulsed (CFSElo) CD45.1 cells. 18 hours later CFSEhi cells were specifically reduced in the ID93+GLA-SE immunized recipients. (B) The frequency of in vivo killing of CFSEhi ID93-pulsed was determined for immunized animals relative to the unimmunized controls. (C) The frequency of granzyme A, granzyme B, and perforin was determined for naïve (CD44lo) and memory (CD44hi) CD8 T cells from unimmunized (black bars) and immunized (white bars) animals N=3–5 animals/group. Data are representative of eight experiments with similar results. Bars represent mean.
Figure 2. ID93+GLA-SE immunization elicits granzyme A expressing CD4 T cells.
(A) ID93-specific CD4 T cells were identified by tetramer staining after immunization. The frequency of (B) granzyme A, (C) granzyme B, and (D) perforin or (E) MFI of Eomes was determined for naïve (CD44lo), memory (CD44hi) or Rv3619 tetramer specific CD4 T cells from unimmunized (black bars) and immunized (white bars) animals. N=5 animals. Data are representative of three experiments with similar results. Bars represent mean + s.d.
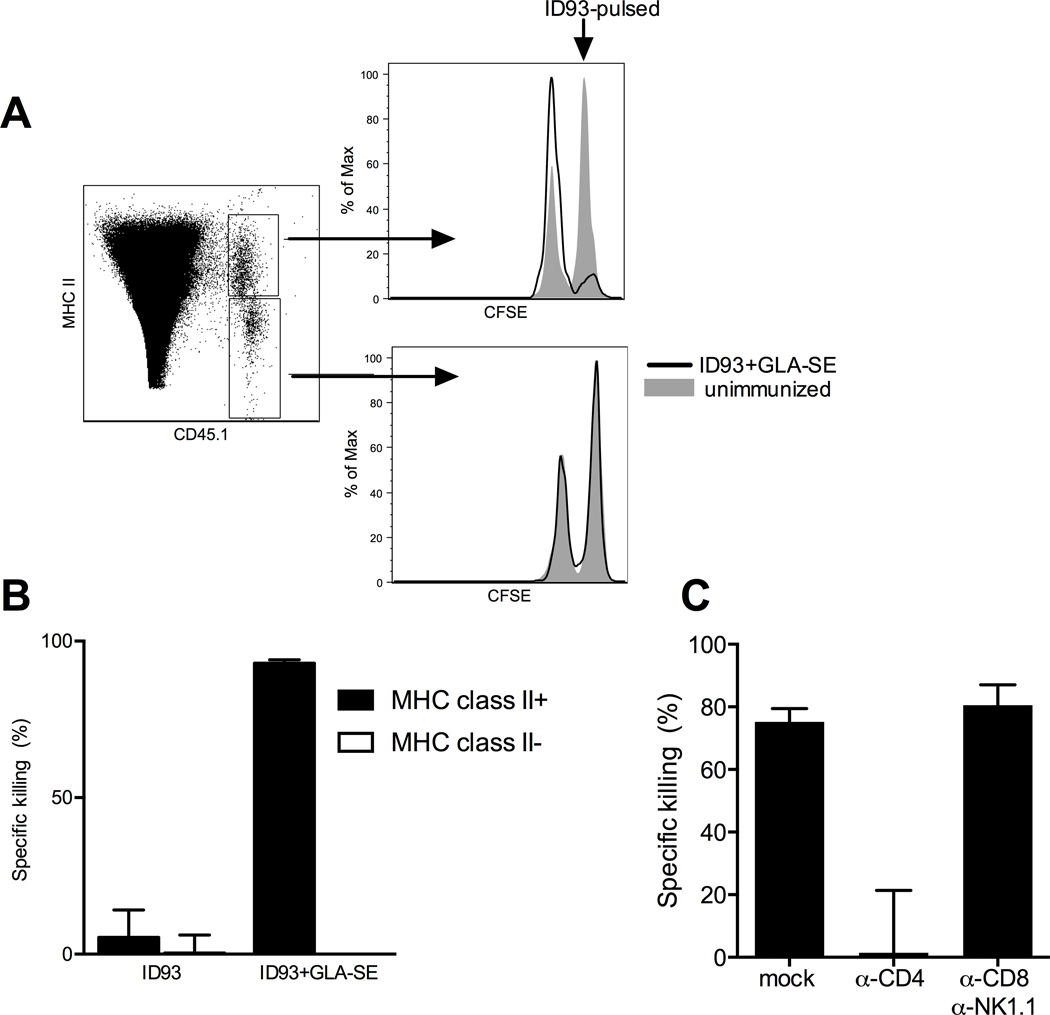
CD4 cells mediate MHCII-restricted cytotoxicity
To determine whether MHCII-restricted CD4 T cells were mediating the in vivo cytotoxicity we observed after immunization we assessed the killing of MHCII+ and MHCII− target cells pulsed with ID93. ID93 pulsed MHCII+ cells were almost completely ablated in vaccinated recipients whereas there was minimal killing of MHCII- ID93 pulsed cells (Figure 3A and B). Induction of these MHCII-restricted CTLs largely depended on the GLA-SE adjuvant, as immunization with ID93 alone elicited very little specific in vivo killing, in line with the paucity of ID93-specific CD4 T cells generated by immunization with ID93 alone (Figure 3B) (24). Similar results of adjuvant dependent induction of MHCII-restricted in vivo killing were obtained with a second fusion protein, F3, consisting of two Leishmania proteins, adjuvanted with GLA-SE (data not shown).
Figure 3. ID93+GLA-SE immunization produces cytolytic CD4 T cells.
(A) ID93+GLA-SE (black line) and unimmunized (gray fill) mice received a 1:1 mix of ID93 pulsed (CFSEhi) and unpulsed (CFSElo) cells. 18 hours later MHCII+ CFSEhi cells were specifically eliminated in the ID93+GLA-SE immunized recipients. (B) The frequency of in vivo killing of MHCII+ and MHCII− cells was determined for animals immunized with ID93 alone or ID93+GLA-SE. (C) Depletion of CD4 T cells, but not CD8 T cells and NK cells following vaccination with ID93+GLA-SE was sufficient to ablate the in vivo killing of ID93 pulsed MHCII+ cells. N=5 animals/group. Data are representative of three experiments with similar results. Bars represent mean + s.d.
To confirm that this was mediated by CD4 T cells and not canonical cytolytic cell populations such as CD8 T cells or NK cells we depleted CD4 T cells or CD8 T cells and NK cells after vaccination. Depletion of CD4 T cells completely abolished the in vivo killing of MHCII+ cells in vaccinated animals, whereas CD8 T cell and NK cell depletion had no effect (Figure 3C). Taken together we find that ID93+GLA-SE produces a novel population of CD4 T cells that expresses granzyme A and Eomes and kills ID93-pulsed cells in vivo in an MHC II restricted manner.
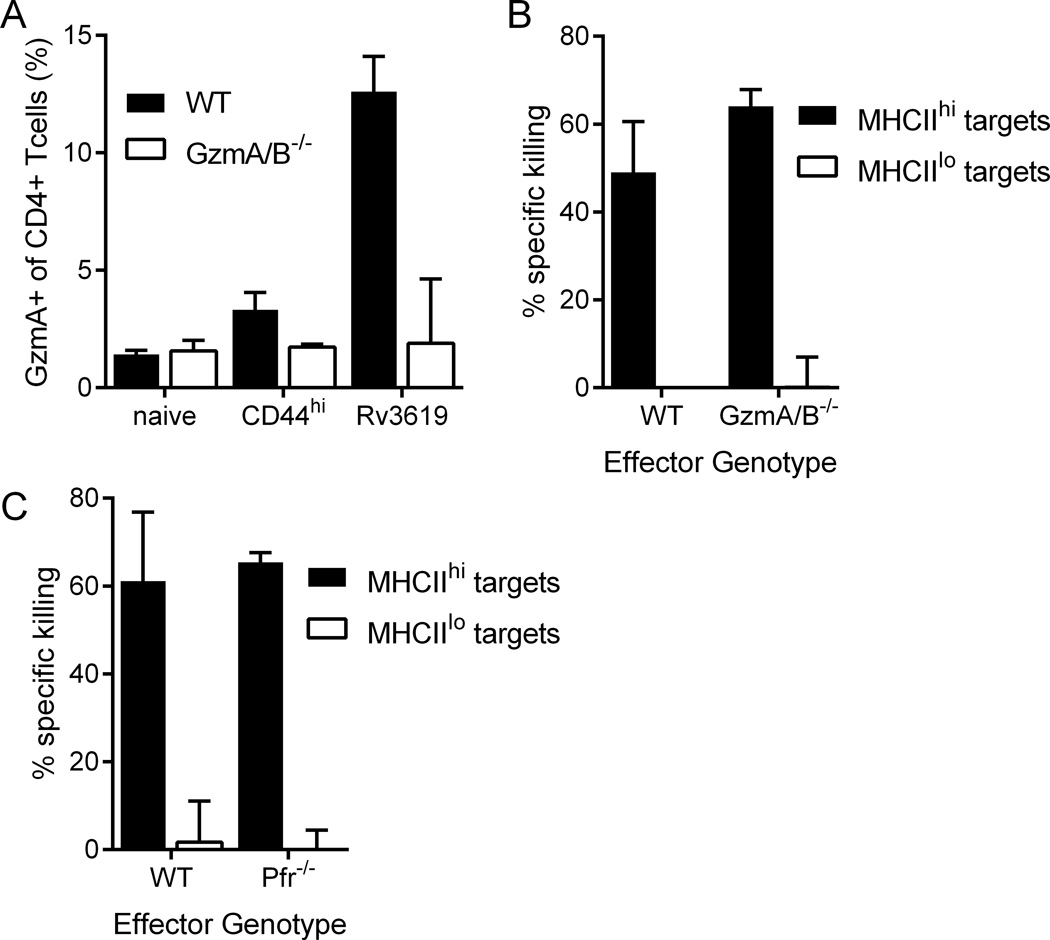
CD4 cytotoxicity is independent of granzyme, perforin, Eomes, FasL, TRAIL, and canonical death signaling pathways
The lack of perforin expression by ID93-specific CD4 T cells suggested that the in vivo cytolytic activity may not depend on the expression of granzyme A. To test this hypothesis we immunized WT and granzyme A/B deficient mice. Granzyme A staining in memory and ID93-specific CD4 T cells was abolished in the granzyme A/B deficient animals, confirming the specificity of the staining (Figure 4A). Despite the loss of granzyme A expression, the in vivo cytolytic capacity was not diminished in the immunized GzmA/B−/− mice (Figure 4B). Further the in vivo killing was not dependent on perforin expression as killing was not ablated in vaccinated Pfr−/− animals (Figure 4C). Thus the in vivo CD4 T cell mediated cytotoxicity does not depend on exocytosis of lytic granules containing granzymes or perforin.
Figure 4. In vivo cytotoxicity does not require granzyme A/B or perforin.
(A and B) WT and GzmA/B−/− mice were immunized with ID93+GLA-SE or left unimmunized. (A) The frequency of granzyme A expression by memory (CD44hi) and Rv3619 tetramer-specific CD4 T cells was increased in vaccinated WT but not GzmA/B−/− immunized animals. In vivo cytotoxicity of MHCII+ cells was not impaired by (B) granzyme A/B or (C) perforin deficiency relative to WT. N=5 animals/group. Data are representative of (A and B) two or (C) four experiments with similar results. Bars represent mean + s.d.
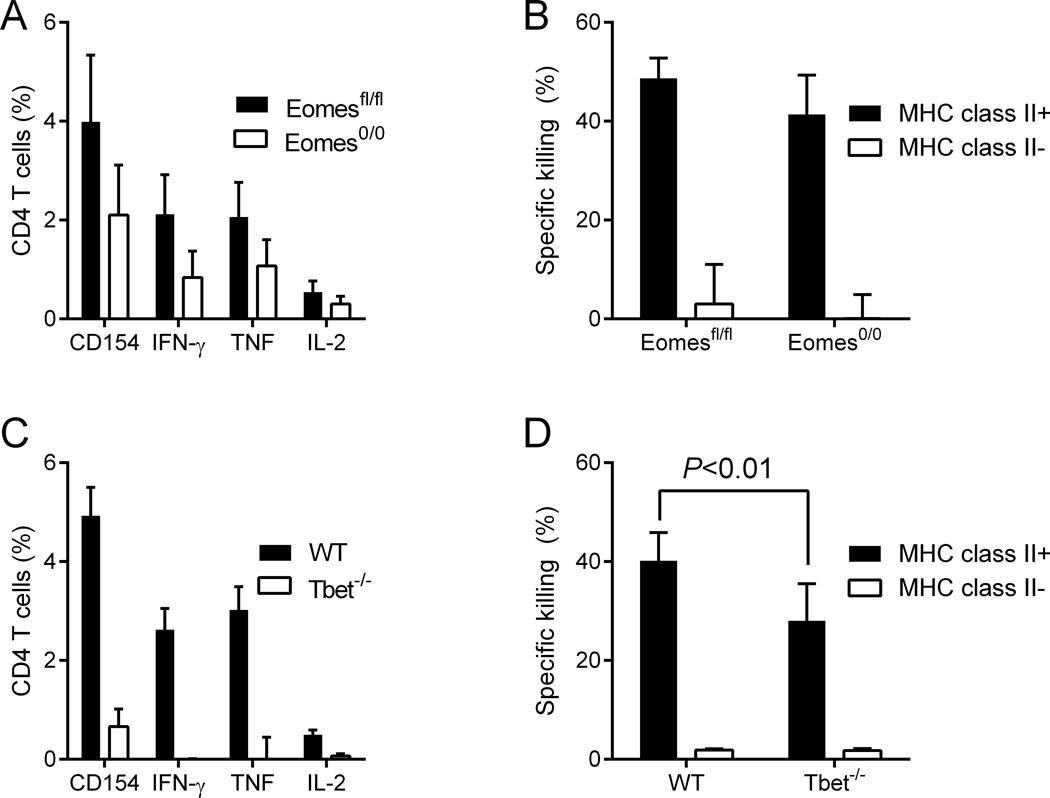
The Tbox transcription factor Eomes is critical for the development of cytolytic CD4 T cells by CD134 and CD137 stimulation (20) and is upregulated along with Tbet in ID93-specific CD4 T cells following vaccination with ID93+GLA-SE (24)and Figure 2E). As global Eomes deficiency is embryonic lethal, we bred Eomesfl/fl and CD4-Cre mice to generate either CD4-Cre− Eomesfl/fl (Eomes fl/fl) or CD4-Cre+ Eomesfl/fl (Eomes 0/0) which lack Eomes expression in all T cells (30). Eomes-deficiency reduced the frequency of ID93-specific TH1 cells by ~50% following vaccination (Figure 5A). To our surprise Eomes was not necessary for the killing of MHCII+ target cells in vivo (Figure 5B). To determine whether the residual TH1 cells were sufficient to mediate in vivo cytotoxicity we immunized WT and Tbet−/− mice. As expected Tbet was necessary for the generation of ID93-specific TH1 cells, however there was a modest frequency of ID93-specific cells remaining in the vaccinated Tbet−/− mice as determined by CD154 expression upon ID93 stimulation (Figure 5C). Tbet-deficiency resulted in a modest, but significant (P<0.01) reduction in specific lysis of MHCII+ cells in vivo (Figure 5D). However there was substantial residual in vivo cytotoxicity activity indicating that neither transcription factor was essential for the development of CD4 CTL following vaccination and that CD4 CTL activity did not depend on TH1 differentiation controlled by Tbet.
Figure 5. Development of cytolytic CD4 T cells by vaccination is independent of the Tbox transcription factors Eomes and Tbet.
(A) The frequency of ID93 specific TH1 cells and (B) in vivo cytotoxicity against MHCII+ ID93 pulsed target cells were determined in ID93-GLA-SE immunized Eomesfl/fl and Eomes0/0 mice. (C and D) WT and Tbet−/− mice were immunized with ID93+GLA-SE. (C) Only the WT animals developed ID93-specific TH1 cells, (D) but both populations killed MHCII+ ID93 pulsed target cells in vivo. N=5 animals/group . Data are representative of (A and B) four or (C and D) two experiments with similar results. Bars represent mean + s.d.
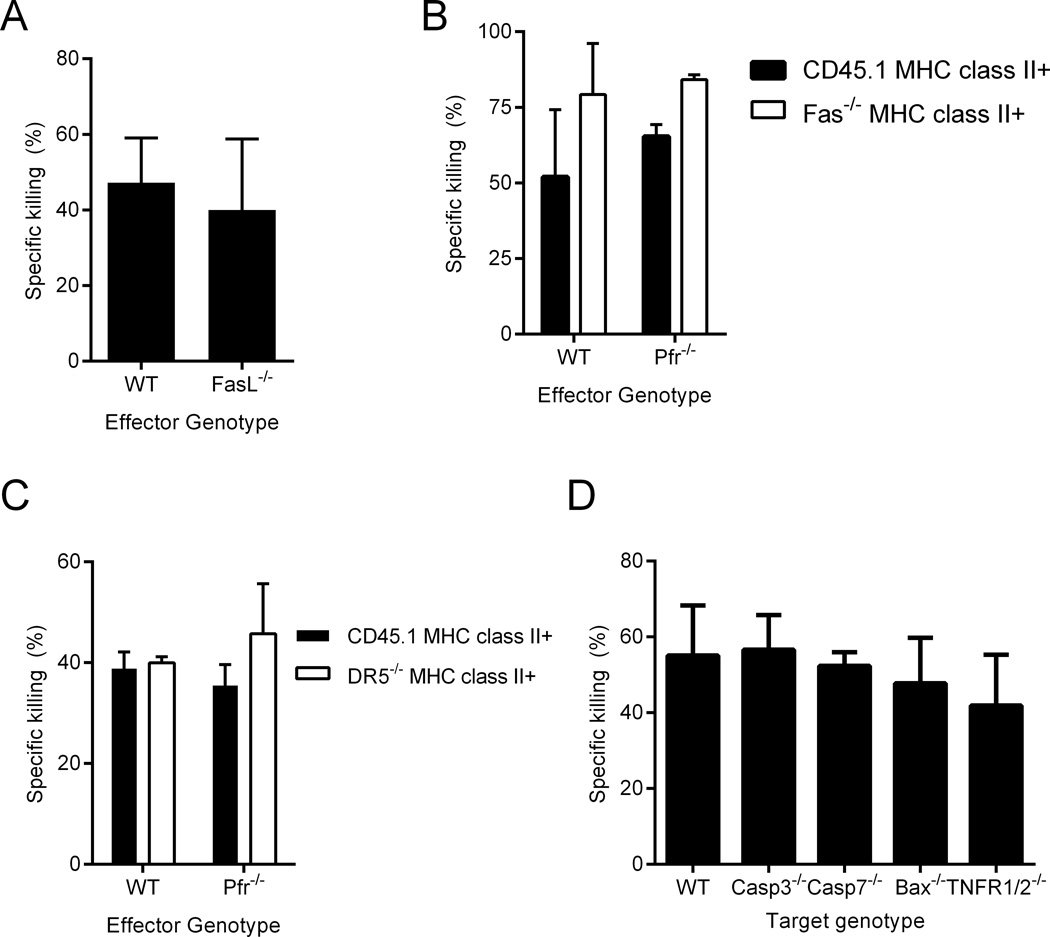
In addition to producing perforin and granzyme-containing lytic granules, CTLs can kill target cells via cell-cell interactions, primarily FasL on the CTL binding to Fas on the target cell. To test this possibility we immunized WT and FasL−/− mice with ID93+GLA-SE. One week after immunization WT and FasL−/− cohorts were able to kill MHCII+ target cells with similar efficiency (Figure 6A). To determine whether this killing was mediated by redundant lytic granule and Fas-FasL pathways we examined the killing of a mixture of CD45.1 and Fas−/− target cells in WT and perforin-deficient recipients. Immunized perforin-deficient recipients killed Fas−/− targets more efficiently than CD45.1 targets, indicating that perforin-dependent cytolytic granules and the Fas-FasL pathway are dispensable for CD4 CTL activity following vaccination (Figure 6B).
Figure 6. In vivo CD4 T cell cytotoxicity is independent of canonical cytolytic pathways.
(A) WT and FasL−/− immunized mice both kill MHCII+ CD45.1 cells. (B) WT and Pfr−/− immunized mice both kill CD45.1 and Fas−/− MHCII+ WT cells. (C) WT and Pfr−/− immunized mice kill CD45.1 and DR5−/− MHCII+ target cells. (D) WT immunized mice kill WT, Casp3−/−, Casp7−/−, Bax−/− and TNFR1/2−/− MHCII+ targets. N=5 animals/group. Data are representative of two to five experiments with similar results. Bars represent mean + s.d.
The TNF super receptor family member death receptor 5 (DR5) can also initiate cell death on target cells when engaged by its ligand TRAIL on cognate CTLs (31). To test this possibility we transferred a 1:1 mix of CD45.1 and DR5−/− target cells into immunized WT and Pfr−/− recipients. As with Fas-deficiency we found that neither DR5 nor perforin were necessary for in vivo cytotoxicity (Figure 6C). Based on these results none of the canonical CTL effector pathways are necessary for CD4 CTL activity after vaccination.
To determine whether these cells kill via one of the canonical cell death signaling pathways or a unique pathway we immunized a cohort of WT mice and transferred in either WT targets or targets lacking a canonical cell death signaling component, either caspase 3, caspase 7, Bax, or TNFR1/2 (1, 32). Surprisingly none of these signaling components were necessary for the killing of MHCII+ targets in vaccinated recipients (Figure 6D). These data suggested a novel killing pathway that was independent of the known effector and signaling pathways associated with CTL activity.
CD4 cytotoxicity depends on CD40 and CD154
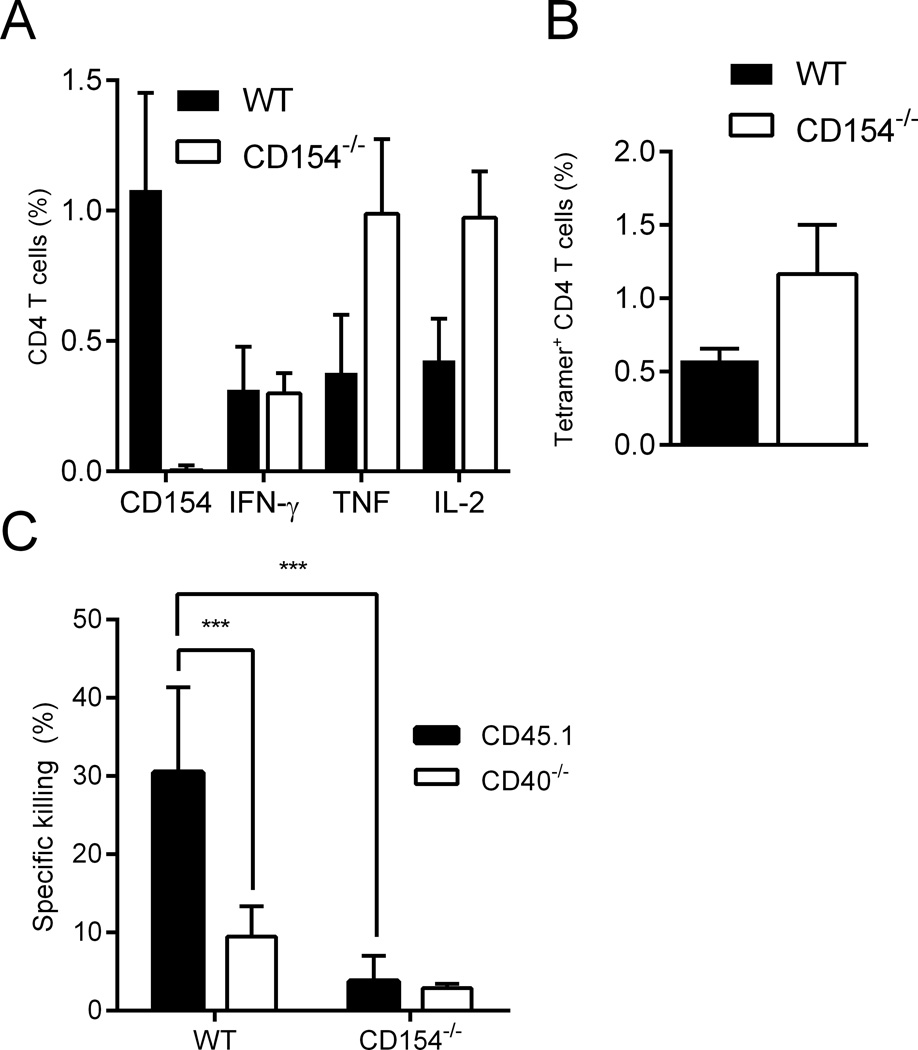
In the absence of Tbet there was a partial loss of in vivo cytotoxicity, along with a complete loss of TH1 cytokine production (Figure 5C and D). However even in these animals substantial CTL activity remained, along with some ID93-specific CD154 expression by CD4 T cells. B cells are the most prevalent MHCII-expressing splenocytes in our target cell population and they constitutively express CD40. To determine whether CD154 was necessary for in vivo killing of MHCII+ cells, we immunized WT and CD154−/− animals and transferred in a 1:1 mix of CD45.1 and CD40−/− target cells. Immunization of CD154−/− mice generated a greater frequency of ID93-specific CD4 T cells as determined by the frequency of CD4 T cells producing IFN-γ, TNF, or IL-2 upon ID93 stimulation or by I-Ab tetramer labeling (Figure 7A and B). Unlike the other knockouts tested CD154−/− mice failed to kill WT MHCII+ target cells. Similarly WT mice were unable to kill CD40−/− MHCII+ target cells (Figure 7C). Thus ID93+GLA-SE immunization produces CD4 CTLs that express CD154 that binds CD40 on MHCII+ cells presenting the cognate antigens resulting in death of the target cell.
Figure 7. In vivo CD4 T cell cytotoxicity depends on CD40-CD154 interactions.
Immunization with ID93+GLA-SE produced similar frequencies of ID93-specific CD4 T cells as determined by the frequency of (A) IFN-γ, TNF, or IL-2 producing cells upon ID93 stimulation or (B) staining with an I-Ab tetramer. (C) CD154−/− immunized mice do not develop cytolytic CD4 T cells upon vaccination. Similarly immunized WT mice develop cytolytic CD4 T cells that kill WT but not CD40−/− MHCII+ target cells. N=5 animals/group . Data are representative of four experiments with similar results. Bars represent mean + s.d
Discussion
The TLR4 agonist adjuvant GLA-SE augments a robust TH1 and antibody response when paired with recombinant protein vaccine antigens. (24, 26) We now find that this combination also induces CD4 T cells with the ability to kill cells bearing cognate MHCII:peptide complexes. Although CD4 CTLs have been noted for more than three decades, to our knowledge reports of vaccine-elicited CD4 CTLs have been rare. Unlike previous reports we find that this CD4 CTL activity does not rely on any of the canonical CTL mechanisms including perforin, granzymes, Fas-FasL, or TRAIL-DR5; rather the CD4 CTLs induce cell death by ligating CD40 on target cells via expression of CD154. Unlike other CD4 CTLs these cells do not require the expression of the transcription factor Eomes for their cytolytic activity.
CD4 T cells have traditionally been characterized by their ability to provide help to a number of different immune responses including augmenting B cell production of antibodies and formation of memory cells, modifying APCs to more efficiently prime CD8 T cells, and activating macrophages to resist intracellular infections. In addition direct cytolytic activity of CD4 T cells was noted in cell lines, but largely dismissed as an artifact of the long-term stimulation and culturing needed to detect the cytolytic activity (2). More recently CD4 CTLs specific for a number of viral infections have been isolated directly ex vivo. These include CD4 CTLs that recognize HIV, influenza, EBV, and many other pathogens (reviewed in (4, 33)). By adapting an in vivo killing assay, Jellison et al. demonstrated that CD4 CTLs arise during murine LCMV infection (5). Notably these CD4 CTLs are almost as efficient at killing target cells in vivo as the canonical CD8 CTLs, although the kinetics of killing is slower for CD4 CTLs (34). CD4 CTLs likely play an important role in controlling pathogens that infect macrophages and other APCs as well as other cell types that express MHC class II either constitutively or upon infection. In mice perforin expression by CD4 T cells is important for the control of both influenza and ectromelia virus (6, 8). In humans the frequency of malaria-specific CD4 T cells that degranulate upon stimulation correlates with protection against experimental malaria challenge, whereas the frequency of malaria-specific IFN-γ-producing CD4 or CD8 T cells do not correlate with protection, suggesting a role for CD4 CTLs in controlling malaria infection (35). In HIV the frequency of Gag-specific CD4 T cells that express granzyme A and other cytolytic markers correlates with suppression of viral load and prevention of disease progression in patients not undergoing anti-retroviral therapy (11). Although the granzyme A expression by CD4 T cells elicited by immunization that we observed following vaccination was not necessary for CD4 CTL activity in this study, it is intriguing to speculate about its potential benefits. Induction of granzyme A expressing CD4 T cells via the GLA-SE adjuvant may be a beneficial component of an efficacious vaccine.
Human and murine CD4 CTLs have been shown to kill via perforin or Fas-dependent pathways, or both. The usage of perforin or Fas depends on the identity of the pathogen driving CTL development as well as antigen abundance and concentrations of IL-2 (4, 16, 22, 33). However these pathways are not sufficient to explain the activity of some CD4 CTLs. Using an in vivo killing assay similar to the one we use here, Woodworth et al. found that M.tb.-specific CD4 CTLs arise during infection of mice (19). This activity was independent on perforin, Fas-FasL, and TNF, leading the authors to conclude there was a novel, but undefined mechanism of CD4 CTL. Similarly perforin- and Fas-independent CD4 CTLs have been observed in murine γ-herpesvirus 68 infection (36). Based on our observations here we speculate that these CD4 CTL activities may also depend on CD154 engagement of CD40 on target cells. Granzyme/perforin- and Fas-dependent cell death require the activation of executioner caspase pathways including caspase 3 and 7 or mitochondrial cytochrome c efflux mediated by Bad/Bax (1). We found that these pathways are dispensable for CD4 CTL activity in this system. CD40 signals through a variety of TRAF molecules to engage a number of downstream signaling pathways (reviewed in (37)). It is possible that one or more of these TRAF pathways may be engaged to drive target cell death in this system.
CD4 CTL activity has been associated with IFN-γ-producing CD4 T cells, suggesting that they are not a unique lineage, but rather a subset of TH1 cells. However recent work by Brown and Swain demonstrated that non-polarizing conditions (i.e. TH0) are more effective for producing CD4 CTLs than TH1 or TH2 polarizing conditions (22). Additionally expression of the T-box transcription factor Eomes is essential for the expression of granzyme B by CD4 T cells stimulated via CD134 and CD137 (20). Ectopic expression of Eomes was sufficient to convert non-cytolytic Th2 cells into CTLs, suggesting that Eomes-dependent CD4 CTLs represent a unique differentiation pathway (21). Type I interferons may also play a role in some CD4 CTL development by acting on the transcription factors Blimp-1 and Tbet (38). The CD154-dependent CD4 CTLs we report here do not require Tbet expression, strongly suggesting that they are not a subset of TH1 cells. To our surprise they also did not require Eomes expression, indicating that they develop via a unique differentiation mechanism. CD154 expression upon antigen stimulation has been proposed as an indicator of CD4 T cell specificity regardless of TH subset differentiation, thus CD4 CTL activity via this pathway may be common to all antigen-experienced CD4 T cells (39).
CD4 T cell expressed CD154 ligation of CD40 on B cells and APCs plays a central role in many adaptive immune responses. CD40 is constitutively expressed on B cells throughout development. CD40 signaling is essential for the development of T dependent antigen responses including germinal center formation, somatic hypermutation, isotype switching, memory B cell differentiation, and generation of plasma cells (reviewed in (37)). Defects in CD154-CD40 result in one of several types of Hyper-IgM syndrome (X linked in the cases of CD154 deficiency) marked by a lack of class switching and somatic hypermutation (40). CD40 engagement on APCs upregulates MHC and co-stimulatory molecules necessary for T cell priming, augments IL-12 secretion, and promotes cross presentation. CD40 engagement on both B cells and APCs can promote survival by upregulating the pro-survival genes Bcl-XL and c-FLIP (41–43). Considering this pro-survival effect of CD154-CD40 signaling we were surprised to find that CD154 and CD40 were essential for the in vivo CTL activity observed after vaccination. However CD40 has also been implicated in cell death under certain circumstances. For example CD40 signaling can increase expression of Fas on tumor cells, increasing susceptibility to FasL-mediated killing (44–46). Engagement of CD40 on tumor cells can inhibit proliferation via an undefined mechanism (47, 48). More directly human trimeric CD154 can induce apoptosis of CD40-expressing solid tumors (46). Finally the CD40 agonist antibody dacetuzumab is used to treat metastatic melanomas and B cell lymphomas by inducing apoptosis (49, 50). Thus the ability to induce CD154-dependent CD4 CTLs by vaccination with an adjuvanted protein may be of particular benefit for therapeutic cancer vaccines.
In summary we find that the TLR4 agonist adjuvant GLA-SE induces CD4 CTLs with the ability to kill autologous B cells that present a cognate MHCII:peptide complex. This activity is independent of all previously defined CTL mechanisms and instead depends on CD154 engagement of CD40 on target cells. This represents a newly defined function for this receptor-ligand pair and may be related to the observations of anti-tumor activity of CD40 engagement. Future efforts will be dedicated to defining the signaling mechanisms necessary for CTL activity and application of this to the development of effective CTL vaccines.
Acknowledgments
We thank Stephen Schoenberger for provision of DR5−/− splenocytes, Chris Fox for provision of GLA-SE and Chris Wilson for helpful discussions. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC class II tetramers.
Footnotes
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases National Institutes of Health, Department of Health and Human Services, under Award Number AI101488 to M.T.O. and Contract No. HHSN272200800045C to R.N.C.. The content of this publication is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health, nor policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984;308:365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- 3.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. Journal of biomedicine & biotechnology. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soghoian DZ, Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines. 2010;9:1453–1463. doi: 10.1586/erv.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 7.Brien JD, Uhrlaub JL, Nikolich-Zugich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J Immunol. 2008;181:8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang M, Siciliano NA, Hersperger AR, Roscoe F, Hu A, Ma X, Shamsedeen AR, Eisenlohr LC, Sigal LJ. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc Natl Acad Sci U S A. 2012;109:9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 10.Zheng N, Fujiwara M, Ueno T, Oka S, Takiguchi M. Strong ability of Nef-specific CD4+ cytotoxic T cells to suppress human immunodeficiency virus type 1 (HIV-1) replication in HIV-1-infected CD4+ T cells and macrophages. J Virol. 2009;83:7668–7677. doi: 10.1128/JVI.00513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soghoian DZ, Jessen H, Flanders M, Sierra-Davidson K, Cutler S, Pertel T, Ranasinghe S, Lindqvist M, Davis I, Lane K, Rychert J, Rosenberg ES, Piechocka-Trocha A, Brass AL, Brenchley JM, Walker BD, Streeck H. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003165. 123ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long HM, Leese AM, Chagoury OL, Connerty SR, Quarcoopome J, Quinn LL, Shannon-Lowe C, Rickinson AB. Cytotoxic CD4+ T cell responses to EBV contrast with CD8 responses in breadth of lytic cycle antigen choice and in lytic cycle recognition. J Immunol. 2011;187:92–101. doi: 10.4049/jimmunol.1100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikiforow S, Bottomly K, Miller G, Munz C. Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2003;77:12088–12104. doi: 10.1128/JVI.77.22.12088-12104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen EM, Remmerswaal EB, Heemskerk MH, ten Berge IJ, van Lier RA. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood. 2006;108:3121–3127. doi: 10.1182/blood-2006-03-006809. [DOI] [PubMed] [Google Scholar]
- 15.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167:2734–2742. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 16.Lewinsohn DM, Bement TT, Xu J, Lynch DH, Grabstein KH, Reed SG, Alderson MR. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–2379. [PubMed] [Google Scholar]
- 17.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–924. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 19.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, Adler AJ. CD134 Plus CD137 Dual Costimulation Induces Eomesodermin in CD4 T Cells To Program Cytotoxic Th1 Differentiation. J Immunol. 2011;187:3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshima K, Chiba S, Suzuki H, Kokubo K, Kobayashi H, Iizuka M, Iwabuchi K, Shinohara N. Ectopic expression of a T-box transcription factor, eomesodermin, renders CD4(+) Th cells cytotoxic by activating both perforin- and FasL-pathways. Immunology letters. 2012;144:7–15. doi: 10.1016/j.imlet.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257:69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A Dual TLR Agonist Adjuvant Enhances the Immunogenicity and Protective Efficacy of the Tuberculosis Vaccine Antigen ID93. PLoS One. 2014;9:e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, Vedvick TS, Duthie MS, Clegg CH, Van Hoeven N, Reed SG. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001094. 53ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin SL, Ching LK, Pine SO, Moutaftsi M, Lucas E, Vallur A, Orr MT, Bertholet S, Reed SG, Coler RN. Protection against tuberculosis with homologous or heterologous protein/vector vaccine approaches is not dependent on CD8+ T cells. J Immunol. 2013;191:2514–2525. doi: 10.4049/jimmunol.1301161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185:3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 31.Dorothee G, Vergnon I, Menez J, Echchakir H, Grunenwald D, Kubin M, Chouaib S, Mami-Chouaib F. Tumor-infiltrating CD4+ T lymphocytes express APO2 ligand (APO2L)/TRAIL upon specific stimulation with autologous lung carcinoma cells: role of IFN-alpha on APO2L/TRAIL expression and -mediated cytotoxicity. J Immunol. 2002;169:809–817. doi: 10.4049/jimmunol.169.2.809. [DOI] [PubMed] [Google Scholar]
- 32.Tite JP. Evidence of a role for TNF-alpha in cytolysis by CD4+, class II MHC-restricted cytotoxic T cells. Immunology. 1990;71:208–212. [PMC free article] [PubMed] [Google Scholar]
- 33.Brown DM. Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildemann SK, Eberlein J, Davenport B, Nguyen TT, Victorino F, Homann D. High efficiency of antiviral CD4(+) killer T cells. PLoS One. 2013;8:e60420. doi: 10.1371/journal.pone.0060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bijker EM, Teirlinck AC, Schats R, van Gemert GJ, van de Vegte-Bolmer M, van Lieshout L, IntHout J, Hermsen CC, Scholzen A, Visser LG, Sauerwein RW. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis. 2014;210:1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuller KA, Flano E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83:4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hua L, Yao S, Pham D, Jiang L, Wright J, Sawant D, Dent AL, Braciale TJ, Kaplan MH, Sun J. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J Virol. 2013;87:11884–11893. doi: 10.1128/JVI.01461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 40.Bhushan A, Covey LR. CD40:CD40L interactions in X-linked and non-X-linked hyper-IgM syndromes. Immunol Res. 2001;24:311–324. doi: 10.1385/IR:24:3:311. [DOI] [PubMed] [Google Scholar]
- 41.Hennino A, Berard M, Krammer PH, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J Exp Med. 2001;193:447–458. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuscano JM, Druey KM, Riva A, Pena J, Thompson CB, Kehrl JH. Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood. 1996;88:1359–1364. [PubMed] [Google Scholar]
- 43.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Molecular and cellular biology. 2000;20:5503–5515. doi: 10.1128/mcb.20.15.5503-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Seki N, Sayers TJ, Subleski J, Gruys EM, Murphy WJ, Wiltrout RH. Constitutive expression of functional CD40 on mouse renal cancer cells: induction of Fas and Fas-mediated killing by CD40L. Cell Immunol. 2005;235:145–152. doi: 10.1016/j.cellimm.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 46.Alexandroff AB, Jackson AM, Paterson T, Haley JL, Ross JA, Longo DL, Murphy WJ, James K, Taub DD. Role for CD40-CD40 ligand interactions in the immune response to solid tumours. Mol Immunol. 2000;37:515–526. doi: 10.1016/s0161-5890(00)00079-1. [DOI] [PubMed] [Google Scholar]
- 47.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, Stone MJ. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:691–703. [PubMed] [Google Scholar]
- 48.von Leoprechting A, van der Bruggen P, Pahl HL, Aruffo A, Simon JC. Stimulation of CD40 on immunogenic human malignant melanomas augments their cytotoxic T lymphocyte-mediated lysis and induces apoptosis. Cancer Res. 1999;59:1287–1294. [PubMed] [Google Scholar]
- 49.Lewis TS, McCormick RS, Stone IJ, Emmerton K, Mbow B, Miyamoto J, Drachman JG, Grewal IS, Law CL. Proapoptotic signaling activity of the anti-CD40 monoclonal antibody dacetuzumab circumvents multiple oncogenic transformation events and chemosensitizes NHL cells. Leukemia. 2011;25:1007–1016. doi: 10.1038/leu.2011.21. [DOI] [PubMed] [Google Scholar]
- 50.Lewis TS, McCormick RS, Emmerton K, Lau JT, Yu SF, McEarchern JA, Grewal IS, Law CL. Distinct apoptotic signaling characteristics of the anti-CD40 monoclonal antibody dacetuzumab and rituximab produce enhanced antitumor activity in non-Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4672–4681. doi: 10.1158/1078-0432.CCR-11-0479. [DOI] [PubMed] [Google Scholar]