Abstract
Glucose concentration changes in the nucleus tractus solitarius (NTS) affect visceral function and metabolism by influencing central vagal circuits, especially inhibitory, GABAergic NTS neurons. Acutely elevated glucose can alter NTS neuron activity, and prolonged hyperglycemia and hypoinsulemia in animal models of type 1 diabetes results in plasticity of neural responses in the NTS. NTS neurons contributing to metabolic regulation therefore act as central glucose sensors and are functionally altered in type 1 diabetes. Glucokinase (GCK) mediates cellular utilization of glucose, linking increased glucose concentration to excitability changes mediated by ATP-sensitive K+ channels (KATP). Using quantitative RT-PCR, Western blot, and in vitro electrophysiology, we tested the hypothesis that changes in GCK expression in the NTS accompanied development of diabetes symptoms in the streptozotocin (STZ)-treated mouse model of type 1 diabetes. After several days of hyperglycemia in STZ-treated mice, RNA expression of GCK, but not Kir6.2 or SUR1, was decreased versus controls in the dorsal vagal complex. Electrophysiological recordings in vitro indicated that neural responses to acute hyperglycemia, and synaptic responsiveness to blockade of GCK with glucosamine, were attenuated in GABAergic NTS neurons from STZ-treated mice, consistent with reduced molecular and functional expression of GCK in the vagal complex of hyperglycemic, STZ-treated mice. Altered autonomic responses to glucose in type 1 diabetes may therefore involve reduced functional GCK expression in the dorsal vagal complex.
Keywords: GABA neuron, hyperglycemia, KATP channel, nucleus tractus solitarius, postsynaptic current, vagus
INTRODUCTION
Diabetes mellitus, defined by unequivocally elevated blood glucose levels, affects over 29 million people in the United States (Centers for Disease Control and Prevention, 2014). Some of the serious complications of diabetes include heart disease, stroke, hypertension, blindness, nervous system damage, and gastrointestinal dysfunction. Treatments for the disease remain inadequate, despite substantial investment to reduce symptoms and complications of the disease. Multiple ‘preautonomic’ areas of the brain contribute to systemic glucose homeostasis (Zsombok and Smith, 2009, Kalsbeek et al., 2010, Yi et al., 2010) and are also affected by elevated blood glucose levels. In particular, neural circuits in the hindbrain play a critical role in regulating plasma glucose and insulin levels. More specifically, vagally-mediated parasympathetic output critically regulates visceral functions related to metabolic homeostasis, and abundant evidence indicates that the brainstem dorsal vagal complex plays a primary and critical role in glucose-sensitive modulation of plasma glucose and insulin levels, feeding, and energy balance (Ritter et al., 1981, Laughton and Powley, 1987, Ritter et al., 2000, Zsombok and Smith, 2009).
Neurons in the brainstem nucleus of the solitary tract (NTS) receive glutamatergic, primary vagal afferent synaptic input from the gut and other thoracic and abdominal viscera. Vagal afferents rapidly convey information about gastrointestinal distention and nutrient content to the NTS, where that information is processed, integrated with neuronal and humoral signals, and transmitted to other brain areas, including to vagal motor neurons of the dorsal motor nucleus of the vagus (DMV). Neurons in the NTS respond to acutely altered glucose concentration with either increases or decreases in neural excitability and altered synaptic input (Oomura et al., 1974, Balfour et al., 2006, Wan and Browning, 2008, Lamy et al., 2014, Boychuk et al., 2015a), which are glucokinase (GCK)-dependent. The depolarizing response is mediated by inactivation of ATP-sensitive K+ (KATP) channels (Balfour et al., 2006, Boychuk et al., 2015a) and KATP channel modulation prevents the glucose-induced, GABA mediated inhibition of vagal motor neurons (Ferreira et al., 2001). Type I diabetes is characterized by uncontrolled hyperglycemia due to reduced insulin secretion from pancreatic beta cells. Synaptic and other cellular responses in the dorsal vagal complex are altered in models of type 1 diabetes, even after normalizing glucose concentration (Zsombok et al., 2011, Browning, 2013, Blake and Smith, 2014, Bach et al., 2015, Boychuk et al., 2015b). Vagal reflexes are often blunted during chronic hyperglycemia, and altered vagal function may contribute to diabetes-associated visceral dysfunction (Saltzman and McCallum, 1983, Undeland et al., 1998), suggesting that chronically-elevated glucose alters responsiveness of neurons in the dorsal vagal complex.
Because of the involvement of GCK and KATP channel modulation in the neuronal response to glucose, and the altered responsiveness of NTS neurons in animal models of type 1 diabetes, we tested the hypothesis that GCK or KATP channel expression is altered after several days of chronic hyperglycemia/hypoinsulemia in the streptozotocin (STZ)-treated mouse. Understanding how glucose sensitivity in the dorsal brainstem is altered in diabetes may offer hypotheses to guide development of alternative therapies for the disease.
EXPERIMENTAL PROCEDURES
Animals
Mice were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Kentucky Animal Care and Use Committee (Animal Welfare Assurance Number A3336–01). Euthanasia was accomplished by anesthesia with isoflurane to effect (IsoThesia; Henry Schein, Melville, NY), followed by decapitation while anesthetized. Juvenile and young adult (28–42 days) male CD-1 (Harlan Laboratories, Indianapolis, IN) or transgenic ‘GIN’ mice (FVB-Tg (GadGFP) 4570Swn/J; The Jackson Laboratory, Bar Harbor, ME) were used for all experiments and housed under a standard 14-h light-10-h dark cycle, with food and water provided without restriction. The GIN mice express EGFP in a subset of GABA neurons in the NTS, which comprise a large proportion of NTS neurons (Oliva et al., 2000, Williams and Smith, 2006, Glatzer et al., 2007, Boychuk et al., 2015a).
Streptozotocin Injection
Intra-peritoneal injection of STZ (200mg/kg in 0.9% NaCl), which kills insulin-secreting pancreatic β cells, was used to induce chronic hyperglycemia in mice. Blood glucose concentration (non-fasted) was measured by tail puncture using a Nova Max PLUS glucometer from normal mice, which were then fasted for 4–6 h prior to STZ or vehicle injection. Control mice were either injected with saline (0.9% NaCl) or untreated. No differences in electrophysiological parameters were observed between normoglycemic saline-injected and untreated mice; they were therefore pooled and considered as a single control group. Blood glucose levels (non-fasted) were measured daily. Onset of hyperglycemia (i.e., blood glucose concentration above 300mg/dl) varied between animals, but occurred between 1 and 3 days post-STZ injection and remained elevated until the day of the experiment. Animals were used for electrophysiological recordings and molecular analyses after 3–4 days of continuous hyperglycemia.
Brain slice preparation
On-cell and whole-cell voltage-clamp recordings were made using brain stem slices prepared from male GIN mice, 4–5 weeks of age. Animals were deeply anesthetized by isoflurane inhalation (IsoThesia; Henry Schein) and decapitated while anesthetized. The brain was rapidly removed and blocked to isolate the brainstem and then glued to a sectioning stage. Transverse (coronal) brain stem slices (300 μm) containing the caudal NTS (i.e., ±600 μm rostral and caudal to area postrema) were made in ice cold, oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) using a vibrating microtome (Vibratome Series 1000; Technical Products, St. Louis, MO). The ACSF contained (in mM): 124 NaCl, 3 KCl, 2 CaCl2, 1.3 MgCl2, 1.4 NaH2PO4, 26 NaHCO3, and 11 or 2.5 glucose (pH 7.15–7.3); osmolality was adjusted to 290–310 mOsm/kg with sucrose; equimolar ACSF was made with by substitution sucrose for glucose substitution experiments. Slices were incubated for an equilibration period for ≥1 h in warmed (30–33°C), oxygenated ACSF prior to recording.
Electrophysiology
A single brain slice was transferred to a recording chamber mounted on a fixed stage under an upright microscope (BX51WI; Olympus, Melville, NY), where it was continually perfused by warmed (30–33°C), oxygenated ACSF. EGFP-labeled NTS neurons were targeted for recording under a 40x water-immersion objective with fluorescence and infrared-differential interference contrast (IR-DIC) optics, as previously described (Williams and Smith, 2006, Glatzer et al., 2007, Gao et al., 2009, Boychuk et al., 2015a). For recordings from EGFP-labeled NTS neurons, initial visualization was made briefly under epifluorescence using a fluorescein isothiocyanate (FITC) filter set (excitation filter wavelengths: 450–490 nm).
On-cell and whole-cell patch-clamp recordings were obtained in the NTS using pipettes pulled from borosilicate glass (Garner Glass, Claremont, CA; open tip resistance 4–6 MΩ) using a Sutter P-87 horizontal puller (Sutter Instrument Co., Novato, CA). Pipettes were filled with a solution containing (in mM): 130 K+-gluconate (or Cs+-gluconate), 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH (or CsOH), 2–4 ATP (pH 7.15 – 7.3). Electrophysiological signals were recorded using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 3 kHz, and recorded onto a PC-style computer (Digidata 1440A, Molecular Devices) using pClamp 10.2 software (Molecular Devices). A 2–4 GΩ seal was maintained for on-cell recordings. For whole-cell voltage-clamp recordings, series resistance was monitored throughout the recordings and data were used for analysis if the series resistance remained <25 MΩ and changed by ≤20% during the recording. Once a recording was obtained, cells were allowed to equilibrate for ~10 min before beginning data collection.
In whole-cell voltage-clamp recordings, resting potential was determined by monitoring the voltage at which no current was injected. Spontaneous EPSCs (sEPSC) were examined at a holding potential of −65 mV and IPSCs (sIPSCs) were examined at 0 mV using pipettes containing Cs-gluconate to block K+ currents, thereby improving voltage control and reducing noise. Miniature EPSCs and IPSCs (mEPSCs and mIPSCs) were recorded in the presence of tetrodotoxin (2 μM). The GCK inhibitor glucosamine (GA; 5μM; MP Biomedical, Santa Ana, CA) was applied with the ACSF for 10 min. For effects of elevated glucose concentration, slices were incubated in ACSF containing 2.5 mM glucose for at least 1 hr prior to on-cell recording. Spontaneous action potential activity was recorded for 10 min prior to changing to ACSF containing 15 mM glucose for 10 min before returning to 2.5 mM glucose solution (washout). Recordings of 2–3 min epochs were made prior to and after 10 min of GA- or 15 mM glucose-containing ACSF application. Washout was at least 15 min.
RNA isolation
Brainstem slices (300μm) from male CD-1 or GIN mice were isolated in cold ACSF, as described for electrophysiological recordings. The dorsal vagal complex, including most of both the DMV and NTS, was visualized under a dissecting microscope and excised from the rest of the brainstem with a 1 mm diameter biopsy punch (Miltex, Inc. York, PA). Resulting punched-tissue was then suspended in 500 μL of TRIzol (Sigma-Aldrich) and gently shaken periodically for 5min. Chloroform (100–250 μl) was added and tubes were vortexed for 15 s, maintained at 4°C for 20 min, and subsequently centrifuged at 12,000 rpm for 15 min at 4°C. The pellet was discarded and the supernatant containing RNA was transferred into fresh 1.5 ml centrifuge tubes, mixed with 500 μL of ice-cold propanol, incubated at room temperature for 10 min, and centrifuged at 12,000 rpm for 10 min at 4°C. Propanol was decanted and RNA was washed by re-suspension in 500 μL 75% ethanol followed by centrifugation at 7500–12,000 rpm for 10 min at 4°C. The wash step was repeated, the ethanol decanted, and RNA samples were air-dried for 10–20 min. RNA samples were re-suspended in 8–10 μL RNAse-free water and stored at −80°C or immediately reverse-transcribed into cDNA.
TaqMan QPCR
RNA samples were reverse transcribed in reverse-transcription master mix containing: 1 μl random nonamers (50 μM; Sigma-Aldrich), 5 μl MMLV RT buffer (5x) (Fisher Scientific, Pittsburgh, PA), 5 μl dNTPs (10 mM; Fisher), 2 μl DEPC-treated H2O (Fisher), 1 μl reverse transcriptase (Fisher), and RNAse inhibitor (1 μl; Fisher) in a thermocycler (Mastercycler, Eppendorf) at 42°C for 90 min followed by 5 min at 95°C. Negative controls included no template and RNAse-free sterile water instead of template. No RNA was detected in these controls. Primers and probes were: β-actin, Bactin, NM_007393 Actb; SUR1, Abcc8 NM_011510; and Kir6.2, Kcnj11, NM_001204411 (Integrated DNA Technologies, Coralville, IA) and GCK, NM_010292.4 (Sigma). Master mix included 10 ul of TaqMan Universal master mix II (Applied Biosystems, Life Technologies, Grand Island, NY), 1 ul of primer/probe mix and 9 μl Of cDNA+RNAse-free sterile H2O. Samples were loaded into a 96-well plate (Bio-Rad, Hercules, CA). Samples were centrifuged for 2 min at 1000 RPM and placed in an Applied Biosystems thermocylcer (ABI 7500) for PCR analysis. Samples were held at 95°C 2 min and cycled 50 times at 95°C for 30 s, 60°C for 15 s and at 72°C 15 s. Gene transcript level from each mouse was measured in triplicate; target transcript cycle threshold (CT) value was normalized to the CT value for β-actin for each sample; resulting difference CT (dCT) values were compared statistically. Results were also analyzed by the 2ddct method (Livak and Schmittgen, 2001). Differences of p<0.05 were considered significant.
Western Blots
Brainstem slices (300 μm) were cut as described above, the dorsal vagal complex was isolated from the slice, and tissue punches containing the DMV and NTS were immediately transferred to 40–60 μl of lysis buffer consisting of 0.15M NaCl, 5mM EDTA (pH 8), 1% Triton X-100, 10mM Tris-HCl (pH 7.4), 10μl/ml of 100mM PMSF (174.2 mg/10ml in methanol), and 100μl/ml of 0.5M NaF (pH 10). Each sample was sonicated and centrifuged immediately at 12,000 RPM for 3 min. Supernatant was aspirated, aliquoted, and stored at −80°C until further use. Protein concentration was measured using a Bradford Protein Assay and 20 μg of protein was loaded per lane for Western blot analysis. The appropriate volume of sample together with equal amounts of loading buffer was boiled in water for 2 min. Samples and ladder were loaded into precast SDS polyacrylamide gels and electrophoresed at 50 mA for 45–80 min. Proteins were then transferred at 200 mA for 2 hours onto polyvinylidene difluoride membranes for Western blot analysis. Membranes were blocked in 1:1 Odyssey (Li-Cor Biosciences, Lincoln, NE ) blocking buffer/TBS/0.1% Tween 20 for 1 hr at room temperature. Due to well-separated molecular weights of the GCK (band at 65 kD) and β-actin (band at 40–45 kD) protein membranes were cut in half and incubated overnight at 4°C, with a rabbit monoclonal anti-GCK (1:1000; Abcam, Cambridge, MA) and a rabbit monoclonal anti-β-actin (1:10000; Abcam) antibody in Odyssey blocking buffer/TBS/0.1% Tween 20. Membranes were washed 4 times (5 min) with TBS on a shaker and treated for 1 hr with fluorescence-conjugated anti-rabbit IgG (IRDye 680RD; Li-Cor Biosciences). Membranes were then washed (4 × 5 min) and scanned on a densitometer (Odyssey model 9120, Li-Cor Biosciences) to quantify band density. Background density was subtracted from the GCK band density and normalized to β-actin, which was used as a loading control.
Data analysis
Recordings were analyzed using Clampfit 10.2 (Molecular Devices) and MiniAnalysis 6.0.7 software (Synaptosoft, Decatur, GA). At least two minutes of continuous recording under each condition was used to assess mean action potential frequency or EPSC and IPSC frequency and amplitude. The Kolmogorov-Smirnov (K-S) intra-assay test was used to determine statistical significance of drug- or glucose-induced changes in the frequency of action potentials, EPSCs, or IPSCs within a recording. For all electrophysiological experiments, a two-tailed Student’s t-test was used to identify effects within neurons; a two-tailed Chi-square test was used to compare proportions of responding cells between STZ-treated and saline-treated mice (Graphpad Prism, San Diego, CA). For quantitative RT-PCR and Western blots, an unpaired Student t-test was used to detect differences between samples from each group. Values are presented as mean ± SEM; statistical significance for all measures was set at p<0.05.
RESULTS
Molecular expression of GCK and KATP channels
Punches of tissue containing the dorsal brainstem were collected from normoglycemic (n=8; glucose index 180 ± 4 mg/dl) and STZ-treated CD-1 mice that were hyperglycemic for 3–4 days (n=8; 469 ± 5 mg/dl). All target transcript measurements were normalized to β-actin expression. Quantitative RT-PCR revealed a significant decrease in GCK expression in the dorsal vagal complex from STZ-treated hyperglycemic CD1 mice versus controls (p<0.05; Fig. 1). GCK expression was similarly reduced in the vagal complex of GIN mice (n=5 control, n=7 STZ-treated; p<0.05). No significant expression differences were detected for Kir6.2 or SUR1 transcripts between normoglycemic and hyperglycemic CD-1 mice (p>0.05; Fig. 1). Molecular expression of GCK, but not components of the KATP channel, was reduced in STZ-treated, hyperglycemic mice relative to control mice.
Figure 1.
Expression of glucokinase (GCK) and ATP-sensitive K+ (KATP) channels in the dorsal vagal complex of normoglycemic control and hyperglycemic, streptozotocin (STZ)-treated mice. A. Molecular expression of GCK mRNA was significantly reduced in the dorsal vagal complex of STZ-treated mice after 3–4 days of chronic hyperglycemia versus control mice (p<0.05; n=8 for each group). Expression of Kir6.2 and SUR1, components of the KATP channel, were not significantly altered (p>0.05). B. Western blot analysis indicated that GCK protein expression was lower in the dorsal vagal complex of STZ-treated mice (n=6) than controls (p<0.05; n=8). Inset: Sample blot of β-actin and GCK protein expression from control and STZ-treated mice; center lane is protein ladder. Asterisks indicate p<0.05.
To determine if the decrease in mRNA transcription correlated with decreased protein expression, Western blots were performed on punches from an additional 8 control and 8 STZ-treated hyperglycemic CD-1 mice. Western blot analysis indicated that GCK protein expression was significantly reduced in the dorsal vagal complex of STZ-treated hyperglycemic mice (p<0.05; Fig. 1).
Effect of GCK inhibition on synaptic input to NTS neurons
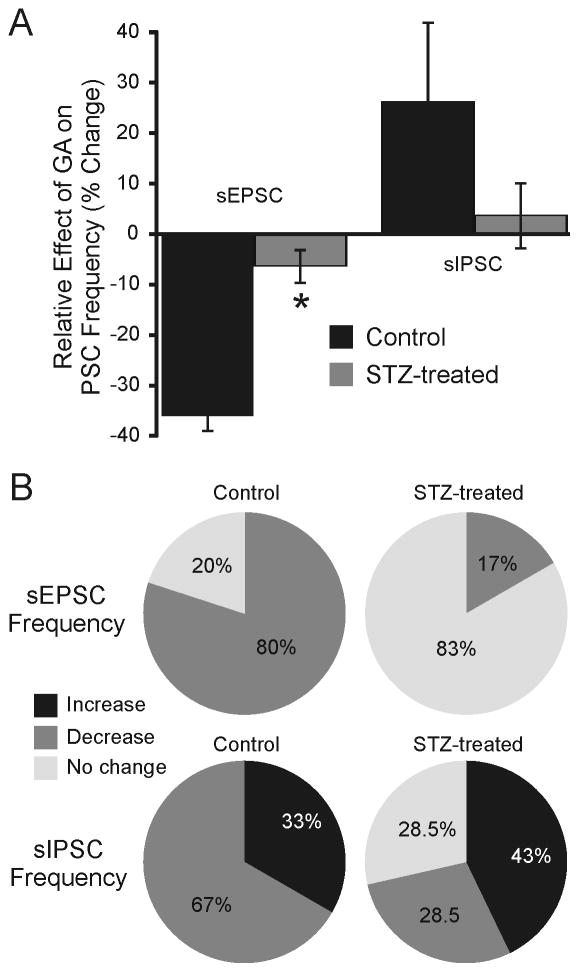
Glucokinase inhibition prevents responses to acute hypoglycemia in a subset of GABAergic NTS neurons (Lamy et al., 2014). Since GCK expression was reduced in the NTS of STZ-treated mice after several days of hyperglycemia, responses to the GCK inhibitor, GA (5 μM) were determined in GABAergic medial NTS (mNTS) neurons, identified by expression of EGFP in acute slices from normoglycemic control (n=5) and STZ-treated GIN mice (n=5). In GABAergic mNTS neurons from normal mice, GA application decreased the frequency of sEPSCs by ≥20% in 80% of neurons (12 of 15 neurons; 2.83 ± 0.34 Hz control ACSF; 1.72 ± 0.22 Hz in GA; n=15; p<0.05; Fig. 2), with no change in sEPSC frequency in the remaining three neurons. No effect on synaptic current amplitude was detected (p>0.05). In the presence of TTX (2 μM) to prevent action potential firing, there was no significant effect of GA on miniature EPSC (mEPSC) frequency in control mice (p>0.05); mEPSC frequency was decreased slightly in only one of five neurons from three mice, so effects in STZ-treated mice were not assessed. These findings are consistent with an effect of GCK in mediating spontaneous excitatory synaptic responses to ambient glucose levels in NTS neurons (Wan and Browning, 2008) and indicated that GCK blockade inhibited excitatory, glutamatergic synaptic input to most GABA neurons.
Figure 2.
Modulation of synaptic responses in NTS neurons by glucosamine (GA). A. Graph showing the relative overall effect of GA on sEPSCs and sIPSCs in GABAergic mNTS neurons from control and STZ-treated mice (n=6–15). sEPSC frequency was suppressed in the presence of GA in 80% of neurons from control mice, but GA was generally without effect on sEPSC frequency in most neurons (83%) from STZ-treated mice. Asterisk indicates significant difference from control group for sEPSC frequency. Effects of GA on sIPSCs were variable in both control and STZ-treated mice. B. Pie graphs indicating the percentage of neurons in which GA application evoked a ≥20% change in frequency of either sEPSCs or sIPSCs in mice from each treatment group.
In GABAergic mNTS neurons from STZ-treated mice after 3–5 days of hyperglycemia (n=5), effects of GA on sEPSC frequency were significantly less robust than in controls (p<0.05; Chi-square), being reduced (−22%) in only one of six neurons and unchanged in the remaining five cells (Fig. 2). Overall, mean sEPSC frequency was unchanged in the presence of GA (7.63 ± 1.04 Hz in control ACSF; 7.04 ± 0.83 Hz in GA; n=6; p>0.05). Consistent with the decreased GCK expression in the vagal complex, modulation of excitatory synaptic activity by GA was reduced in GABAergic mNTS neurons from STZ-treated, hyperglycemic mice.
Effects of GA application on sIPSC frequency and amplitude were also determined. In GABAergic mNTS neurons from control mice (n=5), GA application was without effect on the overall population (1.22 ± 0.2 Hz control, ACSF; 1.25 ± 0.30 Hz, GA; n=9; p>0.05), but was either increased (n=3) or decreased (n=6) in individual neurons (Fig. 2). The amplitude of sIPSCs was unchanged by GA (p>0.05). Similar to results in control mice, there was no overall effect on sIPSC frequency in neurons from STZ-treated mice (1.04 ± 0.30 Hz control ACSF; 1.01 ± 0.31 Hz GA; n=7; p>0.05). sIPSC amplitude was also unchanged (p>0.05). In neurons from STZ-treated mice, GA application either increased (n=3), decreased (n=2) or was without effect (n=2) on sIPSC frequency (Fig. 2). There was no significant effect of GA on mIPSC frequency in control mice; mIPSC frequency was increased slightly in only two of five neurons from four mice, so effects in STZ-treated mice were not assessed. Although sIPSC frequency in GABAergic mNTS neurons was usually altered by GA application, robust differences between responses in neurons from control and STZ-treated mice were not observed (p>0.05; Chi-square).
Glucose effect on Action Potentials
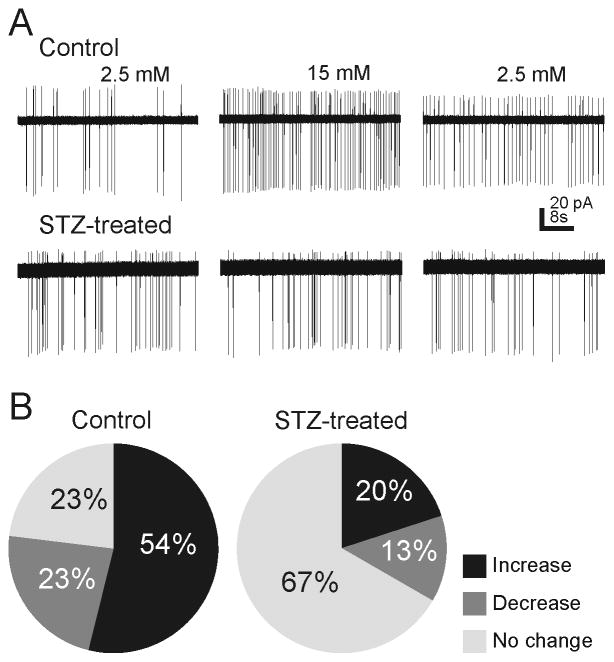
Neurons in the NTS are glucose sensors, and this sensitivity may be especially prominent in GABAergic NTS neurons (Ferreira et al., 2001, Lamy et al., 2014, Boychuk et al., 2015a). Since GCK expression was reduced in the NTS of STZ-treated mice after several days of hyperglycemia, we examined action potential frequency of GABAergic NTS neurons in response to elevating glucose from 2.5 to 15 mM using on-cell recordings. Increasing glucose concentration resulted in a >20% change in action potential frequency in 77% of neurons (10 of 13 cells) in neurons from normoglycemic control mice (n=6). Increasing glucose concentration resulted in an increase in action potential frequency in seven of 13 neurons (122 ± 57% increase; Fig. 3), a decrease in three neurons (−27 ± 2% decrease), and no change in three neurons. In neurons from STZ-treated, hyperglycemic mice (n=6), elevating glucose resulted in a change in action potential frequency in only five of 15 neurons (33%), a significantly lower percentage of responses than in neurons from control mice (p<0.05, two-tailed Chi-square). The frequency of action potentials was increased in three neurons (111 ± 22%), decreased in two cells (−55 ± 10%), and was unaffected in the remaining 10 cells. Whereas increasing glucose concentration resulted in a significant and large change in action potential frequency in the majority of neurons in normoglycemic mice, action potential frequency in most neurons from hyperglycemic mice was unaffected by increasing glucose concentration.
Figure 3.
Effects of elevated glucose on sodium currents (action potentials) in NTS neurons from control and STZ-treated mice. A. Examples of on-cell recordings in GABAergic mNTS neurons from control (upper) and STZ-treated (lower) mice showing spontaneous action potential (sodium current) activity in ACSF containing 2.5 mM glucose, 15 mM glucose, and after 15 min wash to 2.5 mM glucose. B. Pie graphs indicating the proportion of neurons (n=13 control; n=15 STZ-treated mice) that responded with an increase, decrease, or no change in action potentials when glucose concentration was elevated. Glucose elevation most often increased action potential firing in neurons from control mice, whereas firing rate was unchanged in most neurons from STZ-treated mice.
DISCUSSION
Neurons in the NTS receive direct input from the primary viscerosensory vagal afferents and comprise the initial response component of central parasympathetic regulatory circuits. Subsets of these neurons, and GABA neurons in particular, are known to be glucose-sensitive (Oomura et al., 1974, Balfour et al., 2006, Wan and Browning, 2008, Browning, 2013, Lamy et al., 2014, Boychuk et al., 2015a). Several physiological aspects of central vagal circuitry are altered functionally in diabetes (Zsombok et al., 2011, Browning, 2013, Blake and Smith, 2014, Bach et al., 2015, Boychuk et al., 2015b), consistent with modified parasympathetic regulation of the viscera concurrent with the disease (Saltzman and McCallum, 1983). Glucose sensing in NTS neurons involves GCK, which catalyzes the conversion of glucose to glucose-6 phosphate in neurons and other cells (Balfour et al., 2006, Briski et al., 2009), resulting in increased ATP/ADP ratio. In several neural systems, membrane responses after increased glucose concentration are caused by ATP binding to KATP channels to affect changes in membrane potential. Diabetes induces changes in GCK or KATP channel expression in specific hypothalamic nuclei (Levin and Dunn-Meynell, 1998, Nishio et al., 2006), which are consistent with altered or compensatory neuronal responses to chronically-elevated glucose or hypoinsulinemia in STZ-treated rats. Altered electrophysiological responsiveness of NTS neurons in type 1 diabetes has been demonstrated (Browning, 2013, Bach et al., 2015), which could contribute to visceral dysregulation in diabetes, but the mechanisms of this plasticity are unknown. Here, we found that molecular and functional expression of GCK—but not components of the KATP channel—were diminished in the vagal complex of mice with type 1 diabetes. Consistent with decreased mRNA transcription, GCK protein levels were reduced, neuronal and synaptic responses to GCK blockade were attenuated, and neuronal activity responses to increased glucose concentration were diminished. A similar decrease in GCK expression and activity has been reported in the arcuate nucleus, but not in other glucose-responsive hypothalamic nuclei, of STZ-treated rats (Nishio et al., 2006). The present findings suggest that responses of NTS neurons to increased glucose concentration may be altered as a consequence of chronic hyperglycemia or hypoinsulinemia in a GCK-dependent manner, similar to glucose-sensitive neurons of the arcuate nucleus.
Neurons in the NTS normally respond to elevated or reduced glucose concentration with either increases or decreases in excitability (Oomura et al., 1974, Balfour et al., 2006, Wan and Browning, 2008, Browning, 2013, Lamy et al., 2014, Boychuk et al., 2015a), which are often GCK-dependent. Glucose-induced increases in excitation are mediated by inactivation of KATP channels in NTS neurons (Balfour et al., 2006, Boychuk et al., 2015a) and KATP channel modulation prevents the glucose-induced, GABA mediated inhibition of vagal motor neurons (Ferreira et al., 2001). We tested the hypothesis that expression of molecular components of the KATP channel was altered after several days of hyperglycemia. KATP channels in central neurons are mainly composed of SUR1 and Kir6.2 subunits and SUR1 is expressed by glucose-sensing NTS cells (Balfour et al., 2006). In STZ-treated rats, SUR binding is increased in neurons of the dorsomedial, ventromedial, and lateral—but not paraventricular—hypothalamic nuclei after seven days of hyperglycemia (Levin and Dunn-Meynell, 1998), suggesting increased KATP channel expression that may serve a compensatory function in specific neurons. We found that molecular expression of SUR1 and Kir6.2 was unchanged in the vagal complex of mice with type 1 diabetes, which is inconsistent with the hypothesis that SUR1 activity increases in the NTS after several days of chronic hyperglycemia.
Since KATP channel-mediated responses to glucose in the NTS require GCK, the decrease in GCK expression suggests a mechanism for blunted responsiveness to glucose that has been proposed to occur in chronically hyperglycemic mice (Browning, 2013). The decreased molecular and protein expression of GCK we observed was consistent with an attenuation of the electrophysiological response to GCK inhibition. Blockade of GCK activity resulted in altered synaptic excitability of most GABAergic NTS neurons from normoglycemic mice, and this effect was mainly action potential-dependent, consistent with tonic GCK-mediated effects on neuronal activity in the slice. Notably, it is conceivable that blockade of GCK activity might deplete energy supplies. This potential confound is mitigated, however, by the fact that application was limited to 10 min in this study. Further, postsynaptic current amplitude was not altered by GA, suggesting that neural responsiveness was not overtly affected. The effects of blocking GCK were reduced in neurons from mice with type 1 diabetes, especially on action potential-dependent glutamate release, suggesting that GABAergic NTS neurons may be less responsive to excitatory synaptic input in mice with type 1 diabetes. Moreover, responsiveness of GABAergic NTS neurons to increased glucose concentration was attenuated in STZ-treated, hyperglycemic mice. This further implies that synaptic activity in the NTS normally occurs in the context of glucose concentration, since GCK mediates glucose-responsiveness in these neurons.
Previous studies indicated that responses of NTS neurons to acute hypoglycemia required GCK activity (Balfour et al., 2006, Lamy et al., 2014). Responses to transient hypoglycemia were previously reported in NTS neurons that expressed glucose transporter 2 (GLUT2) and the expression of the transporter was colocalized in a subset of GABA neurons (Lamy et al., 2014). We recently showed that GABA neurons were either depolarized or hyperpolarized by glucose, and the depolarization in particular was sensitive to blockade of GCK activity or by blocking KATP channels (Boychuk et al., 2015a). Here, we found that attenuated neuronal responses to transient hyperglycemia in mice with type 1 diabetes coincided with reduced molecular, protein, and functional GCK expression in the vagal complex, suggesting that prolonged hyperglycemia affects glucose responsiveness in the vagal complex.
Elevated glucose concentration increases glutamatergic synaptic transmission from vagal afferents in rats and mice, and these effects were reported to be attenuated in a model of type 1 diabetes (Browning, 2013). Our results are consistent with this report, and offer a mechanistic explanation for the loss of response. The glucoregulatory response to nutritive substances applied in the intestine is prevented by blocking ionotropic glutamate receptors in the NTS (Wang et al., 2008, Cheung et al., 2009), suggesting that glutamatergic, vagal afferent activation of NTS neurons is required for this response. Blockade of NMDA receptors in the vagal complex prevents the positive effect of bariatric surgery on systemic blood glucose levels, an effect hypothesized to occur by inhibiting a “gut-brainstem-liver” circuit in the vagal complex (Verberne et al., 2014).
Our results support the hypothesis that diminished GCK expression in the vagal complex of mice with type 1 diabetes results in reduced responsiveness of NTS neurons to glucose, including the response to synaptic glutamate release. It is likely that glutamatergic, visceral afferent synaptic input to the NTS, including input mediating mechano- and chemoreceptor activity in the gut (Barber and Burks, 1987), occurs in the context of glucose concentration. Restoring GCK expression may help restore physiological responsiveness of NTS neurons to glucose, thereby helping to normalize parasympathetic regulation of visceral function, including glucose homeostasis, in diabetes.
Highlights.
In STZ-treated, hyperglycemic mice:
Expression of glucokinase (GCK) in the dorsal vagal complex was significantly reduced, relative to control mice;
Molecular expression of KATP channel components was unchanged;
Synaptic responses of NTS neurons to GCK inhibition were significantly altered;
Effects of increased [glucose] on action potential firing was attenuated.
Reduced GCK dependent, glucose-mediated responses in the NTS likely contribute to autonomic dysregulation in diabetes.
Acknowledgments
Supported by NIH grants R01 DK056132 and R21 HD079256.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach EC, Halmos KC, Smith BN. Enhanced NMDA receptor-mediated modulation of excitatory neurotransmission in the dorsal vagal complex of streptozotocin-treated, chronically hyperglycemic mice. PloS one. 2015;10:e0121022. doi: 10.1371/journal.pone.0121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. The Journal of physiology. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber WD, Burks TF. The response of brainstem neurons to chemical activation of gastric sensory receptors. Gastroenterology clinics of North America. 1987;16:521–524. [PubMed] [Google Scholar]
- Blake CB, Smith BN. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307:R711–720. doi: 10.1152/ajpregu.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarius. Journal of neurophysiology jn 00310 02015. 2015a doi: 10.1152/jn.00310.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Halmos K, Smith BN. Diabetes induces GABA receptor plasticity in murine vagal motor neurons. Journal of neurophysiology jn 00209 02015. 2015b doi: 10.1152/jn.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski KP, Cherian AK, Genabai NK, Vavaiya KV. In situ coexpression of glucose and monocarboxylate transporter mRNAs in metabolic-sensitive caudal dorsal vagal complex catecholaminergic neurons: transcriptional reactivity to insulin-induced hypoglycemia and caudal hindbrain glucose or lactate repletion during insulin-induced hypoglycemia. Neuroscience. 2009;164:1152–1160. doi: 10.1016/j.neuroscience.2009.08.074. [DOI] [PubMed] [Google Scholar]
- Browning KN. Modulation of gastrointestinal vagal neurocircuits by hyperglycemia. Front Neurosci. 2013;7:217. doi: 10.3389/fnins.2013.00217. eCollection 02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention A, GA. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. US Department of Health and Human Services; 2014. [Google Scholar]
- Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. The Journal of physiology. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 2009;1291:40–52. doi: 10.1016/j.brainres.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. Journal of neurophysiology. 2007;98:1591–1599. doi: 10.1152/jn.00336.2007. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci. 2010;1212:114–129. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- Lamy CM, Sanno H, Labouebe G, Picard A, Magnan C, Chatton JY, Thorens B. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab. 2014;19:527–538. doi: 10.1016/j.cmet.2014.02.003. doi:510.1016/j.cmet.2014.1002.1003. [DOI] [PubMed] [Google Scholar]
- Laughton WB, Powley TL. Localization of efferent function in the dorsal motor nucleus of the vagus. Am J Physiol. 1987;252:R13–R25. doi: 10.1152/ajpregu.1987.252.1.R13. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Effect of streptozotocin-induced diabetes on rat brain sulfonylurea binding sites. Brain research bulletin. 1998;46:513–518. doi: 10.1016/s0361-9230(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nishio T, Toyoda Y, Hiramatsu M, Chiba T, Miwa I. Decline in glucokinase activity in the arcuate nucleus of streptozotocin-induced diabetic rats. Biological & pharmaceutical bulletin. 2006;29:216–219. doi: 10.1248/bpb.29.216. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- Saltzman MB, McCallum RW. Diabetes and the stomach. The Yale journal of biology and medicine. 1983;56:179–187. [PMC free article] [PubMed] [Google Scholar]
- Undeland KA, Hausken T, Gilja OH, Aanderud S, Berstad A. Gastric meal accommodation studied by ultrasound in diabetes. Relation to vagal tone. Scandinavian journal of gastroenterology. 1998;33:236–241. doi: 10.1080/00365529850170784. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Sabetghadam A, Korim WS. Neural pathways that control the glucose counterregulatory response. Front Neurosci. 2014;8:38. doi: 10.3389/fnins.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Browning KN. D-glucose modulates synaptic transmission from the central terminals of vagal afferent fibers. Am J Physiol Gastrointest Liver Physiol. 2008;294:G757–763. doi: 10.1152/ajpgi.00576.2007. [DOI] [PubMed] [Google Scholar]
- Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. The Journal of physiology. 2006;573:395–412. doi: 10.1113/jphysiol.2006.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, La Fleur SE, Flier JS, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802:416–431. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14024–14031. doi: 10.1523/JNEUROSCI.2081-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta. 2009;1792:423–431. doi: 10.1016/j.bbadis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]