Abstract
Background
Quality of oncologic outcomes is of paramount importance in the care of patients with non-small cell lung cancer (NSCLC). We sought to evaluate the relationship of hospital volume for lobectomy on cancer-specific survival in NSCLC patients treated in California, as well as the influence of Committee on Cancer (CoC) accreditation.
Methods
The California Cancer Registry was queried from 2004–2011 for cases of Stage I NSCLC and 8,345 patients were identified. Statistical analysis was used to determine prognostic factors for cancer-specific survival.
Results
7,587 patients were treated surgically. CoC accreditation was not significant for cancer-specific survival, but treatment in high volume centers was associated with longer survival when compared to low and medium volume centers (HR 1.77, 1.474–2.141 and HR 1.23, 1.058–1.438).
Conclusions
These data suggest that surgical treatment in high volume hospitals is associated with longer cancer-specific survival for early-stage NSCLC, but that CoC accreditation is not.
Keywords: Non-small cell lung cancer, Cancer specific survival, Lobectomy, Sublobar resection, Commission on Cancer accreditation, Thoracic Surgery
Lobectomy with mediastinal lymphadenectomy is the standard of care for the treatment of early-stage non-small cell lung cancer (NSCLC). Although previous studies show a relationship between hospital procedural volume and perioperative outcomes, we sought to evaluate the relationship of hospital volume on cancer-specific survival in early-stage NSCLC patients treated in California, as well as the influence of facility American College of Surgeons Commission on Cancer (CoC) accreditation on long-term outcomes.
Background
In the modern era, where perioperative outcomes are scrutinized and publicized no one single measure of quality exists. Many studies have evaluated the relationship between hospital procedural volume and perioperative outcomes and have attempted to use hospital volume as a surrogate for quality [1–9]. In the United States and England, there is a trend of improved perioperative complications and mortality in high volume centers [1–6]. European studies, in contrast, find that surgeon volume rather than hospital volume contributes to perioperative morbidity and that hospital volume should not be considered a substitute for quality outcomes [7–9]. Cancer-specific survival is rarely considered in these studies; however cancer-specific survival is a key metric for all concerned with providing quality oncologic care.
Like hospital procedural volume, CoC accreditation is a measureable hospital characteristic; however unlike volume, the relationship between CoC accreditation and patient outcomes has not been well studied. The CoC is a conglomerate of over 50 organizations that focus on improving oncologic outcomes and quality of life for cancer patients [10–13]. The CoC provides standards, prevention, research, education and quality monitoring and accreditation is based on compliance and adherence to these guidelines[10]. CoC accredited hospitals are more likely to have oncology-related services including screening programs, chemotherapy, radiation, survivorship and hospice services and they are required to report to the National Cancer Data Base [11]. CoC accreditation is one method patients can use to assess healthcare quality. However, the association between performance on national quality indicators and CoC accreditation has not been firmly established [11–13].
In order to provide patients with additional information from which to make informed healthcare decisions regarding the quality of oncologic outcomes in NSCLC, we analyzed data from the California Cancer Registry (CCR). We hypothesized that increased cancer-specific survival would be seen in high volume centers and centers with CoC accreditation.
Methods
This was a University of California, Davis Institutional Review Board (IRB)-approved, retrospective cross-sectional study of patients diagnosed with NSCLC through the CCR. Consent was waived because only de-identified data were included in the study. The CCR, a program of the California Department of Public Health, is a population-based registry that has collected cancer incidence and mortality data for the entire population of California since 1988. By law (Health and Safety Code, Section 103885), all new reportable cancer cases diagnosed in California residents must be provided to the CCR, and data are collected from diagnostic and treatment facilities[14]. To ensure current follow up for vital status and cause of death, the CCR database is linked annually to death certificates, hospital discharge data, Medicare files, the Department of Motor Vehicles, Social Security, and other administrative databases. Linkage to the National Death Index ensures capture of deaths occurring outside California as well as cause of death, and follow up is over 96% for patients diagnosed since 2000. The CCR is a participant in both the Centers for Disease Control National Program of Cancer Registries and the National Cancer Institute Surveillance Epidemiology and End Results (SEER) program, which requires the highest standards of data quality, as judged by completeness, accuracy, and timeliness.
Data extracted from medical records include patient demographics (age, gender, race, socioeconomic status), year of diagnosis, tumor characteristics, stage at diagnosis, and hospital and physician information. Race/ethnicity in the CCR is based on information collected from medical records supplemented with linkage to algorithms to better identify Hispanics and Asian/Pacific Islanders. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and non-Hispanic Asian/other. Patient address at diagnosis is assigned to a census tract, and neighborhood socioeconomic status (SES) was based on U.S. Census characteristics combined into the summary Yost index[15], categorized as low, medium, and high SES.
Only patients for whom NSCLC was the first or only cancer diagnosis were included. Patients diagnosed at autopsy were excluded from analysis. Stage at diagnosis was defined based on the SEER modification of the AJCC staging system, and only patients diagnosed as Stage I and treated with surgery were included in this analysis. Hospitals where definitive surgery was performed were categorized by Commission on Cancer (CoC) accreditation and average number of lobectomies or sublobar resections performed during the study period (Low < 20, Medium (20–50), High (> 50). Patients treated at major hospitals that had previously been CoC accredited were categorized as CoC accredited. Hospitals with invalid codes were excluded. Patients were followed through December 31, 2011.
Patient’s demographic information, hospital, and tumor characteristics were summarized using descriptive statistics. Two-sided Chi-square tests were used to determine whether baseline characteristics were significantly different between the two treatment groups. The overall survival was estimated using Kaplan-Meier method within hospital volume categories and CoC accreditation. Log-rank tests were conducted to examine whether the unadjusted differences in survival between the two groups were statistically significant. Multivariable PH Cox regression analysis was performed for the cause-specific survival analysis with hospital volumes and CoC accreditation as the main predictors of interest, adjusting for patient’s age, gender, race, Socio-economic status (SES), tumor stage, and types of treatment. We considered p-values less than 0.05 as statistically significant. All statistical analyses were conducted using SAS for Windows, version 9.3 (SAS Institute Cary, NC).
Results
The California Cancer Registry identified 8,345 patients with Stage 1 NSCLC who were treated from 2004–2011. Of these, 7,587 were treated with surgical resection consisting of either lobectomy or sublobar resection. The patients were a median age of 70, 56% were women, and 73% were white (Table 1). Seventy-four percent of patients were treated in a CoC accredited facility. Thirty-four percent (2575/7587) of patients were treated in high volume centers for lobectomy, 48% (3610/7587) were treated in medium volume centers and 18% (1392/7587) were treated in low volume centers. Most patients underwent lobectomy (82%) as opposed to sublobar resection (18%). A majority of hospitals were low volume and most hospitals were non-teaching, urban, not-for-profit hospitals.
Table 1.
Patient Characteristics
| Characteristic | N | % |
|---|---|---|
| Age | ||
| <50 | 231 | 3.0 |
| 50–64 | 1934 | 25.5 |
| >65 | 5422 | 71.5 |
|
| ||
| Sex | ||
| Male | 3359 | 44.3 |
| Female | 4228 | 55.7 |
|
| ||
| Race | ||
| Non-Hispanic, White | 5585 | 73.6 |
| Non-Hispanic, Black | 441 | 5.8 |
| Hispanic | 646 | 8.5 |
| Asian/Pacific Islander | 870 | 11.5 |
| Other | 45 | 0.6 |
|
| ||
| SES | ||
| Lowest/lower-middle | 2365 | 31.1 |
| Middle | 1668 | 22.0 |
| Higher-middle/Highest | 3287 | 43.3 |
| Unknown | 267 | 3.5 |
|
| ||
| Stage of disease | ||
| Stage 1A | 4682 | 61.7 |
| Stage 1B | 2905 | 38.3 |
|
| ||
| Surgery in a CoC accredited Hospital | ||
| Yes | 5620 | 74.1 |
| No | 1967 | 26.0 |
|
| ||
| Hospital Volume | ||
| Low <20 | 1392 | 18.3 |
| Medium 20–50 | 3610 | 47.6 |
| High >50 | 2575 | 33.9 |
| Unknown | 10 | 0.1 |
|
| ||
| Type of Surgery | ||
| Sublobar resection | 1363 | 17.9 |
| Lobectomy | 6224 | 82.0 |
To illustrate the effects of hospital volume and CoC accreditation on cancer-specific survival, Kaplan-Meier plots are shown in Figure 1a and b. As shown in Figure 1a, cancer-specific survival was significantly longer for patients treated in high volume centers (log-rank test, p<0.0001). Figure 1b demonstrates better cancer-specific survival in hospitals with CoC-accreditation (log-rank test, p<.001).
Figure 1. Kaplan-Meier Cancer Specific Survival Curves.
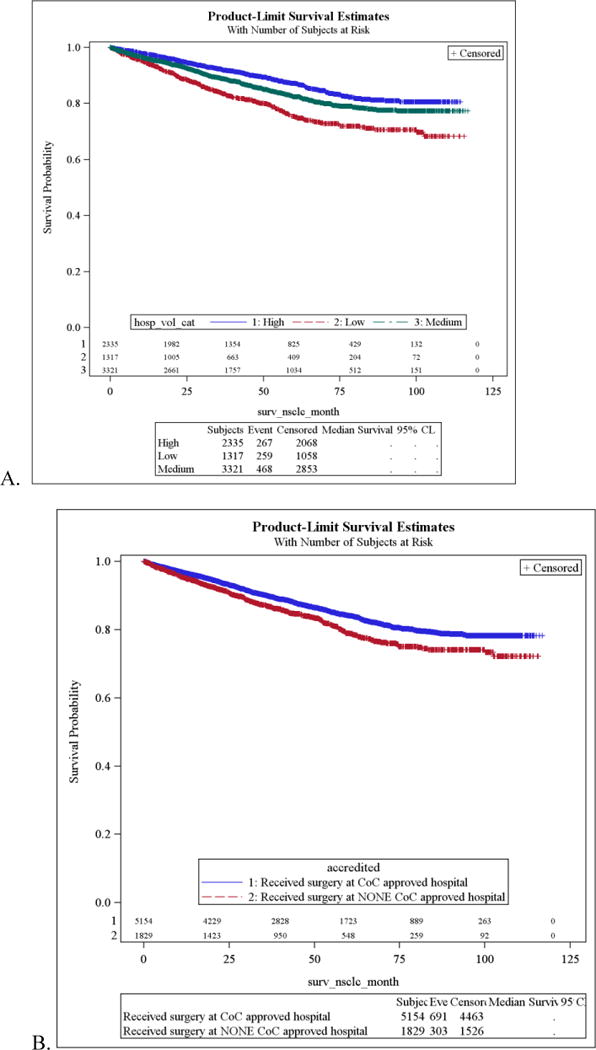
A. Hospital Volume, (log-rank test, p<0.0001) B. CoC Accreditation (log-rank test, p<.001)
Lobectomy, female gender, high SES, and Stage 1A disease were predictive of increased cancer-specific survival after adjustment for other factors. (Table 2) Age >65 was associated with decreased cancer-specific survival. Hispanic and Asian/Pacific Islanders demonstrated longer cancer-specific survival when compared to Non-Hispanic whites. There was no statistical difference between cancer-specific survival for Caucasians and African-Americans. Treatment in a high volume center was associated with longer cancer-specific survival when compared to low and medium volume centers. CoC accreditation was not a significant predictor of cancer-specific survival. When the analysis was confined to patients treated in low volume hospitals, CoC accreditation continued to have a non-significant effect on survival (HR=1.201, CI=0.930–1.552).
Table 2.
Cause-specific survival analysis for patients diagnosed with Stage I NSCLC, California, 2004–2011
| Variables | Hazard ratio (95% Confidence interval) |
|---|---|
|
| |
| Age | |
| <50 | Reference |
| 50–64 | 1.025 (0.652, 1.610) |
| 65+ | 1.616 (1.046, 2.496) |
| Sex | |
| Male | 1.237 (1.091, 1.041) |
| Female | Reference |
| Race | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 0.908 (0.695, 1.186) |
| Hispanic | 0.743 (0.580, 0.951) |
| Asian/Pacific Islander | 0.706 (0.0.567, 0.879) |
| SES | |
| Low SES | 1.239 (1.069, 1.436) |
| Medium SES | 1.141 (0.972, 1.340) |
| High SES | Reference |
| SEER stage | |
| IA | Reference |
| IB | 1.895 (1.670, 2.150) |
| CoC accreditation | |
| Yes | Reference |
| No | 1.047 (0.904, 1.213) |
| Hospital volume | |
| Low (<20) | 1.777 (1.474, 2.141) |
| Medium (20–50) | 1.234 (1.058, 1.438) |
| High (>50) | Reference |
| Treatment | |
| Lobar resection | 0.614 (0.528, 0.713) |
| Sublobar resection | Reference |
Comment
When facing a diagnosis of early-stage NSCLC, patients must make an educated decision about where to obtain treatment. To make these decisions, they must weigh perioperative outcomes and long-term oncologic outcomes. However, patients commonly rely on hospital characteristics and other third party designations as surrogate markers for outcomes and quality of care in order to inform their medical decision-making. Many studies have focused on the relationships between hospital volume and perioperative outcomes, but few have found that hospital volume impacts long-term cancer specific survival [16]. Our data are novel by demonstrating increased cancer-specific survival for early-stage NSCLC patients treated surgically in high volume centers. Although some have suggested that the early benefits seen by patients in high volume centers do not result in long-lasting effects on oncologic outcomes, our data suggest otherwise.
In addition to hospital procedural volume, we chose to examine the effect of CoC accreditation, another hospital characteristic, on cancer-specific survival. The Commission on Cancer is a program of the American College of Surgeons, established for the purpose of recognizing cancer programs for providing high-quality cancer care. Knutson et al, demonstrate the value that physicians and administrators place on CoC accreditation, with over 90% of respondents agreeing that accreditation improves patient care and outcomes[12]. However, data are not clear regarding the correlation between accreditation status and outcomes. Merkow et al demonstrated that CoC accredited facilities were likely to perform less well than non-accredited centers on venous thromboembolism prophylaxis, serious complication composite scores, catheter-associated bloodstream and urinary tract infection, as well as glycemic control[13]. In our series, we did not observe an independent effect of CoC accreditation on cancer-specific survival; and on subset analysis of low volume hospitals, we did not observe a significant effect of CoC accreditation on survival. Therefore these data suggest that CoC accreditation may reflect the ability of a facility to adhere to guidelines and standards, rather than its clinical outcomes; and that outcomes from thoracic surgery are related more strongly to surgeon and hospital experience.
The search for adequate measures of quality for surgical outcomes is ongoing. Kozower et al performed a large analysis of the Society of Thoracic Surgeons (STS) General Thoracic Database examining over 18,000 lung cancer resections performed in 111 facilities[17]. Significant differences were found between the hospitals that performed the best and worst among 30-day outcomes, but the predictors of improved hospital performance were poorly understood and long-term outcomes were not measured because of STS database limitations. The CCR has limited clinical information and therefore perioperative outcomes are not available for analysis, but cancer-specific survival can be examined. The lack of detailed clinical data in the CCR makes risk-adjustment impossible in our series. However, there are notable strengths to this analysis, including the large number of patients diagnosed with Stage 1 NSCLC, the diversity of the patient cohort, and the ability to measure long-term cancer specific mortality.
Many series have established a relationship between high surgical volume and improved perioperative outcomes across cancer specialties, but the use of hospital volume as a measure of quality remains controversial because of lack of consistency in the measurement of hospital volume [1, 3–6, 18–20]. Most series, including ours, categorize hospital volume into tertiles or quintiles. Kozower et al compared 3 techniques for calculation of hospital volume and suggest that the impact of hospital volume is dependent on how volume is defined and entered into the regression equation[21]. Despite these findings, the analysis of hospital volume as a nonlinear function using restricted cubic splines has not been widely adopted.
Conclusion
In the era of transparency of outcomes and public reporting, these reported outcomes are challenging for patients and physicians to interpret. Hospital volume should not be viewed as the sole determinant of quality, but it should be recognized as an important and significant factor contributing to decreased perioperative morbidity and mortality as well as increased cancer-specific survival. CoC accreditation may reflect a measure of adherence to standards and processes of care, but the lack of correlation with oncologic outcomes in this series is notable and warrants further investigation.
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Funding source: This work was supported by the NCI Comprehensive Cancer Center Support Grant, (P30CA93373)
References
- 1.Bach PB, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 2.Chang MY, Sugarbaker DJ. Surgery for early stage non-small cell lung cancer. Semin Surg Oncol. 2003;21(2):74–84. doi: 10.1002/ssu.10024. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 4.Park HS, et al. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg. 2012;93(2):372–9. doi: 10.1016/j.athoracsur.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Luchtenborg M, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol. 2013;31(25):3141–6. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- 6.Little AG, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80(6):2051–6. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 7.Falcoz PE, et al. The impact of hospital and surgeon volume on the 30-day mortality of lung cancer surgery: A nation-based reappraisal. J Thorac Cardiovasc Surg. 2014;148(3):841–8. doi: 10.1016/j.jtcvs.2014.01.030. discussion 848. [DOI] [PubMed] [Google Scholar]
- 8.Wouters MW, et al. Variation in treatment and outcome in patients with non-small cell lung cancer by region, hospital type and volume in the Netherlands. Eur J Surg Oncol. 2010;36(Suppl 1):S83–92. doi: 10.1016/j.ejso.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Sioris T, et al. Effect of surgical volume and hospital type on outcome in non-small cell lung cancer surgery: a Finnish population-based study. Lung Cancer. 2008;59(1):119–25. doi: 10.1016/j.lungcan.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Commission on Cancer. 2015 Jan 31; Available from: https://www.facs.org/quality-programs/cancer.
- 11.Bilimoria KY, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–81. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 12.Knutson AC, et al. The role of the American College of Surgeons’ cancer program accreditation in influencing oncologic outcomes. J Surg Oncol. 2014;110(5):611–5. doi: 10.1002/jso.23680. [DOI] [PubMed] [Google Scholar]
- 13.Merkow RP, et al. Relationship between cancer center accreditation and performance on publicly reported quality measures. Ann Surg. 2014;259(6):1091–7. doi: 10.1097/SLA.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 14.California Cancer Registry. 2015 Jan 31; Available from: http://www.ccrcal.org/
- 15.Yost K, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 16.Etzioni DA, et al. Patient survival after surgical treatment of rectal cancer: impact of surgeon and hospital characteristics. Cancer. 2014;120(16):2472–81. doi: 10.1002/cncr.28746. [DOI] [PubMed] [Google Scholar]
- 17.Kozower BD, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90(3):875–81. doi: 10.1016/j.athoracsur.2010.03.115. discussion 881–3. [DOI] [PubMed] [Google Scholar]
- 18.Dimick JB, et al. Hospital volume and surgical outcomes for elderly patients with colorectal cancer in the United States. J Surg Res. 2003;114(1):50–6. doi: 10.1016/s0022-4804(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 19.Dimick JB, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199(1):31–8. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Taub DA, et al. Impact of surgical volume on mortality and length of stay after nephrectomy. Urology. 2004;63(5):862–7. doi: 10.1016/j.urology.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Kozower BD, Stukenborg GJ. The relationship between hospital lung cancer resection volume and patient mortality risk. Ann Surg. 2011;254(6):1032–7. doi: 10.1097/SLA.0b013e31821d4bdd. [DOI] [PubMed] [Google Scholar]


