Abstract
Background and Aims
Regeneration of the hepatic mass is crucial to liver repair. Proliferation of hepatic parenchyma is intimately dependent on angiogenesis and resident macrophage-derived cytokines. However the role of circulating monocyte interactions in vascular and hepatic regeneration is not well-defined. We investigated the role of these interactions in regeneration in the presence and absence of intact monocyte adhesion.
Methods
Partial hepatectomy was performed in wild-type mice and those lacking the monocyte adhesion molecule CD11b. Vascular architecture, angiogenesis and macrophage location were analyzed in the whole livers using simultaneous angiography and macrophage staining with fluorescent multiphoton-microscopy. Monocyte adhesion molecule expression and sprouting-related pathways were evaluated.
Results
Resident macrophages (Kupffer cells) did not migrate to interact with vessels whereas infiltrating monocytes were found adjacent to sprouting points. Infiltrated monocytes colocalized with Wnt5a, angiopoietin 1 and Notch-1 in contact points and commensurate with phosphorylation and disruption of VE-cadherin. Mice deficient in CD11b showed a severe reduction in angiogenesis, liver mass regeneration and survival following partial hepatectomy, and developed unstable and leaky vessels that eventually produced an aberrant hepatic vascular network and Kupffer cell distribution.
Conclusions
Direct vascular interactions of infiltrating monocytes are required for an ordered vascular growth and liver regeneration. These outcomes provide insight into hepatic repair and new strategies for hepatic regeneration.
Keywords: Angiogenesis, immunology, innate immune system, hepatic repair
Introduction
The liver is a unique organ in not only its capacity to regenerate but in the complex sequential activation of multiple pathways and cell types involving the entire remaining organ in recovery of mass.1,2 What has not yet been fully delineated is how these processes interact and in particular the converging roles of angiogenesis3 and circulating monocytes. After hepatectomy, injured hepatocytes, liver progenitor cells and resident macrophages, that is, Kupffer cells (KCs) from portal areas recruit circulating immune cells such as monocytes to damaged liver through release of monocyte chemotactic protein 1 (MCP-1)4–5. Infiltrating monocytes can activate c-Met and Tie-2 pathways by direct interaction,6–8 and trigger the paracrine release of different cytokines and endothelial growth factors important to liver regeneration such as the family of molecules Notch and Wnt.9,10,11 Either disruption of the canonical Wnt/β-catenin signaling pathway in macrophages 12 or depletion of systemic macrophages reduces liver regeneration.13,14 KCs in particular, as resident macrophages play an elaborate control role of sinusoidal endothelium.2 Priming factors (e.g., IL-6 and TNFα) by KCs and hepatocytes themselves15 stimulate hepatocytes to respond to growth factors (e.g., HGF, TGF-β and EGF).16 Hepatocytes undergo DNA synthesis that peaks at ∼36h post-hepatectomy in mice16 followed by three additional smaller waves of proliferation to eventually restore the original number of hepatocytes.17 Proliferation of hepatocytes sequentially advances from periportal to pericentral areas of the lobule, as a wave of mitosis under circadian control.18
Simultaneously there arises a vascular response that begins with endothelial budding, and is facilitated by vasodilation and uncoupling of interendothelial contacts, which allows extravasation of plasma proteins and extracellular matrix components to set down an initial scaffold for migrating ECs.19 The vasodilator and pro-angiogenic substance nitric oxide (NO)20 is the main factor responsible of the initial vascular effects, while Wnt and Notch family proteins provide proliferating and non-proliferating control of tip and stalk cells, respectively.21 These factors help overcome the forces that provide vascular mechanical strength and stiffness. At the molecular level, the disruption of EC assembly includes the phosphorylation of adherens junctions such as vascular endothelial (VE)-cadherin, platelet endothelial cell adhesion molecule-1, and their corresponding cytoskeleton-linking molecules. Moreover, endothelial and inducible NO synthases are up-regulated after partial hepatectomy by c-Met activation and released NO facilitates the uncoupling of endothelial junctions.22–23 Likewise, c-Met receptor has recently been described as a co-receptor of the leukocyte Intercellular Adhesion Molecule 1 (ICAM-1)24 and this latter signaling pathway triggers liver regeneration by stimulating Kupffer cells to release tumor necrosis factor α (TNF-α) and IL-6 in mice.25
The regulation of interactions of infiltrating monocytes with endothelial cells is critical to optimization of angiogenesis in order to synchronize vascular growth to parenchymal expansion during hepatic regeneration and it is these interactions we describe herein.
Materials and Methods
Animal experiments
Male C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA)) and mice lacking CD11b (Strain: B6.129S4-Itgamtm1Myd/J) from the Jackson Laboratory (Bar Harbor, ME). All animals were maintained in a temperature-controlled room (22°C) on a 12-h light-dark cycle under institutional and NIH guidelines. After arrival, mice were continuously fed ad libitum until euthanasia. Partial hepatectomy was performed as described26 and approved by the Animal Ethics Committee at Massachusetts Institute of Technology, MA, USA. The regenerating right lobe was used for all analyses at different time points. Liver restoration rate was calculated as liver weight/body weight × 100.
Whole-mount multiphoton imaging of macrophage presence and angiography in liver
Mice (9–12 weeks old) were anesthetized with isoflurane and then injected with 100 µL of 20 mg/mL 70-kDa Texas red-dextran in Dulbecco's PBS into the tail vein in order to load macrophages by phagocytosis. After 2 hours the animals were euthanized by overexposure to CO2. Then, mice were perfused via the left ventricle with phosphate buffered saline (PBS) followed by an injection of fluorescein isothiocyanate-labeled dextran (FITC-dextran, MW 2×106 Da., Sigma, St. Louis, MO). Finally, vascular and macrophage fluorescence was visualized under a multiphoton microscope (Leica Microsystems, Heerbrugg, Switzerland). Vascular analysis and macrophage presence were determined by capturing 10 µm z-series of whole liver with a 25 x, N.a. 1.05 objective, Olympus FV-1000 MP (Olympus, America Inc, Center Valley, PA, USA) in which the viewing field is 512 × 512 µm. Number of macrophages, vascular diameter and anastomosis quantification were analyzed in all of the z-images with ImageJ manually or using the tool “angiogenesis analyzer” when appropriate. Intrasinusoidal or extrasinusoidal macrophages attached to vessel walls were quantified as positive macrophage-endothelial cell interactions. Dual staining (in yellow) did not interfere with the identification of intravascular staining of attached macrophages (highlighted in red) which were not stained by FITC-dextran at every focal plane. The initial total number of macrophages (sham) was considered as the original number of resident macrophages (KCs). After hepatectomy, the number of KCs was calculated as: total number of macrophages – (number of macrophage-EC interactions – original number of macrophage-EC interactions).
Tissue and cell analysis
Tridimensional reconstruction software analysis, gene expression analysis by Real-time PCR, immunofluorescent staining, Western blotting and “In vitro” studies are all described in the supporting Materials and Methods.
Statistical analysis
Data are expressed as mean ± standard error. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA), the Newman-Keuls test, and the unpaired Student’s t test when appropriate. Differences were considered to be significant at a p value of 0.05 or less. Data sampled from Gaussian populations were used for the calculation of Pearson Correlation Coefficient. A Pearson correlation coefficient (r) value of >0.75 was considered to exhibit strong positive correlation, a value between 0.50 and 0.75 was considered as a moderate correlation whereas value less than 0.5 was considered to demonstrate a weak correlation between two variables.
Results
Vascular vasodilation and angiogenesis are progressive from portal to central areas after hepatectomy
To evaluate and quantify the vascular and angiogenic effects after hepatectomy we performed a whole-mount fluorescent angiography with multiphoton microscopy and analyzed the tridimensional segmentation of the bidimensional stack of images. Significant vascularization was comprised of immediate angiogenesis and delayed vasodilation (Figure 1A). The number of vascular anastomosis rose 7-fold at regeneration compared to immediate post-hepatectomy (Figure 1A). There was as well distension of the hepatic and sinusoidal collagen fibers that serve as the scaffold for normal liver, commensurate with expansion of the injured mass to make space for vascular and parenchymal growth (Supplementary Figure 1). Vasodilation followed 16 hours after liver excision originating in portal areas and expanding to central areas with progressive tissue regeneration (Figure 1A). Portal areas were easily identified by tridimensional segmentation as convergent areas of major vessels (Supplementary Figure 2). We quantified inducible nitric oxide synthase (iNOS) expression along the regeneration process to assess the relationship between induction of this enzyme and vasodilation. iNOS expression tracked with vasodilation (Supplementary Figure 3) and indeed there was a correlation of 0.995 between the two during liver regeneration (Supplementary Figure 3).
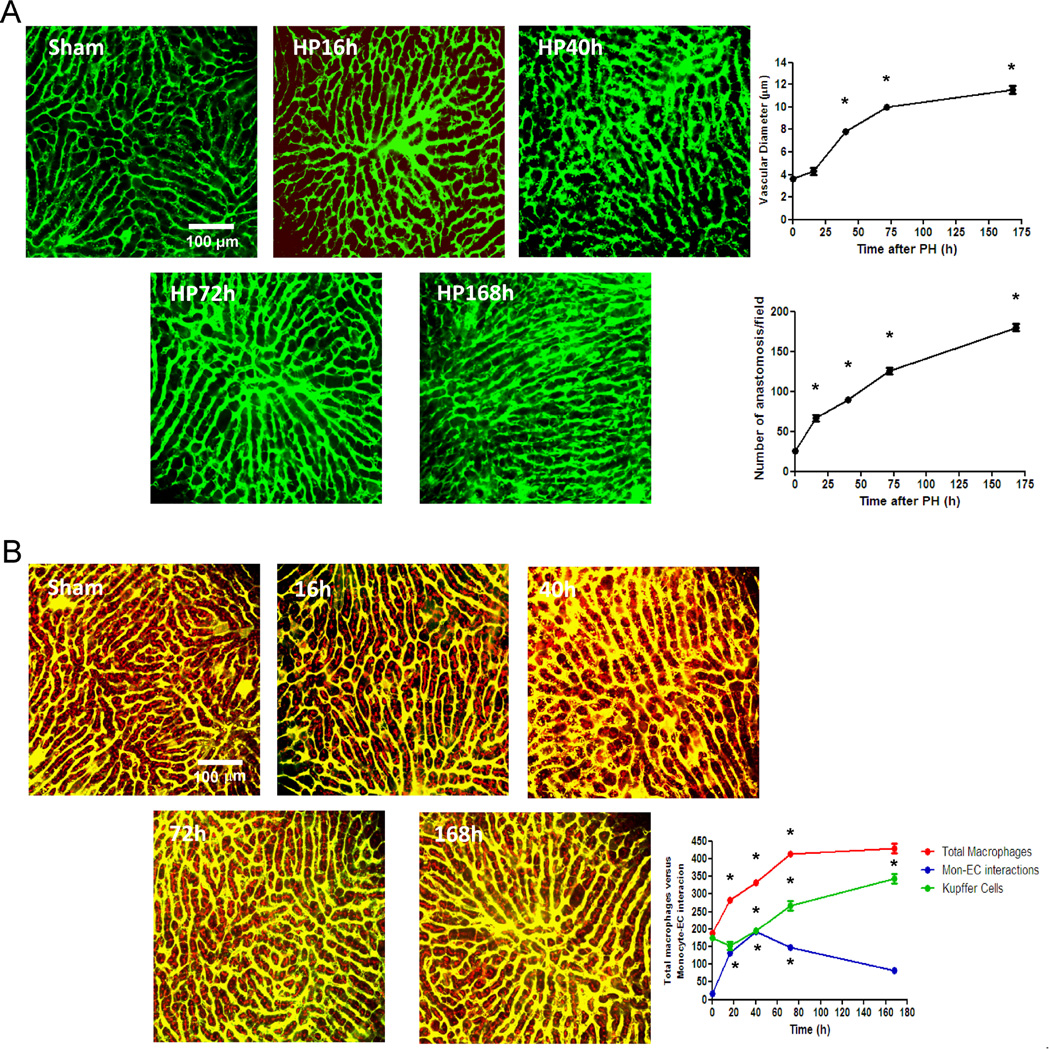
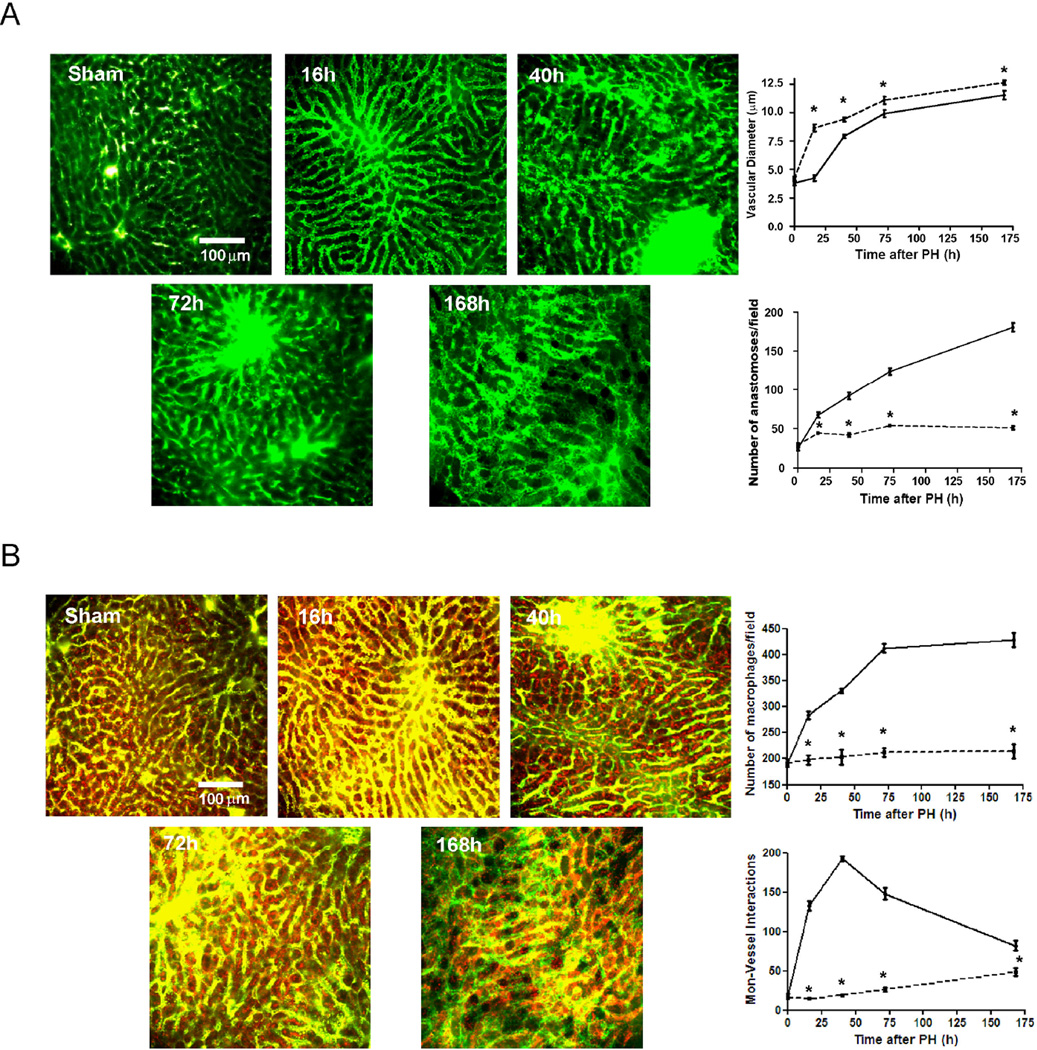
Figure 1. Monocyte/macrophage interactions with liver sinusoidal endothelial cells during liver regeneration.
C57BL/6 mice underwent 70% hepatectomy and samples of liver right lobe were examined at sequential time points (0, 16, 40, 72, 168 hours post-op). Vasodilation (initiated in portal areas) and anastomoses were analyzed by angiography (vascular perfusion of FITC-dextran, MW 2×106 Da) using multiphoton microscopy (A). The total number of macrophages and monocyte-vascular interactions were quantified in whole liver images obtained from mice intravenously injected with 70 kDa Texas red-dextran 2 hours before angiography with FITC-dextran and multiphoton microscopy, and sacrifice. Intravascular and extravascular monocyte-endothelium interactions and non-interacting macrophages highlighted in red were quantified in every z-stack from every field. (B); one representative Z image of liver from every time point is shown n=10 at every time point; *, p<0.05 vs. former time point.
Infiltrating monocytes but not resident Kupffer Cells interact directly with sinusoid after partial hepatectomy
To analyze the possible role of the interactions of circulating monocytes with angiogenesis during liver regeneration we visualized the emerging hepatic vascular network using angiography in concert with fluorescent staining of macrophages in whole mouse liver. Quantification of z-stack images obtained by multiphoton mi croscopy in sham samples revealed that most of resident macrophages (i.e. KCs) were located in close contact with hepatocytes within the space of Disse with no direct interaction with sinusoids (Figure 1B). Only 9% of resident macrophages were attached to the hepatic vascular network in normal conditions (Figure 1B). These findings were further visualized by analysis of tridimensional segmentation as above (Supplementary Figure 4). After hepatectomy, the number of direct contacts of macrophages with hepatic vasculature progressively increased peaking at 40 h and then gradually decreasing thereafter (Figure 1B). The number of KCs, as identified as the total number of resident macrophages at time zero, however rose slightly or remained constant in the first post-op day and then doubled progressively thereafter (Figure 1B). Thus, monocyte recruitment occurs early after hepatectomy but the expansion of the KC population needs more than 1 day to begin to synchronize their replication with the growing parenchyma. Indeed, the number of KCs correlates perfectly (r=0.997) with liver restoration rate (Supplementary Figure 5).
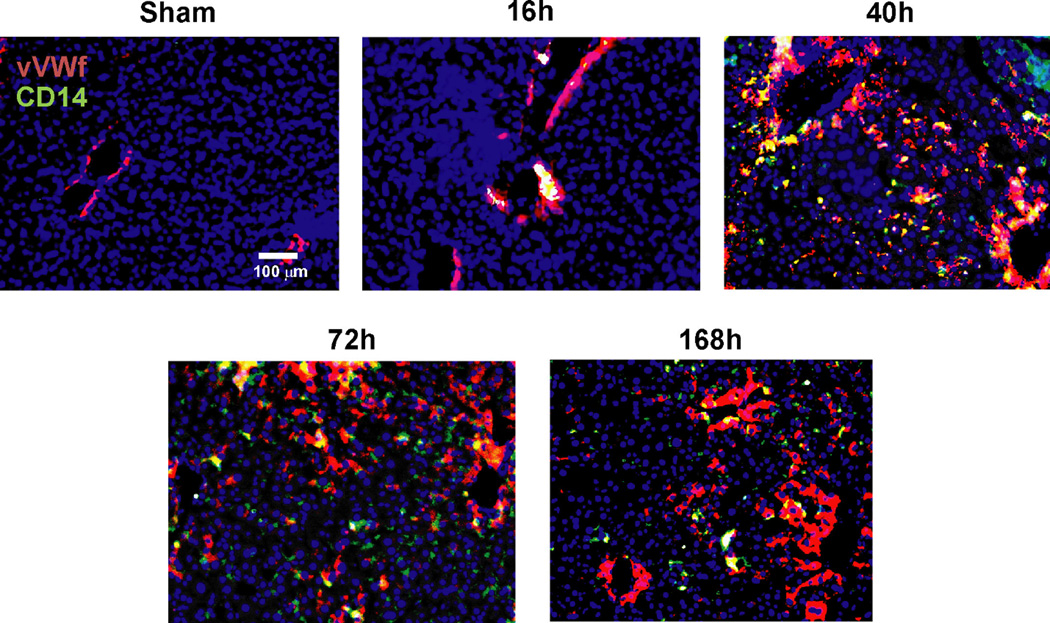
To determine whether macrophages in contact with liver vessels were recruited from circulating monocytes we stained liver sections for CD14 a marker highly expressed in macrophages derived from infiltrated monocytes27, at different time points after hepatectomy. The number of macrophages derived from circulating monocytes in direct contact with liver endothelium followed the pattern quantified by multiphoton microscopy, emerging from the portal space to the centrolobulillar areas until 40h post-op and then progressively declining afterwards (Figure 2).
Figure 2. Interactions between circulating monocytes and endothelial cells after partial hepatectomy are initiated in portal space.
Representative images of liver sections identified vessels by staining for von Willebrand factor (in red) and recruited monocytes by staining for CD14 (in green). Nuclei were stained by DAPI (blue). Initial contacts of recruited monocytes (in yellow) take place in portal areas and then expand to the rest of liver sinusoid as regeneration progresses peaking at 40h post-hepatectomy.
Macrophage activation by endothelial contact is associated with VE-cadherin disruption and the delivery of factors to select vascular sprouting points
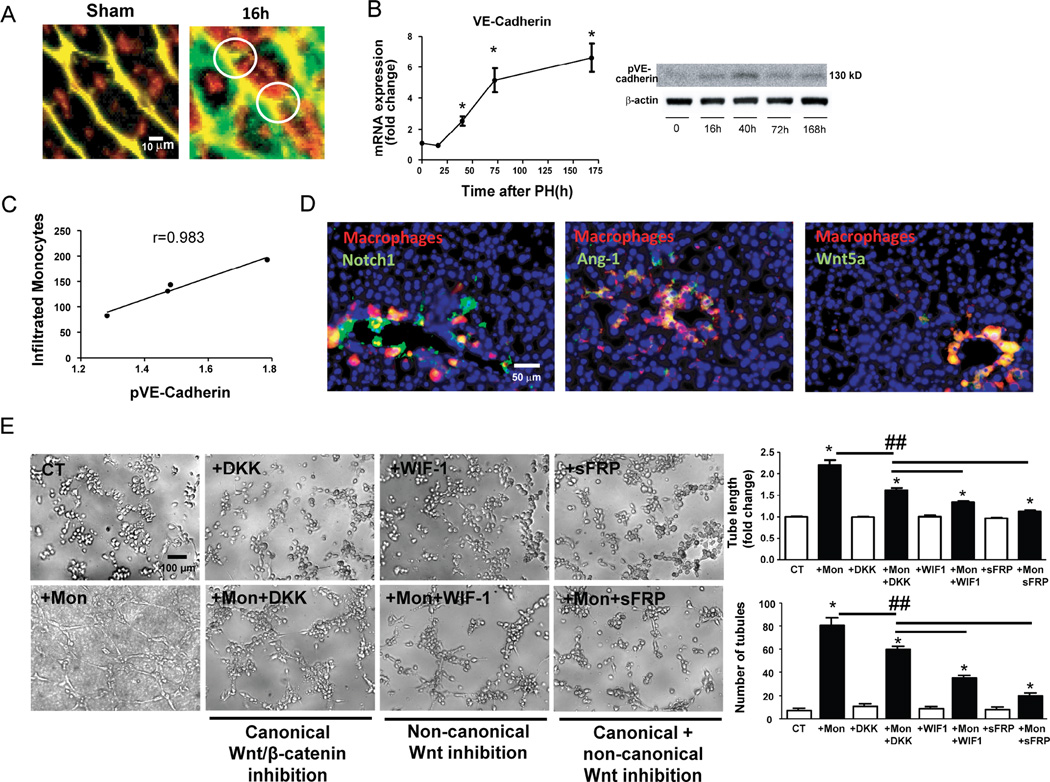
Multiphoton microscopy studies also provided clear images showing that recruited monocytes spread and site adjacent to small vascular budding structures (Figure 3A). To assess whether interactions of infiltrated monocytes can be associated with vascular sprouting along the liver regeneration process we quantified the phosphorylated form of vascular endothelial cadherin (VE-cadherin) as a marker of endothelial disruption leading to vascular sprouting.28 Expression of VE-cadherin progressively rose 24h after hepatectomy and continued and plateaued (Figure 3B) but phosphorylation of VE-Cadherin fell after peaking interestingly at the time that monocyte-endothelial interactions were also on the decline (Figure 3B). Indeed there is an excellent correlation (r=0.983) between the number of monocyte-EC contacts and phosphorylation of VE-Cadherin suggesting a reflection of influence on vascular sprouting (Figure 3C).
Figure 3. Infiltrated macrophages stimulate vascular sprouting in contact points with vessels.
Amplification of multiphoton images (vessels in green and yellow, macrophages in red), visualized vascular buds (white circles) surrounded by spread recruited macrophages as early as 16 hours after hepatectomy, none of which were identifiable in comparison to sham samples (A) mRNA levels of VE-cadherin (indicator of vascular proliferation) after hepatectomy showed that angiogenesis begins after 16 hours and progressively increases until 168 hours (B) In contrast phosphorylation of VE-cadherin (Western blot) and therefore disruption of endothelial junctions to allow the sprouting process increases from 16 to 40 hours post-op and then decreases until the end of liver regeneration (B). The pattern of phosphorylation of VE-cadherin evaluated by Western Blot shows excellent correlation with the number of infiltrated monocytes during liver regeneration (C) Hepatic staining of these recruited macrophages (CD14, in red) enhanced the local expression of sprouting-related factors Wnt5a, Notch1 and Ang-1 (in green) in contact points of vessels (yellow) in portal areas as early as 16 hours post-op (D). Tube formation assay of HUVEC inflamed by TNF-α (5 ng/mL) in the presence or absence (Control, CT) of THP-1 monocytes (Mon) or Wnt inhibitors (DKK for canonical Wnt pathway; WIF-1 for non-canonical Wnt pathway; sFRP for both Wnt pathways). Representative phase contrast images and quantification of tube length and number of tubules are shown (E). n=10 at every time point; *, p<0.05 vs. former time point; ##, p<0.01.
In addition to the possible angiogenic effects of direct interactions of monocytes with adhesion molecules we also analyzed factors locally released by recruited macrophages that can stimulate proliferation of tip cells (such as Wnt5a) or select stalk cells (such as Notch1 or Ang-1). In this regard, we found that monocytes recruited at an early phase of liver regeneration (16h post-op) are attached to vessels and colocalize with Wnt5a, Notch1 and Ang-1 in the portal space (Figure 3D). The liver expression of these factors progressively increases up to a maximum at 72h (Supplementary Figure 6A) when endothelial proliferation is described to reach a peak as well.29 In vitro, THP-1 cells (a monocyte cell line) also deliver Wnt5a (non-canonical Wnt ligand) and Notch-1 as soon as they interact with HUVECs inflamed by TNF-α (Supplementary Figure 6B). Wnt ligands delivered by THP-1 after interacting with HUVECs demonstrated to play an important role inducing endothelial tube formation since total blockade of Wnt signaling by sFRP drastically reduced the formation of tubules (Figure 3E). Indeed, inhibition of the effects of non-canonical ligands such as Wnt5a using WIF1 resulted in a higher inhibition of tube formation than inhibiting the canonical Wnt/β-catenin pathway using DKK (Figure 3E)
ICAM-1 and MCP-1 expression is sequentially activated during liver regeneration
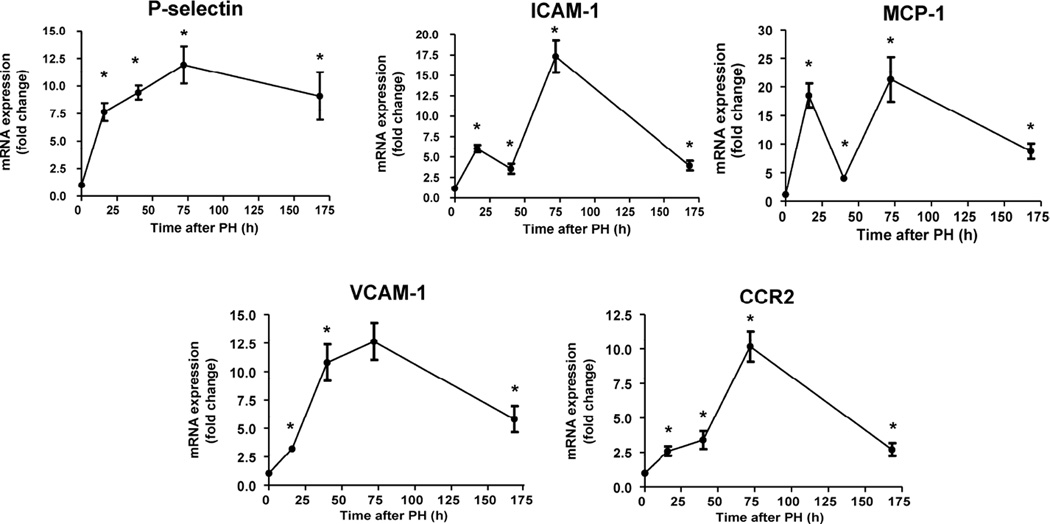
To establish which endothelial adhesion molecules are involved in the recruitment of circulating monocytes and the sequential waves of sprouting during liver regeneration we quantified the gene expression of the most important monocyte adhesion molecules: P-selectin, CCR2, VCAM-1 and ICAM-1. Expression of the three of these was increasingly up-regulated with liver regeneration, with a maximum peak of expression 72 h post-hepatectomy (Figure 4). Surprisingly, gene expression of ICAM-1 showed two sequential waves of up-regulation, one at 16 h and one at 72 h after hepatectomy with down-regulation at 40 h (Figure 4), the time when monocyte interactions peak. In this context expression of monocyte chemotactic protein (MCP-1) exhibited two waves of expression that parallel ICAM-1 gene expression (Figure 4), suggesting synergistic or concomitant contributions to control of signaling pathways activated by the interaction of monocytes with ECs.
Figure 4. Sequential gene activation of ICAM-1 and MCP-1 during liver regeneration.
Real-time PCR analysis of gene expression of vascular adhesion molecules (P-selectin, ICAM-1, VCAM-1 and CCR2) and the monocyte chemotactic MCP-1 showed an increasing up-regulation of P-selectin, VCAM-1 and CCR2 throughout the whole process of liver regeneration. In contrast two sequential activations of gene expression of ICAM-1 and MCP-1 were found at 16 and 72 hours post-op and a down-regulation of their transcripts at 40h thus coinciding with the peak of monocyte-EC interactions; n=10 at every time point; *, p<0.05 vs. previous time point.
Suppression of CD11b–mediated binding of monocytes to endothelial ICAM-1 impairs vascular sprouting leading to vascular leakage
To investigate the direct relationship of ICAM-1 activation by monocyte binding and hepatic sprouting process we analyzed the effects of gene suppression of the monocyte counter-receptor that mediates ICAM1 leukocyte adhesion (i.e. CD11b) in vascular and liver regeneration after partial hepatectomy. Two groups of mice of the same age were analyzed: wild-type and KO for CD11b. The elimination of monocyte interactions in KO mice for CD11b abolished sprout formation and angiogenesis and promoted a significant and sustained increase in vascular diameter (Figure 5A). Portal areas showed leaky blood vessels between 40–72 h post-op that was obliterated after 7 days. The vascular network that formed had aberrant branching and shunts and lost the KC distribution observed in normally regenerating livers (Figure 5A). The KO of CD11b abrogated stable interactions and recruitment of monocytes in liver sinusoids and portal space after partial hepatectomy that was only partially compensated after 7 days pos-top by the mobilization of KC from the hepatocyte vicinity to the surface of blood vessels (Figure 5B).
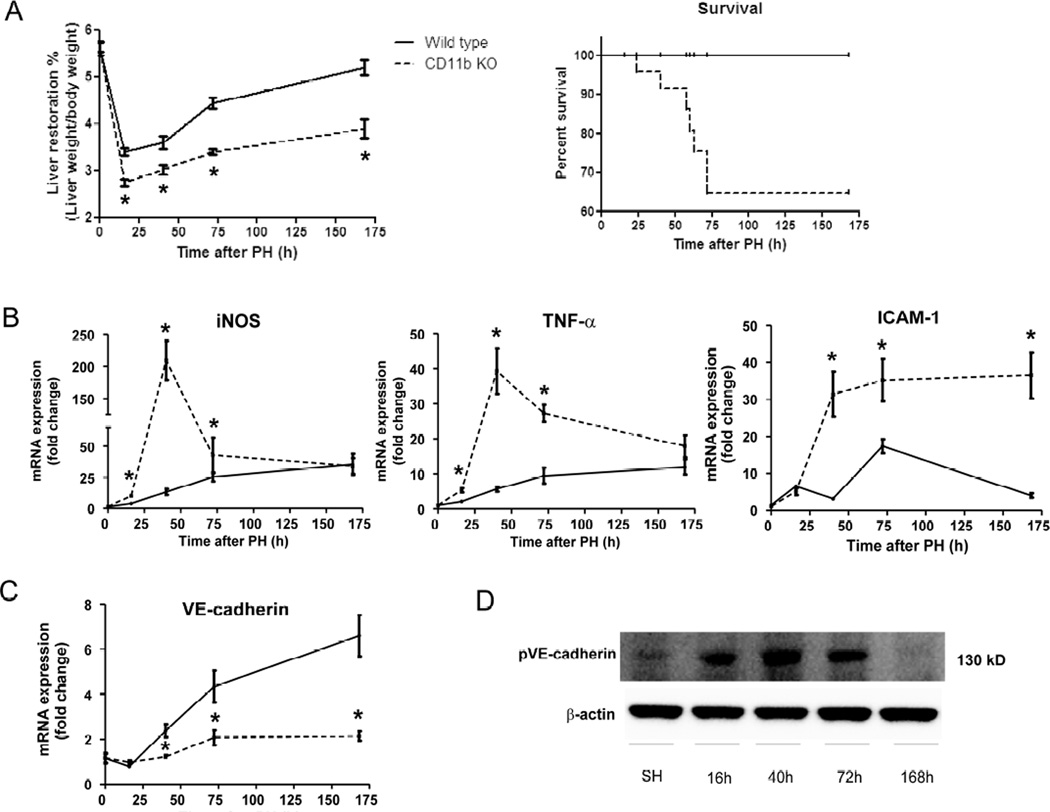
Figure 5. Gene suppression of CD11b disrupts vascular growth, promotes a higher vasodilation and permeability and alters KC distribution after partial hepatectomy.
Two groups of C57BL/6 mice (wild-type and CD11b KO) underwent 70% hepatectomy. The nature and form of the vascular network formed in the right lobe of the liver were examined sequentially (0, 16, 40, 72, 168 hours post-op) by angiography using multiphoton microscopy. Vasodilatation was present in CD11b KO mice (dashed lines) just after hepatectomy and greater than observed in wild-type mice (solid lines) during liver regeneration, but vascular growth was drastically reduced and vascular leakage visualized in CD11b KO mice as compared with wild-type mice (A). The total number of macrophages increased minimally after hepatectomy in CD11b KO mice as compared with wild-type mice. KCs migrated to interact with blood vessels after 72 hours post-op replacing the absent monocyte-ECs interactions in CD11b KO mice. (B); One representative Z image of liver from every time point in CD11b KO mice is shown; the mean values ± standard error for the groups are indicated for wild-type (solid lines) and CD11b KO (dashed lines); n=10 at every time point; *, p<0.05 vs. wild-type mice.
Mice lacking CD11b display impaired liver regeneration and survival
Aberrant vascular growth of mice lacking CD11b resulted in a drastic reduction of hepatic mass regeneration and reduction in survival by 35% within the first 72h (Figure 6A). During the critical period of 40–72h gene expression of iNOS, TNF-α and ICAM-1 were upregulated (Figure 6B). Conversely VE-cadherin gene expression was down-regulated pointing to a reduction in angiogenesis (Figure 6C) but phosphorylation was augmented in association with increased TNF-α and iNOS during periods of maximal vascular leak (Figure 6D). This disruption of vascular integrity cannot be attributed to an increase of other well-known permeability factors such as VEGF-A or Ang-230 whose mRNA levels were also drastically decreased in CD11b KO mice (Supplementary Figure 7A). Furthermore, gene expression of sprouting factors Wnt5a, Ang-1 and Notch1 was down-regulated in CD11b KO mice and progressively recovered after 72h in correlation with the recruitment of KCs to interact with hepatic vessels (Supplementary Figure 7B). Hepatic proliferation (PCNA expression) and expression of known mediators of liver regeneration (IL-6 and hepatocyte growth factor) was also found drastically reduced in CD11b KO mice ((Supplementary Figure 7C).
Figure 6. Mice lacking CD11b show a reduction in liver regeneration and survival after hepatectomy commensurate with up-regulation of iNOS and TNF-α.
Liver samples from C57BL/6 mice subjected to 70% hepatectomy demonstrated significantly reduced hepatic mass regeneration in CD11b KO mice and as a consequence, a drop in survival in comparison to wiild-type mice (A). The absence of interactions between CD11b and ICAM-1 in blood vessels of KO mice promoted significant up-regulation of iNOS, TNF-α and ICAM-1 peaking at 40 and 72 hours post-op as compared with wild-type mice (B). Moreover mice deficient in CD11b showed a drastic reduction of VE-cadherin gene expression assessed by Real-time PCR (C) in comparison to wild-type mice and in concordance with the results showing a decrease of angiogenesis in CD11b KO mice. The up-regulation pattern of TNF-α was associated with enhanced levels of phosphorylation of VE-cadherin in CD11b KO mice peaking at 40 hours after hepatectomy (D); n=10 at every time point; *, p<0.05 vs. wild-type mice.
Discussion
Ordered angiogenesis has long been considered to be critical for optimal wound healing. The formation of new, functional blood vessels requires the sprouting of preexisting blood vessels and their subsequent fusion with other blood vessels.31 Little is known about the mechanisms by which leading ECs at vascular sprouts (endothelial “tip” cells) are selected to proliferate and elongate to form new functional blood vessels. Some investigations point to direct interactions of monocytes with ECs as a driving force to stimulate endothelial proliferation6–7 and to mediate the fusion of endothelial tip cells.32 We demonstrate that circulating monocytes are selectively recruited to specific areas of regenerating livers, especially surrounding sprouting spots. This recruitment begins in portal areas and expands to the rest of hepatic tissue commensurate with vasodilation and the hepatic expression of iNOS. As iNOS is up-regulated in injury, thus inducing synthesis of the vasodilator and proangiogenic substance NO33, our findings suggest that hepatocytes and resident KCs locally deliver paracrine factors to regulate vascular tone and growth during liver regeneration. Released substances serve not only to coordinate vessel and tissue growth but cellular interactions as well, attracting circulating monocytes (e.g. MCP-1) and stimulating nearby ECs to expose monocyte adhesion molecules (e.g. TNF-α)4,34 (Figure 7A). Indeed, the number of interactions of recruited monocytes with liver vascular network directly associated with phosphorylation and disruption of VE-cadherin connections. This uncoupling of inter-EC connectivity mediated by VE-cadherin is essential to the plasticity of the selected endothelial tip cell as it drives migration and elongation.35 Non-resident macrophages also serve as chaperones of endothelial sprouting by locally secreting proliferative factors such as Wnt5a and Ang-1 and the stalk cell stabilizer Notch1. The final outcome of this collaboration between recruited monocytes and signals from hepatocytes and KCs is that vascular anastomosis increase proportionally to hepatic mass growth and KC number until total liver mass is restored. In this regard, we show that Wnts release from infiltrating monocytes and namely the contribution of Wnt5a and non-canonical ligands is especially important to drive angiogenesis in those inflamed vessels.
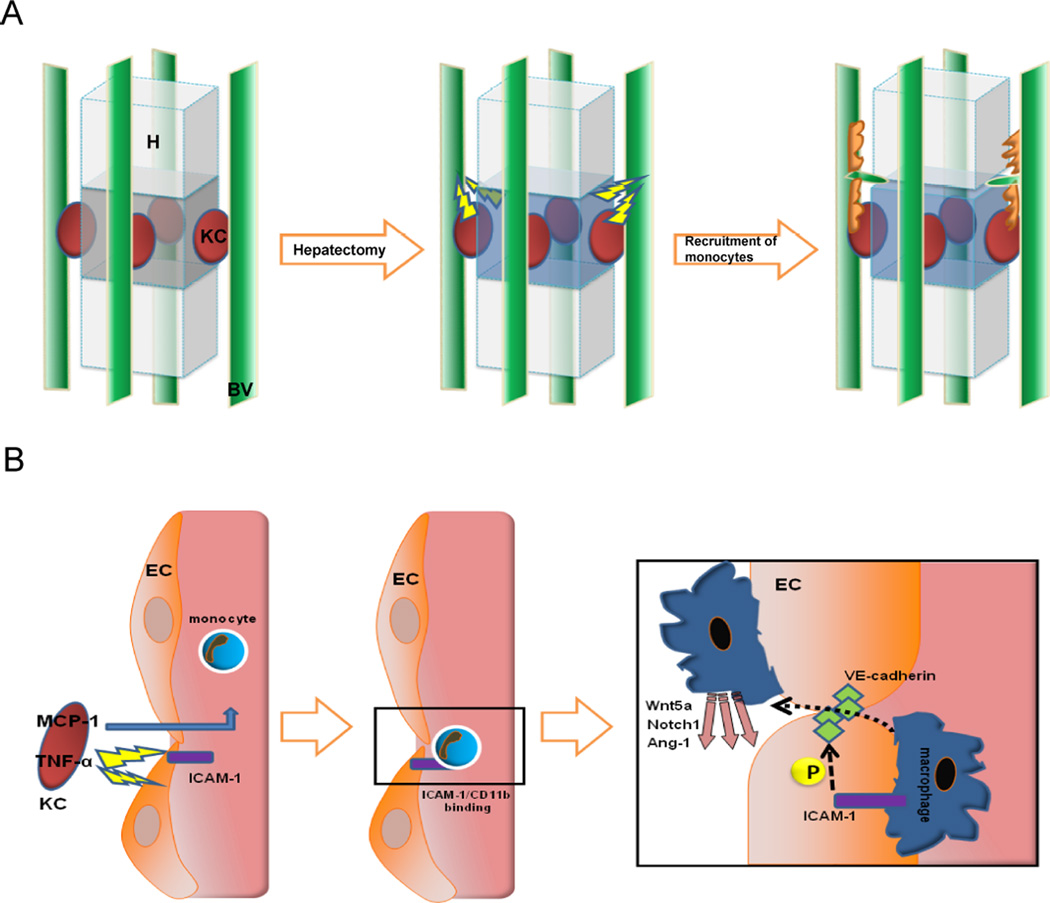
Figure 7. Proposed model of the role of circulating monocytes in liver regeneration.
Tridimensional schematic structure of KC (in red), hepatocytes and blood vessels (in green) following the pattern obtained by multiphoton microscopy indicates that signals from injured liver stimulate the release of chemotactics and the induction of monocyte adhesion molecules on the endothelium. These signals recruit monocytes to selected areas, driving the sprouting and angiogenic process (A). KCs deliver MCP-1 to blood stream and TNF-α towards closer ECs to promote the expression of ICAM-1 which bind attracted monocytes which will phosphorylate interendothelial VE-cadherin to allow them to migrate throughout the vessel and locally deliver sprouting factors (B).
Current investigations in the field of liver regeneration have focused on the priming phase of DNA synthesis of hepatocytes and essential transcription factors in the first 4 hours after hepatectomy where CD14+ infiltrated monocytes are seen.36 We now demonstrate that recruited monocytes are important beyond the priming phase and throughout the process of liver regeneration. Indeed, leukocyte adhesion molecules such as P-selectin, CCR2 and VCAM-1 up-regulated after 24 h post-hepatectomy maintain the number of rolling interactions and the likelihood of recruiting monocytes. Interestingly, the adhesion molecule ICAM-1 was also up-regulated after hepatectomy but its gene expression was significantly down-regulated when monocyte interactions and VE-cadherin phosphorylation were maxima and just preceding the two main waves of hepatocyte proliferation as reported.37 Altogether monocyte adhesion molecule expression seems to be regulated by monocyte interactions in accordance with the requirements of endothelial sprouting and the subsequent hepatic mass expansion. Actually ICAM-1 activation is an important signaling pathway to trigger vascular sprouting38–39. Therefore ICAM-1 appeared as a good candidate to understand the role of direct interactions of monocytes with hepatic endothelium to regulate vascular and liver regeneration.
Monocyte adhesion to activated endothelium promotes EC proliferation6–8 and is an essential step for arteriogenesis via ICAM-1/Mac-1.40 Gene suppression of any of the subunits of the monocyte receptor Mac-1 (CD11b/CD18) disrupts wound healing in mice.41–42 However the role of this monocyte receptor has not been analyzed in liver regeneration after partial hepatectomy. We now show that suppression of the gene for CD11b reduced survival of mice undergoing partial hepatectomy in concert with reduction in recruitment of circulating monocytes into the hepatic vascular network and impaired vascular and liver mass regeneration. The suppression of monocytes adhesion to ICAM-1 in CD11b KO mice promotes a sequence of gene up-regulation that compensates for reduced role of monocyte-EC interactions in liver regeneration. These compensatory effects include increase in ICAM-1 and TNF-α expression in an effort to attract monocytes and moreover up-regulation of iNOS to drive an alternative angiogenic process via NO. Interestingly, since TNF-α can also directly phosphorylate VE-cadherin43 the increase of TNF-α expression in CD11b KO mice was followed by an increased visual vascular leakage and a disorganized and aberrant vascular network after hepatectomy. In this regard we observed a significant reduction in the ratio VE-cadherin expression/VE-cadherin phosphorylation which can explain the vascular permeability effects that arise with imbalance in ECs junction stability. In fact, the exacerbated and uncontrolled increase of TNF-α is known to display suppressive effects on wound healing in mice.44
To regulate these vascular disorders KCs were progressively recruited into vessel walls from their static, basal physiological state and location in an attempt to replace the role of monocyte-EC interactions in the control of vascular and liver growth in KO mice. Thus, macrophage interaction with ICAM-1 in liver endothelium appears to be important for the regulation of the endothelium integrity but also in the control of TNF-α production during angiogenesis after hepatectomy.
In physiological liver regeneration then, MCP-1 and TNF-α released from KCs attract circulating monocytes to the vicinity and stimulate ECs to express ICAM-1 near endothelial junctions. The interaction of recruited monocytes with ICAM-1 promotes the phosphorylation of VE-cadherin only in selected locations that govern the passage of the leukocyte which will deliver growth factors to stimulate vascular sprouting (Figure 7B). In liver regeneration after chronic injury and fibrosis, macrophages display dual and differentiated roles. During the advance of liver fibrosis while the injury remains, accumulated macrophages stimulate inflammation and scarring via activation of myofibroblasts.45 In contrast recovery is marked by matrix degradation and repair.45 Hence, the balance between stimuli from milieu and the presence of different subsets of macrophages46 may determine the final behavior of these macrophages towards liver disease or repair.
In summary, liver regeneration is a complex process that requires the communication, coordination and interaction of different cell types to successfully recover hepatic mass. Our findings support a significant role for circulating monocytes in driving the sprouting of endothelial cells by direct interaction with liver vascular network. The binding of circulating monocytes to endothelial ICAM-1 is essential for selecting areas for vascular sprouting and sequentially coordination and stimulation of physiological vascular growth in accordance with hepatocyte and KC proliferation. These findings identify new mechanisms and potential targets for liver repair.
Supplementary Material
Acknowledgments
Financial support:
This work was supported by a grant from the NIH (R01 GM 49039). P.M-L was supported by a post-doctoral fellowship from the Fundacion Alonso Martin Escudero program 2012 and then by the Beatriu de Pinós Program, Modalitat-A awarded by AGAUR (fellowship number: 2013 BP_A 00051).
We acknowledge support provided by the David H. Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology for providing access to multiphoton microscopy used for this study.
Abbreviations
- EC
Endothelial cell
- KC
Kupffer Cells
- MCP-1
monocyte chemotactic protein 1
- NO
nitric oxide
- ICAM-1
Intercellular Adhesion Molecule 1
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 3.Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, et al. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002;236:703–711. doi: 10.1097/00000658-200212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diehl AM. Neighborhood watch orchestrates liver regeneration. Nat Med. 2012;18:497–409. doi: 10.1038/nm.2719. [DOI] [PubMed] [Google Scholar]
- 5.Viebahn CS, Benseler V, Holz LE, Elsegood CL, Vo M, Bertolino P, et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Schubert SY, Benarroch A, Ostvang J, Edelman ER. Regulation of endothelial cell proliferation by primary monocytes. Arterioscler Thromb Vasc Biol. 2008;1:97–104. doi: 10.1161/ATVBAHA.107.157537. [DOI] [PubMed] [Google Scholar]
- 7.Schubert SY, Benarroch A, Monter-Solans J, Edelman ER. Monocyte activation state regulates monocyte-induced endothelial proliferation through Met signaling. Blood. 2010;115:3407–3412. doi: 10.1182/blood-2009-02-207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schubert SY, Benarroch A, Monter-Solans J, Edelman ER. Primary monocytes regulate endothelial cell survival through secretion of angiopoietin-1 and activation of endothelial Tie2. Arterioscler Thromb Vasc Biol. 2011;4:870–875. doi: 10.1161/ATVBAHA.110.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monsalve E, Pérez MA, Rubio A, Ruiz-Hidalgo MJ, Baladrón V, García-Ramírez JJ, et al. Notch-1 up-regulation and signaling following macrophage activation modulates gene expression patterns known to affect antigen-presenting capacity and cytotoxic activity. J Immunol. 2006;176(9):5362–5373. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammator y responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108(3):965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 11.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45(5):1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation. Hepatology. 2014;60(3):964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1570–G1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- 14.Amemiya H, Kono H, Fujii H. Liver regeneration is impaired in macrophage colony stimulating factor deficient mice after partial hepatectomy: the role of M-CSF-induced macrophages. J Surg Res. 2011;165(1):59–67. doi: 10.1016/j.jss.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Norris CA, He M, Kang LI, Ding MQ, Radder JE, Haynes MM, et al. Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS One. 2014;24(4):e96053. doi: 10.1371/journal.pone.0096053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Bao Q, Kumar S, Hu M, Wang GY, Dai G. Four waves of hepatocyte proliferation linked with three waves of hepatic fat accumulation during partial hepatectomy-induced liver regeneration. PLoS One. 2012;7:e30675. doi: 10.1371/journal.pone.0030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 19.Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 20.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet P, De Smet F, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol. 2009;6:315–326. doi: 10.1038/nrclinonc.2009.64. [DOI] [PubMed] [Google Scholar]
- 22.Mei Y, Thevananther S. Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology. 2011;54:1777–1789. doi: 10.1002/hep.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olaku V, Matzke A, Mitchell C, Hasenauer S, Sakkaravarthi A, Pace G, et al. c-Met recruits ICAM-1 as a coreceptor to compensate for the loss of CD44 in Cd44 null mice. Mol Biol Cell. 2011;22:2777–2786. doi: 10.1091/mbc.E11-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 27.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 28.Hatanaka K, Simons M, Murakami M. Phosphorylation of VE-cadherin controls endothelial phenotypes via p120-catenin coupling and Rac1 activation. Am J Physiol Heart Circ Physiol. 2011;300:H162–H172. doi: 10.1152/ajpheart.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 30.Melgar-Lesmes P, Tugues S, Ros J, Fernández-Varo G, Morales-Ruiz M, Rodés J, et al. Vascular endothelial growth factor and angiopoietin-2 play a major role in the pathogenesis of vascular leakage in cirrhotic rats. Gut. 2009;58(2):285–292. doi: 10.1136/gut.2008.155028. [DOI] [PubMed] [Google Scholar]
- 31.Ribatti D, Crivellato E. "Sprouting angiogenesis", a reappraisal. Dev Biol. 2012;372:157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, et al. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am J Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, et al. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–674. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 36.Su AI, Guidotti LG, Pezacki JP, Chisari FV, Schultz PG. Gene expression during the priming phase of liver regeneration after partial hepatectomy in mice. Proc Natl Acad Sci U S A. 2002;99:11181–11186. doi: 10.1073/pnas.122359899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 38.Holland J, Owens T. Signaling through intercellular adhesion molecule 1 (ICAM-1) in a Bcell lymphoma line. The activation of Lyn tyrosine kinase and the mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:9108–9112. doi: 10.1074/jbc.272.14.9108. [DOI] [PubMed] [Google Scholar]
- 39.Wimmer R, Cseh B, Maier B, Scherrer K, Baccarini M. Angiogenic sprouting requires the fine tuning of endothelial cell cohesion by the Raf-1/Rok-α complex. Dev Cell. 2012;22:158–171. doi: 10.1016/j.devcel.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, et al. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94(9):1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Burns AR, Smith CW. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am J Pathol. 2006;169(5):1590–1600. doi: 10.2353/ajpath.2006.060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters T, Sindrilaru A, Hinz B, Hinrichs R, Menke A, Al-Azzeh EA, et al. Wound-healing defect of CD18(−/−) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J. 2005;24(19):3400–3410. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, et al. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1232–L1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- 44.Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119(12):3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinoshita M, Uchida T, Sato A, Nakashima M, Nakashima H, Shono S, et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.