Abstract
The prognostic impact of body mass index (BMI) in patients with upper tract urothelial carcinoma (UTUC) is an ongoing debate. Our study aimed to investigate the prognostic role of BMI in patients treated with radical nephroureterectomy (RNU) for UTUC from a multi-institutional Korean collaboration. We retrospectively reviewed data from 440 patients who underwent RNU for UTUC at four institutions in Korea. To avoid biasing the survival estimates, patients who had previous or concomitant muscle-invasive bladder tumors were excluded. BMI was categorized into approximate quartiles with the lowest quartile assigned to the reference group. Kaplan-Meier and multivariate Cox regression analyses were performed to assess the influence of BMI on survival. The lower quartile BMI group showed significantly increased overall mortality (OM) and cancer specific mortality (CSM) compared to the 25%-50% quartiles and upper quartile BMI groups. Kaplan-Meier estimates showed similar results. Based on multivariate Cox regression analysis, preoperative BMI as a continuous variable was an independent predictor for OM and CSM. In conclusion, preoperative underweight patients with UTUC in Korea survive less after RNU. Preoperative BMI may provide additional prognostic information to establish risk factors.
Graphical Abstract
Keywords: Body Mass Index; Carcinoma, Transitional Cell; Nephroureterectomy; Upper Urinary Tract; Survival
INTRODUCTION
Upper tract urothelial carcinoma (UTUC) is a relatively rare neoplasm and accounts for 5%-6% of all urothelial tumors. Approximately 20%-40% of patients initially present with locally advanced disease and lymph node metastases at the time of diagnosis (1,2,3,4). Radical nephroureterectomy (RNU) is the surgical standard of care for the treatment of patients with UTUC (5). However, contemporary oncologic outcomes remain poor because of the high risk of systemic recurrence following surgery (6). The 5-yr survival rates for patients classified with pT2 and pT3 stage disease are 73% and 40%, respectively, and the median survival for patients with T4 stage disease is estimated to be 6 months (7,8). Tumor stage, grade, lymph node status, lymphovascular invasion (LVI), multifocality, and histologic tumor necrosis are pathological variables identified as prognostic factors in UTUC following RNU (9,10). More accurate predictors of clinical outcomes could possibly lead to better identification and counseling for patients with potentially unfavorable outcomes who may benefit from adjuvant chemotherapy (11,12,13). Currently, knowledge of preoperatively assessable prognostic factors in UTUC is still limited. Preoperative prognostic factors including patients' body composition, age, gender, and previous bladder cancer history may offer the opportunity for more convenient stratification prior to operation, compared to conventional pathological risk factors (14). The relationship among obesity, cancer risk, and prognostic influence of body mass index (BMI) have been reported for numerous malignancies (15,16,17), but the impact of BMI on the prognosis of patients with UTUC is still poorly defined. Previous studies revealed contradictory results regarding the prognostic value of BMI in patients with UTUC after RNU. Most studies on Western cohort have shown that higher BMI is associated with worse survival outcomes (18,19), whereas studies of Asian population noted that increased BMI was an independent predictor for favorable survival (14,20). Asian populations have a higher percentage of body fat for a given BMI than Caucasians and the Asia Cohort Consortium proposed a new BMI cutoff for public health action in Asia (21). Difference in body composition profiles and inclusion criteria between ethnic groups may explain these discrepancies. However, only two Asian population studies examining the effect of BMI on the oncological outcomes after RNU have thus far been reported and limited by its small sample size. Moreover, no study in Korea has been reported yet. Therefore, we investigated the prognostic role of BMI in patients treated with RNU for UTUC from a large, multi-institutional Korean collaboration.
MATERIALS AND METHODS
Patients
After obtaining institutional review board (IRB) approval, a database of 505 patients with UTUC who underwent either open or laparoscopic RNU between 2001 and 2013 at four academic centers was reviewed. The database listed patient characteristics including age, gender, BMI, history of bladder cancer, pre-operative American Society of Anesthesiologists Physical Status (ASA-PS) score, surgical approach (open vs. laparoscopic), tumor pathology (stage, grade, lymph node status, and lymphovascular invasion), tumor necrosis, concomitant carcinoma in situ, tumor location, use of adjuvant chemotherapy, prior endoscopic therapy, unifocal or multifocal disease (UTUC multifocality was defined as the synchronous presence of multiple tumors in the renal pelvis or ureter), disease recurrence, mortality from urothelial carcinoma. All of the patients had complete follow-up data available and were considered for the analysis. A computerized databank was generated for the data transfer. After combining the data sets, reports were generated for each variable to identify data inconsistencies and other data integrity problems. Prior to final analysis, the database was frozen, and the final data set was produced for the current analysis. To avoid biasing the survival estimates, patients who had previous or concomitant muscle-invasive bladder tumors treated by cystectomy were excluded. Patients with distant metastasis at diagnosis and those who received neoadjuvant therapies were also excluded and 440 patients remained eligible.
Surgery was performed according to the standard criteria for RNU: extrafascial dissection of the kidney with the entire length of the ureter and the adjacent segment of the bladder cuff. The hilar and regional lymph nodes adjacent to the ipsilateral great vessel were generally resected if they were palpable intraoperatively or enlarged during preoperative axial imaging. All surgical specimens were processed according to the standard pathological procedures at each institution. The tumor was assessed according to the 2002 American Joint Committee on Cancer/Union Internationale Contre le Cancer TNM classification. Tumor grading was assessed using the 1998 World Health Organization/International Society of Urologic Pathology consensus classification.
Each patient was monitored according to the standard guidelines. In general, patients were evaluated every 3-4 months for the 1st year following nephroureterectomy, every 6 months from the second through the 5th year, and annually thereafter. Follow-up consisted of a history taking, physical examination, routine blood and serum chemistry laboratory work, urine cytology, chest radiography, cystoscopic evaluation of the urinary bladder, and radiographic evaluation of the upper urinary tract. Bone scans, chest computed tomography, or magnetic resonance imaging was performed when clinically indicated.
In this study, disease recurrence was defined as a locoregional recurrence or a new distant metastasis based on clinical and radiographic findings. Recurrence-free survival was defined as the period between surgery and the detection of recurrence, distant metastasis, or the study's endpoint. Time to cancer-specific survival was calculated as the time from surgery to the date of cancer-attributable mortality.
Statistical analysis
Preoperative BMI was classified by quartiles of the range: quartile 1, <21.7 kg/m2; quartile 2, 21.7-23.7 kg/m2; quartile 3, 23.8-25.7 kg/m2; quartile 4, ≥25.8 kg/m2. Continuous variables are shown as the median and interquartile range (IQR). Differences in variables with continuous distributions across dichotomous categories were assessed using ANOVA. The Fisher's exact and chi-square tests were used to evaluate the association between categorical variables. The statistical endpoints for our analysis were recurrence-free, cancer-specific, and overall survival of patients. Survival analyses were conducted according to the Kaplan-Meier method and survival characteristics were compared using the log-rank test. Univariate and multivariate survival analyses were performed using the Cox proportional hazard regression model. Statistical significance was considered to be P<0.05 and all reported P values are 2-sided. Analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was carried out in agreement with the applicable laws and regulations, good clinical practices, and ethical principles as described in the Declaration of Helsinki. The institutional review board of Yonsei University College of Medicine approved this study protocol (Approval number: 4-2014-0634). Informed consent was waived by the board.
RESULTS
Clinicopathological characteristics according to BMI categories
Table 1 lists the demographics and clinicopathological characteristics of the study population categorized by preoperative BMI quartiles. There were no significant differences among the subsets of patients in terms of clinicopathological characteristics including age, gender, history of previous or concomitant bladder cancer, ASA-PS score, pathologic stage, pathologic grade, lymph node status, lymphovascular invasion, surgical margin status, concomitant carcinoma in situ, tumor location, adjuvant chemotherapy, or multifocality (all P>0.05).
Table 1. Comparison of the demographics and clinicopathological characteristics according to BMI subgroup.
| Parameters | BMI quartiles | ||||
|---|---|---|---|---|---|
| 0%-25% (<21.7 kg/m2) |
25%-50% (21.7-23.7 kg/m2) |
50%-75% (23.8-25.7 kg/m2) |
75%-100% (≥25.8 kg/m2) |
P value | |
| No. of patients | 110 | 110 | 110 | 110 | |
| Age (yr, mean ± SD) | 68.0 ± 11.4 | 65.3 ± 10.8 | 65.6 ± 10.0 | 65.5 ± 10.3 | 0.182* |
| ASA-PS score | 0.153† | ||||
| 1 | 31 (25.9) | 29 (28.7) | 32 (31.4) | 26 (24.5) | |
| 2 | 59 (56.2) | 67 (66.3) | 65 (63.7) | 69 (65.1) | |
| 3 | 15 (14.3) | 5 (5.0) | 5 (4.9) | 11 (10.4) | |
| Smoking history (%) | 41 (37.3) | 25 (22.7) | 34 (30.9) | 25 (22.7) | 0.066† |
| Gender (%) | 0.666† | ||||
| Male | 80 (72.7) | 78 (70.9) | 75 (68.2) | 72 (65.5) | |
| Female | 30 (27.3) | 32 (29.1) | 35 (31.8) | 38 (34.5) | |
| Previous or concomitant NMIBC (%) | 18 (16.4) | 19 (17.3) | 30 (27.3) | 21 (19.1) | 0.164† |
| Tumor location (%) | 0.456† | ||||
| Renal pelvis | 46 (41.8) | 38 (34.5) | 41 (37.3) | 34 (30.9) | |
| Ureter | 49 (44.5) | 55 (50.0) | 51 (46.4) | 64 (58.2) | |
| Both | 15 (13.6) | 17 (15.5) | 18 (16.4) | 12 (10.9) | |
| Pathologic T stage (%) | 0.578† | ||||
| Tis | 1 (0.9) | 2 (1.8) | 2 (1.8) | 3 (2.7) | |
| Ta | 3 (2.7) | 6 (5.5) | 5 (4.5) | 9 (8.2) | |
| T1 | 34 (30.9) | 34 (30.9) | 32 (29.1) | 35 (31.8) | |
| T2 | 29 (26.4) | 21 (19.1) | 29 (26.4) | 32 (29.1) | |
| T3 | 40 (36.4) | 46 (41.8) | 41 (37.3) | 28 (25.5) | |
| T4 | 3 (2.7) | 1 (0.9) | 1 (0.9) | 3 (2.7) | |
| Pathologic N stage (%) | 0.486† | ||||
| Nx | 46 (41.8) | 55 (50.0) | 50 (45.5) | 61 (55.5) | |
| N0 | 59 (53.6) | 49 (44.5) | 54 (49.1) | 46 (41.8) | |
| N1-2 | 5 (4.5) | 6 (5.5) | 6 (5.5) | 3 (2.7) | |
| Tumor grade (%) | 0.463† | ||||
| Low | 23 (20.9) | 25 (22.7) | 30 (27.3) | 32 (29.1) | |
| High | 87 (79.1) | 85 (77.3) | 80 (72.7) | 78 (70.9) | |
| Multiplicity (%) | 30 (27.3) | 32 (29.1) | 27 (24.5) | 22 (20.0) | 0.434† |
| Concomitant CIS (%) | 4 (4.6) | 8 (8.4) | 7 (7.6) | 11 (11.5) | 0.403† |
| Lymphovascular invasion (%) | 19 (17.3) | 20 (18.2) | 19 (17.3) | 18 (16.4) | 0.988† |
| Positive surgical margin (%) | 8 (7.3) | 8 (7.3) | 3 (1.8) | 5 (4.5) | 0.209† |
| Adjuvant chemotherapy | 23 (20.9) | 21 (19.1) | 22 (20.0) | 12 (10.9) | 0.187† |
P value was based on the *ANOVA and †Fisher exact test. BMI, body mass index; SD, standard deviation; BC, bladder cancer; ASA-PS, American Society of Anesthesiologists Physical Status; NMIBC, non-muscle invasive bladder cancer; CIS, carcinoma in situ.
Locoregional recurrence/distant metastasis by BMI categories
The median follow-up period of the study cohort was 31 months (IQR 15 to 57 months). Overall, 111 patients (25.2%) experienced locoregional recurrence/distant metastasis. The recurrence/metastatic free survival at 3 and 5 yr were 72% and 67%, respectively. Compared to the lower quartile BMI group, the 25%-50% quartiles was associated with approximately 50% reduced odds of locoregional recurrence/distant metastasis (Table 2). Kaplan-Meier analyses did not exhibit significantly different recurrence free survival between BMI grouping (Figure not shown).
Table 2. Oncologic outcomes among the BMI quartiles in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma.
| BMI quartiles† | Recurrence (%) | OR (95%, CI)* | P | CSM (%) | OR (95%, CI)* | P | OM (%) | OR (95%, CI)* | P |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 (31.8) | 1 | 34 (30.9) | 1 | 29 (26.4) | 1 | |||
| 2 | 22 (20.0) | 0.479 (0.243-0.942) | 0.033 | 15 (13.6) | 0.327 (0.159-0.674) | 0.002 | 11 (10.0) | 0.282 (0.126-0.630) | 0.002 |
| 3 | 30 (27.3) | 0.757 (0.398-1.441) | 0.397 | 22 (20.0) | 0.566 (0.294-1.090) | 0.089 | 17 (15.5) | 0.521 (0.255-1.067) | 0.075 |
| 4 | 24 (21.8) | 0.711 (0.367-1.376) | 0.311 | 16 (14.5) | 0.385 (0.187-0.795) | 0.010 | 12 (10.9) | 0.358 (0.160-0.799) | 0.012 |
*ORs were calculated by unconditional logistic regression analysis and adjusted for pathologic stage and grade; †Preoperative BMI was classified by quartiles of the range: quartile 1, <21.7 kg/m2; quartile 2, 21.7-23.7 kg/m2; quartile 3, 23.8-25.7 kg/m2; quartile 4, ≥25.8 kg/m2. BMI, body mass index; CSM, cancer specific mortality; OM, overall mortality; CI, confidence interval; OR, odds ratio.
Overall mortality and cancer specific mortality by BMI categories
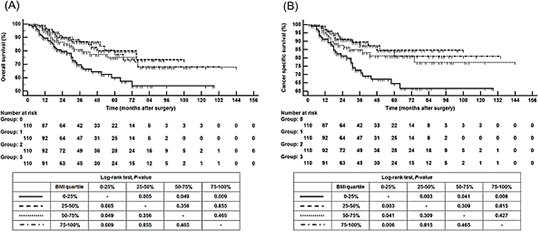
During follow up, 69 patients (15.7%) died of UTUC. The cancer specific survival (CSS) at 3 and 5 yr was 82% and 76%, respectively. The 25%-50% quartiles and upper quartile BMI groups were associated with approximately 60% reduced odds of overall mortality (OM) and cancer specific mortality (CSM) than the lower quartile BMI group following adjustment for pathologic T stage and pathologic grade (Table 2). Kaplan-Meier estimates showed that the lower quartile BMI group had poorer overall survival (OS) and CSS compared to the middle two quartiles and the upper quartile BMI groups (Fig. 1). Using multivariate Cox regression analysis, preoperative BMI as a continuous variables (HR, 0.922; CI, 0.853-0.998, P=0.045), ASA-PS score (≥3) (P=0.033), locally advanced stage or node positive state (P=0.047), lymphovascular invasion (P<0.001), and margin status (P=0.008) were independent predictors of OM (Table 3). On the other hand, preoperative BMI as a continuous variables (HR, 0.906; CI, 0.830-0.990, P=0.028), ASA-PS score (≥3) (P=0.025), locally advanced stage or node positive state (P=0.027), lymphovascular invasion (P<0.001), surgical margin (P=0.010) were independent predictors of CSM (Table 4).
Fig. 1. Kaplan-Meier survival curves. (A) Overall survival. (B) Disease-specific survival categorized by BMI in patients with upper tract urothelial carcinoma following radical nephroureterectomy.
Table 3. Univariate and multivariate Cox regression models for the prediction of overall mortality in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%, CI) | P value | HR (95%, CI) | P value | |
| Age (continuous) | 1.027 (1.004-1.050) | 0.021 | 1.019 (0.994-1.045) | 0.139 |
| Gender (female) | 1.000 (0.635-1.576) | 1.000 | ||
| ASA-PS score (≥ 3) | 2.084 (1.150-3.776) | 0.015 | 1.981 (1.057-3.711) | 0.033 |
| BMI (continuous) | 0.914 (0.853-0.979) | 0.010 | 0.922 (0.853-0.998) | 0.045 |
| Smoking history (yes) | 0.994 (0.612-1.614) | 0.980 | ||
| Tumor size (continuous) | 1.009 (1.003-1.015) | 0.004 | 1.005 (0.998-1.012) | 0.199 |
| Multiplicity (yes) | 1.590 (1.028-2.458) | 0.037 | 1.665 (0.939-2.952) | 0.081 |
| Pathologic T stage (≥ T3) and/or N stage (N1-2) | 3.025 (1.734-5.278) | < 0.001 | 1.964 (1.009-3.824) | 0.047 |
| Grade (high) | 2.723 (1.478-5.018) | 0.001 | 1.774 (0.861-3.658) | 0.120 |
| Concomitant CIS (yes) | 0.757 (0.275-2.085) | 0.591 | ||
| Lymphovascular invasion (yes) | 3.198 (2.061-4.960) | < 0.001 | 2.400 (1.469-3.921) | < 0.001 |
| Margin status (positive surgical margin) | 4.178 (2.256-7.738) | < 0.001 | 2.478 (1.261-4.871) | 0.008 |
| Adjuvant chemotherapy (yes) | 1.799 (1.108-2.921) | 0.017 | 1.407 (0.776-2.550) | 0.261 |
HR, hazard ratio; CI, confidence interval; ASA-PS, American Society of Anesthesiologists Physical Status; BMI, body mass index; CIS, carcinoma in situ.
Table 4. Univariate and multivariate Cox regression models for the prediction of cancer specific mortality in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%, CI) | P value | HR (95%, CI) | P value | |
| Age (continuous) | 1.015 (0.991-1.040) | 0.227 | ||
| Gender (female) | 1.192 (0.726-1.956) | 0.487 | ||
| ASA-PS score (≥ 3) | 2.069 (1.051-4.071) | 0.035 | 2.251 (1.107-4.579) | 0.025 |
| BMI (continuous) | 0.905 (0.838-0.977) | 0.011 | 0.906 (0.830-0.990) | 0.028 |
| Smoking history (yes) | 0.788 (0.444-1.397) | 0.414 | ||
| Tumor size (continuous) | 1.008 (1.001-1.015) | 0.021 | 1.006 (0.998-1.014) | 0.143 |
| Multiplicity (yes) | 1.566 (0.958-2.559) | 0.074 | ||
| Pathologic T stage (≥ T3) and/or N stage (N1-2) | 4.859 (2.325-10.154) | < 0.001 | 2.789 (1.125-6.914) | 0.027 |
| Grade (high) | 4.385 (1.895-10.146) | 0.001 | 2.720 (0.933-7.928) | 0.067 |
| Concomitant CIS (yes) | 0.706 (0.220-2.269) | 0.559 | ||
| Lymphovascular invasion (yes) | 4.176 (2.588-6.737) | < 0.001 | 3.097 (1.810-5.297) | < 0.001 |
| Margin status (positive surgical margin) | 4.559 (2.383-8.721) | < 0.001 | 2.530 (1.246-5.138) | 0.010 |
| Adjuvant chemotherapy (yes) | 2.355 (1.409-3.937) | 0.001 | 1.697 (0.904-3.187) | 0.100 |
HR, hazard ratio; CI, confidence interval; ASA-PS, American Society of Anesthesiologists Physical Status; BMI, body mass index; CIS, carcinoma in situ.
DISCUSSION
The current study investigated the prognostic role of BMI in a large, multi-institutional cohort of patients treated with RNU for UTUC. We categorized the patients' cohorts into quartiles rather than using established BMI cut-off points, and demonstrated that the preoperative BMI offers additional prognostic information.
Numerous studies have detected a significant association between preoperative BMI and cancer-susceptibility or aggressiveness in several hormone-related cancers, such as breast and endometrial cancer (22,23). BMI strongly correlates with densitometry estimates of body fat composition in adults, and excess body fat is associated with increased insulin, which increases insulin-like growth factor-I (IGF-I). IGF-I stimulates cell proliferation and suppresses apoptosis (24,25). Excess body fat is also associated with systemic inflammation. Adipose tissue produces a variety of inflammatory factors, including leptin, adiponectin, and cytokines (26,27). It is increasingly apparent that cancer progression depends on a complex interaction between the tumor and host inflammatory responses. On the other hand, there is a paucity of data on the influences of BMI in hormone-independent cancers such as urothelial carcinoma of the upper or lower urinary tract. There is no doubt that obesity strongly correlates with perioperative complications and morbidity or mortality; however, little is known about the prognostic value of BMI in patients with UTUC after radical surgery (28). Previous studies revealed contradictory prognostic outcomes associated with BMI in UTUC patients. Ehdaie et al. (18) showed that increased BMI adversely impacts oncological outcomes in patients with UTUC. They found a 37% difference in both 5-yr disease-free survival and CSS rates between patients with BMI ≥30 kg/m2 (49%, 47%) and patients with BMI <25 kg/m2 (86%, 84%). They also found that patients with a higher body mass index were more likely to have infiltrative architecture and lymphovascular invasion. In our cohort, none of clinicopathologic variables shows interesting differences between BMI quartiles. Regard to locoregional recurrence/distant metastasis, the 25%-50% quartile group have an approximately 50% reduced odds of locoregional recurrence/distant metastasis compared to the lower quartile BMI group, however, there was no significant differences among other subsets of patients. Thus we could not sure the potential association between BMI and aggressive tumor characteristics. A Canadian multicenter collaboration study reported that patients with higher BMI (BMI≥30 kg/m2) had worse RFS in patients undergoing RNU (1). However, they did not found any significant differences in OS or CSS among subsets of patients (BMI<25, 25 ≤BMI<30, and BMI ≥30 kg/m2). In contrast, using a cohort of 103 Japanese UTUC patients, Inamoto et al. (14) employed original BMI cut-off (22 kg/m2) and showed that increased BMI (BMI≥22 kg/m2) was an independent predictor for favorable OS and CSS. More recently, Liu et al. (20) analyzed single center data for 236 Chinese UTUC patients. In this study, the mean CSS time was 42.7 months for underweight patients, 55.1 months for normal patients and 61.2 months for obesity patients. They also demonstrated that a preoperative underweight was an independent predictor of unfavorable survival in patients with UTUC. They postulated three hypotheses to explain the improved outcomes for obese patients, including a functional barrier, increased energy sources, and multiple hormonal, endocrine, or nutritional factors. Although our data was similar to previous Asian cohort studies, it is hard to direct comparison of prognostic influence of BMI among studies because we did not use the established BMI criteria for Asians, issued by the Asia Cohort Consortium. Because there were only 16 patients (4% of total cohort) with underweight defined by Asia Cohort Consortium (BMI lower than 18.5 kg/m2) in our cohort, we categorized the patients into approximate quartiles instead of using established BMI cut-off points. By the definition of the Asia Cohort Consortium, preoperative underweight may overlap with tumor cachexia (29). Tumor cachexia has long been postulated to be a key determinant of cancer-related death. However, this hypothesis is applicable only for small part of the whole, actually, underweight defined by WHO criterion for the Asia Pacific Region accounts for approximately 4% of whole population, and 2.5% of men over thirties in Korea (30). In our preliminary analyses, optimal BMI cut-off value which gave optimal sensitivity and specificity for cancer specific mortality in patients treated with RNU for UTUC was 21.61 kg/m2 (data not shown). To determine the optimal BMI cut-off point can stratify patients with UTUC after RNU into unfavorable or favorable prognostic groups, additional collaboration studies in other populations are inevitable.
Our study had several inherent weaknesses. First, standardization of surgical techniques or practice guidelines such as adjuvant chemotherapy and follow-up protocols was impossible because of the multi-institutional and retrospective nature of the study. Relatively short periods of follow-up and its retrospective nature also warrant consideration. In addition, databank from four institutions did not handle the weight change. Weight loss has been shown to be an independent prognostic indicator of decreased survival in cancer patients. However, our study cohort has a relatively localized stage and metastatic cancer patients with severe cachexia were excluded.
In conclusion, preoperative underweight patients with UTUC in Korea survive less after RNU. Current high volume, multi-center study reconfirmed the adverse prognostic impact of preoperative underweight in Korean UTUC patients following RNU. International collaboration studies are needed to give an account for ethical contradictory results between Asia and the West.
Footnotes
DISCLOSURE: The authors have no potential financial conflicts on this subject.
AUTHOR CONTRIBUTION: Conception and design of the study: Kang HW, Ha YS. Acquisition of data: Kang HW, Ha YS, Byun SS, Yun SJ. Statistical analysis: Jung HD, Kim TH, Yun SJ. First draft of manuscript: Kang HW. Revision and critical review of the manuscript: Byun SS, Kwon TG, Kim WJ, Choi YD. Manuscript approval: all authors.
References
- 1.Ehdaie B, Shariat SF, Savage C, Coleman J, Dalbagni G. Postoperative nomogram for disease recurrence and cancer-specific death for upper tract urothelial carcinoma: comparison to American Joint Committee on Cancer Staging Classification. Urol J. 2014;11:1435–1441. [PubMed] [Google Scholar]
- 2.Tawfiek ER, Bagley DH. Upper-tract transitional cell carcinoma. Urology. 1997;50:321–329. doi: 10.1016/S0090-4295(97)00230-6. [DOI] [PubMed] [Google Scholar]
- 3.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG, et al. The Upper Tract Urothelial Carcinoma Collaboration. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 4.Cho YH, Seo YH, Chung SJ, Hwang I, Yu HS, Kim SO, Jung SI, Kang TW, Kwon DD, Park K, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J Urol. 2014;55:453–459. doi: 10.4111/kju.2014.55.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen E, et al. European Association of Urology. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka N, Kikuchi E, Shirotake S, Kanao K, Matsumoto K, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol. 2014;65:227–234. doi: 10.1016/j.eururo.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Salamanca JI, Shariat SF, Rodriguez JC, Chromecki TF, Ficarra V, Fritsche HM, Kassouf W, Matsumoto K, Cabello LO, Seitz C, et al. Prognostic role of ECOG performance status in patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int. 2012;109:1155–1161. doi: 10.1111/j.1464-410X.2011.10479.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 9.Xylinas E, Kluth L, Mangal S, Roupret M, Karakiewicz PI, Shariat SF. Predictive tools for clinical decision-making and counseling of patients with upper tract urothelial carcinoma. World J Urol. 2013;31:31–36. doi: 10.1007/s00345-012-0947-5. [DOI] [PubMed] [Google Scholar]
- 10.Seisen T, Colin P, Hupertan V, Yates DR, Xylinas E, Nison L, Cussenot O, Neuzillet Y, Bensalah K, Novara G, et al. Postoperative nomogram to predict cancer-specific survival after radical nephroureterectomy in patients with localised and/or locally advanced upper tract urothelial carcinoma without metastasis. BJU Int. 2014;114:733–740. doi: 10.1111/bju.12631. [DOI] [PubMed] [Google Scholar]
- 11.Krabbe LM, Bagrodia A, Lotan Y, Gayed BA, Darwish OM, Youssef RF, John G, Harrow B, Jacobs C, Gaitonde M, et al. Prospective analysis of Ki-67 as an independent predictor of oncologic outcomes in patients with high grade upper tract urothelial carcinoma. J Urol. 2014;191:28–34. doi: 10.1016/j.juro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, Fajkovic H, Lotan Y, Raman JD, Zigeuner R, et al. French National Database on Upper Tract Tumors; Upper Tract Urothelial Carcinoma Collaboration. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189:1662–1669. doi: 10.1016/j.juro.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Lin YK, Kaag M, Raman JD. Rationale and timing of perioperative chemotherapy for upper-tract urothelial carcinoma. Expert Rev Anticancer Ther. 2014;14:543–551. doi: 10.1586/14737140.2014.882774. [DOI] [PubMed] [Google Scholar]
- 14.Inamoto T, Komura K, Watsuji T, Azuma H. Specific body mass index cut-off value in relation to survival of patients with upper urinary tract urothelial carcinomas. Int J Clin Oncol. 2012;17:256–262. doi: 10.1007/s10147-011-0284-5. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiler G, Königsberg R, Fesl C, Mlineritsch B, Stoeger H, Singer CF, Pöstlberger S, Steger GG, Seifert M, Dubsky P, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol. 2011;29:2653–2659. doi: 10.1200/JCO.2010.33.2585. [DOI] [PubMed] [Google Scholar]
- 16.Simkens LH, Koopman M, Mol L, Veldhuis GJ, Ten Bokkel Huinink D, Muller EW, Derleyn VA, Teerenstra S, Punt CJ. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur J Cancer. 2011;47:2560–2567. doi: 10.1016/j.ejca.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 17.de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjöld B, Gutiérez J, Andersson M, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119:145–153. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 18.Ehdaie B, Chromecki TF, Lee RK, Lotan Y, Margulis V, Karakiewicz PI, Novara G, Raman JD, Ng C, Lowrance WT, et al. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol. 2011;186:66–72. doi: 10.1016/j.juro.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachir BG, Aprikian AG, Izawa JI, Chin JL, Fradet Y, Fairey A, Estey E, Jacobsen N, Rendon R, Cagiannos I, et al. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: results from a Canadian multicenter collaboration. Urol Oncol. 2014;32:441–448. doi: 10.1016/j.urolonc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Liu JY, Li YH, Liu ZW, Zhang ZL, Ye YL, Yao K, Jiang LJ, Han H, Qin ZK, Zhou FJ. Influence of body mass index on oncological outcomes in patients with upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. Int J Urol. 2014;21:136–142. doi: 10.1111/iju.12208. [DOI] [PubMed] [Google Scholar]
- 21.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 22.von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a Gynecologic Oncology Group study. Cancer. 2006;107:2786–2791. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 23.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 24.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 25.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 27.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, Cleary MP. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 28.Youssef RF, Gayed BA, Margulis V. Prognostic factors and oncological outcomes after radical nephroureterctomy for upper tract urothelial carcinoma: review of contemporary multi-center series. Open J Urol. 2012;2:246–252. [Google Scholar]
- 29.Boffetta P, McLerran D, Chen Y, Inoue M, Sinha R, He J, Gupta PC, Tsugane S, Irie F, Tamakoshi A, et al. Body mass index and diabetes in Asia: a cross-sectional pooled analysis of 900,000 individuals in the Asia cohort consortium. PLoS One. 2011;6:e19930. doi: 10.1371/journal.pone.0019930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korea Centers for Disease Control and Prevention. The Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-3) 2012. Cheongwon: Korea Centers for Disease Control and Prevention; 2013. [Google Scholar]