Abstract
Objective
Development of brown-like/beige adipocytes in white adipose tissue (WAT) helps to reduce obesity. Thus, we investigated the effects of resveratrol, a dietary polyphenol capable of preventing obesity and related complications in humans and animal models, on brown-like adipocyte formation in inguinal WAT (iWAT).
Methods
CD1 female mice (5-month-old) were fed a high-fat diet with/without 0.1% resveratrol. In addition, primary stromal vascular cells separated from iWAT were subjected to resveratrol treatment. Markers of brown-like (beige) adipogenesis were measured and the involvement of AMP-activated protein kinase (AMPK) α1 was assessed using conditional knockout.
Results
Resveratrol significantly increased mRNA and/or protein expression of brown adipocyte markers including uncoupling protein 1 (UCP1), PR domain-containing 16 (PRDM16), Cell death-inducing DFFA-like effector A (Cidea), elongation of very long chain fatty acids protein 3 (Elovl3), peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), cytochrome C and pyruvate dehydrogenase (PDH) in differentiated iWAT stromal vascular cells (SVC), suggesting that resveratrol induced brown-like adipocyte formation in vitro. Concomitantly, resveratrol markedly enhanced AMPKα1 phosphorylation and differentiated SVC oxygen consumption. Such changes were absent in cells lacking AMPKα1, showing that AMPKα1 is a critical mediator of resveratrol action. Resveratrol also induced beige adipogenesis in vivo along with the appearance of multiocular adipocytes, increased UCP1 expression and enhanced fatty acid oxidation.
Conclusion
Resveratrol induces brown-like adipocyte formation in iWAT via AMPKα1 activation and suggest that its beneficial anti-obesity effects may be partly due to the browning of WAT and as a consequence, increased oxygen consumption.
Keywords: beige adipocytes, obesity, resveratrol, stromal vascular cells, uncoupling protein 1, white adipose tissue
Introduction
Mammals have two morphologically and functionally distinct types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), both of which are involved in energy homeostasis. WAT mainly stores energy in the form of lipids (triglycerides) in unilocular white adipocytes and secretes a number of adipokines and other factors, such as leptin, adiponectin, tumor necrosis factor α (TNF-α), and interleukin-6 (IL-6), to regulate energy metabolism and immune function 1, 2. Excessive WAT accumulation that occurs in obesity is a major risk factor for developing insulin resistance, type 2 diabetes mellitus and cardiovascular diseases 3, 4. In contrast, BAT specializes in dissipating energy as heat due to its high mitochondrial content and expression of uncoupling protein 1 (UCP1) 5, 6. However, the amount of BAT found in adults is typically quite low7. Recently, brown-like adipocytes were discovered in WAT, so called beige adipocytes 8, 9. Similar to brown adipocytes, beige adipocytes express UCP1 to dissipate energy. Thus, stimulating the development of beige adipocytes in WAT, so called ‘browning’, might reduce adverse effects of WAT and could help to improve metabolic health 10-12.
There are many transcriptional regulators including PRDM16, PGC1α, C/EBPα and PPARγ, as well as various secreted mediators, such as bone morphogenetic protein 7 (BMP7), Irisin, fibroblast growth factor 21 (FGF21), atrial and brain natriuretic peptides, that can induce the formation of brown-like adipocytes 6, 10, 11, 13, 14. Meanwhile, certain pharmacological and nutritional agents, are also involved in promoting WAT browning 15, 16 by activating transcription factors or related regulatory signaling pathways 17. As a nutritional or dietary supplement, resveratrol, a natural polyphenol present in the skin of grapes and other plants, has remarkable beneficial effects on energy metabolism and related disorders in mammals 18, 19. It has been reported that resveratrol protects against high-fat diet induced obesity in mice 20, 21 and elicits beneficial effects on obese persons 22, 23. Resveratrol also inhibits adipogenesis 24-26 and enhances fat mobilization 27-29. Resveratrol increased UCP1 expression in 3T3-L1 cells 25, and enhanced the mitochondrial DNA content and UCP1 expression in primary mouse embryonic fibroblasts (MEF)-derived adipocytes 30. To date, studies regarding resveratrol in adipose tissue mainly focus on the white adipogenesis and lipid metabolism, and the effects of resveratrol on the formation of brown-like or beige adipocytes remains sparsely studied. To our knowledge, there is no report about mechanisms in which resveratrol induces the formation of brown-like adipocytes.
The effects of resveratrol on metabolic health are due, at least in part, to its ability to activate the AMP-activated protein kinase (AMPK), a master regulator of energy metabolism 15, 19. AMPK consists of one α-catalytic subunit and two regulatory subunits, β and γ. The catalytic subunit of AMPK has two isoforms, α1 and α2, which have different tissue expression patterns. In adipose tissue, the α1 catalytic subunit is the predominant isoform expressed 31, 32, while the α2 isoform is highly expressed in muscle and liver but at a low level in adipose tissue 33, 34. The measurement of AMPK activity indicates that the α1 isoform accounts for the majority of the total activity of this kinase in adipose tissue 32, 35. Although there are a number of studies on the role of AMPK in adipose tissue metabolism 21, 36, 37, it is unclear whether AMPK is involved in the browning of white adipocytes.
In the present study, we sought to elucidate the role of resveratrol in brown-like adipocyte formation in WAT and to explore the mechanism underlying this process. Our data show that resveratrol induces browning of white fat, a process mediated by AMPKα1.
Materials and methods
Animals
Twelve adult CD1 female mice (5-month-old) were randomly divided into 2 groups: a control group, which was fed a high-fat diet (HFD; 45% energy from fat, D12451, Research Diet, New Brunswick, NJ), a resveratrol (Resv) group, which was fed a HFD containing 0.1% (w/w) resveratrol. Mice were housed in environmentally controlled rooms on a 12-h light-dark cycle with free access to food and water. Before and after the treatment, we measured the basal metabolic rate (BMR) (oxygen consumption (VO2), CO2 production (VCO2) and respiratory exchange ratio (RER)) of mice during the day (quiescent phase) using a CLAMS (Columbus Instruments, Columbus, OH) indirect open circuit calorimetry system. We deprived the mice of food for 4 h prior to measurement and continuously measured for 3 h (with water provided), taking a measurement every 30 s 38, 39. We used the lowest 10 consecutive measures (5 min) as the estimate of BMR.
Body weight and food intake were measured weekly. At the end of 4 weeks of treatment, mice were sacrificed by carbon dioxide anesthesia. Inguinal WAT (iWAT) was rapidly isolated and weighed. One side of the adipose tissues were frozen in liquid nitrogen and stored at -80°C until further analyses. A middle portion of the other side was fixed in 4% paraformaldehyde for sectioning and staining. Another portion of the other side was cultured in DMEM/F12 medium for tissue oxygen consumption measurement. Wild type and RosaCre/AMPKα1flox/flox C57BL/6 mice (Jackson Lab, Bar Harbor, Maine) were housed in environmentally controlled rooms on a 12-h light-dark cycle with free access to food and water. All animal experiments and care procedures were performed according to protocols pre-approved by the Institutional Animal Care and Use Committees (IACUC) at Washington State University.
Antibodies and chemicals
Antibodies against AMPKα (#2532), phospho-AMPKα at Thr172 (#2535), pyruvate dehydrogenase (PDH) (#2784), cytochrome c (#4280), β-tubulin (#2146) and goat anti-rat antibody Alexa Fluor 488 (#4416) were purchased from Cell Signaling (Danvers, MA). Anti-PRDM16 polyclonal antibody (#ABD130) was purchased from Millipore (Billerica, MA). Anti-UCP1 polyclonal antibody (#sc28766) was bought from Santa Cruz Biotechnology (Dallas, TX). Goat anti-rabbit IRDye 800CW (#926-32211) and goat anti-rabbit IRDye 680RD (#926-68070) secondary antibodies for western blot were purchased from LI-COR (Lincoln, NE). Fluoro-Gel II with 40, 6-diamidino-2-phenylindole (DAPI) (#17985-50) was purchased from Electron Microscopy Sciences (Hatfield, PA). Insulin, dexamethasone, indomethacin, 3-isobutyl-1-methylxanthine (IBMX), Triiodothyronine (T3), Oil-Red O, and compound C were purchased from Sigma (St. Louis, MO). Collagenase D and dispase II were purchased from Roche Diagnostics (Indianapolis, IN). DMEM/F12 and fetal bovine serum (FBS) were purchased from Life Technologies (Grand Island, NY).
Stromal vascular cell (SVC) isolation and in vitro differentiation
SVC were isolated from iWAT as previously described 40. The medium was changed every other day41. To induce brown adipogenic differentiation of SVC, confluent SVC were cultured in DMEM/F12 containing 10% FBS, 1% penicillin-streptomycin solution with 5 μg/ml insulin, 1 nM T3, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) and 0.125 mM indomethacin for 2 days. The cells were then switched to DMEM/F12 supplemented with 10% FBS and 5 μg/ml insulin for 5 more days and the medium was changed every other day 42. For SVC from iWAT of weaning Rosa26Cre/AMPKα1flox/flox mice, confluent SVC were treated with 250 nM 4-hydroxytamoxifen (4-OHT) for 2 days to delete AMPKα1 before being induced to undergo brown adipogenic differentiation 43.
In vitro O2 consumption assay
In vitro O2 consumption measurement was performed with Thermo Scientific Orion 3-Star Dissolved Oxygen meter and probe (Thermo Electron Corporation, Madison, WI) 44. Equal numbers of iWAT SVC were seeded and treated with vehicle (control) or 10 μM resveratrol (Resv) to induce differentiation. On day 7, the differentiated SVC were changed to fresh DMEM/12 for 30 min. The dissolved oxygen (DO) in the medium were measured at the start and end of incubation. For the tissues, a thin slice (50 mg) of iWAT from control and resveratrol fed mice were cultured in medium for 1 h, and DO was measured before and after incubation. O2 consumption of differentiated SVC or iWAT were calculated as the rate of decrease in DO 45.
Oil-Red O staining
Differentiated cells were subjected to Oil-Red O staining as previously described 46.
Immunostaining of cells and tissue sections
Immunofluorescence staining of cells was conducted as previously described 43. Fluorescence was examined and images were acquired using an EVOS fl fluorescence microscope (Advanced Microscopy Group, Bothell, WA). As for iWAT, paraffin-embedded iWAT sections (5 μm thick) were either stained with hematoxylin and eosin (H&E) 47 or used for UCP1 immunohistochemical (IHC) staining 48. Adipocyte diameters were analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD).
Real-time quantitative PCR
Total RNA was extracted from differentiated iWAT SVC cells using TRIzol reagent (Sigma, St. Louis, MO) according to the manufacturer’s protocol and cDNA was synthesized from 0.5 μg of total RNA using a reverse transcription kit (Bio-Rad, Hercules, CA). Real-time quantitative PCR was carried out in the final 10 μl volume of the amplification mixture containing 2x Qprecise Green Master Mix (EarthOx, LLC, San Francisco, CA), primers, and cDNA using a CFX RT-PCR detection system (Bio-Rad). Δ cycle threshold (CT) was used to calculate the differences between the target CT value and the control (18S) for each sample: Δ CT = CT (target)-CT (control). The relative expression level was calculated using 2−ΔCT. The following cycle parameters were used: 40 two-step cycles of 95°C for 15 s, 58°C for 60 s 46. Primer sequences (with their respective PCR fragment lengths) were shown in Table 1.
Table 1. The primer sequences used for real-time quantitative PCR.
| Gene | Forward (5’-3’) | Reverse (3’-5’) | Amplicon size (bp) | Gene access number |
|---|---|---|---|---|
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 | NR_046233.2 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | 136 | NM_007702.2 |
| CD137 | GTCGACCCTGGACGAACTGCTCT | CCTCTGGAGTCACAGAAATGGTGGTA | 132 | NM_001077509.1 |
| Elovl3 | GATGGTTCTGGGCACCATCTT | CGTTGTTGTGTGGCATCCTT | 73 | XM_006526624.1 |
| PGC1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC | 161 | XM_006503779.1 |
| PRDM16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG | 87 | NM_001291029.1 |
| Tbx1 | TGAAGAAGAACCCGAAGGTGG | ACTTGGAACGTGGGGAACATT | 133 | XM_006536887.1 |
| TMEM26 | GAAACCAGTATTGCAGCACCCAAT | AATATTAGCAGGAGTGTTTGGTGGA | 205 | NM_177794.3 |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG | 123 | NM_009463.3 |
Western blot analysis
Western blot was conducted as previously described 46. Immunoreactive proteins in the membrane were scanned and analyzed by Odyssey Infrared Imaging System (LI-COR, Inc., Lincoln, NE). Band density was normalized according to the β-tubulin content.
Statistical analysis
The in vitro data were generated from three independent experiments and 3 parallels were used in each experiment. The in vivo data were obtained from one experiment, with six mice in each treatment. Data are presented as means ± standard error of the means (SEM). Statistical analysis was performed using Sigmaplot 12.5 (Systat Software, Inc., San Jose, CA). Differences between means were determined using Student’s t-test or one way analysis of variance (ANOVA) followed by Duncan’s multiple test when appropriate and a confidence level of P < 0.05 was considered to be statistically significant.
RESULTS
Resveratrol exerts dose dependent effects on brown adipogenic differentiation of iWAT SVC
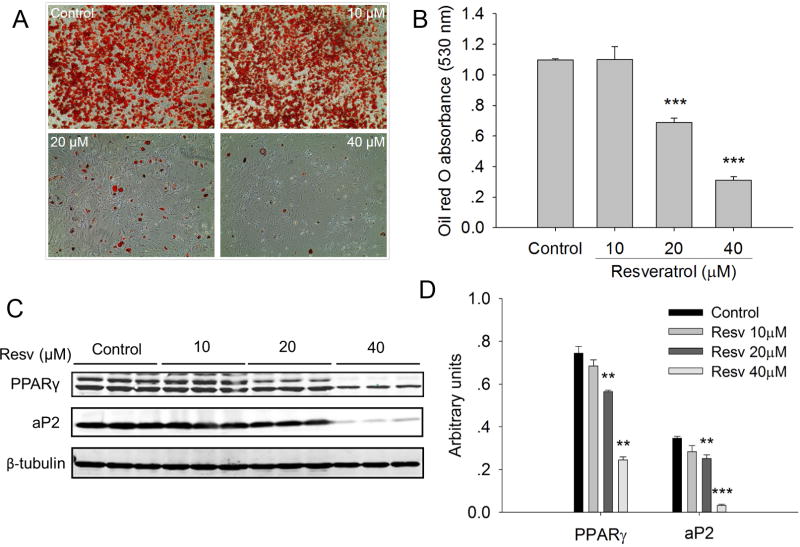
First, we investigated the effects of resveratrol on brown adipogenic differentiation of iWAT SVC. The result of Oil Red O staining demonstrated that the higher concentrations (20 μM or 40 μM) of resveratrol significantly (P < 0.001) inhibited lipid accumulation in the differentiated iWAT SVC after 7-day brown adipogenic differentiation (Fig. 1A, B) and suppressed the expression of adipogenic markers PPARγ and aP2 (Fig. 1C, D). Similar inhibitory effects of resveratrol on white adipogenesis were observed previously 24, 25. On the other hand, at the concentrations 10 μM or lower, resveratrol had no effect on lipid accumulation.
Figure 1.
Effects of resveratrol on the lipid accumulation and the expression of adipogenic marker genes in differentiated inguinal WAT (iWAT) SVC. A) Oil-Red O staining was conducted in the differentiated iWAT SVC after 7-day brown adipogenic differentiation. Microscopic pictures were taken on day 7 with × 100 magnification. B) The stained Oil-Red O was extracted with isopropanol. The absorbance of the extracted Oil-Red O was spectrophotometrically determined at 530 nm to measure triglyceride (TG) accumulation. C, D) Western blot analysis of adipogenic marker genes (PPARγ and aP2) in the differentiated iWAT SVC after 7-day brown adipogenic differentiation, and β-tubulin was used as a loading control (C). Mean ± SEM of immunoblotting bands of PPARγ and aP2 (D). The intensities of the bands were expressed as arbitrary units. ** P < 0.01 and *** P < 0.001 versus control.
Resveratrol promotes formation of brown-like adipocytes in differentiated iWAT SVC cells
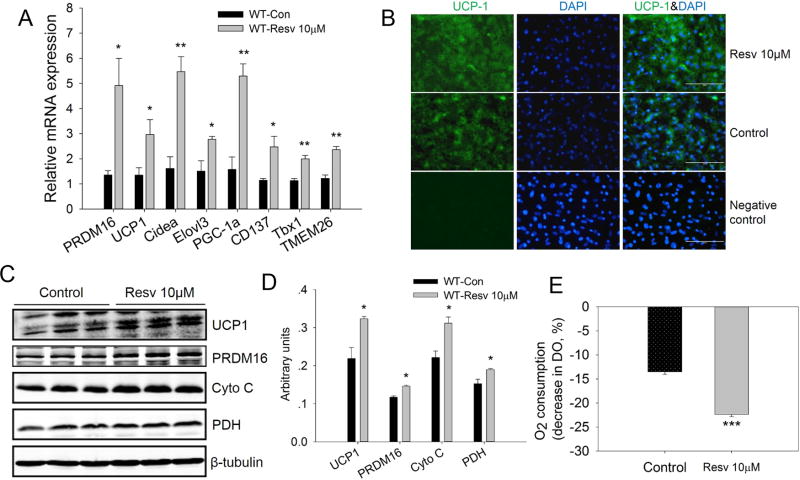
Although low concentrations of resveratrol had no effect on lipid accumulation during brown adipogenic differentiation of iWAT SVC, we further determined whether resveratrol stimulated the generation of brown-like adipocytes by analyzing the mRNA expression of brown adipocyte specific genes. As shown in Fig. 2A, resveratrol increased the mRNA level of PRDM16 (3.6-fold versus control, P < 0.05), a key transcription factor regulating brown adipogenesis. UCP1 expression, which is specific to brown adipocytes and does not occur in white adipocytes, was also markedly elevated (2.2-fold versus control, P < 0.05). In addition, expression of Cidea, a gene predominantly expressed in brown adipocytes, and Elovl3, a very long chain fatty acid elongase that is expressed in brown but not white fat, increased 3.4-fold (P < 0.01) and 1.8-fold (P < 0.05), respectively, in resveratrol group. Moreover, resveratrol increased the mRNA expression of PGC1α (3.3-fold, P < 0.01), the master regulator of mitochondrial biogenesis and oxidative phosphorylation. Finally, resveratrol promoted the mRNA expression of beige adipocytes selective markers such as CD137 (1.8-fold, P < 0.05), Tbx1 (1.9-fold, P < 0.01), and TMEM26 (2.1-fold, P < 0.01).
Figure 2.
Resveratrol promoted formation of brown-like adipocytes in the differentiated iWAT SVC cells from wild-type mice after 7-day adipogenic differentiation. A) Relative mRNA levels of brown adipocytes selective genes (PRDM16, UCP1, Cidea, Elovl3 and PGC-1a) and beige adipocytes selective genes (CD137, Tbx1 and TMEM26). B) UCP1 immunofluorescence staining for UCP1 in the differentiated iWAT SVC. Nuclei were stained with DAPI (scale bar, 100 μm). C, D) Western blot analysis of brown adipocytes selective genes (UCP1, PRDM16, Cyto C, and PDH) in the differentiated iWAT SVC, and β-tubulin was used as loading control (C); Mean ± SEM of immunoblotting bands of UCP1, PRDM16, Cyto C and PDH (D). The intensities of the bands were expressed as arbitrary units. E) Basal O2 consumption of differentiated iWAT SVC from control and resveratrol treated groups. *P < 0.05, **P < 0.01 and *** P < 0.001 versus control.
Immunostaining results showed that the expression of UCP1 in the resveratrol-treated group was higher than that of control group (Fig. 2B) and this was confirmed by western blotting as the UCP1 protein level in resveratrol treated cells was 1.5-fold higher than that of control cells (P < 0.05). Consistent with mRNA expression, the protein level of PRDM16 was also markedly increased in the resveratrol treatment group (1.2-fold versus control, P < 0.05). Moreover, the protein levels of cytochrome C (Cyto C) (1.4-fold versus control, P < 0.05) and pyruvate dehydrogenase (PDH) (1.2-fold versus control, P < 0.05), which represent the mitochondrial content, were also elevated by resveratrol (Fig. 2C, D). The mRNA and protein expression data together provided evidence that resveratrol promoted the formation of brown-like adipocytes of iWAT SVC.
Browning of WAT is expected to similarly increase cellular respiration. To investigate whether resveratrol elevated cellular respiration, O2 consumption of differentiated iWAT SVC was measured after 7 days of treatment. Consistent with increased browning, the basal oxygen consumption in resveratrol group was 1.6-fold higher than that of control cells (P < 0.001) (Fig 2E).
Resveratrol stimulates the phosphorylation of AMPKα in SVC
In order to determine whether AMPKα was involved in the resveratrol-mediated browning effects, we examined the effects of resveratrol on the phosphorylation of AMPKα (p-AMPKα). As shown in Fig. 3, resveratrol increased the phosphorylation of AMPKα in differentiated wild-type iWAT SVC cells (1.3-fold versus control, P < 0.05), with no effect on total AMPKα (t-AMPKα). In addition, the ratio pAMPKα/ t-AMPKα was elevated (1.2-fold versus control, P < 0.01) in resveratrol treated group. Furthermore, the protein level of Sirt1 was also higher (1.5-fold versus control, P < 0.05) due to resveratrol treatment. When the confluent iWAT SVC was treated with 4-OHT to knockout AMPKα1 acutely before brown adipogenic differentiation, the expressions of p-AMPKα, t-AMPKα and Sirt1 in differentiated iWAT SVC were much lower than seen in wild type cells. Moreover, we found that resveratrol had no effect on the protein levels of p-AMPKα, t-AMPKα or Sirt1 in AMPKα1 knockout SVC cells.
Figure 3.
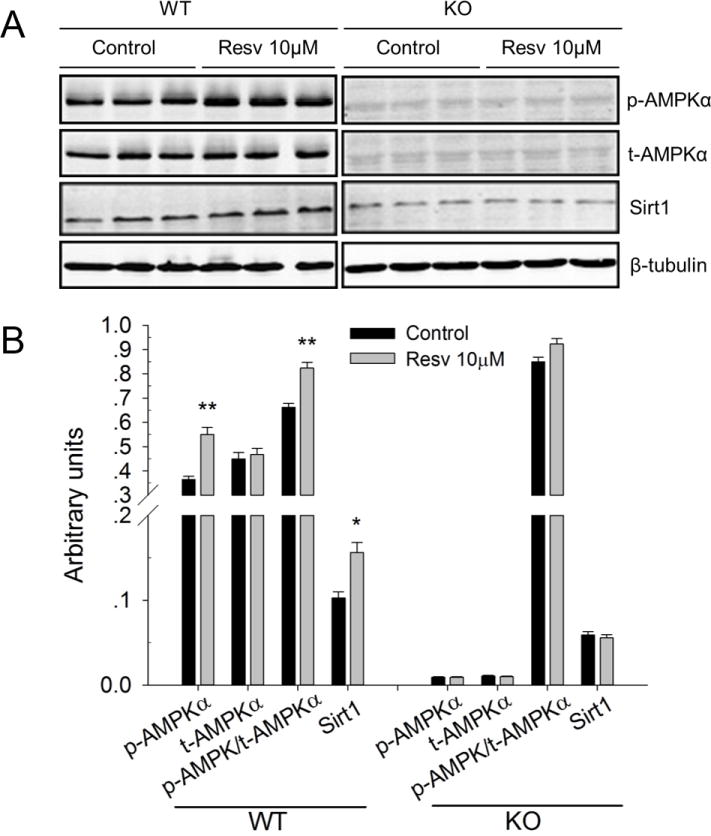
Effects of resveratrol on the phosphorylation of AMPKα and Sirt1 in wild type and AMPKα1 knockout iWAT SVC. A) Western blot analysis of phospho-AMPKα (p-AMPKα), t-AMPKα (t-AMPKα) and Sirt1 in the differentiated iWAT SVC of wild type (left part) and AMPKα1 deletion (right part). β-Tubulin was used as loading control. B) Mean ± SEM of immunoblotting bands of p-AMPKα, t-AMPKα, p-AMPKα/t-AMPKα and Sirt1 in wild type and AMPKα1 knockout cells. The intensities of the bands were expressed as arbitrary units. *P < 0.05 versus control.
AMPK inhibition or AMPKα1 deletion eliminate the browning effects of resveratrol on mouse iWAT SVC
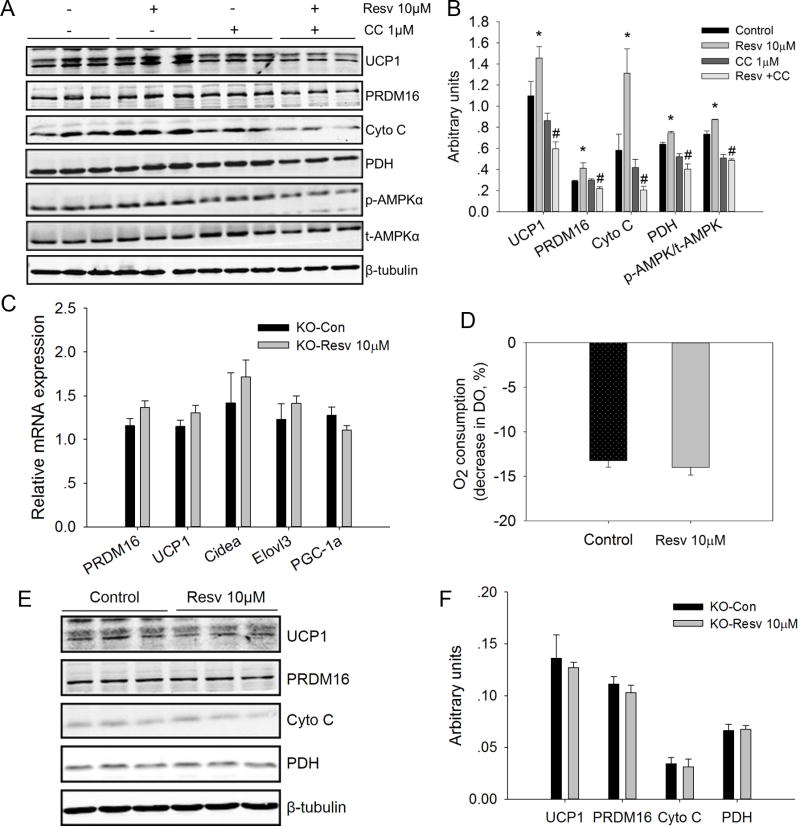
AMPK inhibitor Compound C was used to examine the effects of AMPK inhibition on the resveratrol mediated browning effects on mouse iWA SVC. We found that Compound C (1μM) did inhibit the activation of AMPK (p-AMPK/t-AMPK) (Fig. 4A, B). Although Compound C had no effects on the expression of UCP1, PRDM16, Cyto C and PDH. However, Compound C inhibited the promotional effects of resveratrol on the expression of these genes (Fig. 4A, B).
Figure 4.
AMPK inhibition or AMPKα1 deletion eliminated the browning effects of resveratrol on mouse differentiated iWAT SVC. A) Effects of AMPK inhibitor Compound C (CC) in the protein contents of UCP1, PRDM16, Cyto C, PDH, phospho-AMPKα (p-AMPKα), and t-AMPKα (t-AMPKα) in the differentiated iWAT SVC after 7-day brown adipogenic differentiation. β-Tubulin was used as loading control. B) Mean ± SEM of immunoblotting bands of UCP1, PRDM16, Cyto C, PDH, p-AMPKα/t-AMPKα. The intensities of the bands were expressed as arbitrary units. *P < 0.05 versus control, #P < 0.05 versus Resv 10 μM. C) Relative mRNA levels of brown adipocyte selective genes (PRDM16, UCP1, Cidea, Elovl3, and PGC1α) in the differentiated SVC after 7-day differentiation with classical brown adipogenic induction cocktails. SVC cells from iWAT of weaning Rosa26Cre/AMPKα1flox/flox mice were treated with 4-hydroxytamoxifen (4-OHT) to delete AMPKα1 before being induced to undergo brown adipogenic differentiation. D) Basal O2 consumption of differentiated AMPKα1 knockout iWAT SVC from control and resveratrol treated groups. E, F) Western blot analysis of brown adipocyte selective genes (UCP1, PRDM16, Cyto C, and PDH) in the differentiated SVC after 7-day brown adipogenic differentiation, and β-tubulin was used as loading control (E). Mean ± SEM of immunoblotting bands of UCP1, PRDM16, Cyto C and PDH (F). The intensities of the bands were expressed as arbitrary units.
We also tested whether acute Ampkα1 deletion affected the browning effects of resveratrol on iWAT SVC. To this end, iWAT SVC isolated from weaning Rosacre/Ampkα1flox/flox mice that ubiquitously express a tamoxifen-inducible Cre recombinase were treated with 4-OHT to induce AMPKα1 knockout acutely. In the absence of AMPKα1, resveratrol had no effects on the mRNA expression of PRDM16, UCP1, Cidea, Elovls and PGC1a (Fig. 4C). Consistently, after deletion of AMPKα1, the protein levels of UCP1, PRDM16, Cyto C and PDH in the resveratrol treatment group did not differ from those in the control group (Figure 4E, F). Furthermore, after knocking out AMPKα1, the basal oxygen consumption of differentiated iWAT SVC was not affected by resveratrol treatment (Fig. 4D). These results suggested that AMPKα1 has a major role in mediating the browning effect of resveratrol on iWAT SVC.
Resveratrol reduces body weight, iWAT index and stimulates browning of iWAT
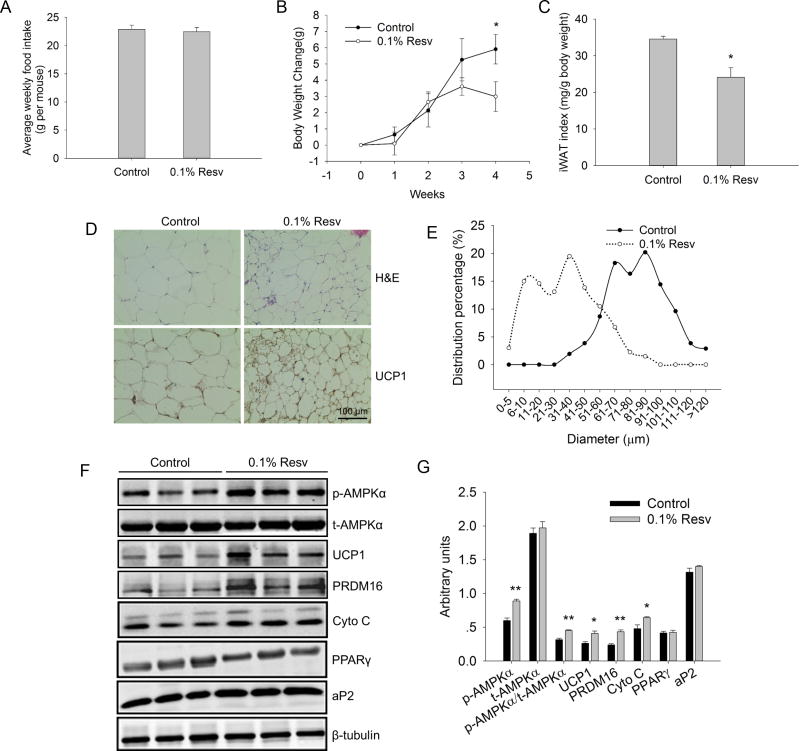
To further analyze browning effects of resveratrol on iWAT in vivo, 5-month-old CD1 mice were treated HFD or HFD containing 0.1% resveratrol for 4 weeks. While there was no apparent difference in food intake (Fig. 5A), the body weight gain in resveratrol supplemented group was lower than that of control group (2.99 ± 0.91 g versus 5.91 ± 0.90 g of control, P < 0.05) (Fig. 5B). In addition, resveratrol markedly reduced the body iWAT index (iWAT mass/body weight) (24.15 ± 2.56 versus 34.57 ± 0.76 of control, P < 0.05) (Fig. 5C), with no effect on blood glucose level. H&E staining results revealed that the average adipocyte diameter in resveratrol supplemented group was much smaller than that of control group (33.29 ± 1.90 μm versus 81.07 ± 1.94 μm of control, P < 0.001) (Fig. 5D). Inspection of the distribution of cell sizes indicated that diameter of most adipocytes (about 70%) in the control group was in the range of 61-100 μm. In contrast, diameter of most adipocytes (about 90%) in the resveratrol supplemented group was smaller than 60 μm (Fig. 5E). Moreover, iWAT from resveratrol treated mice showed the appearance of multiocular adipocytes within white fat, a characteristic of brown adipocytes (Fig. 5D), suggesting that resveratrol induced brown-like remodeling (browning) of iWAT. Subsequently, IHC staining of UCP1 indicated an enhanced UCP1 staining in resveratrol treated mice (Fig. 5D). In agreement, the UCP1 protein content in the resveratrol group was 1.5-fold higher than that of the control group (P < 0.05). Furthermore, resveratrol supplement resulted in increased protein contents of PRDM16 (1.8-fold versus control, P < 0.01) and Cyto C (1.3-fold versus control, P < 0.05), which was accompanied by the elevated expression of p-AMPKα (1.5-fold versus control, P < 0.01) (Fig. 5F, G), suggesting that AMPKα was involved in the resveratrol-induced browning of iWAT. We also found that resveratrol supplement had no effect on the protein expression levels of PPARγ and aP2.
Figure 5.
Resveratrol induced brown-like adipocytes in iWAT. A) Weekly food intake were measured in control (n=6) and 0.1% Resv (n=6) groups. B) Body weight changes were compared between control and 0.1% Resv groups during 4 weeks. C) iWAT index was compared between control and 0.1% Resv groups. D) Representative images of H&E and UCP1 IHC staining in sections of iWAT of control and 0.1% Resv treated mice. All images were obtained at × 400 magnification. E) Distribution percentage of adipocyte diameters from control and 0.1% Resv treated mice. Data analysis from the H&E staining sections. F, G) Western blot analyses of p-AMPKα, t-AMPKα, UCP-1, PRDM 16, Cyto C and adipogenic marker genes (PPARγ and aP2) were performed in iWAT of control and resveratrol treated mice, and β-tubulin was used as the loading control (F). Mean ± SEM of immunoblotting bands of p-AMPKα, t-AMPKα, p-AMPKα/t-AMPKα, UCP-1, PRDM16, Cyto C, PPARγ and aP2 (G). The intensities of the bands were expressed as arbitrary units.*P < 0.05, **P < 0.01 versus control.
We also analyzed the serum profiles (Table 2). While there was no difference in non-fasting glucose, the insulin level was lower in resveratrol treated mice (P < 0.05). In addition, the triglyceride concentration was also reduced in resveratrol supplemented compared to control mice (P < 0.05).
Table 2. Serum profiles of Con and Resveratrol treated CD1 mice fed a high fat diet.
| Treatments | Control (Con) | Resveratrol (Resv) | P-value |
|---|---|---|---|
| Insulin (ng/ml) | 1.48 ± 0.279 | 0.85 ± 0.136 | < 0.05 |
| Triglyceride (mg/dl) | 145.0 ± 28.2 | 87.4 ± 9.33 | < 0.05 |
| Glucose (mg/dl) | 188 ± 16.7 | 196 ± 8.2 | n.s. |
Mice were fed a high energy diet with 45% energy from fat for 4 weeks, and with/without 0.1% resveratrol.
Mice were not fasted before collection of blood samples for analyses. n = 6.
Resveratrol promotes lipid oxidation in iWAT
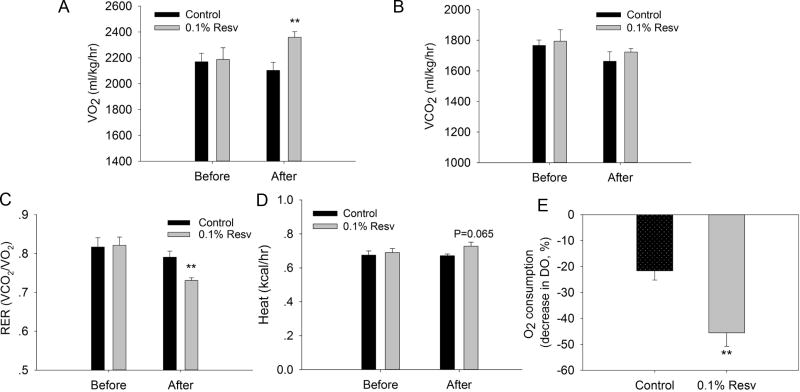
To explore why resveratrol feeding reduced the body weight gain and iWAT mass under HFD, we measured the oxygen consumption of mice. Resveratrol treatment significantly increased the oxygen consumption (VO2) of mice (2,359 ± 43 ml/kg/h versus 2,103 ± 61 ml/kg/h of control, P < 0.01) (Fig. 6A). There was no difference in CO2 production (VCO2) between control and resveratrol group before and after treatment (Fig. 6B). As a result, resveratrol decreased the respiratory exchange rate (RER, VCO2/VO2) (0.731 ± 0.017 versus 0.791 ± 0.038 of control, P<0.01) (Fig. 6C), suggesting that there was a shift to primarily utilize fatty acids for oxidation in the resveratrol group. This was consistent with the reduced serum triglyceride concentration in resveratrol group (Table 2). Furthermore, resveratrol exerted the tendency to increase average heat production (0.727 ± 0.024 versus 0.671 ± 0.010 kCal/h of control, P = 0.065) (Fig. 6D). Moreover, resveratrol increased the basal oxygen consumption (2.1-fold versus control, P < 0.01) (Fig. 6E) of iWAT in vitro.
Figure 6.
Resveratrol promoted the lipid oxidation of iWAT. A) O2 consumption of control and resveratrol treated mice were recorded during a 3-h period. B) CO2 production of control and resveratrol treated mice was recorded during a 3-h period. C) Respiratory exchange ratio (RER) of control and resveratrol treated mice was recorded during a 3-h period. D) Average heat production of control and resveratrol treated mice during a 3-h period. F) O2 consumption of iWAT of control and resveratrol treated mice was measured as the decrease in dissolved oxygen (DO). **P < 0.01 versus control.
Discussion
In this paper, we investigated the effects of resveratrol on the formation of brown-like adipocytes and the mechanism underlying this process. Our results demonstrated that resveratrol induces the browning of mouse iWAT by promoting the expression of brown adipocyte selective genes through the activation of AMPKα1. It has been reported that resveratrol reduces adiposity 19, via inhibiting white adipogenesis 24 and stimulating the lipolysis 27, 28. To date, however, no study assessed resveratrol’s effects on the brown adipogenesis or the formation of brown-like adipocytes. Furthermore, the concentrations used in previous in vitro studies of white adipogenesis 24, 25 are much higher than the plasma concentration 20, 49. These concentrations can stimulated apoptosis 25, 50 and might be less relevant to the physiological effects of resveratrol. In the present study, we found that high concentrations (20 or 40 μM) of resveratrol inhibited lipid accumulation during the brown adipogenic differentiation of iWAT SVC. And these results agreed with previous reports that high concentration (50 μM) of resveratrol inhibits the adipogenic differentiation of 3T3-L1 24, 25 and SGBS preadipocytes 51. However, at the lower concentrations (<= 10 μM), which is closer to the plasma concentration 20, 49, resveratrol did not affect lipid content in induced brown adipocytes. It has been reported that the plasma resveratrol concentration is 1.56 ±0.28 μM in rat fed a high fat diet containing 4 g resveratrol per kg diet 49. While in mice fed high fat diet containing 0.4% resveratrol, the highest plasma resveratrol concentration is about 0.5μM 20. Thus, relative low resveratrol concentration (10 μM) was selected to investigate its role in the formation of brown-like adipocytes during the brown adipogenic differentiation of iWAT SVC.
Our results showed that resveratrol boosts UCP1 mRNA expression in differentiated iWAT SVC, which is consistent with the reports in maturing 3T3-L1 preadipocytes 25 and primary MEF-derived adipocytes 30. In addition, the mRNA expression of other brown adipocytes selective genes such as PRDM16, Cidea, Elovl3, and PGC1α as well as the protein levels of UCP1, Cyto C and PDH were also markedly elevated by resveratrol treatment. Moreover, the expressions of beige adipocyte selective markers such as CD137, Tbx1 and TMEM26 in resveratrol treated group were much higher than those of control group. These data strongly support the notion that resveratrol promotes the formation of brown-like adipocytes in differentiated mouse iWAT SVC.
In vivo studies were conducted to further address the biological effects of resveratrol on the formation of brown-like adipocytes in WAT. We found that resveratrol significantly decreased the body weight gain compared with the control group when challenged with an obesogenic diet. The reduced body weight gain in resveratrol treated mice might be due to the lower body fat accumulation. Our findings confirmed the body fat lowering effects of resveratrol, which have been reported in both animals 21, 29, 52 and humans 22. It has been reported that thermogenesis is involved in the body-fat lowering effects of resveratrol 53, 54. However, in these previous studies, their primary focuses were on the BAT and/or skeletal muscle, but not WAT. In our study, we found that resveratrol resulted in decreased adipocyte size in WAT, which is in agreement with a recent report in humans 23. More importantly, we observed brown-like adipocytes, with an appearance of multiocular lipid droplets, in iWAT, which has not been observed before. The presence of brown-like adipocytes was further confirmed by UCP1 IHC staining. Moreover, the UCP1 protein content was also elevated in the resveratrol group, accompanied with the elevated expression of PRDM16 and Cyto C, two markers of brown adipogenesis 55, 56. These findings strongly suggested the browning effects of resveratrol on iWAT.
Increased browning of iWAT could lead to increased energy expenditure and oxygen consumption. It has been reported that resveratrol improves mitochondrial oxidation function in BAT and skeletal muscle 20, but whether resveratrol elicits similar effects in iWAT has not been evaluated. Our findings indicate that resveratrol increased oxygen consumption (VO2) and decreased respiratory exchange ratio (RER) (CO2 production/O2 uptake) in mice, which is highly consistent with our in vitro data. RER is commonly used to determine the relative contribution of carbohydrate and lipids to overall energy expenditure. A high RER indicates that carbohydrates are being predominantly catabolized, whereas a low RER suggests lipid oxidation 57. Thus, the decreased RER in resveratrol treated mice suggests that a higher ratio of lipids were being oxidized. We also found that resveratrol had the tendency to increase (P = 0.065) the average heat production. It should be noted that the increased oxygen consumption (VO2), heat production and lipid oxidation might be partially due to the activation of BAT by resveratrol 53. Meanwhile, we also found that oxygen consumption of tissue (iWAT) and cells (differentiated iWAT SVF) in resveratrol treated group was higher than that of control group. Thus, a lower iWAT adipocyte size in the HFD-fed mice supplemented with resveratrol might be due to the increase of lipolysis and subsequent elevated fat oxidation and heat production with increased oxygen consumption. Moreover, the expression of genes related to mitochondrial fatty acid oxidation such as PGC1α, PDH, Cyto C was elevated in the resveratrol group. These data were consistent with the enhanced fatty acid oxidation observed in 3T3-L1 and MEF-derived adipocytes following resveratrol treatment 30. Together, our data suggested that the anti-obesity effects of resveratrol at least partially resulted from the enhanced fat oxidation in iWAT.
It has been reported that resveratrol may exert its effects on metabolic health in part through the activation AMPK 49, 58. To investigate whether AMPK was involved in the resveratrol mediated browning of iWAT, we first analyzed the activation of AMPKα (the ratio phosphorylation level of AMPKα (p-AMPKα) to total AMPKα (t-AMPK)) in the differentiated iWAT SVC and found that the ratio p-AMPKα/t-AMPKα was increased in the resveratrol treated group. Meanwhile, AMPKα inhibition by Compound C, which could inhibit the activation of AMPKα, led to the complete elimination of the stimulating effects of resveratrol on the expression of markers of beige adipocytes, including UCP1, PRDM16, Cyto C and PDH. Furthermore, our in vivo study also revealed the increased AMPKα phosphorylation and p-AMPKα/t-AMPKα ratio in iWAT of resveratrol treated mice, in agreement with the previous report 21. These results suggested that AMPKα is involved in the browning effects of resveratrol on iWAT.
Because the predominant isoform of α catalytic subunit expressed in adipose tissue is α1 31, 32, we speculated that AMPKα1 but not AMPKα2 participated in resveratrol induced browning effects. To verify our hypothesis, we acutely delete AMPKα1 by treating the confluent iWAT SVC isolated from Rosa26Cre/Ampkα1flox/flox mice with 4-OHT and, then, induced brown adipogenic differentiation. As expected, only trace amount of p-AMPKα and t-AMPKα was detected in SVC after the acute deletion of AMPKα1, showing that α1 isoform accounts for most of the total activity of this kinase in SVC 32, 35. In the absence of AMPKα1, the effects of resveratrol on the expression of brown adipocyte selective genes were abolished, suggesting that AMPKα1 is the key mediator linking resveratrol to the browning of iWAT. Our study is consistent with a previous study showing that AMPKα1 knockout abolished the effect of resveratrol on metabolic rate in mice 21, though no brown adipogenesis or browning of white adipocytes were examined. Here, building on that study, for the first time, we demonstrate that resveratrol improves metabolism at least partially through enhancing brown-like, or beige adipogenesis in WAT, which is mediated by AMPKα1.
In a recent study, resveratrol was shown to induce thermogenesis by increasing Sirt1 expression 53. And Sirt1 is required for the mitochondrial biogenesis induced by resveratrol 58. Consistently, in our study, the Sirt1 content in SV cells was also activated due to resveratrol treatment, which was absent in AMPKα1 KO cells; in addition, AMPKα1 deficiency dramatically reduced Sirt1 content. These data suggest that AMPK and Sirt1 likely reinforce each other to induce the browning of iWAT. Indeed, AMPK and Sirt1 coordinate to regulate mitochondriogenesis 59.
In conclusion, we provide evidence that resveratrol induces the formation of brown-like adipocytes in mouse iWAT by increasing expression of genes specific to brown adipocytes and stimulating fatty acid oxidation, which appeared to be primarily mediated by AMPKα1. These data demonstrate, in addition to the inhibition of adipogenesis and stimulation of lipolysis, a novel browning role of resveratrol in WAT, which contributes to the beneficial effects of resveratrol in metabolism. Moreover, it extends our knowledge on dietary polyphenols and beige adipogenesis and provides new strategies for the prevention and treatment of obesity and related diseases.
Acknowledgments
Special thanks to Joseph Maricelli for his help in the measurement of basal metabolic rate (BMR). This work was supported by grants from National Institutes of Health (R01HD067449), the National Natural Science Foundation of China (31372397), the Muscular Dystrophy Association (216602) and the National Science Foundation (1147275). This activity was also funded, in part, with an Emerging Research Issues Internal Competitive Grant from the Agricultural Research Center at Washington State University, College of Agricultural, Human, and Natural Resource Sciences.
Abbreviations
- 4-OHT
4-hydroxytamoxifen
- AMPK
AMP-activated protein kinase
- aP2
adipocyte protein 2
- BMR
basal metabolic rate
- BMP7
bone morphogenetic protein 7
- BAT
brown adipose tissue
- CC
Compound C
- Cidea
Cell death-inducing DFFA-like effector A
- Cre
cre recombinase
- Cyto C
cytochrome C
- Elovl3
elongation of very long chain fatty acids protein 3
- FGF21
fibroblast growth factor 21
- H&E
hematoxylin and eosin
- HFD
high fat diet
- IHC
immunohistochemical
- iWAT
inguinal white adipose tissue
- MEF
mouse embryonic fibroblasts
- PDH
pyruvate dehydrogenase
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PPARγ
peroxisome proliferator-activated receptor γ
- PRDM16
PR domain-containing 16
- RER
respiratory exchange ratio
- SVC
stromal vascular cells
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Footnotes
The authors declare no conflicts of interest.
References
- 1.Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Archives of medical research. 2008;39(8):715–28. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Archives of medical science : AMS. 2013;9(2):191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes & metabolism journal. 2013;37(1):22–9. doi: 10.4093/dmj.2013.37.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beranger GE, Karbiener M, Barquissau V, Pisani DF, Scheideler M, Langin D, et al. In vitro brown and “brite”/“beige” adipogenesis: human cellular models and molecular aspects. Biochimica et biophysica acta. 2013;1831(5):905–14. doi: 10.1016/j.bbalip.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 11.Peschechera A, Eckel J. “Browning” of adipose tissue--regulation and therapeutic perspectives. Archives of physiology and biochemistry. 2013;119(4):151–60. doi: 10.3109/13813455.2013.796995. [DOI] [PubMed] [Google Scholar]
- 12.Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World journal of stem cells. 2014;6(1):33–42. doi: 10.4252/wjsc.v6.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo KA, Sun L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Bioscience reports. 2013;33(5) doi: 10.1042/BSR20130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarroya F, Vidal-Puig A. Beyond the Sympathetic Tone: The New Brown Fat Activators. Cell metabolism. 2013;17(5):638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochimica et biophysica acta. 2013;1831(5):969–85. doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Carpene C, Gomez-Zorita S, Deleruyelle S, Carpene MA. Novel Strategies for preventing Diabetes and Obesity Complications with Natural Polyphenols. Current medicinal chemistry. 2014 doi: 10.2174/0929867321666140815124052. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, et al. Novel insights of dietary polyphenols and obesity. The Journal of nutritional biochemistry. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. European journal of pharmacology. 2010;635(1-3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Lam YY, Peterson CM, Ravussin E. Resveratrol vs. calorie restriction: data from rodents to humans. Experimental gerontology. 2013;48(10):1018–24. doi: 10.1016/j.exger.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmers S, Konings E, Bilet L, Houtkooper Riekelt H, van de Weijer T, Goossens Gijs H, et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell metabolism. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. International journal of obesity (2005) 2014;38(3):470–3. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 24.Mitterberger MC, Zwerschke W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(11):1356–76. doi: 10.1093/gerona/glt019. [DOI] [PubMed] [Google Scholar]
- 25.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytotherapy research : PTR. 2008;22(10):1367–71. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutrition research and practice. 2012;6(4):286–93. doi: 10.4162/nrp.2012.6.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simon E, Zechner R, et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. The Journal of nutritional biochemistry. 2012;23(4):379–84. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Zorita S, Treguer K, Mercader J, Carpene C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. Journal of physiology and biochemistry. 2013;69(3):585–93. doi: 10.1007/s13105-012-0229-0. [DOI] [PubMed] [Google Scholar]
- 29.Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, et al. Effect of resveratrol on fat mobilization. Ann N Y Acad Sci. 2011;1215:40–7. doi: 10.1111/j.1749-6632.2010.05845.x. [DOI] [PubMed] [Google Scholar]
- 30.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. The Journal of nutritional biochemistry. 2011;22(9):828–34. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. The Journal of biological chemistry. 2005;280(26):25250–7. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 32.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochemical and biophysical research communications. 2004;316(3):853–8. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, et al. Mammalian AMP-activated protein kinase subfamily. The Journal of biological chemistry. 1996;271(2):611–4. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 34.Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. The Journal of biological chemistry. 2006;281(36):25956–64. doi: 10.1074/jbc.M602992200. [DOI] [PubMed] [Google Scholar]
- 35.Gaidhu MP, Ceddia RB. The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exercise and sport sciences reviews. 2011;39(2):102–8. doi: 10.1097/JES.0b013e31820ac03e. [DOI] [PubMed] [Google Scholar]
- 36.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond) 2013;124(8):491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 37.Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. The Journal of physiology. 2006;574(1):55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarte LC, Vaanholt LM, Sinclair RE, Gamo Y, Speakman JR. Limits to sustained energy intake XII: is the poor relation between resting metabolic rate and reproductive performance because resting metabolism is not a repeatable trait? The Journal of experimental biology. 2010;213(2):278–87. doi: 10.1242/jeb.037069. [DOI] [PubMed] [Google Scholar]
- 39.Speakman JR. Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Frontiers in physiology. 2013;4:34. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aune UL, Ruiz L, Kajimura S. Isolation and differentiation of stromal vascular cells to beige/brite cells. Journal of visualized experiments : JoVE. 2013;(73):50191. doi: 10.3791/50191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Zhou G, Shu G, Wang L, Zhu X, Gao P, et al. Glucose Utilization, Lipid Metabolism and BMP-Smad Signaling Pathway of Porcine Intramuscular Preadipocytes Compared with Subcutaneous Preadipocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;31(6):981–96. doi: 10.1159/000350116. [DOI] [PubMed] [Google Scholar]
- 42.Xue R, Wan Y, Zhang S, Zhang Q, Ye H, Li Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. American journal of physiology Endocrinology and metabolism. 2014;306(4):E363–72. doi: 10.1152/ajpendo.00119.2013. [DOI] [PubMed] [Google Scholar]
- 43.Fu X, Zhao JX, Zhu MJ, Foretz M, Viollet B, Dodson MV, et al. AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. Molecular and cellular biology. 2013;33(22):4517–25. doi: 10.1128/MCB.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diepart C, Verrax J, Calderon PB, Feron O, Jordan BF, Gallez B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Analytical biochemistry. 2010;396(2):250–6. doi: 10.1016/j.ab.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 45.Zhao G, Zhang X, Xu X, Wolin MS, Hintze TH. Depressed modulation of oxygen consumption by endogenous nitric oxide in cardiac muscle from diabetic dogs. American journal of physiology Heart and circulatory physiology. 2000;279(2):H520–7. doi: 10.1152/ajpheart.2000.279.2.H520. [DOI] [PubMed] [Google Scholar]
- 46.Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. Maternal obesity induces epigenetic modifications to facilitate zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62(11):3727–35. doi: 10.2337/db13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor protocols. 2014;2014(6):655–8. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 48.Chi V, Chandy KG. Immunohistochemistry: paraffin sections using the Vectastain ABC kit from vector labs. Journal of visualized experiments : JoVE. 2007;(8):308. doi: 10.3791/308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of Resveratrol and SIRT1 on PGC-1alpha Activity and Mitochondrial Biogenesis: A Reevaluation. PLoS biology. 2013;11(7):e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mader I, Wabitsch M, Debatin KM, Fischer-Posovszky P, Fulda S. Identification of a novel proapoptotic function of resveratrol in fat cells: SIRT1-independent sensitization to TRAIL-induced apoptosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(6):1997–2009. doi: 10.1096/fj.09-142943. [DOI] [PubMed] [Google Scholar]
- 51.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. The American journal of clinical nutrition. 2010;92(1):5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- 52.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food chemistry. 2013;141(2):1530–5. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 54.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. European journal of nutrition. 2014 doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 55.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460(7259):1154–8. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos-Jimenez A, Hernandez-Torres RP, Torres-Duran PV, Romero-Gonzalez J, Mascher D, Posadas-Romero C, et al. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clinical medicine Circulatory, respiratory and pulmonary medicine. 2008;2:1–9. doi: 10.4137/ccrpm.s449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell metabolism. 2012;15(5):675–90. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]