Abstract
Humanized mice represent an important model to study the development and function of the human immune system. While it is known that mouse thymic stromal cells can support human T-cell development, the extent of interspecies cross-talk and the degree to which these systems recapitulate normal human T-cell development remain unclear. To address these questions, we compared conventional and non-conventional T-cell development in a neonatal chimera humanized mouse model with that seen in human fetal and neonatal thymus samples, and also examined the impact of a human HLA-A2 transgene expressed by the mouse stroma. Given that dynamic migration and cell–cell interactions are essential for T-cell differentiation, we also studied the intrathymic migration pattern of human thymocytes developing in a murine thymic environment. We found that both conventional T-cell development and intra-thymic migration patterns in humanized mice closely resemble human thymopoiesis. Additionally, we show that developing human thymocytes engage in short, serial interactions with other human hematopoietic-derived cells. However, non-conventional T-cell differentiation in humanized mice differed from both fetal and neonatal human thymopoiesis, including a marked deficiency of Foxp3+ T-cell development. These data suggest that although the murine thymic microenvironment can support a number of aspects of human T-cell development, important differences remain, and additional human-specific factors may be required.
Humanized mice, in which immune deficient mice are engrafted with human hematopoietic cells, provide a powerful model to study human T-cell development in vivo. Several models of humanized mice have been developed that exhibit de novo thymopoiesis. In the neonatal chimera model, irradiated newborn mice are reconstituted intra-hepatically with cord-blood derived human hematopoietic stem cells,1, 2 and human thymocytes develop within a mouse thymic environment. This approach has been used with multiple immunodeficient mouse strains including the NOD SCID Il2rgtm1Wjll (NSG), NOD SCID Il2rgtm1Sug (NOG) and BALB/c-Rag1null Il2rgtm1Sug (BRG) strains, and these strains, in particular, appear to have comparable thymic reconstitution.3, 4 In another humanized mouse model, adult immunocompromised mice are surgically implanted with human fetal thymus and liver under the kidney capsule and can be later transplanted with autologous human hematopoietic stem cells to prolong thymopoiesis within a human thymic environment.5, 6, 7, 8 One important advantage of the neonatal chimera model is the relative ease with which one can generate these mice in terms of both technical skills and access to tissue. However, to what extent the murine thymic environment can support human T-cell development is not completely understood. To maximize their capacity as a pre-clinical model of human T-cell mediated immunity, it is necessary to understand how human T cells are selected in these systems and the processes that shape the T-cell repertoire.
There are indications that in neonatal chimera NSG mice, T-cell receptor (TCR) selection of human thymocytes may occur via interactions with both murine and human major histocompatibility complex (MHC).9 Thymic reconstitution in neonatal chimera NSG mice reflects all stages of conventional T-cell development and the generation of mature T cells with human leukocyte antigen (HLA)-restricted effector functions.1, 2 These findings suggest that positive selection may be mediated, at least in part, through the occurrence of human HLA-dependent positive selection events, in addition to selection events on murine MHC. In an attempt to increase the efficiency of T-cell generation in the murine thymus of humanized mice, NSG mice expressing human MHC class I molecules on murine thymic epithelial cells (TECs) were generated.10 In these models, peripheral T-cell responses to human-specific pathogens were evaluated, and T-cell development in the presence of a human HLA-A2 transgene appeared to favor development of TCRs with different affinities and specificities.10, 11, 12 Despite these results, it is unclear whether the human HLA transgene affected selection events in the thymus or had an indirect effect on T-cell specificity by influencing peripheral T-cell responses or homeostasis.
In the absence of a human HLA transgene, however, human thymocytes can interact with mouse MHC,9 and we have shown that these interactions can provide tonic signals in the thymus that sustain human thymocyte motility.13 Additionally, human CD4+CD8+ thymocytes express MHC class II molecules,14, 15, 16, 17 and have been implicated in their own positive selection through thymocyte:thymocyte interactions.18, 19 Therefore, it has been proposed that selection of human thymocytes in a mouse thymic environment might be skewed toward atypical TCR:HLA interactions with other human-derived hematopoietic cells due to inefficient selection on mouse MHC.2, 19, 20 This may, in turn, lead to the development of non-conventional T cells with innate-like or regulatory properties that are known to be enriched in the human fetal thymus.19, 21, 22 Additionally, neonatal chimera NSG mice are reconstituted with hematopoietic progenitor cells (HPCs) from human cord blood that likely represent a transitional stage between fetal and adult hematopoietic development and may further contribute to the development of non-conventional T-cell subsets.23
Here, we examined both conventional and non-conventional T-cell development in neonatal chimera NSG mice in the presence or absence of human HLA-A2 transgene expression and in comparison with human fetal and postnatal thymic samples. As T-cell development is intricately linked to thymocyte migration and cellular interactions, we also examined human thymocyte behavior in the neonatal chimera NSG model of humanized mice. We present evidence that the selection and behavior of conventional human thymocytes on mouse stroma resembles, in large part, that in human thymus, and that expression of an HLA-A2 transgene by murine TECs does not dramatically improve T-cell development. Despite visual evidence to suggest human thymocyte interactions with other human hematopoietic-derived cell types in these humanized mice, we did not detect an increase in alternative T-cell lineage development. In addition, non-conventional T-cell differentiation in neonatal chimera NSG mice did not segregate with either fetal or postnatal human thymopoiesis, and the dearth of Foxp3+ thymocytes suggests that other human-specific factors are necessary to fully mimic all aspects of human T-cell development in this model.
RESULTS
Conventional T-cell development in humanized mice recapitulates that in fetal and post-natal human thymus, regardless of an HLA-A2 transgene
The absence of human MHC molecules on murine TECs is thought to limit T-cell development in neonatal chimera humanized mice. In support of this notion, expression of an HLA-A2 human transgene (NSG-HLA-A2 tg) has been reported to alter the TCR repertoire in humanized NSG mice.10, 11, 12 The impact of HLA-A2 tg expression on T-cell development in these mice was inferred from the response of peripheral T cells to infection, and it remains unclear whether the presence of human MHC enhances the overall development of human T cells in humanized neonatal chimera NSG mice. To address this question, we generated humanized mice in which littermate NSG and NSG-HLA-A2 tg neonatal mice were reconstituted with CD34+ HPCs isolated from individual cord blood samples. We found that chimerism in the spleen ranged from 28 to 88% based on the proportion of human CD45+ cells of live mononuclear cells, and was comparable between NSG and NSG-HLA-A2 tg mice (Supplementary Figure 1a). In line with previous studies, we observed similar levels of T-cell reconstitution in the spleen regardless of HLA-A2 transgene expression or HLA-A2 expression in CB donors (Figure 1a and Supplementary Figure 1b and c)10. While the proportion of CD8+ T cells was greater than that of CD4+ T cells in the spleens of both NSG and NSG-HLA A2 tg humanized mice, the absolute numbers of CD4+ and CD8+ T cells did not differ significantly (Figure 1b and Supplementary Figure 1d and f). We also noted that the number of human T cells in the spleen did not correlate with the number of human thymocytes (Figure 1c), suggesting that post-thymic T-cell survival and expansion might have a large impact on the peripheral T-cell populations in these mice. Because the thymocyte populations provide a more direct indication of the efficiency of thymic development, we focused our study of T-cell development in the thymus of humanized mice.
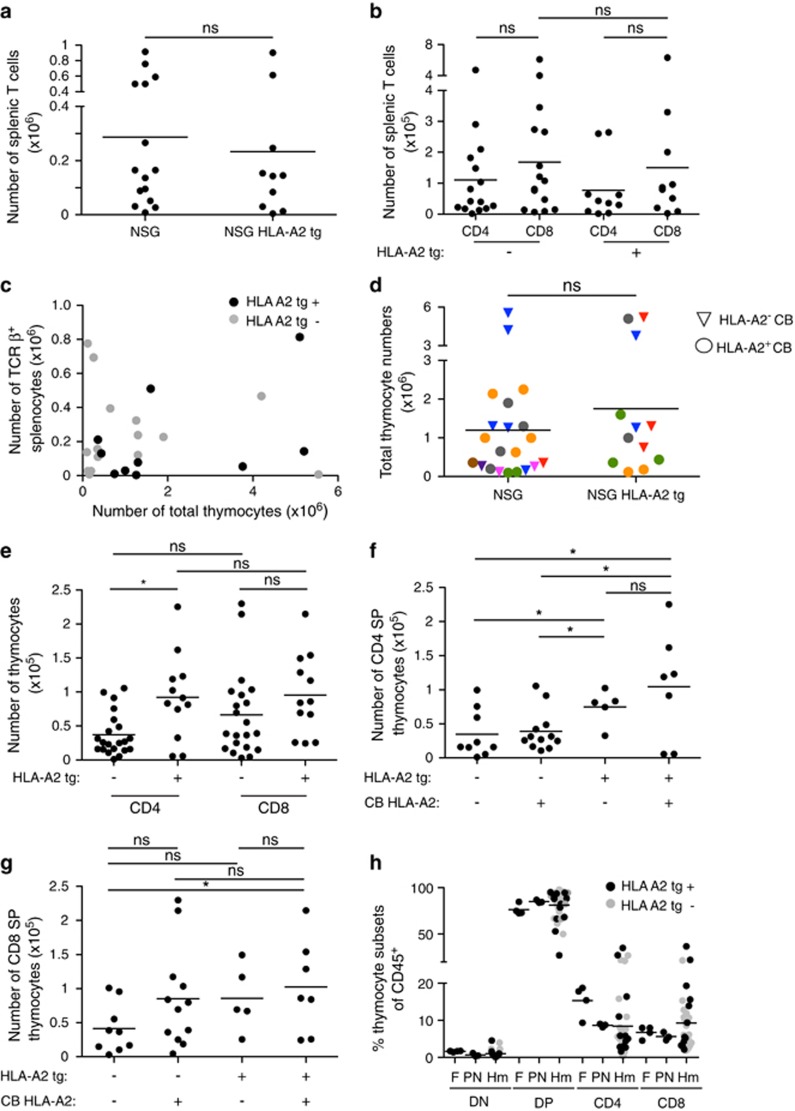
Figure 1.
Development of conventional T cells in humanized mice is comparable to fetal and post-natal human thymus in the presence or absence of an HLA-A2 transgene. (a) Number of human TCRβ+ splenocytes in neonatal chimera NSG and NSG HLA-A2 tg mice at 11–13 weeks post reconstitution. Each dot represents an individual mouse. Line represents average. (b) Number of human CD4+ and CD8+ splenic T cells from neonatal chimera NSG and NSG HLA-A2 tg mice. (c) Number of splenic human T cells versus the number of thymocytes in individual humanized mice. (d) Quantification of total thymocytes in NSG and NSG HLA-A2 tg mice reconstituted with HLA-A2+ and HLA-A2− cord blood (CB). Each color corresponds to an individual CB donor. (e) Number of CD4+ and CD8+ SP thymocytes in neonatal chimera NSG and NSG HLA-A2 tg mice. P=0.2 for CD8+ SP thymocytes. (f) Number of CD4+ SP thymocytes in NSG and NSG HLA-A2 tg mice reconstituted with HLA-A2− or HLA-A2+ CB donors. (g) Number of CD8+ SP thymocytes in NSG and NSG HLA-A2 tg mice reconstituted with HLA-A2− or HLA-A2+ CB donors. P=0.0741 for NSG and NSG HLA-A2 tg mice reconstituted with HLA-A2− CB. (h) Proportion of thymocyte developmental intermediate subsets of CD45+ cells in human fetal (F) (18–20 weeks) and post-natal (PN) (1 week to 2.5 years) human thymic samples and in NSG and NSG HLA-A2 tg mice (Hm) (11–13 weeks post reconstitution). Each dot represents an individual thymus sample. For Hm samples, dots are color coded to distinguish NSG and NSG HLA-A2tg hosts. ns, not statistically significant, * indicates P<0.05. DN indicates CD4−CD8− thymocytes, and DP indicates CD4+CD8+ thymocytes. All data shown in these graphs has been gated on human CD45+ cells.
Cortical TECs have a crucial role in presenting self-peptide-MHC complex for positive selection of conventional T cells. Thus, it seemed likely that expression of a human HLA molecule on murine TECs would result in more efficient human thymocyte selection than on mouse MHC alone and that this, in turn, would lead to enhanced T-cell development. We routinely found that >90% of thymocytes from reconstituted NSG or NSG-HLA-A2 tg mice expressed human CD45 (Supplementary Figure 2a). Expression of an HLA-A2 transgene on murine TECs did not have a major impact on thymic cellularity, and this held true across multiple cord blood donors regardless of donor HLA-A2 expression (Figure 1d and Supplementary Figure 2b).
We predicted that expression of HLA-A2 on murine TECs might enhance development of mature CD8+ single positive (SP) thymocytes. However, while there was no significant difference in the proportion of SP cells, there was an overall trend toward slightly increased numbers of mature thymocytes in NSG HLA-A2 tg mice (Figure 1e and Supplementary Figure 2c). These differences reached statistical significance for CD4+ SP, but not for CD8+ SP thymocytes (Figure 1e). In addition, HLA-A2 expression on CB had only minor effects on SP thymocytes (Figures 1f and g and Supplementary Figures 2d and e). Further, the major thymocyte populations defined by CD4 and CD8 expression were similar in neonatal chimera NSG and NSG-HLA-A2 tg humanized mice, and resembled both fetal and postnatal human thymic samples (Figure 1h). Thus, expression of human HLA-A2 on murine TECs did not dramatically enhance overall T-cell development in humanized mice.
The HLA-A2 transgene is driven by the endogenous human HLA-A2 promoter and is expressed by murine TECs.10 However, the level of the HLA-A2 transgene expression relative to endogenous HLA-A2 on human cells has not been reported. It was therefore possible that lower or less stable HLA-A2 transgene expression was responsible for the lack of striking differences in T-cell development in these mice as compared with non-transgenic controls. To address this issue, we compared cell-surface expression of HLA-A2 on TECs from NSG HLA-A2 tg mice to HLA-A2+ human thymic tissue. Before analysis, NSG HLA-A2 tg mice were reconstituted with BALB/c bone marrow to generate a thymus of sufficient size to analyze HLA-A2 expression on TECs by flow cytometry. We found that the HLA-A2 levels were equivalent (Supplementary Figure 2f). Thus, a defect in HLA-A2 expression on TECs does not account for the lack of dramatic impact of the transgene on overall T-cell cellularity, and supports the notion that mouse MHC is sufficient for the development of conventional human T cells.
Non-conventional T-cell development in humanized mice does not segregate with fetal or post-natal human thymopoiesis
While a murine thymic environment is capable of supporting conventional human T-cell development, it is not clear to what extent it can mimic the development of non-conventional T cells, which may require specialized niches and human-specific growth factors as well as distinct interactions with hematopoietic-derived antigen presenting cells. Innate-like TCRβ+ T cells expressing the transcription factor promyelocytic leukemia zinc finger protein (PLZF) are a non-conventional thymic subset selected by homotypic thymocyte:thymocyte interactions, and it has been suggested that their development may be enhanced in humanized mice due to inefficient selection on mouse MHC.19 However, we found that the proportion of PLZF+ SP T cells in the thymus of humanized mice was comparable to that in fetal samples, and expression of an HLA-A2 transgene on murine stroma did not appear to influence their development (Figures 2a and b and Supplementary Figure 3).19
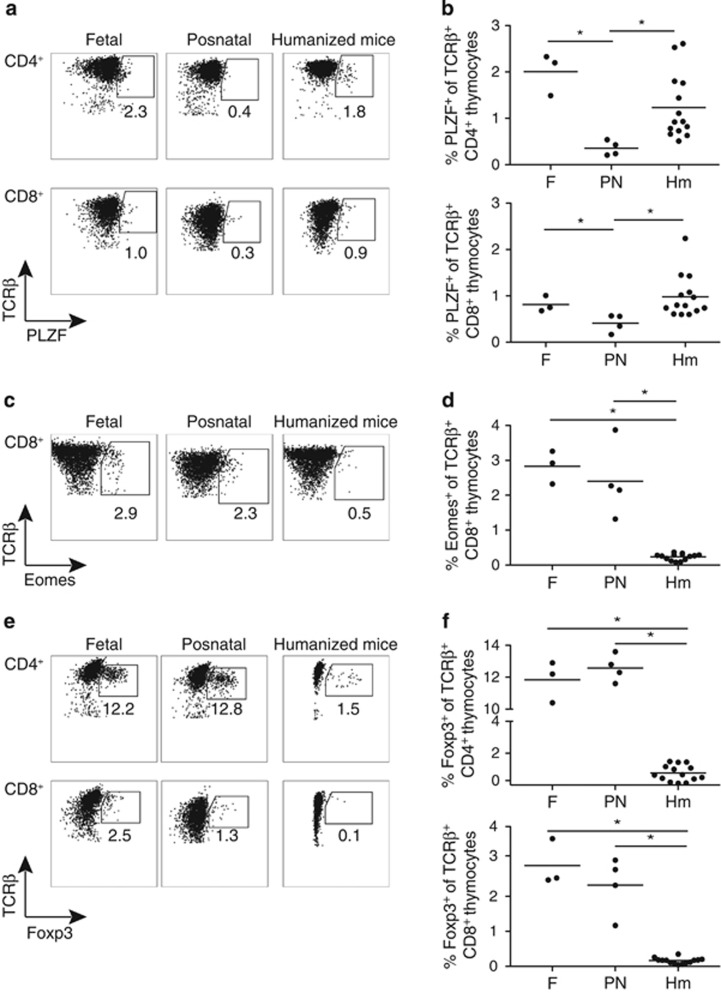
Figure 2.
Non-conventional T-cell development in humanized mice is distinct from fetal and postnatal human thymopoiesis. (a) Representative flow-cytometric analysis of PLZF expression in CD4+ (top panels) and CD8+ (bottom panels) human thymocytes gated on TCRβ+ cells in fetal, postnatal and humanized mice thymic samples. (b) Proportion of PLZF+ cells in CD4+ (top panel) and CD8+ (bottom panel) TCRβ+ human thymocytes in human fetal, postnatal and humanized mice thymic samples. Each dot represents one thymus. (c) Representative flow-cytometric analysis of Eomes expression in CD8+ thymocytes gated on human TCRβ+ cells in human fetal, postnatal and humanized mice thymic samples. (d) Proportion of Eomes+ cells in CD8+ TCRβ+ human thymocytes in human fetal, postnatal and humanized mice thymic samples. (e) Representative flow-cytometric analysis of Foxp3 expression in CD4+ (top panels) and CD8+ (bottom panels) human thymocytes gated on TCRβ+ cells in fetal, postnatal and humanized mice thymic samples. (f) Proportion of Foxp3+ cells in CD4+ (top panel) and CD8+ (bottom panel) TCRβ+ human thymocytes in human fetal, postnatal and humanized mice thymic samples. Line represents average. * indicates P<0.05.
In line with previous reports, the proportion of PLZF+ CD4+ and CD8+ SP T cells was significantly higher in fetal than in postnatal thymus (Figures 2a and b).19 Since HPCs in human cord blood are at the transition between fetal and adult hematopoiesis,23 it was unclear whether thymocytes in humanized mice would be more fetal-like, or more adult-like in their developmental output. The observation that the proportion of PLZF+ thymocytes from humanized mice more closely matched the fetal rather than the postnatal thymus samples suggested that their T-cell development might be more fetal-like. To explore this issue further, we examined additional thymic subsets that might distinguish fetal versus adult human T-cell development, and/or that require specialized thymic microenvironments for their development.
Development of innate-like CD8+ T cells expressing the transcription factor Eomes has been linked both to a fetal development program and to IL-4 production by PLZF+ T cells in mice.22, 24, 25 Additionally, there is evidence that innate-like CD8+ T cells can be selected on hematopoietic cells,26, 27 suggesting that their development might be enhanced in the chimeric mouse–human thymus due to inefficient selection on mouse-MHC. However, we did not observe a significant difference in the proportion of Eomes+ CD8+ SP cells in fetal versus postnatal thymocytes (Figures 2c and d). Moreover, the proportion of Eomes+ CD8+ SP thymocytes was significantly reduced in humanized mice compared with either fetal or postnatal human thymic samples (Figures 2c and d). Thus, the presence of human PLZF+ cells and other hematopoietic cells is not sufficient to support human Eomes+ CD8 T-cell development in humanized mice, suggesting that additional signaling interactions required for their development are not efficiently reproduced in a murine thymic environment.
Like other non-conventional T-cell subsets, TCRγδ T cells elicit a rapid response to foreign antigens and may be of particular importance for immune protection during early life.28, 29 In contrast to their earlier appearance during ontogeny in the mouse, γδ T cells emerge simultaneously with αβ T cells during human fetal thymopoiesis.30, 31, 32 TCRγδ T cells have been shown to develop in the thymus of humanized mice and to populate peripheral organs, but to what extent this mimics their development in humans has not been established.10, 33 Consistent with the lower frequency of TCRγδ T cells in the peripheral blood of fetal samples in comparison with that of adults,34 we found a trend toward a higher proportion of TCRγδ+ thymocytes in postnatal versus fetal samples, although this difference was not statistically significant. However, the proportion of TCRγδ+ thymocytes in humanized mice was comparable to that in fetal thymus and significantly lower than that in postnatal thymus (Supplementary Figure 4).
Finally, we examined the development of T regulatory (Treg) cells expressing the transcription factor Foxp3 in humanized mice. While Treg cells have been described in the thymus and periphery of humanized mice,2, 33, 35 it is unclear whether the murine thymic medulla can recapitulate the specialized niche required to support efficient development of human Foxp3+ Treg cells.36, 37, 38, 39 We found no significant difference in the proportion of Foxp3+ cells among human fetal and postnatal SP thymocytes, consistent with published data (Figures 2e and f).40, 41, 42 However, the proportion of both CD4+ and CD8+ Foxp3+ T cells in the thymus of neonatal chimera NSG mice was decreased compared with human thymus, implying a defect in human Foxp3+ T-cell development or survival within a murine thymic environment (Figures 2e and f). This decrease in the proportion of thymic Foxp3+ T cells was true for both NSG and NSG HLA-A2 tg humanized mice despite the readily detectable presence of Foxp3+ T cells in the periphery (Supplementary Figure 5a and b). These data support the idea that the murine thymic environment cannot mimic all the signaling cues required to guide selection or survival of non-conventional T-cell subsets, in particular Foxp3+ T cells. This contributes to a unique pattern of non-conventional T-cell development in humanized mice that differs from both fetal and postnatal thymopoiesis.
Human thymocyte migration and cellular interactions in a mouse thymic environment
Human T-cell development requires that developmental intermediates undergo specific maturation stages within distinct niches of the thymus, resulting in a tight coupling of thymocyte differentiation with migration and cellular interactions.43 To further examine the degree of interspecies cross-talk that allows human T cells to develop in a mouse thymic environment, we studied the behavior of human thymocytes within the humanized mouse thymus. Neonatal chimera NSG or NSG HLA-A2 tg mice were reconstituted with HLA-A2− cord blood CD34+ hematopoietic progenitors transduced at a low multiplicity of infection with a lentivirus expressing GFP (Lenti-GFP). This resulted in GFP labeling of ~2% of human cells within the murine thymus. We confirmed that GFP+ thymocytes had a normal distribution of developmental intermediates by flow cytometry, with the majority corresponding to relatively immature CD4+CD8+ thymocytes (Figure 3a, top panels). We also identified a substantial population of dendritic cells (DCs) within the GFP+ fraction. Indeed, of the non T-, B-, NK cell (CD3−, CD19−, CD56−) lineage-negative (Lin−) population, ~30% of the remaining GFP+ CD45+ cells expressed CD11c, and ~60% of these expressed high levels of HLA-DR, consistent with previous reports of human thymic DC subsets (Figure 3a, bottom panels).44
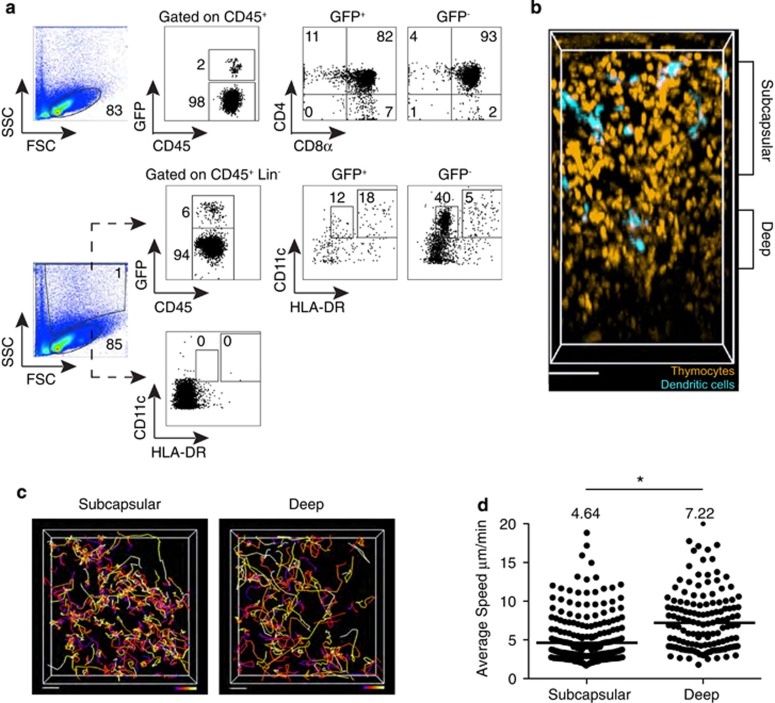
Figure 3.
2-photon imaging of the thymus of humanized mice reconstituted with GFP-expressing human CD34+ hematopoietic progenitors. NSG and NSG HLA-A2 tg mice were reconstituted with GFP-transduced HLA-A2− cord blood progenitors, and all data from NSG and NSG HLA-A2 tg thymi were combined for image analysis. (a) Flow-cytometric analysis of the thymus of a NSG humanized mouse 12 weeks post reconstitution depicting the gating strategy for identification of thymocyte developmental intermediates among human CD45+ cells (top panels), and HLA-DRint CD11c+ and HLA-DRhigh CD11c+ DC subsets among CD45+ Lin− (CD3− CD19−CD56−) cells (bottom panels) within the GFP+ and GFP− fractions of the collagenase digested thymus. (b) 2-photon imaging volume of GFP labeled human thymocytes and DCs within the thymus of an NSG humanized mouse at 12 weeks post reconstitution. For ease of visualization, thymocytes are artificially colored orange and DCs are colored turquoise. Depicted is the compartmentalization of the imaging volume based on distance from the capsule. Scale bar, 70 μm. Thymus was processed for flow cytometry after imaging and data from the same thymus sample are shown in (a). (c) Cell tracks from representative 2-photon time-lapse data sets of human thymocytes within the subcapsular (90 μm in depth, starting 30 μm below the capsule) and deep (30 μm in depth, starting 165 μm below the capsule) portions of the thymus of a humanized mouse (~27 min movies). Tracks are color-coded to represent the passage of time (blue>red>yellow>white). Scale bar 20 μm. (d) Average speed of human thymocytes in the thymus of humanized mice. Each dot represents average speed of an individual tracked cell, and the line represents the average value of compiled track averages for each location. Data represent analysis of two individual imaging volumes obtained from two individual mice. n=300 tracks (subcapsular cells); n=149 tracks (deep cells). * indicates P<0.05.
We next used 2-photon time-lapse microscopy of intact thymic lobes to examine migration of human thymocytes developing in a murine thymic environment. For orientation, we identified the collagen-rich capsule of the thymus by its second-harmonic signal, and the thymus was imaged to a depth of ~300 μm. Cord blood-derived GFP+ cells in the thymus were heterogeneous in size and morphology. The small, predominantly motile thymocytes (pseudo-colored orange) were distinct from the larger, less motile cells with dendritic morphology (pseudo-colored turquoise) (Figure 3b).
We have previously reported that immature human cortical thymocytes exhibit a slower migration pattern compared with more mature thymocytes in the deeper medullary region of the thymus.13 Moreover, there is evidence that the thymus of humanized mice contains distinct cortical and medullary regions.1, 2, 45 To examine regional segregation of thymocyte migration patterns in humanized mice, we divided the imaging volume based on distance from the capsule (Figure 3b). We found that thymocytes within ~90 μm of the capsule migrated with average speeds of 4.6 μm per minute, in close agreement with those reported for human and mouse cortical thymocytes (~4 μm per minute).13, 46, 47, 48 In contrast, thymocytes in the deep volume (~120–190 μm from the capsule) migrated more rapidly with an average speed of ~7 μm per minute (Figures 3c and d). These speeds are slightly slower than those reported for human CD4+ and CD8+ SP thymocytes (~9 and ~12 μm per minute, respectively) on human and mouse thymic slices.13 This may reflect the fact that the 'deep' volume in this system is an approximation for the medulla, and likely represents a mixture of both cortical and medullary thymocytes. These data further support the notion of efficient thymocyte:stromal interspecies crosstalk, reflected in the similar migration speeds of human thymocytes whether they have developed within a murine or a human thymic environment.
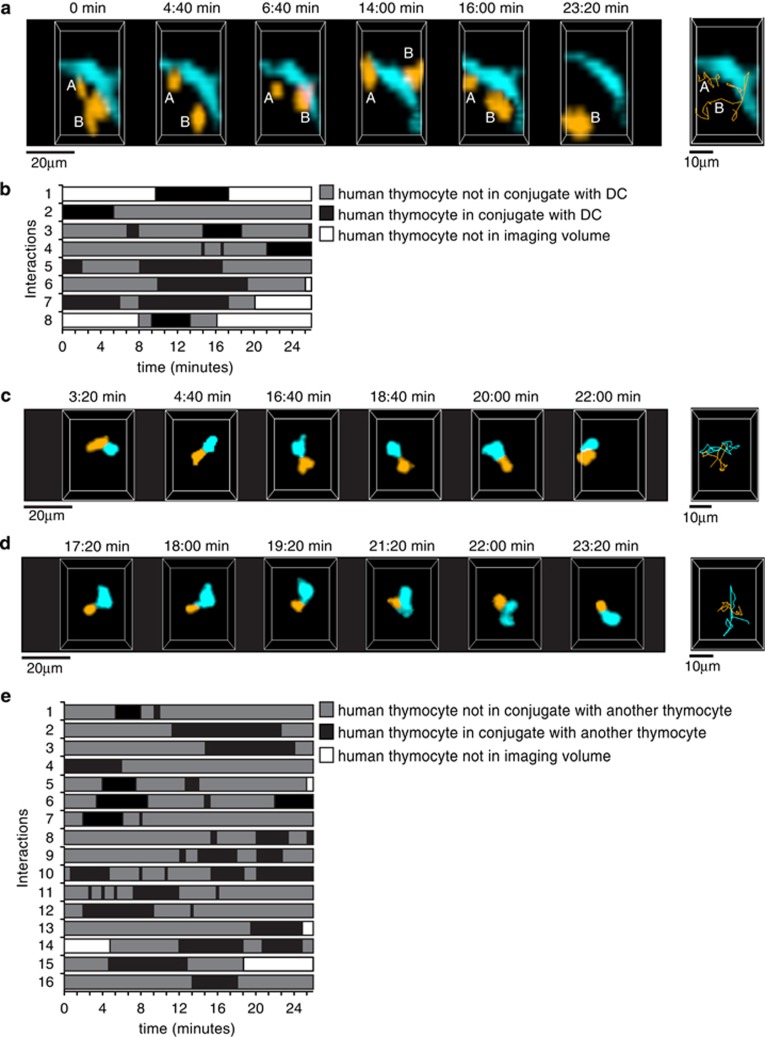
Previous studies have indicated that developing thymocytes in humanized mice may undergo selection by HLA molecules on other human cells.2, 19, 20 We therefore examined cellular interactions between GFP+ human cells in the thymus of humanized mice. We observed multiple examples of labeled thymocytes (~5%) forming dynamic contacts with GFP-labeled cells with DC-like morphology with interactions occurring, on average, during ~30% of the imaging period (Figures 4a and b and Supplementary video 1). Thymocyte speed during these contacts varied, with some cells apparently arrested in contact with the DC-like cell, while others continued to migrate on the surface of the DC-like cell (Supplementary video 1). This behavior is reminiscent of the dynamic and transient contacts described for the non-cognate interaction of a naïve T-cell first encountering a DC in murine lymph nodes.49, 50, 51, 52 We also observed that ~8% of labeled human thymocytes within the thymus of humanized mice came into contact with other labeled human thymocytes during the 20–30 min imaging runs. The thymocyte:thymocyte contacts were dynamic and brief (average duration of 11 time points equivalent to ~7 min) (Figures 4c and e) and in some cases were preceded by the extension of cellular protrusions (Supplementary videos 2 and 3). While some of these contacts may reflect random collisions or associations with a third, unlabeled cell, it is noteworthy that ~50% of contacts were associated with changes in cell shape at the point of contact suggestive of extensive cell:cell interactions (Supplementary videos 2 and 3). Given that only ~2% of the human cells in the thymus of these mice were labeled with GFP (Figure 3a), the proportion of human thymocytes interacting with other human hematopoietic cells in this mouse model is significantly underestimated. Whether these contacts lead to productive TCR signaling that influence selection events in the thymus and lead to the unique non-conventional T-cell developmental program of humanized mice will need to be investigated further.
Figure 4.
Contacts between human thymocytes and DCs in the thymus of humanized mice. NSG and NSG HLA-A2 tg mice were reconstituted with GFP-transduced HLA-A2− cord blood progenitors, and all data from NSG and NSG HLA-A2 tg thymi were combined for image analysis. (a) Cropped representation of 2-photon imaging volumes at indicated time points of human thymocytes contacting a human DC within the thymus of a humanized mouse. Thymocytes and DCs were distinguished based on size and morphology. The thymocytes are color-coded in orange, 'A' and 'B' identify two individual thymocytes. The DC is color-coded in turquoise. Far right panel, thymocyte tracks (orange) for the duration of the movie. Representative of a total of 8 interactions involving 8 different labeled thymocytes/150 thymocytes tracked with an approximate density of 8 DCs/imaging volume. (b) Timeline chart depicting the time of contact with DCs of 8 tracked thymocytes for a total of 40 time points (~27 min). Black boxes indicate periods of thymocyte:DC contacts. (c, d) Cropped representations of 2-photon imaging volumes at indicated time points of human thymocyte:thymocyte contacts within the thymus of a humanized mouse. Thymocytes are color-coded in orange and turquoise for improved visualization. Far right panels, thymocyte tracks for the duration of the movie. (e) Timeline chart depicting the time of contact with other thymocytes of 16 tracked thymocytes for a total of 40 time points (~27 min). For ease of representation, only thymocytes in contact with one other thymocyte are depicted. Black boxes indicate periods of contact between thymocytes.
DISCUSSION
Humanized mice provide an unparalleled opportunity to study human immune development and function in vivo, and have led to the development of important models of human infection, autoimmunity and cancer. Despite extensive interest in using humanized mouse models as a pre-clinical tool, many questions remain, including the extent of interspecies crosstalk.53, 54 The assumption that more efficient selection should occur in the presence of a human HLA transgene led us to directly examine T-cell development and behavior in the thymus of neonatal chimera NSG and NSG-HLA-A2 tg mice reconstituted with human CB HPCs. We demonstrated that conventional human thymocyte development in humanized mice is largely similar to that in human samples. While we predicted enhanced CD8+ T-cell development in the presence of HLA-A2 expression by murine TECs, overall thymic and splenic T-cell reconstitution was similar in humanized NSG and NSG-HLA-A2 tg mice. We also show that the motility of human T cells in humanized mice is similar to that previously described for mouse thymocytes and for human thymocytes within human thymic slices. Despite these similarities, we also demonstrate that the murine thymic environment in humanized mice does not fully recapitulate non-conventional T lineage development.
Overall, low thymic cellularity was a major determinant of our ability to consistently identify and isolate the chimeric human/mouse thymus of humanized mice at 12 weeks post reconstitution. The small size of the chimeric thymus may be a reflection of inefficient homing of human T-cell progenitors to the mouse thymus, a paucity of proliferation of immature human thymocytes, and/or reduced human thymocyte:murine stromal cell cross-talk, among other possibilities. In the thymus, limited niches exist for the development of certain T-cell subsets, and thus, the small size of the humanized mouse thymus may further impede the development of certain thymic subsets.
At first glance, our findings of enhanced CD4+, but not CD8+, SP thymocyte cellularity might seem at odds with the observation that the HLA-A2 transgene leads to enhanced development of HLA-A2 restricted T-cell responses in the periphery of humanized NSG mice.10, 11, 12 However, the HLA-A2 transgene could enhance both negative and positive selection, thus producing a similar overall thymic output, but generating an altered TCR repertoire with a greater ability to recognize foreign peptides presented by HLA-A2. It is interesting to speculate that enhanced positive selection of CD8+ SP thymocytes might support improved thymocyte:stromal cell crosstalk and expansion of the medullary environment that, in turn, would promote CD4+ SP thymocyte cellularity, while the simultaneous improvement in negative selection of CD8+ SP thymocytes might mask any advantages in their development. Additionally, the improvement in CD4+ SP cellularity in the thymus of NSG HLA-A2 tg mice appears to dissipate in the periphery, supporting the notion that the HLA-A2 transgene might also exert its effect in the periphery via the promotion of homeostatic proliferation of HLA-A2 restricted T cells, for example.
Despite comparable conventional T-cell development, we found that the murine thymic environment in humanized mice does not fully recapitulate human non-conventional T lineage development. This difference is most striking in the observed paucity of Foxp3+ CD4+ and CD8+ thymocytes in humanized mice as compared with human thymus samples, despite the presence of Foxp3+ T cells in the periphery. Since development of Foxp3+ T cells is thought to require stronger TCR signals compared with conventional T-cell development, it is possible that interspecies TCR:MHC interactions are unable to provide sufficient TCR signal strength required for efficient Foxp3+ T-cell development.55, 56, 57, 58 Alternatively, the chimeric murine thymus may be unable to provide strong co-stimulatory signals on antigen presenting cells required for the development and/or expansion of Foxp3+ Treg cells.59, 60, 61, 62, 63 We describe the presence of CB-derived human thymic DCs with high MHC class II expression that could provide or cross-present agonist peptide on human HLA molecules. We also provide evidence to suggest that developing thymocytes in humanized mice come in contact with these human hematopoietic-derived thymic DCs. However, the presence of human thymic DCs alone may not suffice to provide appropriate signals for human Treg development. Thymic stromal lymphopoietin (TSLP) conditioning of DCs is required for the development of human Treg cells,39 yet the bulk of TSLP is produced by non-hematopoietic derived TECs.64 The low sequence identity between human and mouse TSLP (43%) and the observation that human cells do not react to mouse TSLP65 may explain the dearth of Foxp3+ CD4+ T cells in the thymus of humanized mice. It remains to be determined whether thymic Foxp3+ cells in neonatal chimera humanized mice have a competitive advantage for survival in peripheral lymphoid organs or whether the increased proportion of Foxp3+ T cells in the spleen is a result of conversion in the periphery.
Human cord blood likely contains a mixture of fetal- and adult-like HPCs,23 leading us to expect that development of non-conventional T-cell lineages in humanized mice might segregate with either fetal or postnatal human T-cell development. Thymic selection of many non-conventional T-cell subsets is thought to be mediated via atypical thymocyte interactions with other thymocytes, or with other bone marrow-derived hematopoietic cells.19, 26, 27 Further, it has been suggested that inefficient selection of human T cells on mouse MHC might skew selection toward non-conventional T-cell populations selected on hematopoietic cells in humanized mice.2, 19, 20 Consistent with this notion, the proportion of PLZF+ cells among TCRβ+ thymocytes in humanized mice was comparable to that of fetal thymocytes. Unlike other innate-like T-cell subsets, human PLZF+ T cells demonstrate diverse Vβ usage comparable to that of conventional CD4 T cells, and are thus unlikely to develop in response to agonist selection.19 This might predict that homotypic thymocyte:thymocyte interactions lead to positive selection signals in response to the additive effect of serial, weak interactions, resulting in a pattern of repeated dynamic contacts observed with low affinity TCR:MHC interactions.66 While our data does not allow us to determine whether the observed thymocyte:thymocyte contacts lead to productive TCR signaling and lineage fate determination, it does suggest that human thymocytes engage in short, serial interactions with other thymocytes in this model.
The well-described signaling inefficiency between mouse cytokines and human receptors is in stark contrast to the interspecies conservation of chemokine signaling.45, 67 We have previously demonstrated that human thymocytes maintain their developmental stage-specific localization and motility within murine thymic stroma.13 In this study, we further these observations by demonstrating that human thymocytes whose maturation has occurred entirely within a murine thymic environment maintain their characteristic patterns of motility. Given the limitations of certain interspecies receptor:ligand interactions, it is remarkable that the T-cell maturation process, which involves the step-wise integration of multiple signaling cues, can proceed with enough efficiency to maintain the characteristic slow migration of immature cortical thymocytes and the faster motility of mature, medullary cells.
Our work adds to the existing data supporting efficient interspecies TCR:MHC encounters and provides new insight into the behavior of human T cells developing in the murine thymus of humanized mice. Differences between mouse and human signaling interactions have led to significant advances in our understanding of the cytokine requirements during human thymopoiesis.68 Similarly, the observed differences in non-conventional T-cell development between humanized mice and human thymus may help to uncover the signaling cues involved in alternate human T-cell lineage fate decisions. Our findings emphasize the importance of studying human immunology in vivo, and may help to design humanized mice that more accurately and efficiently recapitulate human T-cell development and function.
METHODS
Human tissue procurement
Advanced Bioscience Resources (Alameda, CA, USA) and StemExpress (Placerville, CA, USA) procured 18- to 20-week-old fetal thymic tissue and term umbilical cord blood, respectively, after informed consent and in accordance with the Declaration of Helsinki, as well as federal, state and local law. Postnatal thymus samples (1 week to 2.5 years) were collected as surgical byproducts during corrective pediatric cardiac surgeries, and were obtained in accordance with the Declaration of Helsinki and were approved by the institutional review board of UCSF Benioff Children's Hospital Oakland.
Mice
Mice were bred and housed under pathogen-free conditions at the American Association of Laboratory Animal Care approved facility at the Life Sciences Addition at the University of California, Berkeley. Protocols were approved by the University of California, Berkeley Animal Care and Use Committee. NOD SCID γcnull (NSG) and NOD SCID γcnull HLA-A2-tg (NSG-HLA-A2) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).10, 69 At birth to 5 days of age, NSG and NSG-HLA-A2+ littermates underwent total body irradiation (100 rads), followed by intrahepatic injection of 2–6 × 105 lin−CD34+CD38− human hematopoetic progenitors purified from umbilical cord blood (>80% purity, StemExpress, Placerville, CA, USA) or BALB/c bone marrow. Mice were analyzed 11–13 weeks post reconstitution. A total of 71 mice from 7 litters were injected with cord blood from 8 different donors. Of these, 33 (21 HLA-A2 Tg− and 12 HLA-A2 Tg+) mice had an identifiable thymus 11–13 weeks post reconstitution and were used for analysis.
Flow cytometry
Thymus and spleen were prepared as single-cell suspensions with glass tissue grinders, and filtered. For preparation of epithelial cells, thymic tissue was injected with 1 mg ml−1 Collagenase 1A (Sigma-Aldrich, St Louis, MO, USA) and 0.4 mg ml−1 DNase I (Roche, Indianapolis, IN, USA), incubated for 30 min at 37 °C, then pipetted until dissociated. Cells were stained with a fixable viability dye eFluor 506 (eBioscience, San Diego, CA, USA), and then blocked with 24G2 and anti-human CD32/Fc Receptor block (StemCell Technologies, Vancouver, BC, Canada). The following antibodies were used for analysis of cell surface antigens: anti-human CD3-eFluor450, CD4-eFluor780, CD8α-PeCy7, CD19-PerCP, CD45-PerCP or -eFluor450, TCRβ-FITC, -APC, or -biotin, TCRγδ-FITC, EpCAM-FITC (eBioscience), HLA-A2-PE (Abcam, Cambridge, MA, USA), HLA-DR-PE and CD56-PECy5 (BD Bioscience, San Jose, CA, USA). For intracellular staining, cells were fixed and permeabilized following surface staining with the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer's instructions. The permeabilization buffer was supplemented with anti-human CD32/Fc Receptor block (StemCell Technologies), and 5% mouse serum (Jackson ImmunoResearch, West Grove, PA, USA). Following fixation and permeabilization, cells were stained with anti-human PLZF-APC (R&D Systems, Minneapolis, MN, USA), Eomesodermin-PerCP and FoxP3-FITC (eBioscience). Data were acquired with an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Lentiviral transductions
Phoenix cells were transfected with the FUCGW lentiviral vector expressing GFP along with packaging and envelope plasmids using Lipofectamine Plus reagent according to the manufacturer's instructions (Life Technologies, Grand Island, NY, USA). Viral supernatant was harvested 48 h post transfection, and CD34+CD38− cells were transduced in media containing 4 μg ml−1 polybrene (Sigma, St Louis, MO, USA), and 20 ng ml−1 recombinant human IL-7, SCF and TPO (R&D Systems). Transduced progenitors were intra-hepatically injected into newborn NSG and NSG-HLA-A2 tg mice as described above.
2-Photon imaging
Thymus tissue was adhered to a coverslip using Vetbond tissue adhesive (3M, Saint Paul, MN, USA) maintained in warm, oxygenated phenol red-free DMEM, and imaged as previously described.70 Images were acquired using ZEN software on a Zeiss 7 MP microscope (× 20/1.0 Zeiss objective) with a Coherent Chameleon laser tuned to 920 nm (Zeiss, Jena, Germany). Second harmonic and GFP fluorescence were separated using 495 nm, 510 nm and 560 nm dichroic mirrors, and image areas of 175 × 175 μm were scanned every 30–40 s over 40 time points with 3-μm z-steps to depths of up to 400 μm beneath the thymic capsule.
Image analysis
Imaris software (Bitplane Scientific Software, Saint Paul, MN, USA) was used to process the 2-photon movies to obtain migration behavior and interaction data. 2-Photon image data were further analyzed using standard and custom written MATLAB scripts (Mathworks, Natick, MA, USA), Image J and Excel. Custom codes are available upon request. Graphing and statistical analysis was done using GraphPad Prism (La Jolla, CA, USA).
Acknowledgments
We would like to thank Ivan Dzhagalov for critical reading of the manuscript, as well as Seong-Ji Han and Janet Purcell for assistance. This work was supported by a California Institute of Regenerative Medicine grant RM1-01732 (to EAR), clinical fellowship TG2-00164 (to JH), post-doctoral training grant T1-00007 (to HJM), as well as an undergraduate research award (to BY) from private donations to the UC Berkeley Stem Cell Center.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- 1Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005; 106: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004; 304: 104–107. [DOI] [PubMed] [Google Scholar]
- 3Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol 2010; 135: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum Immunol 2009; 70: 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Aryee KE, Shultz LD, Brehm MA. Immunodeficient mouse model for human hematopoietic stem cell engraftment and immune system development. Methods Mol Biol 2014; 1185: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 2006; 108: 487–492. [DOI] [PubMed] [Google Scholar]
- 7McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 1988; 241: 1632–1639. [DOI] [PubMed] [Google Scholar]
- 8Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 2006; 12: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 9Plum J, De Smedt M, Leclercq G, Taghon T, Kerre T, Vandekerckhove B. Human intrathymic development: a selective approach. Semin Immunopathol 2008; 30: 411–423. [DOI] [PubMed] [Google Scholar]
- 10Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci USA 2010; 107: 13022–13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL et al. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS ONE 2009; 4: e7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med 2009; 206: 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Halkias J, Melichar HJ, Taylor KT, Ross JO, Yen B, Cooper SB et al. Opposing chemokine gradients control human thymocyte migration in situ. J Clin Invest 2013; 123: 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Gilhus NE, Matre R. HLA-DR antigens and Fc gamma receptors in fetal and infant thymus, examined by a double-marker technique. Acta Pathol Microbiol Immunol Scand C 1983; 91: 227–232. [PubMed] [Google Scholar]
- 15Marinova T, Altankova I, Dimitrova D, Pomakov Y. Presence of HLA-DR immunopositive cells in human fetal thymus. Arch Physiol Biochem 2001; 109: 74–79. [DOI] [PubMed] [Google Scholar]
- 16Park SH, Bae YM, Kim TJ, Ha IS, Kim S, Chi JG et al. HLA-DR expression in human fetal thymocytes. Hum Immunol 1992; 33: 294–298. [DOI] [PubMed] [Google Scholar]
- 17Thulesen S, Jorgensen A, Gerwien J, Dohlsten M, Holst Nissen M, Odum N et al. Superantigens are presented by and activate thymocytes from infants. Exp Clin Immunogenet 1999; 16: 226–233. [DOI] [PubMed] [Google Scholar]
- 18Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD et al. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity 2005; 23: 387–396. [DOI] [PubMed] [Google Scholar]
- 19Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY et al. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med 2010; 207: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol 2006; 176: 2053–2058. [DOI] [PubMed] [Google Scholar]
- 21Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol 2013; 69: 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC et al. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol 2011; 186: 5749–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 2012; 335: 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES et al. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol 2011; 186: 4573–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol 2010; 11: 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL et al. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 2006; 25: 93–104. [DOI] [PubMed] [Google Scholar]
- 27Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR et al. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity 2007; 27: 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA et al. Ontogeny of gamma delta T cells in humans. J Immunol 2004; 172: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 29Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med 2010; 207: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 1988; 335: 443–445. [DOI] [PubMed] [Google Scholar]
- 31Haynes BF, Heinly CS. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med 1995; 181: 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 1990; 62: 863–874. [DOI] [PubMed] [Google Scholar]
- 33Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2-/- gammac-/- mice: functional inactivation of p53 in developing T cells. Blood 2004; 104: 3886–3893. [DOI] [PubMed] [Google Scholar]
- 34Zhao Y, Dai ZP, Lv P, Gao XM. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin Exp Immunol 2002; 129: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood 2011; 117: 3076–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 2007; 8: 351–358. [DOI] [PubMed] [Google Scholar]
- 37Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 2013; 210: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 2011; 208: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005; 436: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 40Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol 2005; 35: 383–390. [DOI] [PubMed] [Google Scholar]
- 41Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood 2005; 105: 4715–4721. [DOI] [PubMed] [Google Scholar]
- 42Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol 2006; 176: 5741–5748. [DOI] [PubMed] [Google Scholar]
- 43Halkias J, Melichar HJ, Taylor KT, Robey EA. Tracking migration during human T cell development. Cell Mol Life Sci 2014; 71: 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Bendriss-Vermare N, Barthelemy C, Durand I, Bruand C, Dezutter-Dambuyant C, Moulian N et al. Human thymus contains IFN-alpha-producing CD11c(-), myeloid CD11c(+), and mature interdigitating dendritic cells. J Clin Invest 2001; 107: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Plet A et al. Autonomous and extrinsic regulation of thymopoiesis in human immune system (HIS) mice. Eur J Immunol 2011; 41: 2883–2893. [DOI] [PubMed] [Google Scholar]
- 46Ladi E, Schwickert TA, Chtanova T, Chen Y, Herzmark P, Yin X et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol 2008; 181: 7014–7023. [DOI] [PubMed] [Google Scholar]
- 47Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol 2009; 10: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol 2005; 3: e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Azar GA, Lemaitre F, Robey EA, Bousso P. Subcellular dynamics of T cell immunological synapses and kinapses in lymph nodes. Proc Natl Acad Sci USA 2010; 107: 3675–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004; 427: 154–159. [DOI] [PubMed] [Google Scholar]
- 51Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med 2004; 200: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 2007; 129: 773–785. [DOI] [PubMed] [Google Scholar]
- 53Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 2012; 12: 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007; 7: 118–130. [DOI] [PubMed] [Google Scholar]
- 55Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 2002; 3: 756–763. [DOI] [PubMed] [Google Scholar]
- 56Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2: 301–306. [DOI] [PubMed] [Google Scholar]
- 57Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J et al. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol 2002; 168: 4399–4405. [DOI] [PubMed] [Google Scholar]
- 58Ribot J, Romagnoli P, van Meerwijk JP. Agonist ligands expressed by thymic epithelium enhance positive selection of regulatory T lymphocytes from precursors with a normally diverse TCR repertoire. J Immunol 2006; 177: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med 2013; 210: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Legrand N, Cupedo T, van Lent AU, Ebeli MJ, Weijer K, Hanke T et al. Transient accumulation of human mature thymocytes and regulatory T cells with CD28 superagonist in "human immune system" Rag2(-/-)gammac(-/-) mice. Blood 2006; 108: 238–245. [DOI] [PubMed] [Google Scholar]
- 61Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA 2008; 105: 19869–19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000; 12: 431–440. [DOI] [PubMed] [Google Scholar]
- 63Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 2005; 6: 152–162. [DOI] [PubMed] [Google Scholar]
- 64Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3: 673–680. [DOI] [PubMed] [Google Scholar]
- 65Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol 2001; 167: 336–343. [DOI] [PubMed] [Google Scholar]
- 66Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci Signal 2013; 6: ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA 2009; 106: 21783–21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Mention JJ et al. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell-dependent antibody responses in vivo. Proc Natl Acad Sci USA 2011; 108: 6217–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005; 174: 6477–6489. [DOI] [PubMed] [Google Scholar]
- 70Dzhagalov IL, Melichar HJ, Ross JO, Herzmark P, Robey EA. Two-photon imaging of the immune system. Curr Protoc Cytom 2012; Chapter 12: Unit12–Unit26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.