Abstract
BACKGROUND/OBJECTIVES
We examined the chemical composition and the effect of fermented deer antler on hematopoietic factors in bone marrow cells.
MATERIALS/METHODS
For the preparation of fermented deer antler extract (FAB), fermentation was carried out using Bacillus subtilis at 30℃ for 7 days. The hematopoietic effect of FAB was investigated hematopoietic factors in marrow cells.
RESULTS
The contents of total sugar, sulfated glycosaminoglycans, and uronic acid and the dry weight gradually increased with fermentation time. The sialic acid content (from 0.14 mg/mL to 0.54 mg/mL) was the highest on the 4th day of fermentation after which it decreased. The proliferating activity of bone marrow cells increased with fermentation times. The levels of various hematopoietic growth factors were determined to verify the beneficial effect of deer antler extract fermented by B. subtilis on hematopoiesis. FAB increased the number of stem cell factors and granulocyte colony-stimulating factor in bone marrow cells. In addition, FAB augmented the burst-forming unit erythroid and total colonies in splenocyte-conditioned medium compared with non-fermented antler extract (NFA). However, FAB did not affect the mRNA levels of erythropoietin, an important factor for erythropoiesis.
CONCLUSIONS
FAB, like NFA, did not directly affect hematopoiesis, but contributed to hematopoiesis by stimulating the production of hematopoietic factors.
Keywords: Bacillus subtilis, fermented antler, bone marrow
INTRODUCTION
Hematopoiesis is the process of the production of red blood cells (RBCs) and the maturation of the bone marrow. Hematopoiesis accompanies the formation of blood cellular components, which are derived from hematopoietic stem cells (HSCs). RBCs have to be renewed daily at a certain rate for the maintenance of the body in a healthy state [1]. The reduction of RBCs or hemoglobin in the circulation causes serious health problems. In particular, hematopoiesis is an extremely important issue not only for patients who have anemic symptoms but also for patients receiving treatments for clinical cancer [2,3]. Hematopoietic cells in the bone marrow have the ability to self-replicate and differentiate into blood cells, which is required to prevent anemic symptoms. The proliferation and self-renewal of these cells are tightly regulated by hematopoietic growth factors (HGFs). These factors include stem cell factor (SCF), colony-stimulating factors (CSFs), granulocyte-macrophage CSF (GM-CSF), granulocyte CSF (G-CSF), macrophage CSF (M-CSF), erythropoietin (EPO), and various cytokines [4].
Traditionally, deer and deer parts have been popular medicinal sources in Asian countries such as China, Taiwan, Mongolia, and Korea [5]. Deer antler, which is believed to possess restorative and hematopoietic activities [6,7,8,9], is used in several medicinal applications. Recent studies have reported the various biological effects of deer antler extracts. Chen et al., for example, reported that deer antler extract improved muscle fatigue in mice [10]. Li et al. showed that it promotes hair growth [11]. A recent study showed that substances from deer antler could be used as potential medicinal resources as they recovered RBCs, serum EPO levels, and the hepatic activity of δ-aminolevulinic acid dehydratase (ALAD) in anemic animal models [12], suggesting an improvement of hemolytic anemia [13,14]. In another report, deer antler fermented by Cordyceps militaris was found to have physiological effects, including immunomodulatory and antioxidant activities [15]. The primary benefit of fermentation is the conversion of sugars and other carbohydrates into other molecules, thereby enhancing the biological effects. We previously demonstrated that bone growth, immunomodulatory, and antioxidant activities of deer antler could be increased through fermentation [16].
In this study, we tested the effect of deer antler extract fermented by B. subtilis (FAB) on hematopoietic factors in marrow cells, comparing its beneficial effects on hematopoiesis for RBC production with those of non-fermented deer antler (NFA).
MATERIALS AND METHODS
Fermented deer antler extract preparations
Dried antlers from adult male Russian elks (Cervus canadensis) were obtained from Kwang-Dong Pharmaceutical (Gyeonggi-do, Korea). In a previous study in which bacteria strains were isolated for the preparation of FAB to evaluate its physiological activities, Bacillus subtilis KH-15 was used for fermentation [15]. B. subtilis KH-15 isolated from soybean paste was grown on LB broth at 30℃ for 1 day. The stock culture was maintained on LB agar slants and subcultured monthly. The seed culture was grown in a 250-mL flask containing 50 mL of LB broth at 30℃ on a rotary shaker incubator at 150 rpm for 5 days. Flask cultures were incubated in 500-mL flasks containing 100 mL of antler powder medium (15 g of antler powder in 100 mL of distilled water) at 30℃ on a rotary shaker at 150 rpm for 5 days. For the pilot-scale study, cultures were incubated in a 3-L stirred tank fermenter (Fermentech, Gyeonggi-do, Korea) containing 1.8 L of antler powder medium (1.2 kg of antler powder in 1.8 L of distilled water) after inoculation with 2% (v/v) flask culture for 2 days (temperature: 30℃; aeration rate: 1 v/v/min; agitation speed: 150 rpm; initial pH: 5.6). After fermentation, the ferment was centrifuged at 2,800 × g for 20 min. The supernatants were concentrated at 60℃ using a vacuum evaporator and lyophilized to produce FAB. NFA was prepared by soaking 45 g of antler powder in 300 mL of distilled water followed by refluxing for 3 h and cooling. The extracts were centrifuged, concentrated, and lyophilized.
Chemical analysis of the culture media during fermentation
Total sugar and uronic acid contents were determined by the phenol-sulphuric acid method [17] and by mhydroxydiphenyl [18], respectively, using glucose and galacturonic acid as the respective standards. Protein content was assayed using the bicinchoninic acid method, according to the manufacturer's instructions (Pierce Chemicals Ltd, Rockford, USA) using bovine serum albumin as the standard. Dry weight was estimated from the sample weight by heating at 110℃ overnight. Sulfated-glycosaminoglycan (GAG) content was assayed using the modified 1,9-dimethylmethylene blue (DMMB) dye-binding method as previously described [19]. A standard curve was constructed using serial dilutions of chondroitin sulfate (Sigma Chemical Co.). Sialic acid was released by mild acid hydrolysis (0.05 M H2SO4, 80℃) of the glycoprotein, and the free sialic acid content was determined by the thiobarbituric acid method [20].
Proliferation assay of bone marrow cells
Bone marrow cell proliferation induced by FAB or NFA was performed according to the modified procedure of Yu et al. [21]. A diluted antler extract sample (50 µL) was incubated with a bone marrow cell suspension (2.5 × 105 cells/mL in RPMI-1640-FBS, 100 µL) prepared from C3H/He mice for 6 days in a humidified atmosphere of 5% CO2/95% air. After the addition of 20 µL of Cell Counting Kit-8 solution to each well, the cells were continuously cultured for 4-6 h and the fluorescence intensity was measured using a microplate reader set at 450 nm. The bone marrow cell proliferating activity was expressed as the relative percentage of the bone marrow cell growth compared with that of the saline control incubated without the test sample.
Cell preparation and cell cytotoxicity
All surgical and preparative procedures were performed under aseptic conditions. The spleen, bone marrow, and the kidneys were excised from 7-week-old female Balb/c mice. The cell preparation methods were performed according to the method of Liao et al. [22]. Briefly, splenocytes were harvested from the spleen using a 70-µm cell strainer. Marrow cells were separated from the mouse femur and tibia by flushing the bones with RPMI 1640 medium [23]. Kidney cells were harvested from tissue homogenates with 0.1% collagenase. The cytotoxicity of NFA and FAB was measured using the MTT colorimetric assay [24].
Preparation of splenocyte-conditioned medium (SCM)
The spleen was excised from 7-week-old female Balb/C mice and homogenated into single cells. The splenocyte suspension (1 × 107 cells/mL) was cultured in RPMI 1640 medium (10% fetal calf serum) with or without EPO treatments. The supernatant, designated as SCM, was collected after 72 h of incubation and stored at -70℃ until use [22].
Hematopoietic growth factors and hematopoietic stem cell colony-forming unit assay
Cytokine levels were determined from SCM using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN, USA). A colony-forming unit assay with a soft-agar culture method was performed using methylcellulose-based media (R&D Systems, Inc., Minneapolis, MN, USA) to determine the colony-forming potential of HSCs as previously reported [25,26]. Bone marrow cells were harvested from Balb/c mice and dispersed into RPMI1640 medium with 10% FCS after 90 min of culturing at 37℃ in a CO2 incubator. After 14 days of incubation, the colony-forming unit-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM) and burstforming unit erythroid (BFU-E) were determined and expressed as the total colony (GFU-GEMM+ BFU-E) and BFU-E. The effects of interleukin (IL)-3 (base media + IL-3, 10 ng/mL-1), SCF (base media + SCF, 50 ng/mL), EPO (base media + EPO, 5 IU/mL), IL-3 + SCF (base media + IL-3 + SCF), and IL-3 + SCF + EPO (base media + IL-3 + SCF + EPO) were determined by comparing the colony-forming unit to the positive control medium that contained IL-3, IL-6, SCF, and EPO.
Reverse-transcription polymerase chain reaction
The isolation of RNA using TRIzol LS reagent (Invitrogen, Carlsbad, CA, USA) and the synthesis of cDNA using Super Script® III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) were performed according to the manufacturers' instructions. One microgram of cDNA was used as the template in a 20-µL PCR volume with 0.5 mM of each primer, 2.5 mM MgCl2, 0.2 mM of each dNTP, and 0.5 U of Taq polymerase (Promega Corp., Madison, WI, USA) in the presence of 50 mM KCl, 10 mM Tris-HCl (pH 9.0), and 1% Triton X-100. The PCR amplification was performed in a Perkin Elmer 9600 thermal cycler (Fostery, CA, USA) with an initial denaturation of 4 min at 95℃, followed by 25-30 cycles of 30 s at 95℃, 30 s at 55-60℃, 30 s at 72℃, and a final extension of 10 min at 72℃. The PCR products were resolved by electrophoresis on 1.2% agarose gels and were visualized by ethidium bromide staining. The mRNA levels of each gene were normalized to that of the housekeeping gene (β-actin).
Statistical analyses
Data were subjected to statistical analysis using the SPSS software version 12.0 (SPSS, Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) was performed for each response variable. P values < 0.05 were considered significant.
RESULTS
Changes in total sugar, uronic acid, sulfated glycosaminoglycans (S-GAG), sialic acid, protein, and solid contents during antler fermentation using B. subtilis
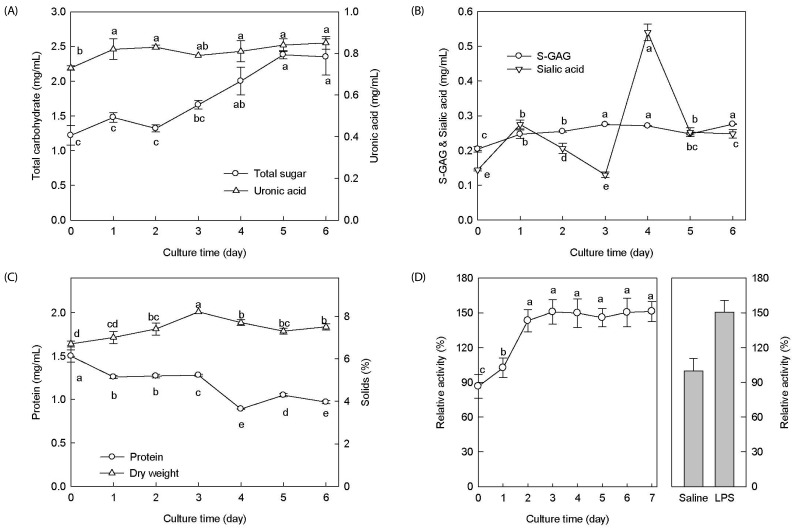
The fermentation of antler using B. subtilis was carried out at 30℃ for 7 days. Fig. 1 shows the changes of the chemical compositions in the culture media during fermentation. As shown in Fig. 1A, the total sugar content continuously increased during the fermentation time. At day 5, the fermented product contained 2.38 mg/mL of total sugar, which was a 2-fold increase compared with the initial amount of 1.22 mg/mL (Fig. 1A). The uronic acid level was slightly increased during fermentation process compared with that measured at the initial time point. The ferment was hydrolyzed in mild sulfuric acid for the analysis of the sialic acid content. The sialic acid level was 4-fold increased at day 4 (0.54 mg/mL) over the initial level, after which it decreased again. (Fig. 1B). The level of ganglioside (S-GAG) gradually increased during fermentation. In contrast, the protein content showed a downward trend over time, while the dry weight was slightly increased during fermentation (Fig. 1C). In addition, FAB prepared in the pilot-scale study had a higher total sugar and sialic acid content (7.84 mg/g and 0.19 mg/g, respectively) than NFA (4.41 mg/g and 0.06 mg/g, respectively) (Table 1). As shown in Fig. 1D, the proliferating activity of the bone marrow cells increased with fermentation time. Especially, cell proliferation was rapidly increased until day 2, after which its levels did not change significantly. This result showed that FAB stimulated bone marrow cells. Since bone marrow hematopoietic stem and progenitor cells have the ability to self-replicate and differentiate into mature blood cells [27], FAB is shown to be able to promote the differentiation of bone marrow cells into leukocytes or erythrocytes in the blood.
Fig. 1. Effect of fermented antler extract on chemical compositions and bone marrow cell proliferation during Bacillus subtilis-mediated fermentation.
Cultures were incubated in a 3 L stirred-tank fermenter containing 1.8 L of the antler powder medium after inoculation with 2% (v/v) flask culture for seven days (temperature, 30℃; aeration rate, 1 vvm; agitation speed, 150 rev/min; initial pH 5.6). Total carbohydrate and uronic acid (A), S-GAG and sialic acid (B), protein and solid content (C) were analyzed, and the proliferative activity of bone marrow cell (D) were determined during fermentation of antler extract. Values are means ± SD (n = 6). Means with different superscript letters within a row are significantly different at P < 0.05 by Duncan's multiple range tests. S-GAG: sulfated glycosaminoglycans.
Table 1. Dry weight, total sugar, uronic acid, S-GAG, and the sialic acid content of the fermented antler [15].

*Contents were expressed mg per g of sample. Reference materials: total sugar: glucose; uronic acid: galacturonic acid; sulfated (S)-GAGs: chondroitin sulfate; sialic acid: N-acetylneuraminic acid. Values are the mean ± SD of triplicate determinations.
The effect of fermented antler extract on SCF, G-CSF, GM-CSF, and IL-3 in SCM
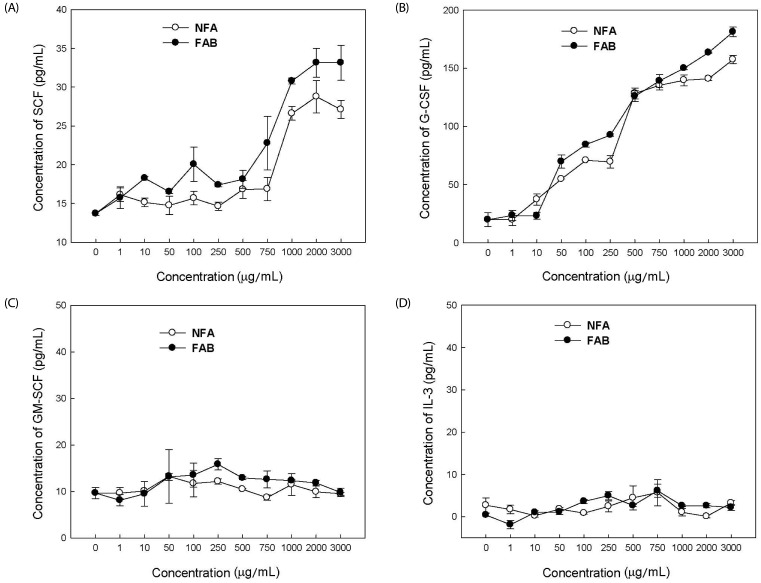
No detectable cytotoxicity of NFA and FAB against splenocytes was observed up to a concentration of 5,000 µg/mL-a concentration that is used in the analysis of hematopoietic factors in splenocytes (Table 2). The hematopoietic factors SCF, G-CSF, and GM-CSF and the cytokine IL-3 in NFA-SCM and FAB-SCM were determined using a commercially available ELISA kit (Fig. 2). The levels of GM-CSF and IL-3 were not significantly changed at the tested doses of both antler extracts, and no significant difference between NFA and FAB was observed. However, both NFA and FAB significantly increased SCF and G-CSF levels in a dose-dependent manner, and their levels were significantly higher after FAB treatment than those observed after NFA treatment at most of the concentrations used.
Table 2. Effect of antler extract on the proliferation of spleen cells.
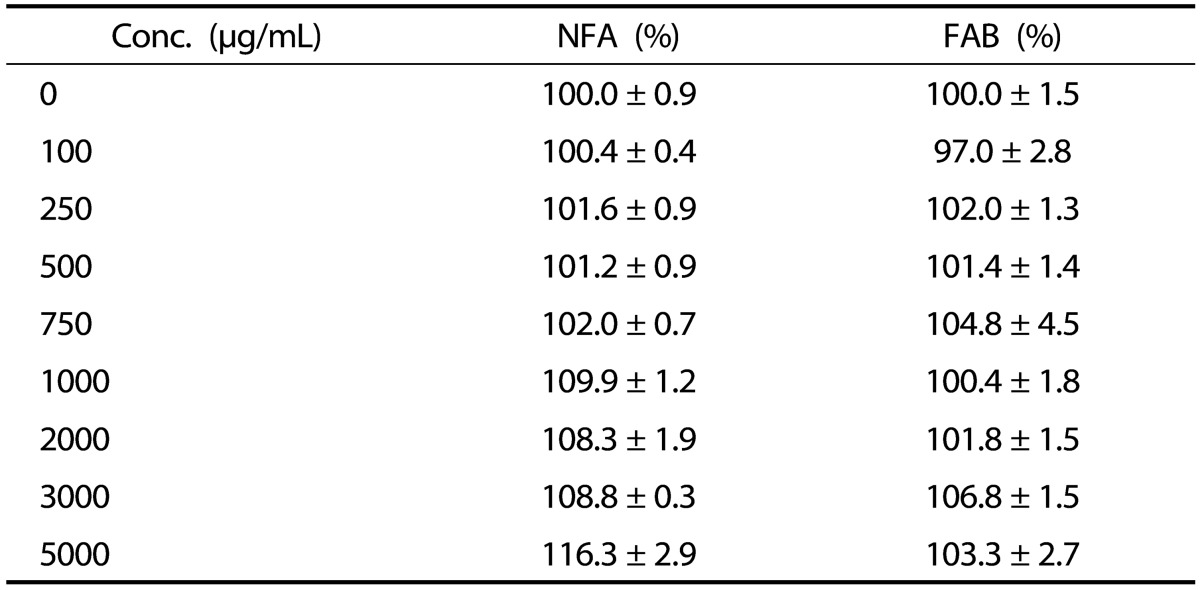
The data are expressed as the mean ± SEM of 3 separate experiments.The data are expressed as percentage of the control. NFA: non-fermented antler extract; FAB: antler extract fermented by B. subtilis.
Fig. 2. Effect of NFA and FAB on hematopoietic growth factors in SCM.
Balb/C mice-derived splenocytes were cultured in RPMI 1640 medium for 72 h in the presence or absence of NFA and FAB. Levels of hematopoietic growth factors including SCF (A), G-SCF (B), GM-SCF (C), and IL-3 (D) were analyzed by ELIZA. The data were expressed as the mean ± SEM of three separate experiments. NFA: non-fermented antler extract; FAB: antler fermented extract by B. subtilis; SCF: stem cell factor; G-CSF: granulocyte colonystimulating factors; GM-SCF: granulocyte-macrophage CSF; IL-3: interleukin-3.
Effect of EPO and cytokines on colony-forming cells
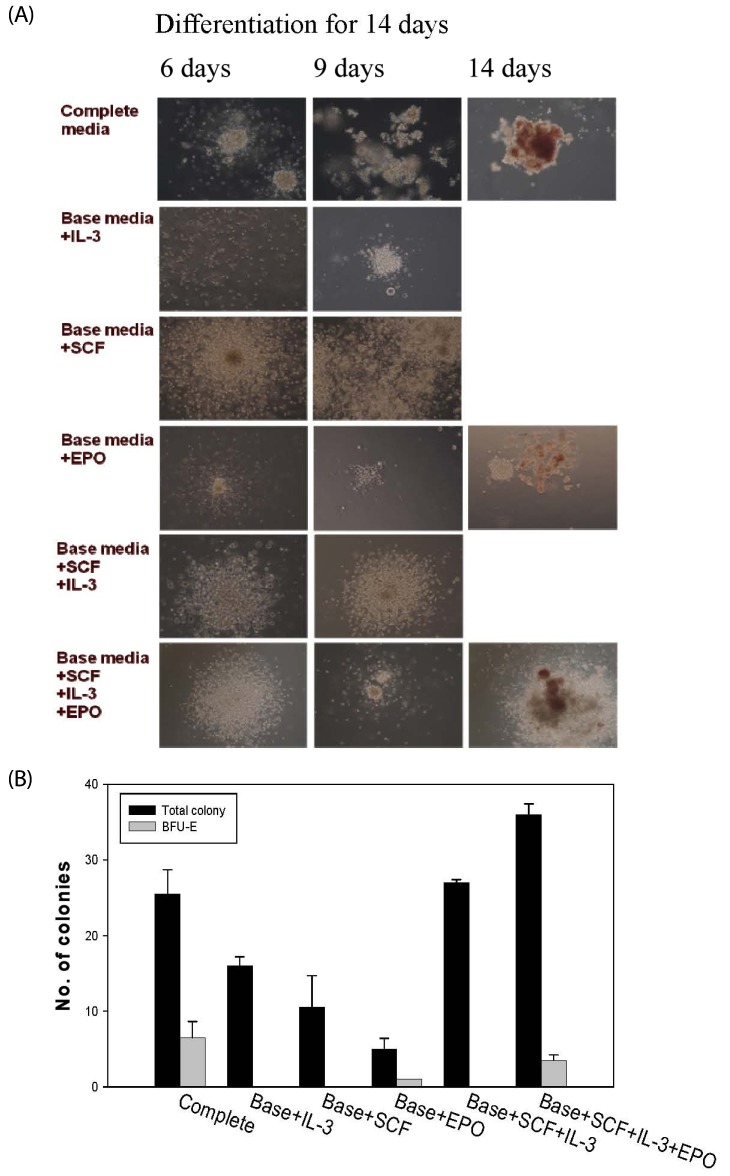
Progenitor cells are known to differentiate into blood cells by the hematopoietic activation of cytokines such as EPO, GM-CSF, G-CSF, IL-3, IL-6, TPO, and SCF [27]. In particular, colony formation in bone marrow cells is a prerequisite for the differentiation into RBCs. We tested the effect of the cytokines IL-3, SCF, and EPO on colony formation in marrow cells. A total colony was formed after treatment with IL-3 and SCF (16.0 and 10.0 colonies, respectively), whereas they failed to form total BFU-E colony, which has the capability of differentiating into RBCs (Fig. 3). On the other hand, EPO-treated cells formed a BFU-E colony, although no clearly detectable total colony was formed. The simultaneous treatment with SCF, IL-3, and EPO led to the formation of both colonies (total and BFU-E, 35.7 and 4.8 colonies, respectively) (Fig. 3), similar to the formation of colonies in complete media. This result showed that EPO played a critical role in the formation of BFU-E colonies.
Fig. 3. Effect of EPO and cytokines on colony-forming cells.
Bone marrow cells derived from Balb/c mice were cultured in RPMI1640 medium with 10% FCS (base media). For colony formation, IL-3, SCF, and EPO were added to the media (completed media), and evaluated. Colony formation was photographed by microscope (A). After 14 days of incubation, the colony-forming unit-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM) and erythroid (BFU-E) were determined (B). Total colony: (GFU-GEMM+BFU-E). The data were expressed as the mean±SEM of three separate experiments. BFU-E had multicentric (burst) erythroid colonies with red or pink color. IL-3: interleukin-3; SCF: stem cell factor; EPO: erythropoietin; GFU-GEMM: colony-forming unit-granulocyte/erythrocyte/macrophage/megakaryocyte; BFU-E: burstforming unit erythroid.
The effect of antler extract on colony formation with or without EPO
Based on the above-mentioned results, we tested the hematopoietic effect of NFA and FAB treatment in marrow cells with or without EPO supplementation. No significant difference in total colony formation was observed between both treatments-with or without EPO supplement (Table 3). In addition, FAB and NFA did not significantly induce the formation of BFU-E colonies, both in the absence and presence of EPO, as compared with that in the control group (Table 3).
Table 3. The effect of EPO and antler extract on colony-forming cells.
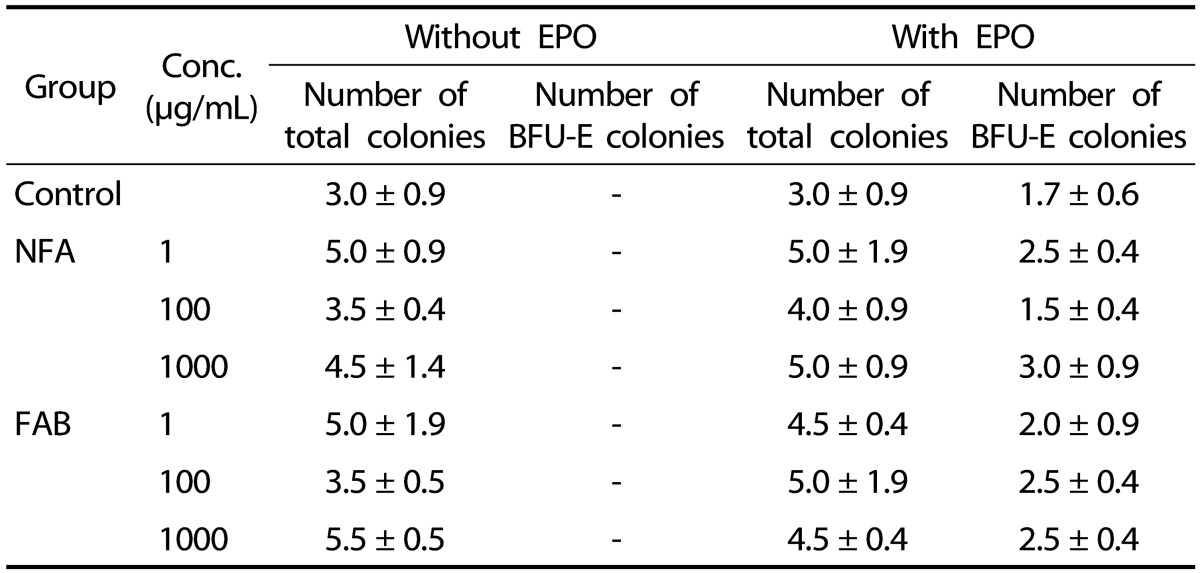
The data are expressed as the mean ± SEM of 3 separate experiments. NFA: non-fermented antler extract; FAB: antler extract fermented by B. subtilis; EPO: erythropoietin; BFU-E: burst-forming unit erythroid.
The effect of EPO and splenocyte-conditioned media on colony formation and the effect of antler extract on EPO mRNA expression in kidney cells
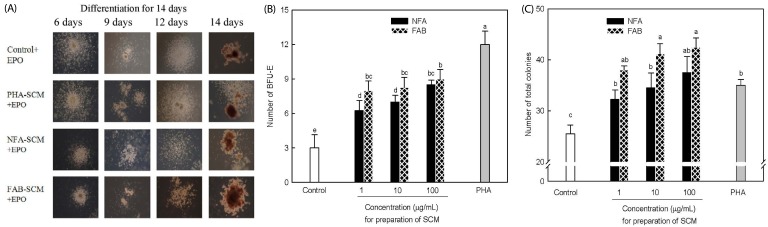
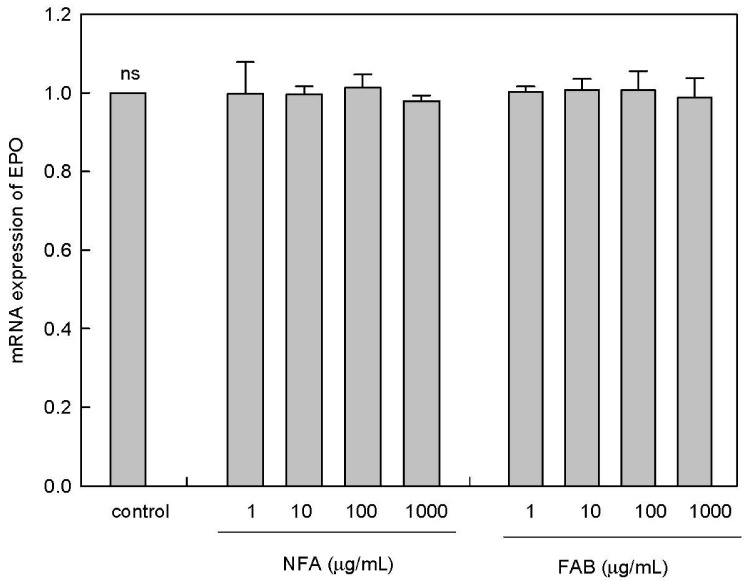
To verify the effect of NFA and FAB on progenitor cell differentiation, we determined colony formation using SCM in marrow cell cultures. The cells were supplemented with EPO and SCM to induce hematopoiesis (Fig. 4). The formation of BFU-E and total colonies was significantly increased by SCM treatment compared with that in the controls (P < 0.05). In particular, PHA, a lymphocyte-activating factor, induced the formation of the highest number of BFU-E colonies in marrow cells. FAB treatment significantly increased the formation of BFU-E and total colonies compared with that after NFA treatment. These results indicated that FAB, more than NFA, effectively stimulated total colony as well as BFU-E colony formation essential for hematopoiesis. Finally, to assess the hematopoietic effect of NFA and FAB, we measured the mRNA level of EPO in kidney cells. There was no significant increase of the EPO mRNA level by both treatments of NFA and FAB (Fig. 5).
Fig. 4. Effect of NFA and FAB on colony formation in splenocyte conditioned media containing EPO.
Bone marrow cells derived from Balb/c mice were cultured in RPMI1640 medium with 10% FCS (base media). Colony formation of bone marrow cells was induced with SCM and EPO. PHA, NFA, and FAB were added to stimulate colony formation, and evaluated (A). The numbers of total colonies and BFU-E were determined in NFA or FAB-treated SCMs containing different concentrations (B and C).Data from four separate experiments are expressed as the mean ± SEM. Means with different superscript letters indicated significant differences at P < 0.05 by Duncan's multiple range tests. NFA: non-fermented antler extract;. FAB: antler extract fermented by B. subtilis ; EPO: erythropoietin; SCM: splenocyte conditioned media; BFU-E: burstforming unit erythroid. PHA was positive control and its concentration was 10 µg/mL.
Fig. 5. Effect of antler extracts on the mRNA expression of EPO in kidney cells.
mRNA expressions of EPO was determined in treated kidney cells treated by NFA and FAB by semi-quantitative RT-PCR. Data from four separate experiments are expressed as the mean ± SEM. Control: saline; NFA: non-fermented antler extract; FAB: antler extract fermented by B. subtilis. Data are expressed as contrast of control, EPO: erythropoietin.
This suggests that FAB positively affected the formation of colonies, which, in turn, is important for hematopoiesis. The stimulating effect of FAB and NFA on colony formation was directly not due to an increase in EPO gene expression. Rather, FAB is shown to promote the EPO-mediated formation of BFU-E colonies via stimulation of hematopoietic factors. However, to understand the erythropoeitic effect of FAB in more detail, late stage factors, such as CFU, in the erythroid development should be investigated in future studies.
DISCUSSION
Natural products with various health claims are currently being re-evaluated as the need for traditional medicinal resources has greatly increased [28]. Antlers are one of the most significant and prominent resources for such traditional medicines [29]. Concerns about the potential side effects from antler consumption have rarely been discussed and reported [29]. Fermented and non-fermented antlers are considered to be safe in vitro and in vivo as reported in a previous study [14].
In the present study, the total sugar, sialic and ganglioside content of FAB increased during fermentation (Fig. 1). We suggest that antler cell membranes were hydrolyzed by fermentation; thus, active compounds, such as proteins and gangliosides, were released into the culture media. Our previous reports were also showed the increase of total sugar, sialic acid and ganglioside content during fermentation with Cordyceps militaris [15]. Our results showed that the cytotoxicity of FAB and NFA was not detected at a concentration less than 5,000 µg/mL in vitro.
In our previous reports, we demonstrated that the fermentation of antlers by Cordyceps militaris enhanced specific physiological effects, including immunomodulatory and antioxidant activities [15], and that fermented antler promoted the differentiation of osteoblasts, which are related to bone formation and growth [16]. Antlers are known to potentiate the recovery of RBCs, serum EPO levels, and the hepatic activity of ALAD in animal models of hemolytic anemia [12]. In this study, the hematopoietic activity of FAB was investigated. Antlers were fermented using B. subtilis and we verified the hematopoietic effect of FAB in bone marrow cells by determining colony formation in vitro.
Hematopoiesis of progenitor cells into RBCs is associated with the hematopoietic activities of various factors, including EPO, GM-CSF, G-CSF, IL-3, IL-6, and SCF [12]. Our data showed that FAB affected the spleen-derived hematopoietic factors such as SCF and G-CSF rather than IL-3 and GM-CSF (Fig. 2). Furthermore, the production of SCF and G-CSF was more stimulated by FAB than by NFA. This result indicated that FAB could more strongly stimulate the differentiation of hematopoietic stem cells from bone marrow cells by promoting the production of SCF and G-CSF.
Erythropoiesis (or hematopoiesis) undergoes several colony-forming stages, including the formation of BFU-E and CFU-E colonies in the spleen and the kidneys, respectively [30]. We tested the effects of the cytokines IL-3, SCF, and EPO on colony formation in marrow cells and observed that EPO played a major role in the formation of BFU-E colonies, which is the earliest stage of erythroid development (Fig. 3). SCF and IL-3 did not lead to the formation of BFU-E colonies, but contributed to the EPO-induced increase in BFU-E colony formation (Fig. 3). Interestingly, in a previous report, it was shown that the cytokines IL-3, IL-6, IL-11, and SCF significantly induced the expansion of marrow cells in vitro [27].
In particular, no hematopoietic effect was observed in NFA- and FAB-treated marrow cells, both in the absence and presence of EPO (Table 3). This result indicated that the hematopoietic effect of antler extract was not directly related to an erythropoietic effect. NFA and FAB-SCM had a significant effect on progenitor cell differentiation in marrow cells by increasing BFU-E and total colonies, with FAB significantly increasing the number of total and BFU-E colonies compared with what was observed for NFA (Fig. 4). This suggests that FAB positively affected the formation of colonies, which, in turn, is important for hematopoiesis. However, to understand the erythropoeitic effect of FAB in more detail, late stage factors, such as CFU, in the erythroid development should be investigated in future studies. Our data verified crucial role of EPO in the formation of BFU-E colonies and that FAB increased the number of colonies (Fig. 3 and 4). Therefore, we tested if FAB could affect the expression of EPO in kidney cells and found that it did not affect the mRNA levels of EPO. Therefore, FAB directly did not regulate EPO for hematopoiesis, but FAB is shown to promote hematopoiesis via activation of hematopoietic factors in the presence of EPO. It is also possible that FAB could support the biological actions of EPO at the cellular level. In particular, EPO is known to play a considerable role in the biological processes underlying wound and neuronal injury healing [31,32]. Whether FAB may affect these biological processes needs to be investigated in the future studies.
We did not address the active compound in FAB that induced the increase of hematopoietic factors. Yang et al. [6] reported that monoacetyldiglycerides isolated from deer antler, Cervus nippon, were potent stimulants of hematopoiesis in vitro. We demonstrated that these active compounds were monoacetyldiglycerides by mass, NMR and IR spectrometry. Su et al. [33] also reported that deer antler base collagens and proteins could enhance the body's hematopoietic function. The mechanism may be that deer antler base collagens and proteins promote the hematopoietic system to secrete hematopoiesis-related factors, improve the hematopoietic micro-environment and stimulate the proliferation, differentiation and maturation of hematopoietic stem cells, and thus elevate the number of red blood cells and the content of hemoglobin in peripheral blood [34]. Another previous study, however, showed that sialic acid played an important role in hematopoietic spleen colony formation [35]. Therefore, based on our data showing highly increased levels of sialic acid on day 4 of fermentation (Fig. 1), sialic acid in FAB is recognized as a major compound responsible for the hematopoietic effect observed in this study. Previous reports can be seen that deer antler base has a very clear hematopoietic modulatory activity. However, determining its exact cellular and molecular mechanisms will require further investigation.
We also previously reported that the changes of some hematopoietic factors, such as EPO and hepatic ALAD activity, induced by phenylhydrazine exposure were restored to within the normal range when anemic rats were treated with antler extract [14]. In the iron deficiency anemic rats, oral administration of the extract for 1 week increased blood hemoglobin, iron contents in liver and spleen, and divalent metal transporter 1 (DMT1) expression in the liver compared to anemic control rats. The fermentation could increase these hematopoietic indexes of deer antler without significant differences [36]. Furthermore, our data showed that FAB did not directly affect EPO production, a crucial factor in hematopoiesis. However, FAB increased the production of spleen-derived hematopoietic factors such as SCF and G-CSF in marrow cells. Therefore, this study shows that FAB has a positive effect on hematopoiesis by promoting the differentiation of hematopoietic stem cells and the production of hematopoietic factors. Taken together, FAB could be a potential remedy for anemic symptoms.
Footnotes
This research was supported by the Technology Development Program for Food, Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea.
References
- 1.Henning GT, Schild SE, Stafford SL, Donohue JH, Burch PA, Haddock MG, Gunderson LL. Results of irradiation or chemoirradiation for primary unresectable, locally recurrent, or grossly incomplete resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:109–118. doi: 10.1016/s0360-3016(99)00379-x. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell SE, Mendenhall WM, Zlotecki RA, Carroll RR. Squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2001;49:1007–1013. doi: 10.1016/s0360-3016(00)01518-2. [DOI] [PubMed] [Google Scholar]
- 3.Ha YW, Jeon BT, Moon SH, Kim YS. Comparison of biochemical components among different fodders-treated antlers. Korean J Pharmacogn. 2003;34:40–44. [Google Scholar]
- 4.Hassan HT, Zander A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol. 1996;95:257–262. doi: 10.1159/000203893. [DOI] [PubMed] [Google Scholar]
- 5.Ivankina NF, Isay SV, Busarova NG, Mischenko T. Prostaglandin-like activity, fatty acid and phospholipid composition of sika deer (Cervus nippon) antlers at different growth stages. Comp Biochem Physiol B. 1993;106:159–162. doi: 10.1016/0305-0491(93)90022-w. [DOI] [PubMed] [Google Scholar]
- 6.Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler) Chem Pharm Bull (Tokyo) 2004;52:874–878. doi: 10.1248/cpb.52.874. [DOI] [PubMed] [Google Scholar]
- 7.Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004;27:1121–1125. doi: 10.1248/bpb.27.1121. [DOI] [PubMed] [Google Scholar]
- 8.Wang BX, Zhao XH, Qi SB, Kaneko S, Hattori M, Namba T, Nomura Y. Effects of repeated administration of deer antler extract on biochemical changes related to aging in senescence-accelerated mice. Chem Pharm Bull (Tokyo) 1988;36:2587–2592. doi: 10.1248/cpb.36.2587. [DOI] [PubMed] [Google Scholar]
- 9.Zhao QC, Kiyohara H, Nagai T, Yamada H. Structure of the complement-activating proteoglycan from the pilose antler of Cervus nippon Temminck. Carbohydr Res. 1992;230:361–372. doi: 10.1016/0008-6215(92)84044-s. [DOI] [PubMed] [Google Scholar]
- 10.Song SK. Influence of deer horn on erythropoietin activity and radioactive iron uptake in rabbits. J Cathol Med Coll. 1970;18:51–60. [Google Scholar]
- 11.Li JJ, Li Z, Gu LJ, Wang YB, Lee MR, Sung CK. Aqueous extract of red deer antler promotes hair growth by regulating the hair cycle and cell proliferation in hair follicles. ScientificWorldJournal. 2014;2014:878162. doi: 10.1155/2014/878162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Park SW. A study on the hemopoietic action of deer horn extract. Korean Biochem J. 1982;15:151–157. [Google Scholar]
- 13.Lee MR, Kim HH, Jo HH, Kang HJ, Gu L, Ly SY, Lee CH, Kim SM, Yang SA, Mo EK, Sung CK. Hemopoietic effect of extracts from four parts of deer antler on phenylhydrazine-induced hemolytic anemia in female rats. J Korean Soc Food Sci Nutr. 2009;38:1718–1723. [Google Scholar]
- 14.Lee SH, Suh HJ, Lee HS, Park Y, Park JW, Jung EY. Hematopoietic effect of Bacillus subtilis-fermented antler extract on phenylhydrazine-induced hemolytic anemia in Sprague-Dawley rats. J Med Food. 2012;15:774–780. doi: 10.1089/jmf.2012.2264. [DOI] [PubMed] [Google Scholar]
- 15.Kim MK, Jung EY, Lee HS, Shin KS, Kim YK, Ra KS, Park CS, Woo MJ, Lee SH, Kim JS, Suh HJ. Isolation of strain for the preparation of the fermented antler and its physiological activities. J Korean Soc Food Sci Nutr. 2009;38:1237–1242. [Google Scholar]
- 16.Lee HS, Kim MK, Kim YK, Jung EY, Park CS, Woo MJ, Lee SH, Kim JS, Suh HJ. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J Ethnopharmacol. 2011;133:710–717. doi: 10.1016/j.jep.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 17.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 18.Chen H, Zhang M, Xie B. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J Agric Food Chem. 2004;52:3333–3336. doi: 10.1021/jf0349679. [DOI] [PubMed] [Google Scholar]
- 19.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 20.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 21.Yu KW, Kiyohara H, Matsumoto T, Yang HC, Yamada H. Intestinal immune system modulating polysaccharides from rhizomes of Atractylodes lancea. Planta Med. 1998;64:714–719. doi: 10.1055/s-2006-957564. [DOI] [PubMed] [Google Scholar]
- 22.Liao HF, Chen YJ, Yang YC. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil- and irradiation-induced myelosuppression. Life Sci. 2005;77:400–413. doi: 10.1016/j.lfs.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 23.Kouro T, Yokota T, Welner R, Kincade PW. Measurement of natural killer cell progenitor activity in culture. Curr Protoc Immunol. 2005;Chapter 22:Unit 22F.3. doi: 10.1002/0471142735.im22f03s66. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang SY, Chen LY, Tsai TF, Su TS, Choo KB, Ho CK. Constitutive production of colony-stimulating factors by human hepatoma cell lines: possible correlation with cell differentiation. Exp Hematol. 1996;24:437–444. [PubMed] [Google Scholar]
- 26.Mecucci C, Cassiman JJ, van der Schueren B, Dewolf-Peeters C, Falini B, van den Berghe H. Phytohemagglutinin-conditioned medium modulates adherence properties and morphology of hairy cells. Leuk Res. 1986;10:1091–1099. doi: 10.1016/0145-2126(86)90054-8. [DOI] [PubMed] [Google Scholar]
- 27.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 28.Brinda R, Parvathy S. Ethnobotanical medicines of anaimalai union pollachi taluk, Coimbatore district, tamilnadu. Anc Sci Life. 2003;22:166–168. [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013;145:403–415. doi: 10.1016/j.jep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Ketley NJ, Newland AC. Haemopoietic growth factors. Postgrad Med J. 1997;73:215–221. doi: 10.1136/pgmj.73.858.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haroon ZA, Amin K, Jiang X, Arcasoy MO. A novel role for erythropoietin during fibrin-induced wound-healing response. Am J Pathol. 2003;163:993–1000. doi: 10.1016/S0002-9440(10)63459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su F, Li H, Wang Y, Huang Y, Xiao S, Xiao Y. Protein component extraction and its bioactivity determination of Sika deer antler base. Anim Sci Vet Med. 2001;18:18–20. [Google Scholar]
- 34.Kong Q, Zhang Y, Wang XJ, Ma M, Chen L, Cui J. A study of IIRWP's increasing effects on WBC number. Chin J Mod Appl Pharm. 1998;15:12–15. [Google Scholar]
- 35.Tonelli Q, Meints RH. Sialic acid: a specific role in hematopoietic spleen colony formation. J Supramol Struct. 1978;8:67–78. doi: 10.1002/jss.400080106. [DOI] [PubMed] [Google Scholar]
- 36.Park SY. Hemato poietic effect of deer antler extract fermented by Bacillus subtilis on primary cell culture of bone marrow and iron deficiency anemic rat [Master's thesis] Seoul: Korea University; 2012. [Google Scholar]