Abstract
Objective
Cartilage oligomeric matrix protein (COMP) is a structural component of cartilage where it catalyzes collagen fibrillogenesis. Elevated amounts of COMP are found in serum during increased turnover of cartilage associated with active joint diseases, such as rheumatoid arthritis (RA) and osteoarthritis (OA). In this study we investigated the ability of COMP to regulate complement. Such capacity was previously shown for some cartilage proteins.
Methods
Regulation of complement by COMP was studied using functional assays in vitro. Interactions between complement proteins and COMP were investigated using direct binding assays and electron microscopy. Circulating COMP and COMP-C3b complexes in serum and synovial fluid from RA and OA patients and healthy controls were measured using a novel ELISA.
Results
We show in vivo evidence of complement activation by released COMP in the general circulation of patients with RA, but not OA patients. We found that COMP induces activation and deposition of C3b and C9 specifically via the alternative pathway of complement, which is attributable to a direct interaction between COMP and properdin. Furthermore, COMP inhibits the classical and the lectin complement pathways due to direct interaction with the stalk region of C1q and mannose-binding lectin, respectively.
Conclusion
COMP is the first extracellular matrix protein for which an active role is demonstrated in inflammation in vivo where it can activate one complement pathway at the same time as it has the potential to inhibit another. The net outcome of these interactions is most likely determined by the type of released COMP-fragments, which may be disease-specific.
Cartilage oligomeric matrix protein (COMP) is a glycoprotein with a predominant expression in cartilage (1) and pressure loaded parts of tendon (2, 3). In addition, some expression has been reported in synovial and dermal fibroblasts (4). COMP is used as an informative molecular marker for ongoing joint destruction, and elevated amounts of COMP can be found both in the synovial fluid and in the serum of patients with active joint disease, e.g. rheumatoid arthritis (RA) and osteoarthritis (OA) (5, 6). One of the main functions of COMP is to catalyze collagen fibrillogenesis by direct interactions with collagens (7, 8) and in the adult to stabilize tissue structure by interaction with molecules bound to the surface of the collagen fibers, such as matrilins (9). COMP is a pentamer consisting of five identical subunits that are linked together by a coiled coil structure in the N-terminus (Fig. 4). The N-terminus is followed by four epidermal growth factor (EGF) domains, eight thrombospondin type 3 (TSP3) repeats and a globular C-terminus (10). Despite the fact that the immune system is regularly exposed to released COMP, injection of rat COMP into rats and mice induces severe, chronic, relapsing arthritis (11, 12) indicating that COMP has the potential to modify joint disease development.
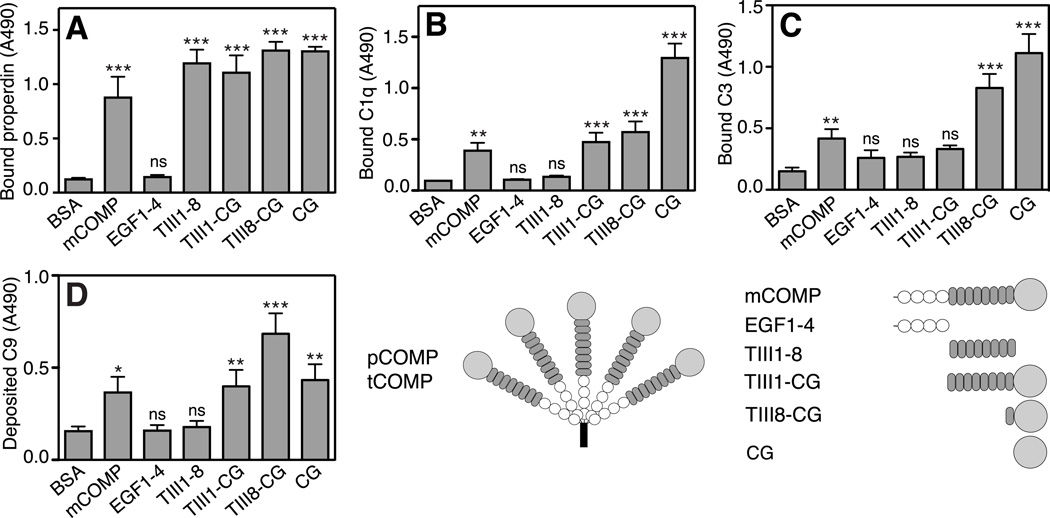
FIGURE 4. Binding of complement factors to COMP constructs.
COMP-constructs were immobilized onto a microtiter plate from solutions of equimolar concentrations and incubated with fluid phase properdin (A), C1q (B) and C3 (C). C9-deposition through the alternative pathway on COMP constructs was measured by immobilizing COMP-constructs and adding NHS diluted in Mg++-EGTA (E). The data are given as the mean and SD of three separate experiments. Statistical significance was calculated with a one-way ANOVA by comparing binding or deposition of complement components onto COMP constructs compared to binding or deposition onto the negative control, BSA. ns, not significant, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
The complement system is a sensor of danger aiding in the removal of dying cells and immune-complexes as well as a defense against foreign pathogens. Uncontrolled complement activation can on the other hand contribute to a wide range of autoimmune disorders and pathological inflammatory conditions such as RA (13). Complement activation products can be found in synovial fluids of patients with active RA, a scenario supported by the protective effect of deficiencies of complement proteins in arthritis mouse models as well as therapeutic effect upon complement inhibition (for review, see (14)).
Complement can be activated through three distinct pathways, which merge at the level of C3-convertases that activate the central complement component C3. The classical pathway is triggered by immune complexes whereas the lectin pathway is initiated by specific carbohydrate structures present on pathogen surfaces. The alternative pathway is constantly activated at low level but it also serves as an amplification loop for the other two pathways (reviewed in (13)). In addition, properdin was shown to directly activate the alternative pathway (15). Complement can also be activated by a number of endogenous ligands, such as members of the small leucine-rich repeat protein (SLRP) family, which are found most abundantly in cartilage (16, 17). During pathogenic cartilage destruction, SLRPs are fragmented and released into the synovial fluid where they can interact with complement. This has been proposed to contribute to the local pro-inflammatory milieu in joints of patients suffering from RA. In this study we explore the role of COMP in complement regulation and show that COMP is able to induce complement activation through the alternative pathway as well as to inhibit the classical and lectin pathways of complement.
Materials and Methods
Patients
Sera and matched synovial fluids were collected from 17 consecutive patients (7 males, 10 females) fulfilling the American College of Rheumatology criteria for RA and seeking care at the Department of Rheumatology at Lund University hospital due to a synovitis in one knee joint (18). Their median age was 64 years (range 25–83). Of these, 15 were positive for IgM rheumatoid factor and 15 for anti-citrullinated peptides (anti-CCP). Five patients were treated with methotrexate, six were treated with prednisolone (<7.5 mg/day), one patient was treated with leflunomide, one with intramuscular gold and one with sulphasalazine. In addition, sera and synovial fluids from 19 patients with radiographically verified, symptomatic knee joint OA (7 males, 12 females) were collected. Five patients were seen at the Department of Rheumatology in Lund, the rest were seen at the Department of Orthopaedic Surgery in Umeå, Sweden. The median age of the OA patients was 63 years (range 38–75). Three of the patients with knee joint OA had other rheumatological conditions, i.e. undifferentiated episodic oligoarthritis, Sjögren´s syndrome and systemic sclerosis. One of the OA patients was rheumatoid factor positive but did not fulfil the criteria for RA. Serum was also collected from 14 healthy age and sex-matched volunteers (6 males, 8 females). The median age of the controls was 60.5 years (range 45–72). The collection of sera and synovial fluids upon informed consent was approved by the Regional Ethical Review Board in Lund, Sweden.
Measurement of COMP-concentration and COMP-C3b complexes in patients
COMP-concentrations in sera and synovial fluids of patients were measured using a commercially available COMP® ELISA (AnaMar). COMP-C3b complexes in serum and synovial fluid were measured by a sandwich ELISA modified from the available COMP® ELISA. Serum or synovial fluid was diluted 1:10 in the sample buffer provided with the kit and a sample (50 µl) was added to the anti-COMP coated plates provided. As a reference sample, 50 µl of the 1.7 U/l calibrator was added to each plate. The plates were incubated for 2 h at room temperature (RT) after which the wells were washed 4 times with the provided washing buffer. A goat anti-C3 antibody (C7761, Sigma) was diluted in the COMP® ELISA conjugate buffer and incubated with the wells for 1 h at RT. Following washing as before, a rabbit anti-goat HRP conjugate (P0449; Dako Cytomation) diluted in conjugate buffer was added to the wells for 1 h at RT. The plates were washed and bound protein was detected according to the protocol of the COMP® ELISA. The obtained absorbance (450 nm, Cary 50 MPR microplate reader, Varian) of the samples was normalized by setting the reference sample absorbance to 1. Each sample was measured twice in duplicate. The results are expressed in arbitrary units.
Proteins and serum
COMP was purified from bovine articular cartilage as described (7). Recombinant COMP and constructs representing specific domains of COMP were expressed and purified as described in the supplementary material. Recombinant bovine decorin was expressed and purified as described (19). Complement components were purchased from Complement Technologies with the exception of C1q (20), Factor H (21) and C4b-binding protein-protein S complex (C4BP-PS) (22), which were purified from human plasma as described. Head-fragments and collagenous stalk regions of C1q were prepared by partial digestion with pepsin (stalks) or collagenase (heads) according to previously published methods (23, 24). Both pepsin and collagenase (from Clostridium histolyticum) were purchased from Worthington. C1s was labeled with 125I using the chloramine T method. Mannose-binding lectin (MBL) was purchased from Statens Serum Institut (Copenhagen, Denmark). C3met, functionally corresponding to C3b, was prepared by treatment of C3 with methylamine as previously described (25). Normal human serum (NHS) was prepared as described previously (26) and was heat inactivated by incubation at +56°C for 30 min.
Direct binding assays
Microtiter plates were coated with recombinant monomeric COMP (mCOMP), recombinant pentameric COMP (pCOMP) and pentameric COMP purified from tissue (tCOMP) at 5.0 µg/ml or BSA at 1% in 75 mM sodium-carbonate buffer, pH 9.6, overnight at +4°C. When comparing binding of complement components to COMP fragments, equimolar amounts of COMP variants were coated onto the plate (100 nM, corresponding to 7.4 µg/ml mCOMP). The plates were blocked with 1% BSA in PBS for 2 h at RT after which properdin, C1, C1q, C3, C3b and MBL in binding buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 50 µg/ml BSA) were added to the plates at increasing concentration. Binding of complement proteins was detected with specific polyclonal antibodies against properdin (A239, Complement Technologies), C1 and C1q (A0136, Dako), C3 and C3b (A0063, Dako) and MBL (AF2307, R&D Systems) followed by HRP-conjugated secondary antibodies (P0399, P0449, Dako). The plates were developed with o-phenylenediamine (OPD) substrate and H2O2 and the absorbance at 490 nm was measured.
Binding of COMP to C1q head and stalk fragments was studied by coating microtiter plates with C1q, C1q heads or C1q stalks at 5 µg/ml. After blocking as above, 20 µg/ml tCOMP was added to the plates and bound protein was detected with a polyclonal rabbit anti COMP antibody. The antibody was raised against human COMP prepared from articular cartilage and used for immunization of rabbits according to the standard protocol. After washing, the plates were incubated with an HRP-conjugated secondary antibody and developed as described above.
Complement activation triggered by COMP
Microtiter plates were coated with tCOMP at 5.0 µg/ml or recombinant COMP constructs at 100 nM in 75 mM sodium-carbonate buffer, pH 9.6, overnight at +4°C. 1% BSA, 2 µg/ml aggregated human IgG (classical pathway) or 2 µg/ml zymosan (alternative pathway) were coated as negative and positive controls. Plates were blocked as above and NHS diluted in GVB2+ (2.5 mM veronal buffer pH 7.3, 150 mM NaCl, 0.1% gelatin, 1 mM MgCl2, 0.15 mM CaCl2) for the classical/lectin pathway or Mg2+-EGTA (2.5 mM veronal buffer pH 7.3, 70 mM NaCl, 140 mM glucose, 10 mM EGTA, 7 mM MgCl2, 0.1% gelatin) for the alternative pathway was added. The plates were incubated at +37°C after which specific polyclonal antibodies against C4c (Q0369, Dako), C3d (A0063, Dako) or C9 (A226, Complement Technologies) were added to the wells followed by HRP-conjugated secondary antibodies. The plates were developed with OPD substrate and H2O2 and the absorbance at 490 nm was measured.
Inhibition of complement activation by COMP
Aggregated human IgG (2 µg/ml for C3b and C4b, 5 µg/ml for C1q) or mannan (100 µg/ml) was coated onto microtiter plates in 75 mM sodium-carbonate buffer, pH 9.6 overnight at +4°C. The plates were blocked and washed as described above. NHS diluted in GVB2+ was incubated with increasing concentrations of pCOMP, BSA, recombinant decorin (classical pathway) or D(+)-mannose (lectin pathway) on ice for 30 min after which the mixtures were added to the plate. The plates were incubated at +37°C and deposited complement components were detected with antibodies against C1q, MBL, C3d and C4c followed by HRP conjugated secondary antibodies as described above.
Hemolytic assay
Inhibition of complement mediated hemolysis of erythrocytes was measured as described in (27) with a final serum concentration of 0.17%.
Electron microscopy
Complexes between pCOMP or mCOMP, and C1q, MBL, properdin and C3met were analyzed by negative staining and electron microscopy as described previously (26). Complement proteins and mCOMP were identified by labeling with colloidal thiocyanate gold (28). Specimens were observed in a Jeol JEM 1230 electron microscope operated at a 60 kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 CCD camera.
Results
COMP activates the alternative pathway
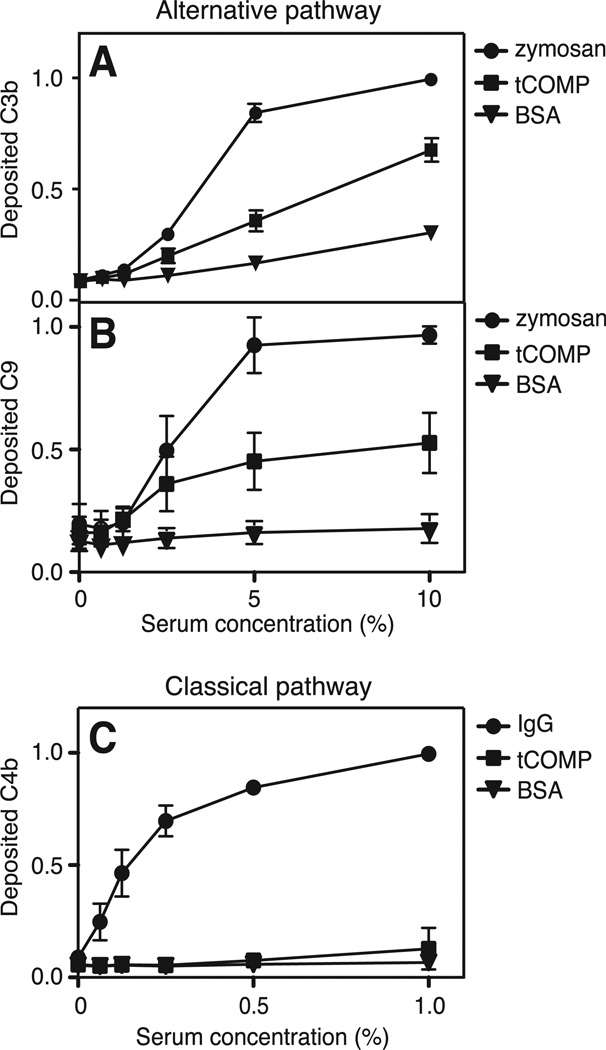
To investigate whether COMP has any complement-regulating properties, we studied the ability of COMP to activate complement in vitro. The different COMP variants used in the assays are illustrated in Fig. 4. tCOMP was coated onto microtiter plates and NHS was added at increasing concentrations. Deposition of C3b and C9 was measured to evaluate activation of the alternative pathway whereas activation of the classical and lectin pathways was measured by detecting the amount of deposited C4b. tCOMP induced both C3b- and C9-deposition through the alternative pathway (Fig. 1A and 1B). COMP was, however, not able to trigger C4b-deposition through the classical or lectin pathway (Fig. 1C).
FIGURE 1. COMP activates the alternative pathway of complement.
tCOMP, zymosan and BSA were coated onto microtiter plates and increasing concentrations of NHS in Mg++EGTA were added. Activation of the alternative pathway was measured by detecting deposited C3b (A) and C9 (B). Activation of the classical and lectin pathways was measured by coating plates with tCOMP, aggregated human IgG or BSA and adding increasing concentrations of NHS in GVB++. Deposition of C4b was measured as an indication of complement activation (C). The graphs show the mean and standard deviation (SD) of three separate experiments. The data in the panels were normalized by setting the highest obtained absorbance of each plate to 1.
COMP inhibits the classical and lectin pathways
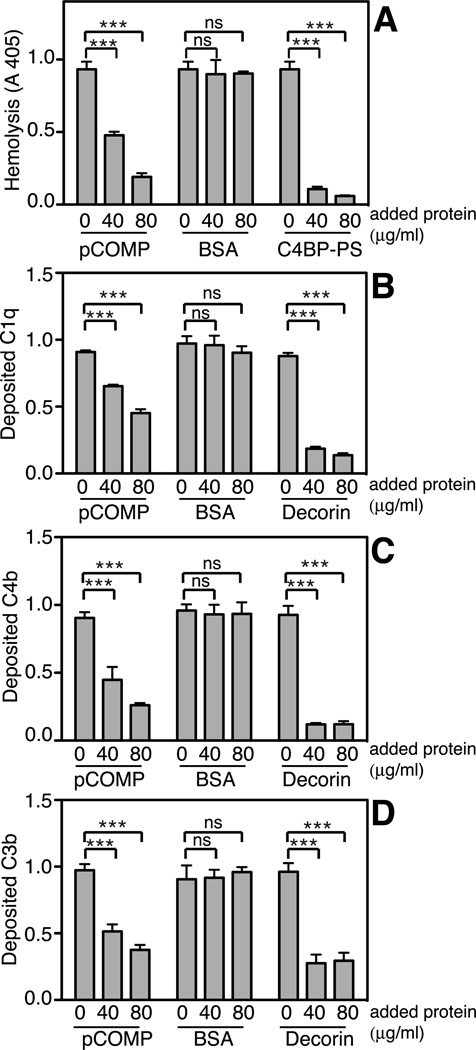
To elucidate whether COMP has any inhibitory effect on the classical pathway, sensitized sheep erythrocytes were incubated with NHS in the presence of increasing amounts of pCOMP, which resulted in dose-dependent inhibition of lysis (Fig. 2A). To investigate at which step in the pathway the inhibition occurred, IgG (for the classical pathway) or mannan (for the lectin pathway) were immobilized onto plates as complement activating agents and NHS incubated with increasing amounts of pCOMP was added. pCOMP inhibited deposition of C1q and further C4b and C3b through the classical pathway (Fig. 2B–D). As a positive control we used decorin known to bind to the collagenous stalks of C1q and inhibit the classical pathway (29). In comparison, pCOMP did not inhibit deposition of MBL through the lectin pathway (Supplemental Fig. 1A) but caused a marked decrease in the deposition of C4b (Supplemental Fig. 1B) and C3b (Supplemental Fig. 1C). As a positive control we used mannose that inhibits binding of MBL to immobilized mannan.
FIGURE 2. COMP inhibits the classical pathway of complement.
The ability of pCOMP to inhibit erythrocyte lysis when pre-incubated with NHS was measured in a hemolytic assay. As the positive control, the main inhibitor of the classical pathway, C4BP-PS, was used (A). Plates were coated with aggregated human IgG and NHS pre-incubated with increas- ing concentrations of pCOMP was added. BSA and decorin were added to NHS as negative and positive controls. The panels show deposition of C1q (B), C4b (C) and C3b (D). The data are given as the mean and SD of three separate experiments and the data in panels B–D were normalized by setting the highest obtained absorbance of each plate to 1. Statistical significance of differences was calculated with a two-way ANOVA. ns, not significant, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
COMP binds to properdin, C1q, C3b and MBL
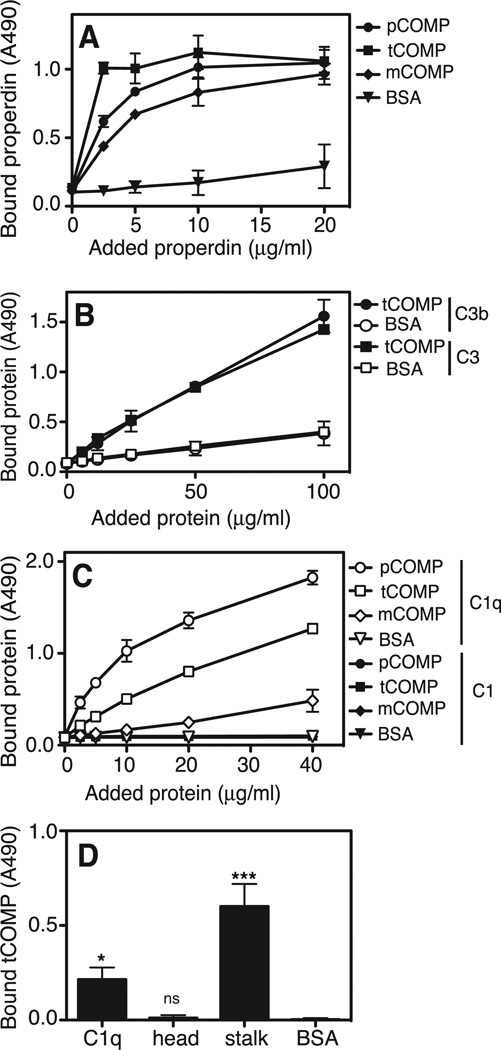
Since COMP was found to activate the alternative pathway, we hypothesized that COMP would interact with the initiating molecules of this pathway, mainly properdin or C3. We found that fluid phase properdin bound to all three forms of immobilized COMP (tCOMP, pCOMP and mCOMP) in a similar manner (Fig. 3A). Importantly, properdin from heat-inactivated NHS bound to immobilized tCOMP (not shown). C3 and C3b were also found to directly bind immobilized tCOMP in a dose-dependent manner (Fig. 3B). C3 also bound immobilized tCOMP directly from heat inactivated NHS, indicating a direct interaction as opposed to C3b-deposition due to complement activation (not shown). Since COMP inhibited the classical pathway already at the level of C1q-deposition, we wanted to study whether COMP could interact directly with C1q. COMP bound C1q but not the intact C1 complex consisting of C1q, associated with a C1r2C1s2-tetramer (30), and the binding was stronger for the polymeric COMP variants (Fig. 3C). Furthermore, tCOMP bound C1q directly from heat inactivated NHS (not shown) indicating the presence of available free C1q in NHS. Moreover, tCOMP bound strongly to the collagenous stalks of C1q but not to the isolated globular head-fragments (Fig. 3D).
FIGURE 3. COMP binds to properdin, C3 and C1q.
tCOMP, pCOMP and mCOMP were coated onto microtiter plates and incubated with fluid phase properdin at increasing concentrations (A). To compare binding of C3 and the C3b activation product to tCOMP, tCOMP was immobilized and increasing concentrations of C3 and C3b were added to the plate. BSA was immobilized as a negative control (B). In order to compare binding of COMP to C1 and C1q, tCOMP, pCOMP and mCOMP were coated onto microtiter plates and fluid phase C1q or C1 at increasing concentrations was allowed to attach (C). To examine the binding region for COMP on C1q, isolated head and stalk fragments of C1q or intact C1q were immobilized and incubated with 20 µg/ml tCOMP (D). The data are given as the mean and SD of three separate experiments. Statistical significance was calculated using a one-way ANOVA in panel D. ns, not significant, *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Since pCOMP inhibited the lectin pathway already at the level of C4b-deposition, we speculated that COMP may interact directly with MBL. When MBL was incubated with immobilized tCOMP, pCOMP or mCOMP, a binding was found for all three variants, although the interaction was stronger for the polymeric forms. MBL also bound tCOMP directly from heat inactivated NHS (not shown).
COMP inhibits the assembly of the C1-complex
Since COMP bound strongly to isolated C1q but not to purified intact C1, we hypothesized that the binding site of COMP would partially overlap with the binding site for the C1r2C1s2-tetramer. To study this, we evaluated the effect of pCOMP on the assembly and dissociation of the C1-complex. We found that the binding of the C1r2C1s2-tetramer to immobilized C1q was inhibited by pCOMP in a dose-dependent manner, although the interaction could not be completely prevented (Supplemental Fig. 2A). pCOMP could also dissociate the preformed C1-complex to some extent, however, the effect was not as strong as that of the positive control, C1-inhibitor (Supplemental Fig. 2B).
Complement proteins bind mainly to the C-terminus of COMP
In order to further characterize interactions, recombinant constructs of COMP (Fig. 4) representing different domains of the molecule were immobilized from equimolar solutions onto microtiter plates and incubated with properdin, C1q, C3 or MBL. Properdin bound fragments TIII1–8, TIII1-CG, TIII8-CG and CG in addition to mCOMP (Fig. 4A). This indicates that properdin has two separate binding sites, one within the TSP3-region and one in the C-terminus of COMP. C1q bound to mCOMP, TIII1-CG, TIII8-CG and CG i.e. all constructs containing the globular C-terminal domain (Fig. 4B). C3 bound to mCOMP, TIII8-CG and CG (Fig. 4C). MBL showed a similar binding pattern as C1q interacting with the constructs containing the C-terminus, however, a possible binding was detected for EGF 1–4 as well (not shown).
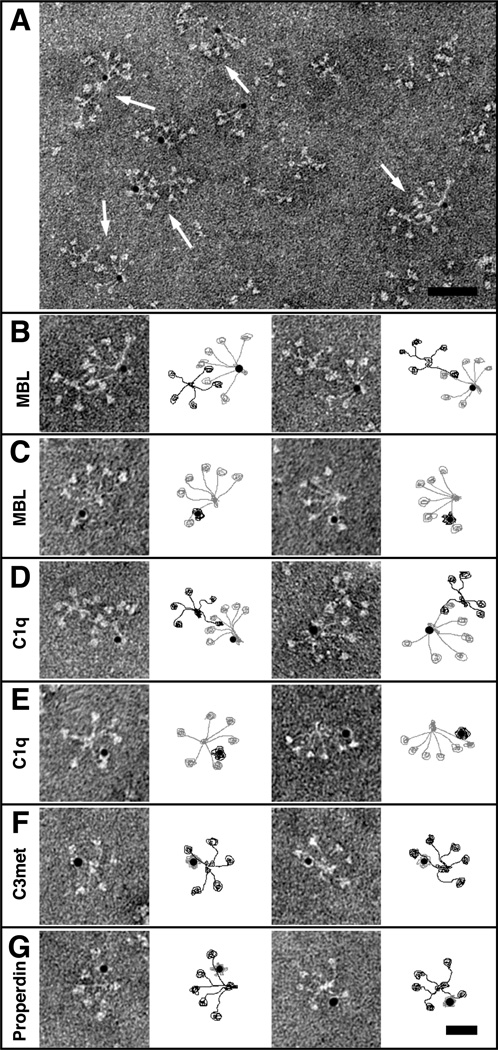
The interaction between pCOMP and C1q, MBL, properdin and C3met was further analyzed by electron microscopy employing gold labeling of the individual components. The interaction between MBL and COMP was localized to the end of the COMP arms (Fig. 5A–B) and was mediated by the neck region of MBL (Fig. 5C). C1q bound pCOMP in a similar manner to MBL, near the C-terminus of pCOMP (Fig. 5D). The binding site of mCOMP on C1q was located to the outermost ends of the collagen-like region, close to the globular heads (Fig 5E). C3met bound to pCOMP close to the C-terminal domain as C1q and MBL (Fig. 5F), whereas properdin seemed to attach also more towards the center of the TSP-region (Fig. 5G). These data confirm the results obtained in binding assays, indicating that the most important binding site for C1q, MBL and C3 on COMP lies within or very close to the C-terminus whereas properdin binds also a bit further in towards the central part of the TSP3-region.
FIGURE 5. Electron microscopy of COMP and complement proteins.
Complexes between pCOMP and gold-labeled complement proteins or gold-labeled mCOMP and complement proteins were visualized by negative staining. A representative field of complexes between pCOMP and gold-labeled MBL is shown in (A). pCOMP in complex with MBL at a higher magnification (B). Selected particles of gold-labeled mCOMP bound to the arms of MBL (C). pCOMP in complex with gold-labeled C1q (D). Gold-labeled mCOMP in complex with C1q (E). Gold-labeled C3met (F) and properdin (G) bound to pCOMP. The schematic illustrations next to each image show COMP in black and complement proteins in grey. The scale bar represents 100 nm in (A) and 50 nm in (B–G).
The C-terminus of COMP activates complement
All immobilized CG-containing COMP constructs induced deposition of C9 from NHS, although deposition was most pronounced on TIII8-GC (Fig. 4E). Neither EGF 1–4 nor TIII1–8 induced C9-deposition, indicating that these fragments do not activate complement. Therefore mainly the C-terminus of COMP is responsible for the complement activating effect, with a contribution from the thrombospondin type III repeat domain, possibly by conformation.
COMP-C3b complexes are found in serum of patients with RA
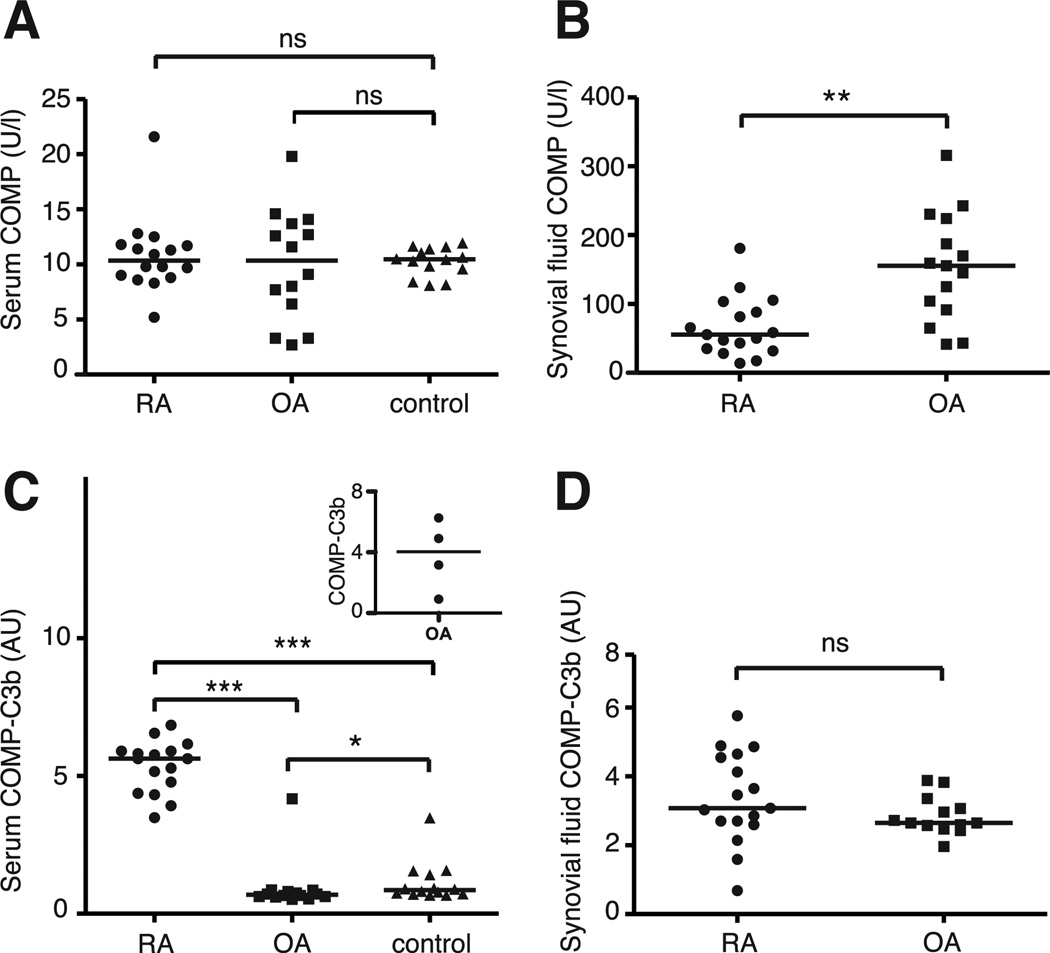
We set out to determine whether there is a link between complement activation and COMP-release in RA patients compared to OA patients and healthy age-matched controls. The mean serum COMP-concentrations in our study did not differ between the studied RA and OA patients and healthy controls (Fig. 6A and Supplemental Table I). The OA patients had significantly higher synovial fluid COMP-concentration than RA patients (Fig. 6B). The relative difference between the COMP-concentrations in serum and synovial fluid between RA and OA is likely a consequence of the larger number of affected joints contributing to the disease in RA patients thereby increasing the serum COMP-concentration. In order to study whether the released COMP can trigger complement activation in vivo, we measured complexes between COMP and the complement activation product C3b in serum and synovial fluid. Such complexes were found in serum of all RA patients but only in one OA patient (Fig. 6C). An elevated COMP-C3b concentration could moreover be found in serum of the three OA patients that had additional rheumatological disorders; one patient with Sjögren’s syndrome, one with systemic sclerosis and one with undifferentiated episodic oligoarthritis (Fig. 6C, inset). In addition, one OA patient who was positive for rheumatoid factor and had a history of undifferentiated arthritis without fulfilling the criteria for RA, had a very low level of COMP-C3b complexes in serum. Although the OA-patients and the major proportion of the controls had extremely low levels of circulating complexes, three of the controls had low but detectable concentrations of COMP-C3b whereas one control was clearly positive for the complexes (Fig. 6C). There was no difference in the amount of COMP-C3b complexes found in synovial fluid between RA and OA patients (Fig. 6D), but in the case of OA they were at least 4–5 times higher than in the circulation. The amount of COMP and COMP-C3b in serum or synovial fluid did not correlate in RA patients (spearman’s rank correlation coefficient (rs)=0.1960, p=0.4669 for serum and rs=−0.2416, p=0.3503 for synovial fluid). In OA patients there was no correlation between serum COMP and COMP-C3b (rs=0.1075, p=0.6710) whereas a weak negative correlation was found between synovial fluid COMP and COMP-C3b (rs=−0.5592, p=0.0196). When excluding the OA patients with additional rheumatological disorders, no correlation between serum or synovial fluid COMP and COMP-C3b was found (for serum rs=0.2031, p=0.4862 and for synovial fluid rs=−0.3659, p=0.2189). The amount of COMP-C3b in serum did not correlate with the erythrocyte sedimentation rate in RA patients (rs=0.0439, p=0.8765), nor did synovial fluid COMP-C3b correlate with the amount of synovial fluid leucocytes in RA patients (rs=0.2413, p=0.3863).
FIGURE 6. COMP and COMP-C3b concentrations in RA and OA patients.
The amount of released COMP in serum of RA patients (n=16) and OA patients (n=14) was measured using a commercial COMP® ELISA (A). COMP-concentrations were also measured in synovial fluid of RA patients (n=17) and OA patients (n=15) (B). Circulating complexes of COMP and C3b were measured using a sandwich ELISA in the serum of 16 RA patients, 14 OA patients and 14 healthy controls (C). The inset shows the levels of COMP-C3b in four OA patients with additional rheumatologi- cal disorders. COMP-C3b concentrations in synovial fluid were measured in 17 RA patients and 13 OA patients (D). The horizontal line in each panel represents the median. Statistical differences between the groups in panels A and C were determined using a Kruskal-Wallis test whereas a Mann-Whitney test was used in panels B and D. AU, arbitrary units; ns, not significant; *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Discussion
Cartilage breakdown may contribute to the inflammatory process in joint diseases and it appears that molecules released into the synovium modulate disease. In this study we show that COMP-C3b complexes are present in both serum and synovial fluid of RA patients as an indicator of COMP-induced complement activation in vivo. No correlation was found between the amount of COMP and COMP-C3b complexes in serum or synovial fluid, indicating that only certain released fragments of COMP have complement activating properties or that there are other molecules competing for the complement-binding sites. That COMP-concentrations in synovial fluid of RA patients were significantly higher than in serum whereas the COMP-C3b concentrations were somewhat lower in the synovial fluid might be explained by the fact that complement as well as total protein concentrations are in general much lower in synovial fluid than in serum. Thereby the availability of properdin and C3 might be a limiting factor (31, 32). The fact that COMP-C3b was found only in synovial fluid and not in serum of OA patients indicate a local formation via complement activation. It is possible that certain types of COMP fragments with complement activating properties are retained within the synovium or that the locally formed complexes cannot be transported into the general circulation in OA patients. In the case of RA, the circulating COMP-C3b complexes might originate from local formation in the synovium and transportation through the inflamed synovial membrane or the complexes might not be formed until in the circulation. There is evidence that the character of COMP fragments released into the synovial fluid during normal cartilage turnover, in OA and in RA is different. Interestingly, the three patients with OA who also were diagnosed with other rheumatic conditions showed serum concentrations of COMP-C3b complexes resembling the ones found in RA patients, suggesting that in these patients, fragments of another type than in uncomplicated OA were released. We plan to evaluate COMP-C3b assay in larger cohorts of patients with various joint diseases as well as those with systemic lupus erythematosus. This will provide information regarding the specificity of the assay as well as on whether interactions between COMP and C3b are involved in these diseases.
We propose that COMP activates the alternative pathway mainly through an interaction with properdin, which might direct complement attack to surfaces with exposed COMP. The complement-activating site was found to be localized within or near the interface between the C-terminus and the last TSP3-repeat. COMP was moreover found to inhibit the classical and lectin pathways of complement, effects that can be attributed to direct interactions with C1q and MBL. Approximately 10% of the total C1 in NHS is present as free C1q and C1r2C1s2 (33) and an excess of free C1q has been demonstrated in synovial fluid in RA (34). By binding to the stalk of C1q, COMP may be able to prevent dissociated C1r2C1s2 from re-associating with free C1q-molecules leading to a local decrease in functional C1-complexes. A similar mechanism has previously been suggested for soluble C1q receptor (35) and pulmonary surfactant protein A (36).
This is the first report showing the in vivo relevance of a released cartilage component for complement activation. In RA, complement has been shown to contribute to the persistent inflammatory state and here we provide one molecular mechanism by which this complement activation may be driven. By detecting COMP-C3b complexes in serum it is possible to distinguish patients suffering from inflammatory arthritis and OA. The assay for COMP-C3b complexes may provide an additional valuable tool to early establish diagnosis, even when levels of tissue markers like COMP are not elevated. This would open up for an early treatment of patients to avoid extensive cartilage damage. With complement-directed therapies being developed for the treatment of inflammatory diseases including RA, an assay measuring pathological complement activation should provide information about which patients would benefit from complement inhibition therapy.
Acknowledgements
We thank prof. Olle Svensson, Department of Surgical and Perioperative Science, Umeå University for providing OA samples. The kits for COMP assay were a kind gift from Anamar. We thank Karin Lindblom, Lund University, for excellent technical assistance.
Financial support: This study was supported by grants from the Swedish Research Council, Swedish Foundation for Strategic Research, NIH (NIAMS), the European Community’s FP6 funding (“Autocure”), Knut and Alice Wallenberg Foundation, Foundations of Österlund, Greta and Johan Kock, Inga-Britt and Arne Lundberg, King Gustav Vth's 80th Anniversary Foundation and grants from the University Hospital in Malmö/Lund.
References
- 1.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6136. [PubMed] [Google Scholar]
- 2.Smith RK, Zunino L, Webbon PM, Heinegård D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16(5):255–271. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 3.DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354(2):237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 4.Dodge GR, Hawkins D, Boesler E, Sakai L, Jimenez SA. Production of cartilage oligomeric matrix protein (COMP) by cultured human dermal and synovial fibroblasts. Osteoarthritis Cartilage. 1998;6(6):435–440. doi: 10.1053/joca.1998.0147. [DOI] [PubMed] [Google Scholar]
- 5.Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 6.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg K, Olsson H, Mörgelin M, Heinegård D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273(32):20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 8.Halasz K, Kassner A, Mörgelin M, Heinegård D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282(43):31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 9.Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279(24):25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- 10.Oldberg A, Antonsson P, Lindblom K, Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267(31):22346–22350. [PubMed] [Google Scholar]
- 11.Carlsen S, Nandakumar KS, Backlund J, Holmberg J, Hultqvist M, Vestberg M, et al. Cartilage oligomeric matrix protein induction of chronic arthritis in mice. Arthritis Rheum. 2008;58(7):2000–2011. doi: 10.1002/art.23554. [DOI] [PubMed] [Google Scholar]
- 12.Carlsen S, Hansson AS, Olsson H, Heinegård D, Holmdahl R. Cartilage oligomeric matrix protein (COMP)-induced arthritis in rats. Clin Exp Immunol. 1998;114(3):477–484. doi: 10.1046/j.1365-2249.1998.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30(2):83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Okroj M, Heinegård D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann Med. 2007;39(7):517–530. doi: 10.1080/07853890701477546. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179(4):2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 16.Sjöberg A, Önnerfjord P, Mörgelin M, Heinegård D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280(37):32301–32308. doi: 10.1074/jbc.M504828200. [DOI] [PubMed] [Google Scholar]
- 17.Sjöberg AP, Manderson GA, Mörgelin M, Day AJ, Heinegård D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46(5):830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Wiberg C, Heinegård D, Wenglen C, Timpl R, Mörgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem. 2002;277(51):49120–49126. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- 20.Tenner AJ, Lesavre PH, Cooper NR. Purification and radiolabeling of human C1q. J Immunol. 1981;127(2):648–653. [PubMed] [Google Scholar]
- 21.Blom AM, Kask L, Dahlbhäck B. CCP1–4 of the C4b-binding protein alpha-chain are required for factor I mediated cleavage of complement factor C3b. Mol Immunol. 2003;39(10):547–556. doi: 10.1016/s0161-5890(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 22.Dahlbäck B. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem J. 1983;209(3):847–856. doi: 10.1042/bj2090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paques EP, Huber R, Priess H, Wright JK. Isolation of the globular region of the subcomponent q of the C1 component of complement. Hoppe Seylers Z Physiol Chem. 1979;360(2):177–183. doi: 10.1515/bchm2.1979.360.1.177. [DOI] [PubMed] [Google Scholar]
- 24.Reid KB, Porter RR. Subunit composition and structure of subcomponent C1q of the first component of human complement. Biochem J. 1976;155(1):19–23. doi: 10.1042/bj1550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom AM, Kask L, Ramesh B, Hillarp A. Effects of zinc on factor I cofactor activity of C4b-binding protein and factor H. Arch Biochem Biophys. 2003;418(2):108–118. doi: 10.1016/j.abb.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Happonen KE, Sjöberg AP, Mörgelin M, Heinegård D, Blom AM. Complement inhibitor C4b-binding protein interacts directly with small glycoproteins of the extracellular matrix. J Immunol. 2009;182(3):1518–1525. doi: 10.4049/jimmunol.182.3.1518. [DOI] [PubMed] [Google Scholar]
- 27.Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, et al. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5(2):e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baschong W, Lucocq JM, Roth J. "Thiocyanate gold": small (2–3 nm) colloidal gold for affinity cytochemical labeling in electron microscopy. Histochemistry. 1985;83(5):409–411. doi: 10.1007/BF00509201. [DOI] [PubMed] [Google Scholar]
- 29.Groeneveld TW, Oroszlan M, Owens RT, Faber-Krol MC, Bakker AC, Arlaud GJ, et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005;175(7):4715–4723. doi: 10.4049/jimmunol.175.7.4715. [DOI] [PubMed] [Google Scholar]
- 30.Arlaud GJ, Gaboriaud C, Thielens NM, Rossi V, Bersch B, Hernandez JF, et al. Structural biology of C1: dissection of a complex molecular machinery. Immunol Rev. 2001;180:136–145. doi: 10.1034/j.1600-065x.2001.1800112.x. [DOI] [PubMed] [Google Scholar]
- 31.Pekin TJ, Jr, Zvaifler NJ. Hemolytic Complement in Synovial Fluid. J Clin Invest. 1964;43:1372–1382. doi: 10.1172/JCI105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killingsworth LM. Clinical applications of protein determinations in biological fluids other than blood. Clin Chem. 1982;28(5):1093–1102. [PubMed] [Google Scholar]
- 33.Ziccardi RJ, Tschopp J. The dissociation properties of native C1. Biochem Biophys Res Commun. 1982;107(2):618–623. doi: 10.1016/0006-291x(82)91536-4. [DOI] [PubMed] [Google Scholar]
- 34.Sjöholm AG, Berglund K, Johnson U, Laurell AB, Sturfelt G. C1 activation, with C1q in excess of functional C1 in synovial fluid from patients with rheumatoid arthritis. Int Arch Allergy Appl Immunol. 1986;79(2):113–119. doi: 10.1159/000233956. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg RH, Faber-Krol M, van Es LA, Daha MR. Regulation of the function of the first component of complement by human C1q receptor. Eur J Immunol. 1995;25(8):2206–2210. doi: 10.1002/eji.1830250814. [DOI] [PubMed] [Google Scholar]
- 36.Watford WT, Wright JR, Hester CG, Jiang H, Frank MM. Surfactant protein A regulates complement activation. J Immunol. 2001;167(11):6593–6600. doi: 10.4049/jimmunol.167.11.6593. [DOI] [PubMed] [Google Scholar]