Abstract
An effective regimen for treatment of tuberculosis (TB) is comprised of multiple drugs that inhibit a range of essential cellular activities in Mycobacterium tuberculosis. The effectiveness of a regimen is further enhanced if constituent drugs act with synergy. Here, we report that faropenem (a penem) or biapenem, doripenem, or meropenem (carbapenems), which belong to the β-lactam class of antibiotics, and rifampin, one of the drugs that forms the backbone of TB treatment, act with synergy when combined. One of the reasons (carba)penems are seldom used for treatment of TB is the high dosage levels required, often at the therapeutic limits. The synergistic combination of rifampin and these (carba)penems indicates that (carba)penems can be administered at dosages that are therapeutically relevant. The combination of faropenem and rifampin also limits the frequency of resistant mutants, as we were unable to obtain spontaneous mutants in the presence of these two drugs. The combinations of rifampin and (carba)penems were effective not only against drug-sensitive Mycobacterium tuberculosis but also against drug-resistant clinical isolates that are otherwise resistant to rifampin. A combination of doripenem or biapenem and rifampin also exhibited synergistic activity against Mycobacterium abscessus. Although the MICs of these three drugs alone against M. abscessus are too high to be of clinical relevance, their concentrations in combinations are therapeutically relevant; therefore, they warrant further evaluation for clinical utility to treat Mycobacterium abscessus infection, especially in cystic fibrosis patients.
INTRODUCTION
Existing treatment of tuberculosis (TB) requires multiple antibiotics to be taken daily for at least 6 months. Historically, β-lactam antibiotics have not been considered for treatment of TB, except for treatment of drug-resistant tuberculosis when available options are exhausted. Reasons that are often cited for lack of activity of β-lactams against Mycobacterium tuberculosis include the chromosomally encoded extended-spectrum class A β-lactamase BlaC and poor permeability across the cell wall (1, 2). Clavulanic acid, by binding to and inhibiting the β-lactamase activity of BlaC, has been shown to render M. tuberculosis susceptible to β-lactams (3). Carbapenems, a subclass of β-lactams with a five-membered ring next to the β-lactam core, are similar to penicillins but with a carbon in the first position and a double bond between C-2 and C-3 (4). They are poor substrates for BlaC and are not affected by its β-lactamase activity (5). Recent reports have demonstrated that carbapenems exhibit high antimicrobial potency against M. tuberculosis; therefore, they may be considered for treatment of tuberculosis (5–9).
Mycobacterium abscessus is a nontuberculous mycobacteria that causes morbid infection in the lungs of a significant percentage of cystic fibrosis patients (10). In a recent study, imipenem, meropenem, ertapenem, and doripenem (all belonging to an older generation of carbapenems and none of which are orally bioavailable) were evaluated against M. abscessus, and it was determined that while imipenem, meropenem, and doripenem have modest activities, other classes of β-lactams (penicillins and cephalosporins except cefoxitin) are ineffective against this organism (11).
In this study, we began by assessing potencies of the carbapenems ertapenem, meropenem, imipenem, doripenem, biapenem, tebipenem, and panipenem and the penem faropenem against M. tuberculosis by determining MICs and minimum bactericidal concentrations (MBCs). We investigated if (carba)penems and isoniazid or rifampin, the two drugs that form the backbone of TB treatment, exhibit any synergy, indifference, or antagonism in activity. We next compared frequencies of spontaneous resistant mutants when M. tuberculosis is exposed to either rifampin or faropenem or a combination of these two drugs. Finally, we studied antimicrobial activities of combinations containing rifampin and (carba)penems against drug-resistant clinical M. tuberculosis isolates. We also evaluated the abovementioned (carba)penems alone and in combination with rifampin for their antimicrobial activities against M. abscessus. Although the MIC of rifampin for M. abscessus is too high, we assessed if (carba)penems and rifampin exhibited synergy and activity at levels that are therapeutically relevant.
MATERIALS AND METHODS
Bacterial strains and in vitro growth conditions.
Drug-susceptible M. tuberculosis H37Rv and drug-resistant M. tuberculosis clinical isolates (with known drug susceptibility profiles and multidrug-resistant [MDR] strains defined as resistant to both rifampin and isoniazid), archived at the All India Institute of Medical Sciences, New Delhi, India, were used. The clinical strains were obtained per institutional ethical guidelines and were deidentified. Strains were grown under standard conditions in Middlebrook 7H9 broth (Difco) supplemented with 0.5% glycerol, 10% oleic acid-albumin-dextrose-catalase (OADC), and 0.05% Tween 80, with constant shaking at 37°C or on Middlebrook 7H10 agar plates at 37°C. M. abscessus ATCC 19977 was grown in Middlebrook 7H9 broth with albumin-dextrose-catalase enrichment with constant shaking at 37°C or on Middlebrook 7H10 agar plates at 37°C. All drugs were obtained from the following commercial vendors: ertapenem (Toronto Research Chemicals); rifampin, isoniazid, meropenem, imipenem, doripenem, biapenem, faropenem, and tebipenem (Sigma-Aldrich); and panipenem (Ochem, Inc.). To assess the quality of these compounds, a few were randomly selected and assessed by liquid chromatography-mass spectrometry. The purity of compounds ranged from 95% to 99%.
MIC.
MICs were determined using standard broth dilution methods (12, 13). Briefly, 105 M. tuberculosis or M. abscessus bacilli grown to exponential phase were inoculated into 15-ml sterile conical tubes containing 2.5 ml of 7H9 broth containing drug at 2-fold dilutions ranging from 80 μg/ml to 0.16 μg/ml. Middlebrook 7H9 broth alone and without drug but inoculated with 105 M. tuberculosis or M. abscessus bacilli were included as negative and positive controls, respectively. Rifampin and isoniazid were used as control drugs. Cultures were incubated at 37°C and evaluated for growth by visual inspection at 14 days for M. tuberculosis and at 3 days for M. abscessus at 30°C per Clinical and Laboratory Standards Institute guidelines (14). Samples with no visible granulations were further assessed by plating on Middlebrook 7H10 plates to verify the lack of bacterial growth. In this method, a single dose of drug is added at the beginning of the assay. We also assessed the MIC by providing two doses of drug, one at the beginning of the assay and one at the midpoint of the assay. Two sets of tubes were used for each drug. In one set, drug was added from 80 μg/ml to 0.16 μg/ml by 2-fold serial dilution. In the next set, half the amount of drug was added in each tube. Therefore, this set started with 40 μg/ml to 0.08 μg/ml drug. At the end of 1 week (midpoint of the assay), the remaining half dose was added with a fresh preparation of the drug. We also assessed MIC90 using the broth microdilution method using 96-well plates per Clinical and Laboratory Standards Institute recommendations (14). Cation-adjusted Mueller-Hinton broth (Becton-Dickinson) was used to assess antimicrobial activities of rifampin and (carba)penems against M. abscessus strains. All MIC assessments were repeated to verify results.
MBC.
Minimum bactericidal concentration (MBC) was determined using a modification of the broth dilution method described above. For M. tuberculosis, samples with no visible growth after 14 days of incubation were plated on Middlebrook 7H10 agar plates, and CFU were enumerated after incubation at 37°C for 21 days. Isoniazid was used as a positive control for bactericidal activity. The input inoculum is the CFU added to each well at the initiation of broth dilution assay and was determined by plating samples at the onset of the assay on Middlebrook 7H10 agar plates. MBC99 refers to the concentration of the drug at which 99% of input bacilli were killed.
Checkerboard titration assay.
The checkerboard titration assay is a modification of the broth dilution assay and was undertaken as described previously (15). Two drugs were added to 2.5 ml of Middlebrook 7H9 broth, each starting at its MIC and serially diluted 2-fold, so all possible 2-fold dilution combinations below the respective MICs are represented. After this step, 105 CFU of M. tuberculosis or M. abscessus was inoculated into each tube. The cultures were incubated at 37°C and evaluated for growth by visual inspection of the broth at 14 days for M. tuberculosis and at 3 days for M. abscessus. The fractional inhibitory concentration (FIC) of each drug in the combination was determined as described previously (15). Briefly, the FIC of a drug in a sample is computed as the concentration of the drug divided by the MIC of the drug when used alone. The FIC index is the sum of the FIC of two drugs in a sample. The FIC index was calculated for each mix of drugs that inhibited M. tuberculosis or M. abscessus growth, and an average FIC index was determined. An average FIC index of ≤0.5 was interpreted as synergy, >0.5 to 2.0 as indifference, and >2 as antagonism.
Time-kill assay.
M. abscessus ATCC 19977 was grown in Middlebrook 7H9 broth to exponential phase, and a suspension at an A600 of 0.01 was prepared by serial dilution in the broth. Two hundred microliters of this suspension or approximately 105 CFU was inoculated into six tubes each with 5 ml of 7H9 broth. These samples contained the following drugs at subinhibitory concentrations (sub-MICs): rifampin (25 μg/ml), doripenem (10 μg/ml), biapenem (10 μg/ml), rifampin and doripenem (25 μg/ml and 10 μg/ml, respectively), rifampin and biapenem (25 μg/ml and 10 μg/ml, respectively), and no drug as a positive control for M. abscessus growth. The samples were cultured in a shaking incubator at 37°C. A 100-μl aliquot was removed from each sample at 0, 3, 6, 12, 24, 48, 72, 96, 144, and 192 h. Following the initiation of this assay, appropriate serial dilutions were plated onto Middlebrook 7H10 agar media, and CFU were enumerated after 3 days of incubation at 37°C.
Determination of frequency of drug resistance emergence.
M. tuberculosis H37Rv was grown in Middlebrook 7H9 broth to exponential phase, a suspension with an A600 of 1.0 was prepared, and 1.0 ml of this suspension was inoculated onto each plate. The Middlebrook 7H10 agar plates were supplemented with a rifampin/faropenem combination at 3.0/0, 6.0/0, 3.0/50, 0.6/5.0, 0.6/0, 0/5.0, and 0/0 μg/ml, respectively, to select resistant mutants that were enumerated by counting CFU on plates after 4 weeks of incubation at 37°C. The frequency of drug-resistant mutants was determined from the numbers of spontaneous mutants observed as a percentage of the input CFU inoculum.
RESULTS
MICs of (carba)penems against M. tuberculosis and M. abscessus.
We evaluated the antimicrobial activity of (carba)penems (i.e., ertapenem, meropenem, imipenem, doripenem, biapenem, faropenem, tebipenem, and panipenem), alone and in combination with clavulanic acid, against M. tuberculosis and M. abscessus using broth dilution assay. For drug-susceptible wild-type M. tuberculosis strain H37Rv, the potencies of these (carba)penems are as follows: tebipenem > faropenem = biapenem = doripenem > meropenem > ertapenem > imipenem > panipenem, with tebipenem having MIC90 of 1.25 to 2.5, faropenem, biapenem, and doripenem having MIC90 of 2.5 to 5.0 μg/ml, and panipenem having a MIC90 of >80 μg/ml (Table 1).
TABLE 1.
Antimicrobial activity of (carba)penems against M. tuberculosis H37Rva
| Drug(s) | MIC90 (μg/ml) | MIC90 (μg/ml) on double dosingb | MBC99 (μg/ml) |
|---|---|---|---|
| Ertapenem | 10–20 | 10–20 | NDc |
| Ertapenem + clavulanic acid | 5–10 | ND | ND |
| Meropenem | 5–10 | 5–10 | 80 |
| Meropenem + clavulanic acid | 2.5–5.0 | ND | ND |
| Imipenem | 40–80 | 40–80 | ND |
| Imipenem + clavulanic acid | 20–40 | ND | ND |
| Doripenem | 2.5–5.0 | 1.25–2.5 | 20 |
| Doripenem + clavulanic acid | 1.25–2.5 | ND | ND |
| Biapenem | 2.5–5.0 | 1.25–2.5 | 20 |
| Biapenem + clavulanic acid | 0.6–1.2 | ND | ND |
| Faropenem | 2.5–5.0 | 2.5–5.0 | 20 |
| Faropenem + clavulanic acid | 2.5–5.0 | ND | ND |
| Tebipenem | 1.25–2.5 | 1.25–2.5 | 10 |
| Tebipenem + clavulanic acid | 0.31–0.62 | ND | ND |
| Panipenem | >80 | ND | ND |
| Panipenem + clavulanic acid | ND | ND | ND |
| Clavulanic acid | >80 | ND | ND |
(Carba)penems were tested alone and in combination with clavulanic acid, a β-lactamase inhibitor.
Double dosing refers to providing half the dose at the onset and the remaining half at the midpoint of the assay.
ND, not determined.
Clavulanic acid is a β-lactamase inhibitor. As M. tuberculosis and M. abscessus possess a chromosomally encoded β-lactamase (1, 2), we hypothesized that the activity of (carba)penems changes if supplemented with clavulanic acid; hence, we assessed MICs of (carba)penems in combination with clavulanic acid. The addition of clavulanic acid did not alter the MIC90 of faropenem, while it reduced the MIC90 of biapenem and tebipenem by 4-fold. A 2-fold reduction in MIC90 was observed for ertapenem, meropenem, imipenem, and doripenem (Table 1).
(Carba)penems are known to undergo slow hydrolysis in aqueous solution as a result of the β-lactam ring opening mediated by nucleophilic attack on C-7 by oxygen from water (16). Due to this instability in an aqueous environment, the effective exposure of (carba)penems to pathogen may change over time. Therefore, we assessed if dividing the drug into two dosages during the course of broth dilution assay affects its MIC. For this, we performed two MIC assessments in parallel: in one we provided the drug in a single full dose at the onset of the assay, and in the second we provided half the dose at the onset and the remaining half at the midpoint of the assay. For instance, in the control set, 200 μg of meropenem was added to a 2.5-ml final volume in the first tube and assayed for 2 weeks. In the test set, 100 μg of meropenem was added at the onset of the assay, and an additional 100 μg was added at the beginning of the second week. If a drug is stable and does not undergo hydrolysis in the culture broth, the first set would provide a superior activity, as M. tuberculosis bacilli would be exposed to a greater dosage over 2 weeks. If the drug is unstable over time, the addition of a fresh dose at the midpoint in the assay may result in superior activity in the second set. A twofold decrease in MIC90 was observed for doripenem and biapenem upon the addition of drug in two doses (Table 1).
We next assessed the activity of the (carba)penems alone and in combination with clavulanic acid against M. abscessus ATCC 19977, a drug-susceptible strain (Table 2). Only doripenem and biapenem exhibited activity with a MIC90 in the range of 10 to 20 μg/ml. The addition of clavulanic acid dramatically reduced the MIC90 of meropenem: when used alone, this drug is not effective up to 80 μg/ml, but in combination with clavulanic acid its MIC90 diminished to 5 to 10 μg/ml, a decrease of >16-fold. A 2-fold reduction in MIC90 was observed for doripenem, biapenem, faropenem, and tebipenem, while the activities for ertapenem, imipenem, and panipenem remained unchanged in the presence of clavulanic acid. Encouraged by these results, we determined MICs of carbapenems against three M. abscessus clinical isolates that are resistant to a range of drugs used to treat M. abscessus infection (Table 3). These strains were susceptible only to doripenem and biapenem, with a MIC90 in the range of 3.12 to 12.5 μg/ml (Table 4). The maximum concentration used in this assessment was 25 μg/ml. Therefore, a MIC90 of >25 μg/ml implies that the actual MIC90 is much higher, as full growth was observed in these wells.
TABLE 2.
Antimicrobial activity of (carba)penems against M. abscessus ATCC 19977
| Drug | MIC90 for M. abscessus (μg/ml) |
|---|---|
| Ertapenem | >80 |
| Ertapenem + clavulanic acid | >80 |
| Meropenem | >80 |
| Meropenem + clavulanic acid | 5–10 |
| Imipenem | >80 |
| Imipenem + clavulanic acid | >80 |
| Doripenem | 10–20 |
| Doripenem + clavulanic acid | 5–10 |
| Biapenem | 10–20 |
| Biapenem + clavulanic acid | 5–10 |
| Faropenem | 40–80 |
| Faropenem + clavulanic acid | 20–40 |
| Tebipenem | 40–80 |
| Tebipenem + clavulanic acid | 20–40 |
| Panipenem | >80 |
| Panipenem + clavulanic acid | >80 |
| Clavulanic acid | >80 |
(Carba)penems were tested alone and in combination with 5 μg/ml clavulanic acid, a β-lactamase inhibitor.
TABLE 3.
Drug susceptibility of selected MDR M. abscessus clinical isolates
| Strain | Level of susceptibility toa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| AMI | CEF | CIP | CLR | LZD | MIN | TOB | TIG | |
| ABS 1 | I | R | R | R | R | R | R | S |
| ABS 2 | R | R | R | R | R | R | R | R |
| ABS 3 | R | R | R | I | R | R | R | R |
AMI, amikacin; CEF, cefoxitin; CIP, ciprofloxacin; CLR, clarithromycin; LZD, linezolid; MIN, minocycline; TOB, tobramycin; and TIG, tigecycline. ABS1, M. abscessus isolate 1; ABS2, M. abscessus isolate 2; ABS3, M. abscessus isolate 3. R, resistant; I, intermediate; S, susceptible (per Clinical and Laboratory Standards Institute cutoffs).
TABLE 4.
Activity of carbapenems against MDR M. abscessus clinical isolatesa
| Strain | MIC90 (μg/ml) |
||||
|---|---|---|---|---|---|
| Meropenem | Imipenem | Doripenem | Biapenem | Ertapenem | |
| ABS 1 | >25 | >25 | 6.25 | 12.5 | >25 |
| ABS 2 | >25 | >25 | 6.25 | 12.5 | >25 |
| ABS 3 | >25 | >25 | 3.12 | 6.25 | >25 |
ABS1, M. abscessus isolate 1; ABS2, M. abscessus isolate 2; ABS3, M. abscessus isolate 3.
MBC.
We evaluated if (carba)penems exhibited bactericidal activity against M. tuberculosis. For this, we selected meropenem, doripenem, biapenem, faropenem, and tebipenem based on their low MIC90 and determined MBC99 to be 80, 20, 20, 20, and 10 μg/ml, respectively (Table 1).
Doripenem, biapenem, faropenem, and meropenem exhibit synergy with rifampin against M. tuberculosis and M. abscessus.
We evaluated the potency of a combination of ertapenem, meropenem, imipenem, doripenem, biapenem, faropenem, and tebipenem with rifampin or isoniazid against M. tuberculosis using checkerboard titration assay (15). The fractional inhibitory concentration (FIC) index of isoniazid and any of the (carba)penems was in the 0.5 to 2.0 range, demonstrating that the activities of the two drugs were indifferent/additive to each other. The assessment of activities of a combination containing two (carba)penems (all three possible pairs among biapenem, faropenem, and tebipenem) revealed FIC indexes in the range of 0.5 to 2.0, thereby demonstrating indifference/additive activity. However, a combination of doripenem and rifampin exhibited a FIC of 0.33, biapenem and rifampin exhibited a FIC of 0.24, meropenem and rifampin exhibited a FIC of 0.4, and faropenem and rifampin exhibited a FIC of 0.19. In these combinations, wells containing either drug at less than 4- to 16-fold the respective MIC90 completely inhibited M. tuberculosis growth. For instance, rifampin/doripenem combinations of 0.06/0.63 or 0.03/2.5 μg/ml, rifampin/biapenem concentrations of 0.06/0.4 or 0.03/1.56 μg/ml, and rifampin/faropenem concentrations of 0.06/0.08, 0.03/0.16, or 0.008/0.3 μg/ml completely inhibited M. tuberculosis growth.
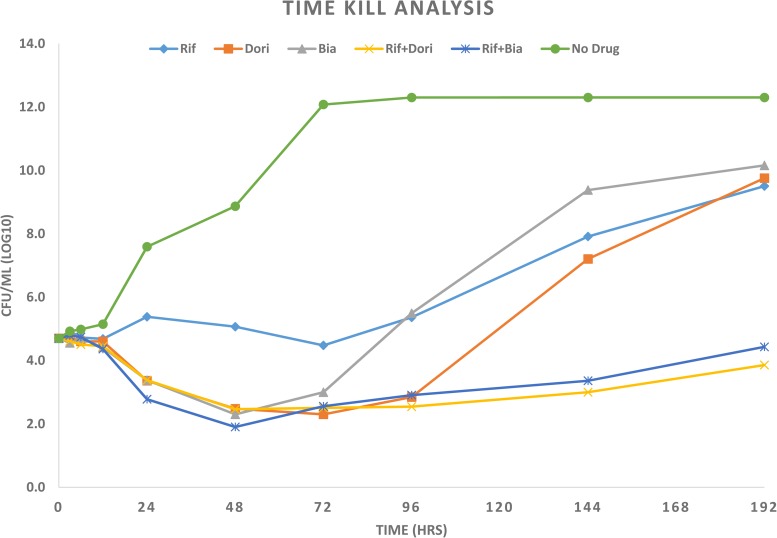
Similarly, we studied if rifampin and doripenem or biapenem, the two carbapenems that we found to exhibit antimicrobial activity against M. abscessus, exhibited synergistic activity against this pathogen. We assessed the MIC90 of rifampin to be 200 to 400 μg/ml, a concentration that is therapeutically irrelevant. However, a combination of rifampin and doripenem or biapenem exhibited synergistic antimicrobial activity against M. abscessus with a FIC of 0.48 and 0.50, respectively. Rifampin/doripenem combinations of 3.1/20, 6.2/20, and 50/10 μg/ml and rifampin/biapenem combinations of 6.2/20 or 25/10 μg/ml completely inhibited M. abscessus growth. The concentrations of rifampin, doripenem, and biapenem in these combinations are therapeutically relevant. We studied kinetics of growth inhibition or killing of M. abscessus ATCC 19977 by rifampin and doripenem or biapenem at sub-MICs to assess the time course activity of synergy between rifampin and doripenem or biapenem. The combinations rifampin/doripenem and rifampin/biapenem yielded ∼6 log10 fewer CFU than when drugs were used individually (Fig. 1).
FIG 1.
Time-kill analysis of M. abscessus ATCC 19977 in Middlebrook 7H9 broth at 37°C when exposed to sub-MICs of drugs. The samples contained 25 μg/ml rifampin (Rif), 10 μg/ml doripenem (Dori), 10 μg/ml biapenem (Bia), 25 μg/ml rifampin plus 10 μg/ml doripenem (Rif+Dori), 25 μg/ml rifampin plus 10 μg/ml biapenem (Rif+Bia), and no drug as a control for growth of M. abscessus.
Mutants resistant to rifampin/faropenem combination were undetectable.
We hypothesized that a synergistic combination would also decrease the frequency of mutants that are resistant to the combination. To test this hypothesis, we determined the frequency of M. tuberculosis resistant mutants in the presence of rifampin and faropenem alone and in combinations (Table 5). At 10 times the MIC90 of rifampin, or 6 μg/ml, the frequency of resistant mutants was 1.8 × 10−8. Similarly, at 10 times the MIC90 of faropenem, or 50 μg/ml, mutants were selected at a frequency of 1 × 10−6. However, we were unable to observe any CFU growth on plates containing a combination of 0.6 μg/ml rifampin and 5.0 μg/ml faropenem, while plates containing either of the drugs at these concentrations produced 1,000 to 5,000 CFU. Despite our repeated efforts in which we increased the number of plates on which to select spontaneous resistant mutants, we were unable to observe any growth in plates containing 0.6 μg/ml rifampin and 5.0 μg/ml faropenem.
TABLE 5.
Frequency of emergence of M. tuberculosis mutants resistant to rifampin and faropenem, alone or in combination
| Concn (μg/ml) of: |
Total no. of resistant mutants | Frequency of resistant mutants | |
|---|---|---|---|
| Rifampin | Faropenem | ||
| 3.0 | 0 | 22 | 2.2 × 10−8 |
| 6.0 | 0 | 18 | 1.8 × 10−8 |
| 0.6 | 0 | 1,000 | 1 × 10−6 |
| 0 | 50 | 1,000 | 1 × 10−6 |
| 0 | 5.0 | 5,000 | 5 × 10−6 |
| 3.0 | 5.0 | 0 | 0 |
| 0.6 | 5.0 | 0 | 0 |
Rifampin/(carba)penem combinations are effective against drug-resistant clinical isolates of M. tuberculosis.
We evaluated the efficacy of rifampin in combination with meropenem, imipenem, doripenem, biapenem, or faropenem for their activities against four clinical isolates of M. tuberculosis: one rifampin monoresistant, one isoniazid monoresistant, and two multidrug-resistant strains (Tables 6 to 8; also see Table S1 in the supplemental material). Rifampin and (carba)penem combinations were growth inhibitory against all strains at sub-MIC levels of the drugs. The MIC90 of rifampin for rifampin monoresistant strains is 0.063 μg/ml when combined with 5 μg/ml meropenem. For multidrug-resistant strains, we assessed the MIC90 of rifampin at 0.25 to 0.5 μg/ml when combined with 5 μg/ml meropenem. Of the (carba)penems that we evaluated, faropenem afforded the most potent synergy with rifampin: when combined with 0.63 μg/ml faropenem, only 0.063 to 0.031 μg/ml of rifampin was required to inhibit growth of both rifampin monoresistant and multidrug-resistant strains (Tables 6 and 7; also see Table S1).
TABLE 6.
Growth profile of rifampin monoresistant clinical isolate of M. tuberculosis in the presence of rifampin and (carba)penemsa
| Drug (concn [μg/ml]) | Growth profile with rifampin at (μg/ml): |
Growth of control |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 0.5 | 0.25 | 0.12 | 0.063 | 0.031 | 0.016 | Positive | Negative | |
| Meropenem (5) | − | − | − | − | − | + | ++ | +++ | |
| Imipenem (40) | − | − | − | − | + | ++ | +++ | +++ | |
| Doripenem (1.25) | − | − | − | − | − | − | + | ||
| Biapenem (1.56) | − | − | − | − | + | ++ | +++ | − | |
| Faropenem (0.63) | − | − | − | − | − | − | + | − | |
| No (carba)penem | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||
For this strain, the rifampin MIC90 is >1 μg/ml. − represents no visible growth, and +++ represents growth equivalent to that of the positive control (M. tuberculosis grown in broth alone).
TABLE 8.
Growth profile of an isoniazid monoresistant clinical isolate of M. tuberculosis in the presence of rifampin and (carba)penemsa
| Drug (concn [μg/ml]) | Growth profile with rifampin at (μg/ml): |
Growth of control |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 0.5 | 0.25 | 0.012 | 0.063 | 0.031 | 0.016 | Positive | Negative | |
| Meropenem (5) | − | − | − | − | − | + | + | +++ | |
| Imipenem (40) | − | − | − | − | + | ++ | ++ | +++ | |
| Doripenem (1.25) | − | − | − | − | − | ++ | +++ | ||
| Biapenem (1.56) | − | − | − | − | − | − | ++ | − | |
| Faropenem (0.63) | − | − | − | − | + | ++ | +++ | − | |
− represents no visible growth, and +++ represents growth equivalent to that of the positive control (M. tuberculosis grown in broth alone).
TABLE 7.
Growth profile of multidrug-resistant clinical isolate 2 of M. tuberculosis in the presence of rifampin and (carba)penemsa
| Drug (concn [μg/ml]) | Growth profile with rifampin at (μg/ml): |
Growth of control |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 | 0.5 | 0.25 | 0.12 | 0.063 | 0.031 | 0.016 | Positive | Negative | |
| Meropenem (5) | − | − | + | ++ | ++ | + | ++ | +++ | |
| Imipenem (40) | − | − | + | ++ | ++ | +++ | +++ | +++ | |
| Doripenem (1.25) | − | − | − | ++ | ++ | +++ | +++ | ||
| Biapenem (1.56) | − | − | − | − | ++ | ++ | +++ | − | |
| Faropenem (0.63) | − | − | − | − | − | − | ++ | − | |
| No carba(penem) | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||
For this strain, the rifampin MIC90 is >1 μg/ml and the isoniazid MIC90 is >0.1 μg/ml. − represents no visible growth, and +++ represents growth equivalent to that of the positive control (M. tuberculosis grown in broth alone).
DISCUSSION
β-Lactams, one of the most widely used classes of antibiotics, are known to inhibit bacterial d,d-transpeptidases (also known as penicillin-binding proteins) (17, 18). It has been demonstrated that β-lactams interfere with peptidoglycan biosynthesis and metabolism in multiple ways and eventually results in cell death (17, 19). Among β-lactams, carbapenems and penems are able to bind and inhibit activities of d,d-transpeptidases, l,d-transpeptidases, and d,d-carboxypeptidases (6, 20–26). This versatile property of (carba)penems to target multiple enzymes prompted the current study to test the clinical utility of commercially available (carba)penems in M. tuberculosis and M. abscessus isolates from patients.
All MIC90 assessments were repeated at least twice to verify results. In addition, for M. abscessus, we repeated the MIC90 assessment using broth microdilution assay in 96-well format per Clinical and Laboratory Standards Institute guidelines (14). Data from these independent repeat assessments were highly consistent. We observed that meropenem in solution is unstable and loses potency even after a single freezing and thawing process. Therefore, only previously unthawed stock solutions of meropenem were used in this study. It was beyond the scope of current study or available resources to assess MBC, activity in combination with rifampin, and frequency of resistant mutants for all commercially available (carba)penems. We also used only a single concentration of clavulanic acid, 5 μg/ml, an optimal concentration derived from our titration of this agent with (carba)penems for activity against M. tuberculosis. This concentration is also within one dilution of the concentration determined in a previous study (5).
With the exception of panipenem, activities of all (carba)penems evaluated in this study are therapeutically relevant, as their MIC90 range from 1.25 to 40 μg/ml. (Carba)penems are known to be either slow substrates or inhibitors of BlaC, a β-lactamase of M. tuberculosis (2, 5, 27–30). A 2- to 4-fold reduction in MIC90 was seen for all (carba)penems except faropenem when supplemented with 5 μg/ml clavulanic acid. The MIC90 of doripenem and biapenem against M. tuberculosis decreased 2-fold when the inoculating dosage was split into two halves. As (carba)penems are known to exhibit time-dependent activity, our data suggest that a proper dosing interval for doripenem and biapenem has the potential to further optimize activities of these drugs. Doripenem, biapenem, faropenem, and tebipenem exhibit bactericidal activity against M. tuberculosis within therapeutically achievable concentrations. Due to the poor oral bioavailability of ertapenem, meropenem, imipenem, and doripenem, these drugs generally are administered intravenously, a route that is not feasible for treatment of TB patients en masse. However, newer (carba)penems, such as faropenem and tebipenem, can be administered orally as prodrugs and have the potential to be of high utility in treating tuberculosis in a regimen containing rifampin, as it is likely that time above MIC would be prolonged due to synergy between these two drugs.
The most significant observation of this study is the synergistic activity of combinations containing a (carba)penem and rifampin. Since only sub-MIC levels are required when combined with rifampin, a further increase in the dosage of (carba)penems may prolong the time above MIC and consequently make this combination therapeutically valuable by enhancing the time-dependent activity of (carba)penems. The combination was effective against drug-susceptible, rifampin monoresistant, isoniazid monoresistant, and two multidrug-resistant M. tuberculosis clinical isolates. As all evaluated strains were susceptible to rifampin and (carba)penems at concentrations that are readily achievable therapeutically, these data warrant studies to evaluate the clinical utility of this combination for the treatment of drug-resistant TB. The drug levels achievable with a 600-mg dose of rifampin is 1.5 μg/ml of free drug, which is capable of inactivating strains with MIC90 of up to 0.375 μg/ml. This study suggests that synergy with (carba)penems could lower the MIC of rifampin significantly. The data for rifampin-resistant strains are extremely promising and suggest that this combination is potent against drug-resistant strains of M. tuberculosis. With the addition of (carba)penems to the regimen, it may be possible to retain the most active mycobactericidal drug in the treatment regimen for MDR TB patients, practically altering the definition of MDR TB. Our inability to obtain spontaneous mutants in the presence of faropenem and rifampin further validates the potential utility of this combination in the treatment of TB, where regimens that can minimize the selection of drug-resistant strains will be highly important in controlling this disease.
(Carba)penems are known to bind and inhibit l,d-transpeptidases, d,d-transpeptidases, and d,d-carboxypeptidases, thereby inhibiting the biosynthesis of peptidoglycan (6, 20–26). Rifampin targets RNA polymerase activity (31, 32). It is reasonable to hypothesize that (carba)penems eventually alter the peptidoglycan layer to the extent that it changes the permeability to rifampin, leading to an increase in effective concentration in the cytosol. It also is possible that inhibition of transcription by rifampin does not permit the cells to upregulate the expression of proteins targeted by (carba)penems. Together, these two mechanisms potentially can lower the concentration of each drug required to exert their antimicrobial activity. Further studies are required to test these hypotheses.
M. abscessus is one of the major opportunistic pathogens that can cause chronic infection in the lungs of cystic fibrosis patients (10). Recently, imipenem was reported to exhibit a MIC90 in the range of 16 to 32 μg/ml against a collection of M. abscessus isolates from cystic fibrosis patients (33). We found doripenem and biapenem to be the most active among carbapenems against M. abscessus ATCC 19977 and three clinical isolates that are resistant to a range of drugs used to treat M. abscessus infection. The addition of clavulanic acid greatly enhanced the activity of meropenem, as its MIC90 decreased from >80 μg/ml to 5 to 10 μg/ml. The antimicrobial activity of doripenem, biapenem, faropenem, and tebipenem was modestly enhanced by clavulanic acid. More important is the synergy in antimicrobial activities of the combination of doripenem or biapenem and rifampin, as the concentrations of these drugs in the combinations are therapeutically significant. As the proportion of drug-resistant M. abscessus strains isolated from cystic fibrosis patients is gradually increasing (34), the potential impact of these synergistic combinations can be significant in the treatment of M. abscessus infection in this patient population.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jacques Grosset for critical discussions.
This study was supported by NIH awards 1R21AI111739-01 and 1DP2OD008459-01 to G.L.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01158-15.
REFERENCES
- 1.Flores AR, Parsons LM, Pavelka MS Jr. 2005. Genetic analysis of the beta-lactamases of Mycobacterium tuberculosis and Mycobacterium smegmatis and susceptibility to beta-lactam antibiotics. Microbiology 151:521–532. doi: 10.1099/mic.0.27629-0. [DOI] [PubMed] [Google Scholar]
- 2.Hugonnet JE, Blanchard JS. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 46:11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tremblay LW, Hugonnet JE, Blanchard JS. 2008. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry 47:5312–5316. doi: 10.1021/bi8001055. [DOI] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, Fischer E, Boshoff HI, Barry CE III. 2012. Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86:367–381. doi: 10.1111/j.1365-2958.2012.08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solapure S, Dinesh N, Shandil R, Ramachandran V, Sharma S, Bhattacharjee D, Ganguly S, Reddy J, Ahuja V, Panduga V, Parab M, Vishwas KG, Kumar N, Balganesh M, Balasubramanian V. 2013. In vitro and in vivo efficacy of beta-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 57:2506–2510. doi: 10.1128/AAC.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalo X, Drobniewski F. 2013. Is there a place for beta-lactams in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis? Synergy between meropenem and amoxicillin/clavulanate. J Antimicrob Chemother 68:366–369. [DOI] [PubMed] [Google Scholar]
- 9.Horita Y, Maeda S, Kazumi Y, Doi N. 2014. In vitro susceptibility of Mycobacterium tuberculosis isolates to an oral carbapenem alone or in combination with beta-lactamase inhibitors. Antimicrob Agents Chemother 58:7010-7014. doi: 10.1128/AAC.03539-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. 2003. Nontuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 167:828–834. [DOI] [PubMed] [Google Scholar]
- 11.Soroka D, Dubee V, Soulier-Escrihuela O, Cuinet G, Hugonnet JE, Gutmann L, Mainardi JL, Arthur M. 2014. Characterization of broad-spectrum Mycobacterium abscessus class A beta-lactamase. J Antimicrob Chemother 69:691–696. doi: 10.1093/jac/dkt410. [DOI] [PubMed] [Google Scholar]
- 12.Gavan TL, Town MA. 1970. A microdilution method for antibiotic susceptibility testing: an evaluation. Am J Clin Pathol 53:880–885. [DOI] [PubMed] [Google Scholar]
- 13.Cynamon MH, Speirs RJ, Welch JT. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob Agents Chemother 42:462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2011. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes. Approved standard M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 15.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. A critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi Y, Sunagawa M, Isobe Y, Hamazume Y, Noguchi T. 1995. Stability of a 1 beta-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem Pharm Bull 43:689–692. doi: 10.1248/cpb.43.689. [DOI] [PubMed] [Google Scholar]
- 17.Walsh C. 2003. Antibiotics: actions, origins, resistance. ASM Press, Washington, DC. [Google Scholar]
- 18.Wivagg CN, Bhattacharyya RP, Hung DT. 2014. Mechanisms of beta-lactam killing and resistance in the context of Mycobacterium tuberculosis. J Antibiot 67:645–654. doi: 10.1038/ja.2014.94. [DOI] [PubMed] [Google Scholar]
- 19.Schoonmaker MK, Bishai WR, Lamichhane G. 2014. Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to beta-lactams. J Bacteriol 196:1394–1402. doi: 10.1128/JB.01396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, Mainardi JL. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J Bacteriol 190:4360–4366. doi: 10.1128/JB.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubee V, Triboulet S, Mainardi JL, Etheve-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt(1) by carbapenems and cephalosporins. Antimicrob Agents Chemother 56:4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdemli SB, Gupta R, Bishai WR, Lamichhane G, Amzel LM, Bianchet MA. 2012. Targeting the cell wall of Mycobacterium tuberculosis: structure and mechanism of L,D-transpeptidase 2. Structure 20:2103–2115. doi: 10.1016/j.str.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Kim J, Im HN, Yoon JY, An DR, Yoon HJ, Kim JY, Min HK, Kim SJ, Lee JY, Han BW, Suh SW. 2013. Structural basis for the inhibition of Mycobacterium tuberculosis L,D-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains. Acta Crystallogr D Biol Crystallogr 69:420–431. doi: 10.1107/S0907444912048998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correale S, Ruggiero A, Capparelli R, Pedone E, Berisio R. 2013. Structures of free and inhibited forms of the L,D-transpeptidase LdtMt1 from Mycobacterium tuberculosis. Acta Crystallogr D Biol Crystallogr 69:1697–1706. doi: 10.1107/S0907444913013085. [DOI] [PubMed] [Google Scholar]
- 25.Li WJ, Li DF, Hu YL, Zhang XE, Bi LJ, Wang DC. 2013. Crystal structure of L,D-transpeptidase LdtMt2 in complex with meropenem reveals the mechanism of carbapenem against Mycobacterium tuberculosis. Cell Res 23:728–731. doi: 10.1038/cr.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordillot M, Dubee V, Triboulet S, Dubost L, Marie A, Hugonnet JE, Arthur M, Mainardi JL. 2013. In vitro cross-linking of peptidoglycan by Mycobacterium tuberculosis L,D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremblay LW, Fan F, Blanchard JS. 2010. Biochemical and structural characterization of Mycobacterium tuberculosis beta-lactamase with the carbapenems ertapenem and doripenem. Biochemistry 49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurz SG, Wolff KA, Hazra S, Bethel CR, Hujer AM, Smith KM, Xu Y, Tremblay LW, Blanchard JS, Nguyen L, Bonomo RA. 2013. Can inhibitor-resistant substitutions in the Mycobacterium tuberculosis beta-lactamase BlaC lead to clavulanate resistance?: a biochemical rationale for the use of beta-lactam-beta-lactamase inhibitor combinations. Antimicrob Agents Chemother 57:6085–6096. doi: 10.1128/AAC.01253-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow C, Xu H, Blanchard JS. 2013. Kinetic characterization of hydrolysis of nitrocefin, cefoxitin, and meropenem by beta-lactamase from Mycobacterium tuberculosis. Biochemistry 52:4097–4104. doi: 10.1021/bi400177y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazra S, Xu H, Blanchard JS. 2014. Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the beta-lactamase from Mycobacterium tuberculosis. Biochemistry 53:3671–3678. doi: 10.1021/bi500339j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, Matter L, Schopfer K, Bodmer T. 1993. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341:647–650. doi: 10.1016/0140-6736(93)90417-F. [DOI] [PubMed] [Google Scholar]
- 32.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912. doi: 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 33.Lavollay M, Dubee V, Heym B, Herrmann JL, Gaillard JL, Gutmann L, Arthur M, Mainardi JL. 2014. In vitro activity of cefoxitin and imipenem against Mycobacterium abscessus complex. Clin Microbiol Infect 20:O297–O300. doi: 10.1111/1469-0691.12405. [DOI] [PubMed] [Google Scholar]
- 34.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.