Abstract
Nosocomial pathogens can be associated with a variety of infections, particularly in intensive care units (ICUs) and in immunocompromised patients. Usually these pathogens are resistant to multiple drugs and pose therapeutic challenges. Among these organisms, Acinetobacter baumannii is one of the most frequent being encountered in the clinical setting. Carbapenems are very useful to treat infections caused by these drug-resistant Gram-negative bacilli, but carbapenem resistance is increasing globally. Combination therapy is frequently given empirically for hospital-acquired infections in critically ill patients and is usually composed of an adequate beta-lactam and an aminoglycoside. The purpose of this study was to evaluate the in vitro activity of plazomicin against carbapenem-resistant Acinetobacter baumannii. Amikacin was used as a comparator. The activity of plazomicin in combination with several different antibiotics was tested by disk diffusion, the checkerboard method, and time-kill studies. Synergy was consistently observed with carbapenems (meropenem and/or imipenem) along with plazomicin or amikacin. When the aminoglycosides were combined with other classes of antibiotics, synergy was observed in some cases, depending on the strain and the antibiotic combination; importantly, there was no antagonism observed in any case. These findings indicate the potential utility of plazomicin in combination with other antibiotics (mainly carbapenems) for the treatment of A. baumannii infections, including those caused by carbapenem-resistant isolates.
INTRODUCTION
Multidrug-resistant (MDR) strains of Acinetobacter baumannii are microorganisms that are being encountered in the clinical setting with an increased frequency and cause serious infections with few viable therapeutic options. A. baumannii has the ability to survive on dry surfaces for prolonged periods of time, whereby it can be easily spread (1) and thus has emerged as an important nosocomial pathogen.
This microorganism is also characterized by its innate and acquired resistance to multiple antimicrobial classes, including carbapenems. There are an increased number of cases of infections due to A. baumannii strains that have resistant or intermediate susceptibility to carbapenems, which previously had excellent clinical and in vitro activity. Carbapenem-resistant A. baumannii is currently included in the Infectious Diseases Society of America (IDSA) list of nosocomial pathogens of particular concern (2). The overproduction of intrinsic chromosomal OXA-51-like enzymes and the production of acquired OXA23, OXA-24/40, and OXA-58 enzymes has been associated with carbapenem-resistant A. baumannii isolates. The use of carbapenems may also be diminished via heteroresistance to these antibiotics, which is a phenomenon that can occur in both carbapenem-susceptible and -resistant isolates (3). As a consequence of limited therapeutic options, drug treatment with newer antimicrobials or antimicrobial combinations has become increasingly important for treating infections caused by these isolates. Furthermore, combination therapy might be the best antimicrobial strategy against microorganisms that exhibit the heteroresistance phenomenon. Combination therapy may minimize the risk of development of resistance and increase the effectiveness of treatment because of the potential synergy of antimicrobials. Combinations, which are usually composed of an adequate β-lactam and an aminoglycoside, are frequently given empirically for severe infections (4–6).
The aminoglycosides are among the oldest classes of antibiotics and demonstrate a broad spectrum of bacterial activity. However, the therapeutic use of aminoglycosides in recent years has been limited by concerns of toxicity (nephrotoxicity and ototoxicity) and increasing antimicrobial resistance. Plazomicin is a next-generation aminoglycoside that was synthetically derived from sisomicin and has enhanced activity against many MDR Gram-negative bacteria. Plazomicin is active against strains possessing a wide range of the most clinically relevant aminoglycoside-modifying enzymes (AMEs). The compound is now being studied in a global phase 3 trial enrolling patients with bloodstream infections or nosocomial pneumonia due to carbapenem-resistant Enterobacteriaceae. Several studies have demonstrated the potential in vitro activity of plazomicin against Gram-positive cocci, mainly Staphylococcus aureus, and against a diverse collection of Gram-negative bacilli (Enterobacteriaceae and Pseudomonas. aeruginosa), but there are few data on the activity of this antibiotic against Acinetobacter baumannii. Preliminary reports suggest that this agent possesses MIC values lower than those of amikacin (7), but more data are necessary to establish the real activity of plazomicin for treatment of carbapenem-resistant A. baumannii.
The purpose of this study was to evaluate the in vitro activity of plazomicin against a collection of MDR A. baumannii isolates obtained from clinical samples at Hospital Clinico San Carlos in Madrid, Spain. Furthermore, because combination therapy is often used for severe nosocomial infections, we examined the activity of plazomicin in combination with other Gram-negative agents against a selection of carbapenem-resistant strains (8–12).
MATERIALS AND METHODS
Bacteria.
A total of 69 imipenem-resistant A. baumannii isolates were included in the study. Of these, 41 were previously characterized (13) and included 9 blaOXA24-like producers, 15 blaOXA58 producers, 1 blaOXA23-like producer, and 16 isolates that only carry the blaOXA51-like β-lactamase gene associated with ISAba1 in the upstream region (7 bp upstream of the promoter). Twenty-six new blaOXA24-like producers, 1 isolate with only blaOXA51-like ISAba, and 1 isolate with blaOXA24-like + blaOXA58 were added to complete the total number. Eight carbapenem-susceptible isolates from the collection of the Department of Microbiology at the Hospital Clínico San Carlos (Madrid, Spain) were also included for comparison analysis.
Susceptibility testing.
Susceptibility testing of a single agent was determined by the agar dilution method according to the Clinical and Laboratory Standards Institute (14). The following antimicrobial agents were used and obtained from the indicated manufacturers: plazomicin from Achaogen (South San Francisco CA); amikacin (A0365900), colistin (C4461), gentamicin (G3632), tobramycin (T4014), imipenem (I0160), and meropenem (M2574) from Sigma-Aldrich (Madrid, Spain); fosfomycin from ERN Laboratories (Barcelona, Spain); and tigecycline from Pfizer (Madrid, Spain). The MIC was defined as the lowest concentration of an antimicrobial agent that completely inhibited the growth of bacteria. The concentrations of antimicrobial agents that were tested against all of the bacteria ranged from 0.06 to 512 μg/ml. Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as the internal controls for each test run. Resistance determination was based on criteria defined by the CLSI, except for the tigecycline and fosfomycin results, which were interpreted according to the European Committee on Antimicrobial Susceptibility Testing for Enterobacteriaceae (15).
Detection of aminoglycoside resistance genes.
All strains were examined by PCR for genes encoding the following aminoglycoside-modifying enzymes (AMEs): phosphotransferase (APH) (3′)-VIa (aphA6), acetyltransferases (AAC) 3-Ia (aacC1), AAC(3)-IIa (aacC2), and AAC(6′)-Ib (aacA4), and nucleotidyltransferase (ANT) (2″)-Ia (aadB). Isolates with high-level (128 μg/ml) resistance to all three traditional aminoglycosides (amikacin, gentamicin, and tobramycin) were also screened for the presence of the ribosomal methylase gene armA. The primers and conditions were those described by Akers et al. (16) for aphA6 and aacA4 and by Haldorsen et al. (17)for aacC1, aacC, aadB, and armA.
Synergy studies.
The in vitro activities of several antibiotic combinations were evaluated against 10 A. baumannii isolates using three different methods: disk diffusion, microdilution checkerboard, and time-kill. Isolates were selected in order to include all of the carbapenemase producer groups. The characteristics of selected isolates appear in Table 2.
TABLE 2.
Characteristics of A. baumannii assessed in synergy studies
| Isolate | MIC (μg/ml)a |
Carbapenemase | AMEb gene(s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PZ | AK | GM | TB | IM | MEM | CST | TG | FOS | |||
| HCSC-Ab2 | 8 | 8 | 512 | 128 | 64 | 4 | 2 | 2 | 128 | OXA58 | aadB + aacA4 |
| HCSC-Ab26 | 16 | 256 | 512 | 256 | >512 | 256 | 1 | 2 | 512 | OXA24 | aadB + aacA4 + aphA6 |
| HCSC-Ab33 | 0.5 | 8 | 8 | 32 | 512 | 256 | 1 | 0.25 | 64 | OXA24 + OXA58 | aacC1 |
| HCSC-Ab59 | 16 | 16 | >512 | 32 | 512 | 128 | 1 | 2 | 128 | OXA51-ISAbaI | None |
| HCSC-Ab66 | 16 | 512 | >512 | 256 | 512 | 64 | 2 | 2 | 512 | OXA24 | aphA6 |
| HCSC-Ab74 | 16 | 16 | >512 | 32 | 2 | 2 | 1 | 1 | 128 | OXA51-ISAbaI | aacA4 + aphA6 |
| HCSC-Ab80 | 32 | 8 | >512 | 256 | 32 | 4 | 1 | 1 | 64 | OXA58 | aphA6 |
| HCSC-Ab102 | 8 | 2 | >512 | 64 | 64 | 8 | 2 | 1 | 128 | OXA24 | aacA4 |
| HCSC-Ab113 | 8 | 2 | 4 | 1 | 32 | 8 | 2 | 2 | 64 | OXA51-ISAbaI | None |
| HCSC-Ab125 | 64 | 16 | 64 | 8 | 256 | 128 | 2 | 2 | 128 | OXA24 | aacC1 + aadB |
PZ, plazomicin; AK, amikacin; GM, gentamicin; TB, tobramycin; IM, imipenem; MEM, meropenem; CST, colistin; TG, tigecycline; FOS, fosfomycin.
AME, aminoglycoside-modifying enzyme.
Disk diffusion test.
A double-disk synergy test was performed for selected isolates to assess the possible interactions between two drugs. Disks of amikacin (30 μg), plazomicin (30 μg), meropenem (10 μg), and imipenem (10 μg) were used. Plazomicin disks were prepared using Whatman paper soaked with the proper amount of antibiotic solution. The distances between the disks were required to be suitably adjusted for each strain in order to accurately detect antibiotic interactions. Disks were placed separately with a distance equal to the sum of the zone radii for each disk tested separately. After 16 to 20 h of incubation at 37°C, the results were read. If the combination resulted in synergism, there was an inhibition zone formed between the disks. If the combination resulted in an additive or indifferent effect, there were two independent zones of clearing. If the combination resulted in antagonism, there would be a reduced zone size compared to the zone sizes of the individual test antibiotics (18).
Checkerboard assay.
The antibacterial effects of a combination of imipenem, meropenem, colistin, fosfomycin, and tigecycline with plazomicin or amikacin were assessed by the checkerboard test as previously described (19). Serial 2-fold dilutions of each antibiotic tested were prepared in Mueller-Hinton broth and mixed in each well of a 96-well microtiter plate so that each row (and column) contained a fixed amount of one agent and increasing amounts of the second agent. The range of drug concentrations used in the checkerboard analysis was such that the dilution range encompassed the MIC for each drug used in the analysis. Broth microdilution plates were inoculated with each strain to yield ∼5× 105 CFU/ml in a 100-μl final volume and incubated for 18 h at 37°C. MICs were determined after overnight incubation, and the fractional inhibitory concentration (FIC) index was calculated for each well.
The FIC index (FICI) was calculated for each combination according to the equation FICI = FICa + FICb = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). The FICI was interpreted as follows: synergy, FICI ≤0.5; indifference, 0.5 < FIC ≤4; and antagonism, FICI >4 (19).
Time-kill experiments.
The activity of amikacin, plazomicin, fosfomycin, meropenem, tigecycline, colistin, and combinations of either amikacin or plazomicin with the other antimicrobials were further evaluated in time-kill experiments for two carbapenem-resistant A. baumannii strains (HCSC-Ab102 and HCSC-Ab113). The time-kill analysis was performed according to previously described methods (19). Experiments were carried out with a starting inoculum of approximately 1 × 107 CFU/ml. Amikacin and plazomicin were added to 0.5× or 0.25× MIC. Other antibiotic concentrations were the mean steady-state concentrations previously published (20). Samples were taken at 0, 3, 6, and 24 h, serially diluted, spread on plates, and incubated at 37°C. Bacterial colonies were counted after 24 h of incubation at 37°C. The limit of detection was 10 CFU/ml. The time-kill curves were constructed by plotting mean colony counts (log10 CFU/ml) versus time. Synergy was defined as a 2-log10 decrease in the colony count of the combination compared with that of the most active antibiotic. An antimicrobial was considered bactericidal when a 3-log10 decrease in the CFU/ml was reached compared with the initial inoculum.
RESULTS
MICs.
The MICs of the study drugs for the 77 A. baumannii isolates are shown in Table 1. None of the A. baumannii isolates was resistant to either tigecycline or colistin, but 46 carbapenem-resistant isolates demonstrated intermediate susceptibility to tigecycline based on EUCAST interpretive criteria. The MICs for plazomicin and amikacin (the most active of the legacy aminoglycosides) ranged from 0.5 μg/ml to 64 μg/ml and from 0.5 μg/ml to 512 μg/ml, respectively, for carbapenem-resistant isolates and ranged from 0.5 μg/ml to 16 μg/ml and from 8 μg/ml to 16 μg/ml, respectively, for carbapenem-susceptible isolates. For the 27 A. baumannii isolates that had high MICs to amikacin (MIC, 256 to 512 μg/ml), the plazomicin MIC values were 16 μg/ml. However, it should be noted that there were eight isolates with plazomicin MICs higher than the amikacin MICs. Four of those isolates were assessed in synergy studies (Table 2).
TABLE 1.
Activity of plazomicin and selected antimicrobial agents against A. baumannii isolates included in the study
| Isolate type and antimicrobial agent | MIC (μg/ml) |
Activity (%)a |
||||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | S | I | R | |
| Carbapenem resistant (n = 69 isolates) | ||||||
| Plazomicin | 16 | 16 | 0.5–64 | |||
| Amikacin | 32 | 256 | 0.5–512 | 46.4 | 4.3 | 49.3 |
| Imipenem | 256 | 512 | 32–>512 | 0 | 0 | 100 |
| Meropenem | 128 | 256 | 4–512 | 0 | 18.8 | 81.2 |
| Gentamicin | >512 | >512 | 1–>512 | 10 | 4.5 | 85.5 |
| Tobramycin | 128 | 256 | 0.25–512 | 20.3 | 2.9 | 76.8 |
| Fosfomycin | 128 | 512 | 8–512 | 1.4 | 98.6 | |
| Tigecycline | 2 | 2 | 0.25–2 | 33.3 | 66.7 | 0 |
| Colistin | 1 | 2 | 1–2 | 100 | 0 | 0 |
| Carbapenem susceptible (n = 8 isolates) | ||||||
| Plazomicin | 1 | 16 | 0.5–16 | |||
| Amikacin | 8 | 16 | 2–16 | 100 | 0 | 0 |
| Imipenem | 1 | 2 | 1–2 | 100 | 0 | 0 |
| Meropenem | 0.5 | 2 | 0.25–2 | 100 | 0 | 0 |
| Gentamicin | 64 | >512 | 0.5–>512 | 37.5 | 0 | 62.5 |
| Tobramycin | 8 | 32 | 0.5–32 | 50 | 0 | 50 |
| Fosfomycin | 128 | >512 | 64–>512 | 0 | 0 | 100 |
| Tigecycline | 0.125 | 2 | 0.125–2 | 100 | 0 | 0 |
| Colistin | 1 | 1 | 1 | 100 | 0 | 0 |
S, susceptible; I, intermediate susceptibility; R, resistant.
A large percentage of isolates were nonsusceptible to gentamicin and tobramycin. However, the overall, resistance to other drugs was higher in the carbapenem-resistant isolates. Fosfomycin was the only exception and had similar MIC values in both groups (resistant and susceptible).
Amikacin was the most active aminoglycoside comparator tested and was selected as the comparator for further synergy studies.
Aminoglycoside-modifying enzymes.
PCR screening for AME genes showed that all isolates except eight (five susceptible and three aminoglycoside resistant) possessed some of the aminoglycoside-modifying enzymes analyzed. All five aminoglycoside-modifying enzymes tested were present singly or in combination. Twenty-six isolates (33.8%) had one AME gene, 31 isolates (40.25%) had two AME genes, 11 isolates (14.3%) had three AME genes, and 1 isolate had 4 different AME genes. aacA4 was the most prevalent AME gene (48 isolates, 12 of which as the only enzyme) followed by aphA6 (33 isolates. 4 alone), and aadB (24 isolates, 3 alone). Eleven isolates were positive for both aacA4 and aphA6, 9 isolates for aacA4 and aadB, and 8 isolates for the tree enzymes (aacA4, aphA6, and aad). There was no evident correlation between the presence of a particular aminoglycoside-modifying enzyme and the MICs of plazomicin. None of the isolates was found to have the ribosomal methylase armA.
Disk diffusion.
Variable synergy was observed between the aminoglycosides and carbapenems by the disk diffusion method against the 10 selected A. baumannii isolates (data not shown) and was strain and antibiotic combination dependent. Thus, according to the strain analyzed, different antibiotic combinations were found synergistic.
Heteroresistance to carbapenems was also detected in several of these isolates. These isolates showed a large number of resistant subpopulations within the inhibition halos around the meropenem and/or imipenem disks. These subpopulations disappeared when a carbapenem disk was in front of an aminoglycoside disk. The results obtained with two different strains appear in Fig. 1.
FIG 1.
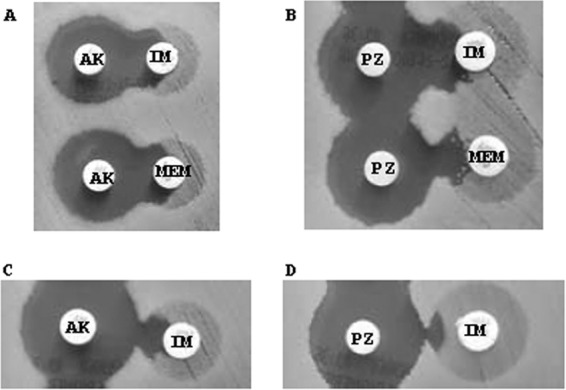
Representative results using the double-disk synergy test with two different carbapenem-resistant A. baumannii isolates: HCSC-Ab102 (A and B) and HCSC-Ab113 (C and D). The synergistic effects of amikacin (A and C) and plazomicin (B and D) can be observed, as well as carbapenem heteroresistance inhibition. (A) HCSC-Ab102: upper disks, amikacin (30 μg) and imipenem (10 μg); lower disks, amikacin (30 μg) and meropenem (10 μg). (B) HCSC-Ab102: upper disks, plazomicin (30 μg) and imipenem (10 μg); lower disks, plazomicin (30 μg) and meropenem (10 μg). (C) HCSC-Ab113: amikacin (30 μg) and imipenem (10 μg). (D) HCSC-Ab113: plazomicin (30 μg) and imipenem (10 μg). PZ, plazomicin; AK, amikacin; IM, imipenem; MEM, meropenem.
Checkerboard assay.
Combinations with carbapenems were the most effective out of all of the antibiotics tested (imipenem, meropenem, colistin, fosfomycin, and tigecycline). Against the isolates included in the study, plazomicin exhibited synergy in combination with imipenem (n = 7), meropenem (n = 6), colistin (n = 4), fosfomycin (n = 1), and tigecycline (n = 1); the greatest number of isolates in which synergism was observed.
Amikacin also was synergistic in several combinations but for fewer isolates than plazomicin, with the exception of amikacin plus imipenem, where the same number of isolates was observed to exhibit synergy. More than 60% of those were observed at physiologically attainable concentration of both drugs although in some of the isolates only for a small portion of the dosing interval of imipenem. It should be noted that all of the combinations that were not synergistic showed FICImin values of >0.5 but ≤1 (Table 3).
TABLE 3.
Checkerboard results of plazomicin and amikacin in double antibiotic combinations against selected Acinetobacter baumannii isolates (n = 10)
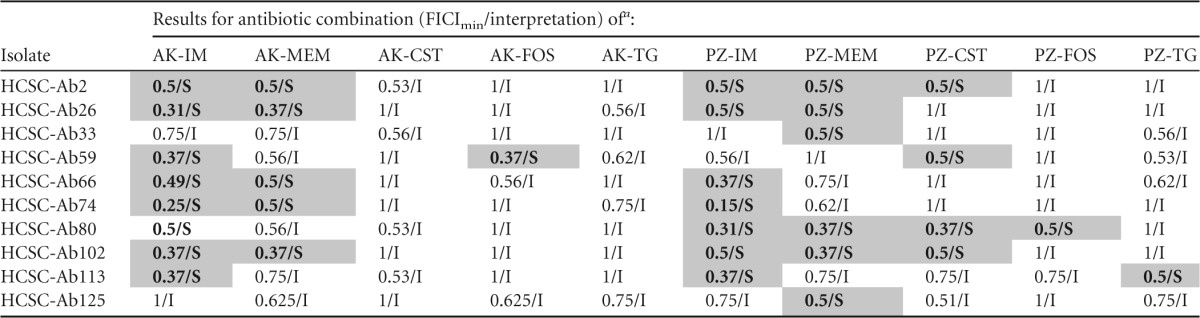
AK, amikacin; IM, imipenem; MEM, meropenem; CST, colistin; FOS, fosfomycin; TG, tigecycline; PZ, plazomicin; S, synergism; I, indifference. Synergism is indicated by gray shading.
Time-kill results.
The synergistic interactions that were inferred from the checkerboard analysis were further assessed by time-kill kinetic experiments with two different isolates. The assays were performed with plazomicin or amikacin in combination with meropenem, tigecycline, fosfomycin, and colistin. All of the antibiotic concentrations used in the time-kill analysis were below their respective MIC values, with the exception of that of colistin.
Both of the strains tested had a colistin MIC value (2 μg/ml) (Table 1) that was lower than the concentration used for time-kill experiments (4 μg/ml). The findings of time-kill studies for each of the two A. baumannii strains during exposure to a single agent or to the combinations tested are summarized in Tables 4 and 5.
TABLE 4.
Change in log10 CFU/ml at 3, 6, and 24 h during time-kill experiments performed against 2 A. baumannii carbapenemase-producing isolates using 0.5× aminoglycoside MIC and steady-state concentration for the other antibiotic
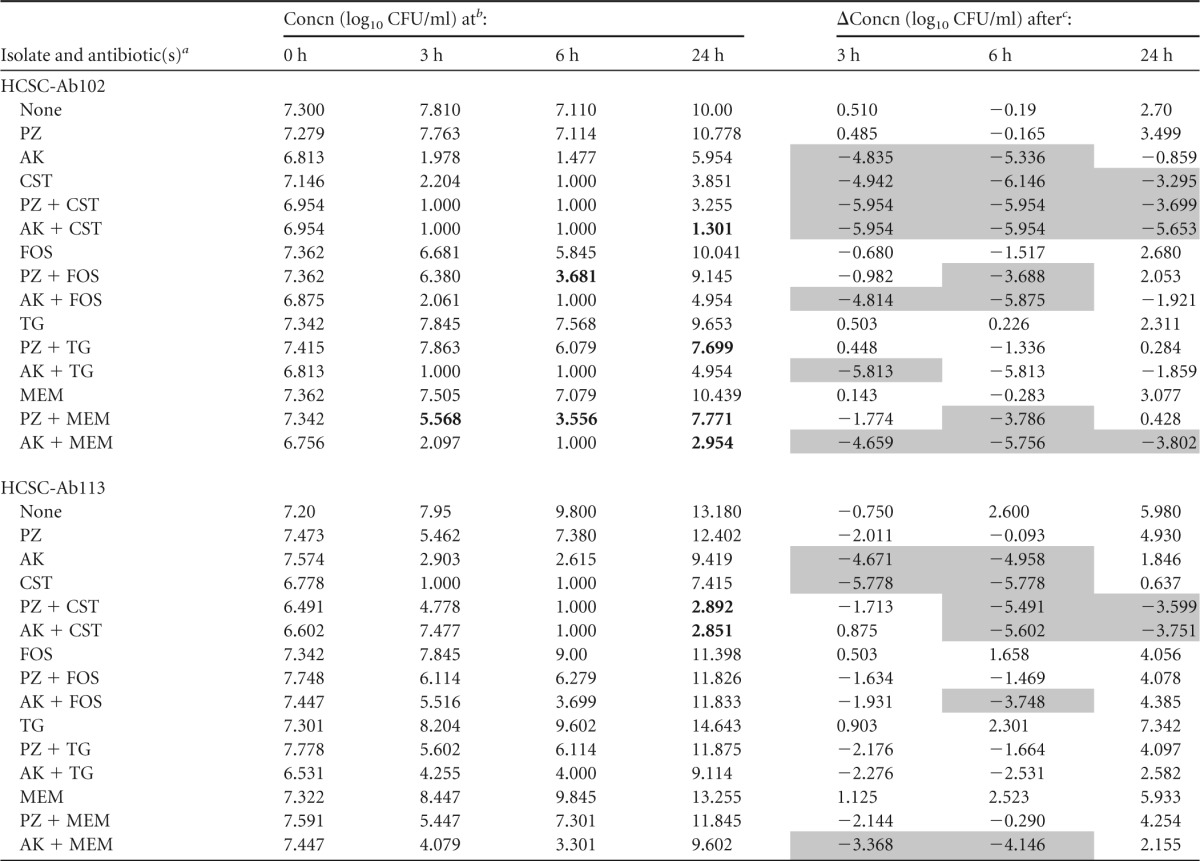
PZ, plazomicin; AK, amikacin; CST, colistin; FOS, fosfomycin; TG, tigecycline; MEM, meropenem.
Synergism is indicated by bold type.
Bactericidal effect is indicated by gray shading.
TABLE 5.
Change in log10 CFU/ml at 3, 6, and 24 h during time-kill experiments performed against 2 A. baumannii carbapenemase-producing isolates using 0.25× aminoglycoside MIC and steady-state concentration for the other antibiotic
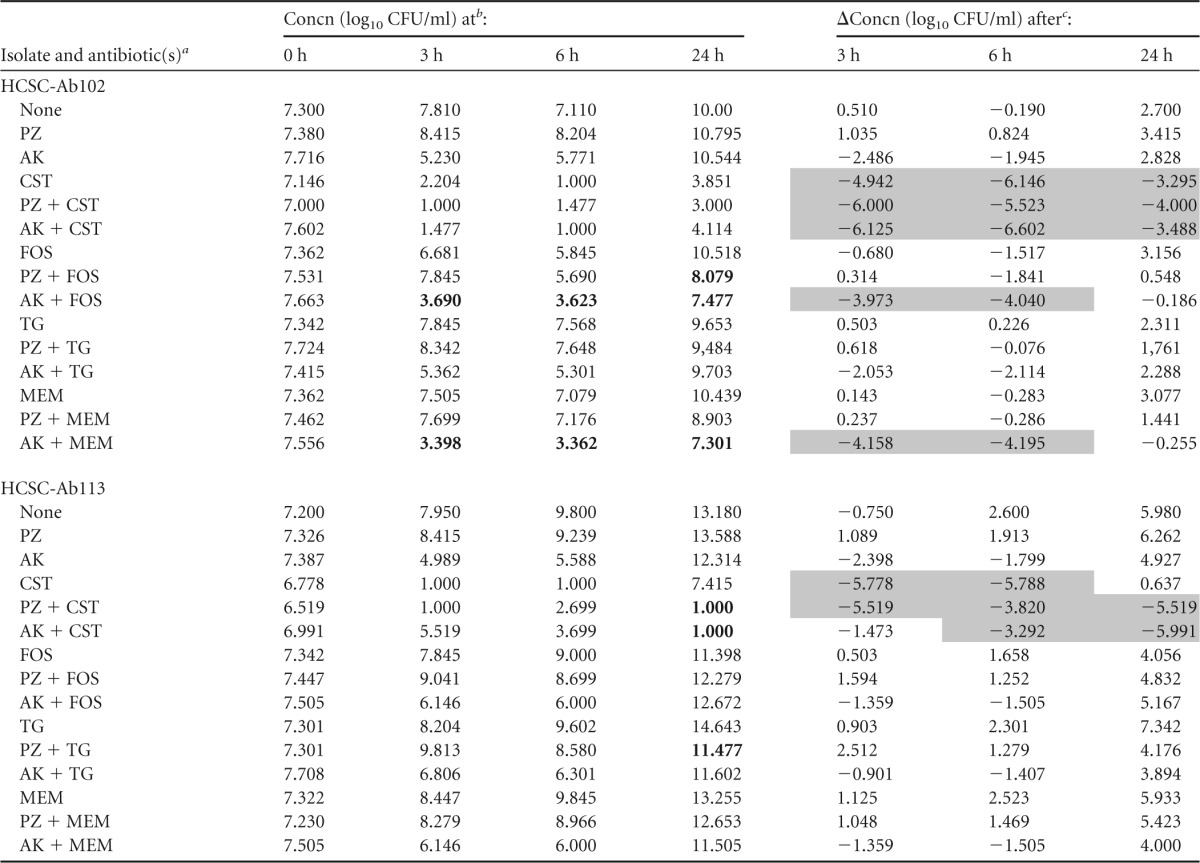
PZ, plazomicin; AK, amikacin; CST, colistin; FOS, fosfomycin; TG, tigecycline; MEM, meropenem.
Synergism is indicated by bold type.
Bactericidal effect is indicated by gray shading.
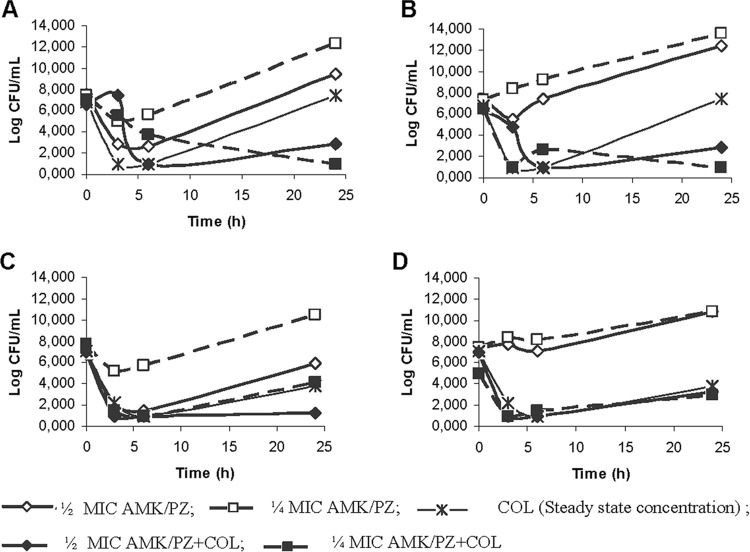
Amikacin-colistin and plazomicin-colistin were bactericidal against both strains (HCSC-Ab102 and HCSC-Ab113) at whatever concentration of aminoglycoside was used (Tables 4 and 5). Colistin alone or in combination with either amikacin (0.5× and 0.25× MIC) or plazomicin (0.5× and 0.25× MIC) was bactericidal within the first 6 h. However, for colistin alone, considerable regrowth occurred after 24 h, while in combination with either amikacin or plazomicin, regrowth was prevented for one isolate (HCSC-Ab113). The effect on the other isolate (HCSC-Ab102) was not as clear. The numbers of CFU/ml were lower in the combinations than for the separate antibiotics, but the difference between these values was practically negligible (Fig. 2).
FIG 2.
Antimicrobial synergy of aminoglycosides and colistin for 2 carbapenem-resistant A. baumannii isolates: HCSC-Ab113 (amikacin [A]and plazomicin [B]) and HCSC-Ab102 (amikacin [C] and plazomicin [D]). PZ, plazomicin; AK, amikacin; COL, colistin.
Amikacin and plazomicin both exhibited a concentration-dependent effect when tested as single agents at concentrations of 0.5× and 0.25× MIC. At both concentrations, the two aminoglycosides allowed regrowth of the two strains evaluated. At 0.5× MIC, the regrowth was detected later than at 0.25× MIC as expected. Regrowth was observed at 24 h with all antibiotics tested.
Synergy was observed after 24 h when plazomicin was combined with colistin (0.25× and 0.5× plazomicin MIC versus strain HCSC-Ab113), with tigecycline (0.25× and 0.5× plazomicin MIC versus strains HCSC-Ab113 and HCSC-Ab102, respectively), with meropenem (0.5× the plazomicin MIC versus HCSC-Ab102), and with fosfomycin (0.25× plazomicin MIC versus HCSC-Ab102). When amikacin was the aminoglycoside used, synergy was noted when it was combined with colistin (0.25× amikacin MIC versus HCSC-Ab113 and 0.5× amikacin MIC versus both strains), with meropenem (0.25× and 0.5× amikacin MIC versus HCSC-Ab102), and with fosfomycin (0.25× amikacin MIC versus HCSC-Ab102). Furthermore, synergy was also observed with some combinations at 3 and 6 h (Tables 4 and 5).
DISCUSSION
In this study, we evaluated the activity of plazomicin against a collection of carbapenem-resistant A. baumannii isolates. Plazomicin demonstrated more potent in vitro activity versus carbapenem-resistant A. baumannii isolates than the other aminoglycosides tested with MIC90 values that were 16-fold and 32-fold lower than those for amikacin/tobramycin and gentamicin, respectively. Genes encoding aminoglycoside-modifying enzymes were detected in most of the study isolates. Aminoglycoside acetyltransferase aacA4, phosphotransferase aphA6, and nucleotidyltransferase aadB have been predominantly identified within our clinical A. baumannii isolates, which frequently contain more than one aminoglycoside resistance gene. The presence of these aminoglycoside-modifying enzymes reduced the efficacy of the traditional aminoglycosides (amikacin, gentamicin, and tobramycin) but had no effect on plazomicin activity.
These results are similar to those previously published (7). To our knowledge, there are no reports about the activity of plazomicin in combination with other antibiotics against A. baumannii isolates. Therefore, we then analyzed the activities of several antibiotics in combination with plazomicin compared to that of amikacin by microdilution checkerboard and time-kill experiments. We used clinically relevant static antibiotic concentrations in the latter analysis. In both assays, we observed synergy for several combinations. The combination of an aminoglycoside plus a carbapenem was the most effective out of all combinations tested. This combination was effective against all strains independent of the carbapenemase possessed. Plazomicin plus imipenem and/or meropenem showed synergy against all of the isolates tested except HCSC-Ab59. Amikacin plus imipenem or meropenem was synergistic against eight out of the 10 A. baumannii isolates tested (except HCSC-Ab33 and HCSC-Ab125).
Presumably, the synergy observed in this combination arises as the result of enhanced intracellular uptake of aminoglycosides caused by the increased permeability of bacteria after incubation with cell wall synthesis inhibitors (21). The inhibition of protein synthesis by the aminoglycoside, which in turn reduces production of the carbapenemase and thus promotes activity of the beta-lactam agent might also have a role in the final effect. This combination presents multiple advantages compared to monotherapy with either of the two antibiotics, even in susceptible isolates. Addition of a carbapenem to an aminoglycoside during therapy is not expected to lead to an increase in toxicity, an important advantage over other combinations (e.g., colistin-aminoglycoside). We found that a carbapenem decreased the active concentration of plazomicin or amikacin by 2- to 64-fold and thus might also contribute to reduce aminoglycoside toxicity. A previous report showed that imipenem, in combination with antimicrobials that have different mechanisms of action, was active against pan-drug-resistant (including imipenem resistant) A. baumannii strains (22). We observed similar results with imipenem via checkerboard analysis as well as with meropenem by both checkerboard and time-kill analysis.
Acinetobacter infections have become more difficult to treat because of the emergence of isolates resistant to multiple antimicrobial drugs. Although colistin, tigecycline, and other classes of antibiotics have been used successfully to treat these infections in a significant number of patients, carbapenems remain the drug of choice. Carbapenems are the standard of care because of their advanced pharmacokinetic/pharmacodynamic (PK/PD) activity in comparison to the remaining antimicrobials like colistin, fosfomycin, and tigecycline. This is cause for concern as carbapenem resistance in A. baumannii becomes more prevalent. Lee et al. (23) showed that carbapenem-susceptible A. baumannii isolates might express inducible resistance to carbapenems following the initiation of carbapenem therapy. Their conclusion was that carbapenem use may lead to the emergence of carbapenem-heteroresistant isolates. Heteroresistance to carbapenems has been described in several other studies (24) and is hypothesized to be a problem for treatment. Combination therapy with a carbapenem and another agent might be considered to prevent treatment failure because of heteroresistance (23). As a first step toward understanding this, we demonstrated that, by disk diffusion, plazomicin and amikacin combination with a carbapenem might avoid this phenomenon; further analysis will be of interest.
In the time-kill experiments, bacterial regrowth occurred at 24 h for most combinations. However, the antibiotic concentrations assessed were primarily below the MICs (except for colistin), and regrowth was less common with combinations than with single antibiotics.
The use of antimicrobial combinations is considered to be one of the best options available to treat infections caused by carbapenem-resistant A. baumannii isolates, despite controversial opinions based on a potential increase in toxicity and a lack of clinical evidence of higher efficiency than monotherapy. Indeed, in addition to the improvement of bactericidality, combination therapy may also reduce the emergence of resistance and improve the spectrum of activity. Numerous antimicrobial combinations have been evaluated in vitro against these pathogens and have included several unconventional antimicrobial agents (25). Furthermore, it has been demonstrated that aggressive treatment involving 2 antimicrobial agents that exhibit synergistic activity improves treatment outcomes in immunocompromised patients with severe multidrug-resistant A. baumannii-related infections (26).
In vitro studies have previously shown the synergy of carbapenems combined with amikacin against MDR A. baumannii strains (27). In this work, we observed similar results and also demonstrated that plazomicin in combination with carbapenems is synergistic. Because the plazomicin MIC90 was lower than that of amikacin, the amount of aminoglycoside needed to display synergy was less with plazomicin, an advantage that may help avoid possible toxicity.
In summary, we have found that plazomicin has the potential to be useful for the treatment of carbapenem-resistant A. baumannii isolates combined with different antibiotics, primarily carbapenems. These combinations can be synergistic at steady-state concentrations and have the potential to overcome heteroresistance to carbapenems, which is frequently observed in this species.
ACKNOWLEDGMENTS
We thank Achagoen for providing plazomicin.
This study was supported by FIS PI13/01471 from the Spanish Ministerio de Economía y Competitividad.
We declare no conflicts of interest.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez Cuenca F, Sanchez Mdel C, Caballero-Moyano FJ, Vila J, Martinez-Martinez L, Bou G, Bano JR, Pascual A. 2012. Prevalence and analysis of microbiological factors associated with phenotypic heterogeneous resistance to carbapenems in Acinetobacter baumannii. Int J Antimicrob Agents 39:472–477. doi: 10.1016/j.ijantimicag.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, Kollef MH. 2010. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 54:1742–1748. doi: 10.1128/AAC.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.America Thoracic Society, Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 6.Motaouakkil S, Charra B, Hachimi A, Nejmi H, Benslama A, Elmdaghri N, Belabbes H, Benbachir M. 2006. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. J Infect 53:274–278. doi: 10.1016/j.jinf.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Landman D, Kelly P, Backer M, Babu E, Shah N, Bratu S, Quale J. 2011. Antimicrobial activity of a novel aminoglycoside, ACHN-490, against Acinetobacter baumannii and Pseudomonas aeruginosa from New York City. J Antimicrob Chemother 66:332–334. doi: 10.1093/jac/dkq459. [DOI] [PubMed] [Google Scholar]
- 8.Walkty A, Adam H, Baxter M, Denisuik A, Lagace-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. 2014. In vitro activity of plazomicin against 5,015 Gram-negative and Gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011-2012. Antimicrob Agents Chemother 58:2554–2563. doi: 10.1128/AAC.02744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. 2012. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 12.Tenover FC, Tickler I, Armstrong ES, Kubo A, Lopez S, Persing DH, Miller GH. 2011. Activity of ACHN-490 against meticillin-resistant Staphylococcus aureus (MRSA) isolates from patients in US hospitals. Int J Antimicrob Agents 38:352–354. doi: 10.1016/j.ijantimicag.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Álvarez-Buylla A, Picazo JJ, Culebras E. 2013. Optimized method for Acinetobacter species carbapenemase detection and identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:1589–1592. doi: 10.1128/JCM.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing. 2015. Clinical breakpoints. http://www.eucast.org/clinical_breakpoints/. [Google Scholar]
- 16.Akers KS, Chaney C, Barsoumian A, Beckius M, Zera W, Yu X, Guymon C, Keen EF III, Robinson BJ, Mende K, Murray CK. 2010. Aminoglycoside resistance and susceptibility testing errors in Acinetobacter baumannii-calcoaceticus complex. J Clin Microbiol 48:1132–1138. doi: 10.1128/JCM.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldorsen BC, Simonsen GS, Sundsfjord A, Samuelsen O. 2014. Increased prevalence of aminoglycoside resistance in clinical isolates of Escherichia coli and Klebsiella spp. in Norway is associated with the acquisition of AAC(3)-II and AAC(6′)-Ib. Diagn Microbiol Infect Dis 78:66–69. doi: 10.1016/j.diagmicrobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Eliopoulos GM, Moellering CR. 1996. Antimicrobial combinations, p 330−396. In Lorian V. (ed), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 19.Petersen PJ, Labthavikul P, Jones CH, Bradford PA. 2006. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother 57:573–576. doi: 10.1093/jac/dki477. [DOI] [PubMed] [Google Scholar]
- 20.Tängdén T, Hickman RA, Forsberg P, Lagerback P, Giske CG, Cars O. 2014. Evaluation of double- and triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother 58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vakulenko SB, Mobashery S. 2003. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev 16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat JM, Pancholi P. 2010. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:4678–4683. doi: 10.1128/AAC.00497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Chen CL, Wang SB, Su LH, Chen SH, Liu SY, Wu TL, Lin TY, Chiu CH. 2011. Imipenem heteroresistance induced by imipenem in multidrug-resistant Acinetobacter baumannii: mechanism and clinical implications. Int J Antimicrob Agents 37:302–308. doi: 10.1016/j.ijantimicag.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Ikonomidis A, Neou E, Gogou V, Vrioni G, Tsakris A, Pournaras S. 2009. Heteroresistance to meropenem in carbapenem-susceptible Acinetobacter baumannii. J Clin Microbiol 47:4055–4059. doi: 10.1128/JCM.00959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galani I, Orlandou K, Moraitou H, Petrikkos G, Souli M. 2014. Colistin/daptomycin: an unconventional antimicrobial combination synergistic in vitro against multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents 43:370–374. doi: 10.1016/j.ijantimicag.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Khawcharoenporn T, Pruetpongpun N, Tiamsak P, Rutchanawech S, Mundy LM, Apisarnthanarak A. 2014. Colistin-based treatment for extensively drug-resistant Acinetobacter baumannii pneumonia. Int J Antimicrob Agents 43:378–382. doi: 10.1016/j.ijantimicag.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Lee NY, Wang CL, Chuang YC, Yu WL, Lee HC, Chang CM, Wang LR, Ko WC. 2007. Combination carbapenem-sulbactam therapy for critically ill patients with multidrug-resistant Acinetobacter baumannii bacteremia: four case reports and an in vitro combination synergy study. Pharmacotherapy 27:1506–1511. doi: 10.1592/phco.27.11.1506. [DOI] [PubMed] [Google Scholar]