Abstract
This study aimed to explore any mutation in the CYP51 gene conferring azole resistance in Aspergillus flavus. Two voriconazole-resistant and 45 voriconazole-susceptible isolates were included in the study. Sequence analysis demonstrated a T1025C nucleotide change in CYP51C, resulting in the Y319H amino acid substitution in one resistant isolate. However, the earlier described T788G mutation in CYP51C conferring voriconazole resistance in A. flavus isolates was present in all isolates, irrespective of their susceptibility status.
TEXT
Aspergillus flavus is the second leading cause of invasive aspergillosis in immunocompromised patients and the predominant causative agent of fungal rhinosinusitis and fungal eye infections (endophthalmitis and keratitis) in tropical countries, like India, Sudan, Kuwait, and Iran (1–8). Voriconazole is used primarily to treat infections caused by A. flavus. Long-term azole therapy may predispose A. flavus to acquire resistance to azoles, including voriconazole
Lanosterol 14 α-demethylase (LDM), which catalyzes the rate-limiting step in the ergosterol biosynthetic pathway, serves as the primary target for azole antifungal drugs. The mechanism of azole resistance in Aspergillus fumigatus is well studied. Missense mutations and alteration of cis regulatory regions in the LDM coding gene CYP51A have been found to be the dominant mechanisms of azole resistance in A. fumigatus (9–12), whereas studies to evaluate the mechanism of azole resistance in A. flavus are sparse (13–15). The present study is an attempt to understand the mechanism of azole resistance in A. flavus.
Two non-wild-type (non-WT) clinical isolates of A. flavus, NCPPF 761157 and NCCPF 760815, with higher MIC values for voriconazole than for the respective wild-type (WT) cutoff value, and 4 WT isolates were initially used (Table 1). The wild type and non-wild type were defined on the basis of epidemiological cutoff values (ECV), with the non-WT having a voriconazole MIC of >1 μg/ml and WT with a voriconazole MIC of ≤1 μg/ml (16). The non-WT strain, NCCPF 761157, was isolated from a sputum sample from a patient with chronic obstructive pulmonary disease, and NCCPF 760815 was isolated from a nasal tissue sample from a patient from India having granulomatous fungal rhinosinusitis. Forty-five additional WT A. flavus clinical isolates were included to screen and validate the mutations (single-nucleotide polymorphisms [SNPs] and indels). Identification of the isolates was done by sequencing partial β-tubulin and calmodulin genes using primers bt2a (GGTAACCAAATCGGTGCTGCTTTC) and bt2b (ACCCTCAGTGTAGTGACCCTTGGC), and cmdA7 (GCCAAAATCT TCATCCGTAG) and cmdA8 (ATTTCGTTCAGAATGCCAGG) (17, 18). Antifungal susceptibility testing was done as per CLSI and EUCAST guidelines (19–22). Coding sequences of the close homologues of CYP51A of A. fumigatus in A. flavus, namely CYP51A (GenBank accession no. XM_002375082.1), CYP51B (GenBank accession no. XM_002379089.1), and CYP51C (GenBank accession no. XM_002383890.1), were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/GenBank), as mentioned by Liu et al. (15). Overlapping primer sets were designed for each homologue, and PCR amplification of each open reading frame and the upstream and downstream regions of each homologue was performed (Table 2). To reduce errors during amplification, two different high-fidelity DNA polymerases (Platinum Taq; Life Technologies, Carlsbad, CA, and KOD Plus-; Toyobo Life Science Department, Osaka, Japan) were used in different sets of experiments (twice). Sequence amplification and analysis were performed using the BigDye Terminator ready reaction kit and a genetic analyzer (Applied Biosystems, Foster City, CA). Consensus of the forward and reverse sequences and the contig assembly of each product from the overlapping fragments were done using the BioNumerics software (Applied Maths, Ghent, Belgium). Sequences were aligned in Clustal X2, and amino acid sequences were deduced using the ExPASy online tool (http://www.expasy.org/translate). To assess the impact of the Y319H substitution on the general structure of A. flavus CYP51C, homology modeling and molecular dynamic simulations were performed for the WT and the Y319H mutant. The amino acid sequence of the query protein was downloaded from UniProt protein sequence database (UniProt ID I8TEB1). The three-dimensional (3D) homology models of the WT and Y319H mutant were generated using the Swiss-Model (http://swissmodel.expasy.org/interactive#sequence) workspace. The LDM (PDB ID 4K0F) structure sharing a sequence identity of 50.51% was used as the template for model building. Models were validated using the Qmean4 score. A production dynamic simulation run was performed using Gromacs 4.6.5 with the GROMOS96 43a1 force field. Molecular dynamics (MD) trajectory analysis was performed using Gromacs utilities, and all the graphs were plotted using Grace. To study the structural and functional effects of the Y319H substitution, the WT and non-WT CYP51C were also analyzed on HOPE (23).
TABLE 1.
Antifungal susceptibility profile of A. flavus isolates for various drugs, performed using CLSI M38-A2 (19)
| Strain | MIC/MEC (μg/ml) fora: |
||||||
|---|---|---|---|---|---|---|---|
| AMB | VORb | ITR | POS | CSP | MCF | ANI | |
| NCCPF 761157 | 2 | 4 (8) | 16 | 0.25 | 0.03 | 0.015 | 0.0075 |
| NCCPF 760815 | 4 | 2 (2) | 1 | 0.5 | 4 | 0.12 | 0.25 |
| NCCPF 760816 | 2 | 0.5 (1) | 0.12 | 0.12 | 0.06 | 0.015 | 0.06 |
| NCCPF 760690 | 4 | 0.125 (0.25) | 0.06 | 0.03 | 0.03 | 0.015 | 0.06 |
| NCCPF 761379 | 1 | 0.5 | 0.12 | 0.06 | 0.06 | 0.015 | 0.06 |
| NCCPF 761425 | 4 | 0.5 | 0.25 | 0.12 | 0.03 | 0.015 | 0.06 |
MEC, minimum effective concentration of echinocandins; AMB, amphotericin B; VOR, voriconazole; ITR, itraconazole; POS, posaconazole; CSP, caspofungin; MCF, micafungin; ANI, anidulafungin.
Values in parentheses for voriconazole are MICs determined by EUCAST method, E.DEF 9.1 (20).
TABLE 2.
Primers used in the study for amplification of homologs of CYP51
| CYP51 homolog | Primer name | Primer sequence (5′–3′) | Position on coordinate (bases) |
|---|---|---|---|
| CYP51A | AflaCYP51A F1 | CAAGAACAGCCTGCACAGAG | 324 |
| AflaCYP51AR1 | GGGTGGATCAGTCTTATTA | 1126 | |
| AflaCYP51AF2 | GCAATCATCGTCCTAAATC | 1066 | |
| AflaCYP51AR2 | CTGTCCATTCTTGTAGGTA | 1899 | |
| AflaCYP51AF3 | GCATGAGGGAGATCTATATG | 1791 | |
| AflaCYP51AR3 | CCTATAATTGCTGGTTTCG | 2649 | |
| AflaCYP51AF4 | TGAAGCTATTCAATGTAGAC | 2480 | |
| AflaCYP51AR4 | ACTGCTGATGGTGTGCTAAG | 3358 | |
| A205T-F | GGAGTCGCATGTACCATTGA | 1510 | |
| A205T-R | TGAAGTTGATCGGAGTGAACC | 1716 | |
| CYP51B | AflaCYP51B F1 | AACACGACTAGGAGCTACAC | 4182 |
| AflaCYP51BR1 | CACCAATCCACTCTATC | 5082 | |
| AflaCYP51BF2 | GATCAGGGAAATGTTCTTC | 4948 | |
| AflaCYP51BR2 | ACGATCGCTGAGATTAC | 5620 | |
| AflaCYP51BF3 | GTTCAGCAAATGTCGAG | 5550 | |
| AflaCYP51BR3 | CCTTTCGTCTACCTGTT | 6344 | |
| AflaCYP51BF4 | AGTGGAGAGCATCCATAGTGA | 6231 | |
| AflaCYP51BR4 | ACAACCCGTTCAAGATATCGG | 7339 | |
| CYP51C | AflaCYP51CF1 | CTGTTGCAGAGCCGTTGATG | 33 |
| AflaCYP51CR1 | CAAAGAGCGACACATAAG | 860 | |
| AflaCYP51CF2 | GGTAATGTCTGGTCATAGG | 751 | |
| AflaCYP51CR2 | ATGAGCTTGGAATTGGG | 1453 | |
| AflaCYP51CF3 | CGAATTCATCCTCAATGG | 1336 | |
| AflaCYP51CR3 | GTCTCTCGGATCACATT | 2137 | |
| AflaCYP51CF4 | GGAACTCTACCAAGAGCA | 2018 | |
| AflaCYP51CR4 | CCTAGATACAGCTAGATACCC | 2819 | |
| AflaCYP51Cdel-F | CCAGCGCTCATAGGTGTATT | 2634 | |
| AflaCYP51Cdel-R | CGTGGTCAGTCAATTGGGTA | 3102 | |
| SNP-F | GCGGTTCTCTACCACGATTTG | 677 | |
| SNP-R | AGGGTCTCTCGGATCACATTT | 1120 |
A comparison of the nucleotide and amino acid sequences of CYP51A homologs of non- WT (NCCPF 761157 and NCCPF 760815) and WT strains (760816, 760690, 760425, and 760379) of A. flavus with a reference sequence (A. flavus strain NRRL3357) showed the G680A transition in CYP51A of strain NCCPF 761157, resulting only in the amino acid change A205T. The upstream (−1,000 bp) and downstream (+1,000 bp) regulatory regions were intact in all strains. In addition, there was no change in the nucleotide or amino acid sequences in CYP51B. However, CYP51C was most polymorphic in nature (Table 3). Six missense nucleotide changes and the resulting amino acid replacements were detected in CYP51A and CYP51C. However, 5 of these substitutions (A205T, M54T, S240A, D254N, and I285V) did not appear to affect the azole susceptibility of the organism, as these changes were also found in WT isolates. Only one nonsynonymous mutation, T1025C, translating to Y319H, was found to be specific to a non-WT isolate (NCCPF 761157). To confirm these findings, we used 45 wild-type isolates to screen for the SNPs and indels coding for these phenotypes of CYP51C in azole-sensitive strains. Tandem duplication of a promoter sequence, TR 34, along with the nonsynonymous point mutation L98H was reported for azole resistance in clinical and environmental isolates of A. fumigatus. However, a mutation of this characteristic was not found in our azole-resistant A. flavus isolates. Nonetheless, a 4-bp deletion was found in the AT-rich intergenic region downstream at position 2734 of CYP51C, which upon screening of the WT collection showed that it was not related to the resistant phenotype. Instead, a compensatory 4-bp insertion mutation was found in the nearby region in those isolates that harbored this deletion (data not shown). Indel mutations usually arise in intergenic regions, which act as mutational hot spots for indels and play a role in purifying selection (24). The present study also contradicts the finding of Liu et al. (15), in which the T788G mutation was implicated in mediating voriconazole resistance in A. flavus. This mutation was not related to voriconazole resistance in our strains, as this SNP was found in all 47 strains tested, irrespective of their susceptibility. Possibly, the T788G mutation is simply a geographical strain variation, as the investigators compared the CYP51C sequence of their resistant strain with the A. flavus NRRL3357 reference sequence only. Alignment of orthologues of cytochrome P450 of different fungal species and of humans showed substitutions, including A205T, M54T, S240A, D254N, and I285V, which were not present in the conserved motifs. (Table 3). The location of the Y319H substitution in a highly conserved position of CYP51C suggests that this might be one of the reasons for azole resistance in our resistant isolates.
TABLE 3.
Mutational analysis of CYP51A, CYP51B, and CYP51C and the corresponding amino acid changes in lanosterol 14 α-demethylase in resistant and sensitive isolates
| Strain | Mutations in CYP51 |
Amino acid change in LDMa |
Regulatory region of CYP51C | ||||
|---|---|---|---|---|---|---|---|
| CYP51A | CYP51B | CYP51C | CYP51A | CYP51B | CYP51C | ||
| NCCPF 761157 | G680A | None | T161C | A205T | None | M54T | 4-bp deletion at bp 2734 |
| T788G | S240A | at bp 2734 | |||||
| G830A | D254N | ||||||
| G923A | I285V | ||||||
| T1025C | Y319H | ||||||
| NCCPF 760815 | None | None | T161C | None | None | M54T | 4-bp deletion at bp 2734 |
| T788G | S240A | ||||||
| G830A | D254N | ||||||
| G923A | I285V | ||||||
| NCCPF 760816 | None | None | T161C | None | None | M54T | None |
| T788G | S240A | ||||||
| NCCPF 760690 | None | None | T161C | None | None | M54T | None |
| T788G | S240A | ||||||
| NCCPF 761379 | None | None | T161C | None | None | M54T | None |
| S240A | |||||||
| NCCPF | None | None | T788G | None | None | M54T | None |
| 761425 | S240A | ||||||
LDM, lanosterol 14 α-demethylase.
As the Y319H substitution is located far from the iron-porphyrin complex, it appears that the substitution indirectly affects drug binding instead of having a direct effect on the docking of azoles at the binding site (Fig. 1). MD simulations revealed that this mutation increases conformational flexibility, as indicated by increased root mean square deviation values (Fig. 2A) and root mean square fluctuation (RMSF) (Fig. 2B); there was a simultaneous decrease in globularity, as depicted by the increase in the radius of gyration of the mutant protein (Fig. 2C). Differences in the radius of gyration between the WT and non-WT CYP51C may be due to a loss of noncovalent interactions, which was caused by the substitution of tyrosine with histidine in the mutant strain. The WT tyrosine residue forms a hydrogen bond with the valine at position 329 and salt bridges with the valine at position 329 and glutamic acid at position 328. Increased flexibility in the non-WT CYP51C may be due to the polar nature of histidine causing interatomic repulsions. On the other hand, tyrosine present in the wild type can form hydrophobic interactions, accounting for the lower RMSF. The structural data for the CYP51C protein of A. flavus is not available to infer the effect of point mutations on the conformations of drug entry channels of orthologous proteins. However, a similar strategy has been applied in earlier studies (25–28). The results from our study provide clues that increased conformational flexibility in the Y319H mutant may be the reason for its reduced drug binding affinity.
FIG 1.
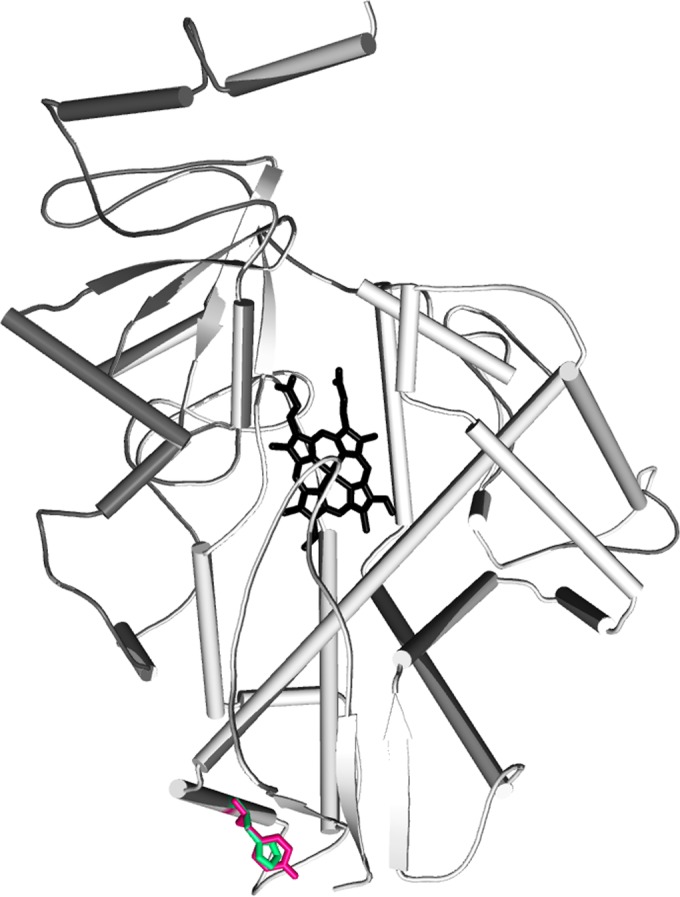
Modeled structure of CYP51C of A. flavus shown in cartoon representation. The porphyrin ring is shown in stick representation in black. The tyrosine residue present in the wild type and the histidine in the mutant are shown in hot pink and green, respectively.
FIG 2.
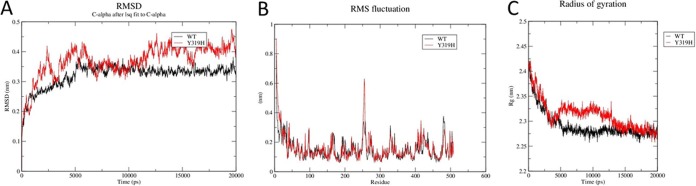
(A) Root mean square deviations of Cα backbones of WT and mutant CYP51C proteins as a function of time (20 ns). (B) Graph shows the average fluctuation of Cα atoms for each residue around the average structure of the protein. The black line stands for the WT molecular dynamics trajectory and the red line for the Y319H mutant dynamics trajectory. (C) Radius of gyration (Rg) of Cα of WT and CYP51C mutant protein as a function of time at 20 ns.
However, the Y319H substitution was not found in the other resistant isolate (NCCPF 760815). The absence of the Y319H substitution in NCCPF 760815 may be due to other mechanisms responsible for the elevated MICs in this isolate. Nonetheless, our findings need to be evaluated in more non-WT A. flavus isolates and by producing a Y319H mutant in a WT background and confirming its azole resistance.
Nucleotide sequence accession numbers.
The nucleotide sequences of CYP51C of NCCPF 761157 and NCCPF 760815 have been submitted to GenBank with the nucleotide accession numbers KR822399 and KR822400, respectively.
ACKNOWLEDGMENTS
We acknowledge the help of Khurram Mushtaq of the Institute of Microbial Technology, Chandigarh, India, during the simulation study and Indian Council of Medical Research for the financial support in conducting the study.
REFERENCES
- 1.Pasqualotto AC. 2009. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med Mycol 47(Suppl 1):S261–S270. [DOI] [PubMed] [Google Scholar]
- 2.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. 2007. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan S, Manavathu EK, Chandrasekar PH. 2009. Aspergillus flavus: an emerging non-fumigatus Aspergillus species of significance. Mycoses 52:206–222. doi: 10.1111/j.1439-0507.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA, Gupta A. 2008. Fungal endophthalmitis: fourteen years' experience from a center in India. Retina 28:1400–1407. doi: 10.1097/IAE.0b013e318185e943. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti A, Rudramurthy SM, Panda N, Das A, Singh A. 2015. Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses 58:294–302. doi: 10.1111/myc.12314. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Singh R. 2011. The emerging epidemiology of mould infections in developing countries. Curr Opin Infect Dis 24:521–526. doi: 10.1097/QCO.0b013e32834ab21e. [DOI] [PubMed] [Google Scholar]
- 7.Khan ZU, Sanyal SC, Mokaddas E, Vislocky I, Anim JT, Salama AL, Shuhaiber H. 1997. Endocarditis due to Aspergillus flavus. Mycoses 40:213–217. doi: 10.1111/j.1439-0507.1997.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan ZU, Ahmad S, Mokaddas E, Said T, Nair MP, Halim MA, Nampoory MR, McGinnis MR. 2007. Cerebral aspergillosis diagnosed by detection of Aspergillus flavus-specific DNA, galactomannan and (1→3)-beta-d-glucan in clinical specimens. J Med Microbiol 56:129–132. doi: 10.1099/jmm.0.46549-0. [DOI] [PubMed] [Google Scholar]
- 9.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother 67:362–366. doi: 10.1093/jac/dkr443. [DOI] [PubMed] [Google Scholar]
- 12.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan-Natesan S, Chandrasekar PH, Alangaden GJ, Manavathu EK. 2008. Molecular characterisation of CYP51A and CYP51B genes coding for P450 14alpha-lanosterol demethylases A (CYP51Ap) and B (CYP51Bp) from voriconazole-resistant laboratory isolates of Aspergillus flavus. Int J Antimicrob Agents 32:519–524. doi: 10.1016/j.ijantimicag.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Natesan SK, Lamichchane AK, Swaminathan S, Wu W. 2013. Differential expression of ATP-binding cassette and/or major facilitator superfamily class efflux pumps contributes to voriconazole resistance in Aspergillus flavus. Diagn Microbiol Infect Dis 76:458–463. doi: 10.1016/j.diagmicrobio.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Sun Y, Chen W, Liu W, Wan Z, Bu D, Li R. 2012. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob Agents Chemother 56:2598–2603. doi: 10.1128/AAC.05477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J Clin Microbiol 49:586–590. doi: 10.1128/JCM.02136-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiser DM, Pitt JI, Taylor JW. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc Natl Acad Sci U S A 95:388–393. doi: 10.1073/pnas.95.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard; 2nd ed CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.EUCAST. 2008. EUCAST definitive document E.DEF 9.1: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/4ESCMID_Library/3Publications/EUCAST_Documents/Other_Documents/EUCAST_moulds_DEFINITIVE_document_V_ISO_April_08%20final.pdf. [Google Scholar]
- 21.Shivaprakash MR, Geertsen E, Chakrabarti A, Mouton JW, Meis JF. 2011. In vitro susceptibility of 188 clinical and environmental isolates of Aspergillus flavus for the new triazole isavuconazole and seven other antifungal drugs. Mycoses 54:e583–e589. doi: 10.1111/j.1439-0507.2010.01996.x. [DOI] [PubMed] [Google Scholar]
- 22.Rudramurthy SM, Chakrabarti A, Geertsen E, Mouton JW, Meis JF. 2011. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn Microbiol Infect Dis 71:370–377. doi: 10.1016/j.diagmicrobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Venselaar H, Te Beek TA, Kuipers RK, Hekkelman ML, Vriend G. 2010. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinformatics 11:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams LE, Wernegreen JJ. 2013. Sequence context of indel mutations and their effect on protein evolution in a bacterial endosymbiont. Genome Biol Evol 5:599–605. doi: 10.1093/gbe/evt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snelders E, Karawajczyk A, Verhoeven RJ, Venselaar H, Schaftenaar G, Verweij PE, Melchers WJ. 2011. The structure-function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: the mechanism of L98H azole resistance. Fungal Genet Biol 48:1062–1070. doi: 10.1016/j.fgb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Ma C, Fiorin G, Carnevale V, Wang T, Hu F, Lamb RA, Pinto LH, Hong M, Klein ML, DeGrado WF. 2011. Molecular dynamics simulation directed rational design of inhibitors targeting drug-resistant mutants of influenza A virus M2. J Am Chem Soc 133:12834–12841. doi: 10.1021/ja204969m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Purohit R. 2014. Use of long term molecular dynamics simulation in predicting cancer associated SNPs. PLoS Comput Biol 10:e1003318. doi: 10.1371/journal.pcbi.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan K, Senapati S. 2012. Understanding the basis of drug resistance of the mutants of αβ-tubulin dimer via molecular dynamics simulations. PLoS One 7:e42351. doi: 10.1371/journal.pone.0042351. [DOI] [PMC free article] [PubMed] [Google Scholar]


