Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a common pathogen associated with nosocomial pneumonia and is an increasing threat for severe community-acquired pneumonia. We have now investigated the role of interleukin-12 (IL-12) in protective immunity against lung infection with MRSA. The importance of IL-12 in protection from pulmonary MRSA infection was demonstrated by the finding that IL-12p35-deficient mice had a lower survival rate, higher bacterial burdens in lung and spleen, and decreased expression of interferon gamma (IFN-γ) in the lung compared to wild-type mice. These effects were completely reversed by replacement intranasal therapy with recombinant IL-12. Furthermore, exogenous IL-12 treatment of wild-type mice 24 h before pulmonary challenge with a lethal dose of MRSA significantly improved bacterial clearance and resulted in protection from death. The IL-12-treated mice had increased numbers of lung natural killer (NK) cells and neutrophils and higher levels of IFN-γ in the lung and serum compared to untreated mice. The major source of IL-12-driven IFN-γ expression in the lung was the NK cell, and the direct target of pulmonary IFN-γ was the lung macrophage, as shown using mice with a macrophage-specific defect in interferon gamma (IFN-γ) signaling (MIIG mice). Importantly, combination therapy with linezolid and IL-12 following intranasal MRSA infection significantly increased survival compared to that of mice receiving linezolid or IL-12 alone. These results indicate that IL-12-based immunotherapy may hold promise for treatment of MRSA pneumonia.
INTRODUCTION
Staphylococcus aureus is an extracellular Gram-positive bacterium that is a major cause of hospital-acquired pneumonia (1–4). More than 50% of Staphylococcus aureus isolates are resistant to various beta-lactam antibiotics and are generally termed methicillin-resistant S. aureus (MRSA). MRSA is an important cause of pneumonia and is responsible for several difficult-to-treat infections in humans. A 2002 to 2003 survey of 59 U.S. hospitals involving 4,543 patients with culture-positive pneumonia identified MRSA in 8.9% of community-acquired pneumonia cases, 26.5% of health care-associated pneumonia cases, 22.9% of hospital-acquired pneumonia cases, and 14.6% of ventilator-associated pneumonia cases (5). Furthermore, over the past 20 years, tertiary medical centers in the United States have experienced a dramatic increase in the percentage of staphylococcal infections caused by MRSA (6). Currently, two antimicrobial agents, linezolid and vancomycin, are approved by the U.S. Food and Drug Administration for the treatment of MRSA pneumonia (7, 8). Linezolid is now felt to be the optimal drug for treating patients with MRSA pneumonia (9). However, even with appropriate antibiotic treatment, MRSA infections in humans are often associated with poor clinical outcomes (3).
Interleukin-12 (IL-12) is a pivotal regulatory cytokine that preferentially activates natural killer (NK) and Th1 cells to produce interferon gamma (IFN-γ) (10). The importance of IFN-γ in phagocyte activation and protection against intracellular bacteria is well accepted, but little is understood about the significance of IL-12 and IFN-γ in protection against extracellular bacteria. Previously, we showed that intranasal (i.n.) administration of exogenous IL-12 provided IFN-γ-dependent protection against subsequent pulmonary infection with the intracellular bacterium Francisella tularensis (11) and the extracellular bacterium Streptococcus pneumoniae (12). In addition, we (11) and others (13) reported that IL-12-deficient mice were more susceptible to F. tularensis and S. pneumoniae lung infections. However, while IL-12 expression can be induced during S. aureus infection (14), IL-17 appears to play a more dominant role in staphylococcal infections and IL-12 is nonprotective, at least for acute disease involving brain abscesses and cutaneous infections (15, 16). In fact, it has been reported that IL-12 and IFN-γ are detrimental for controlling staphylococcal colonization (17) and for recovery from central nervous system (CNS) bacterial-induced inflammation (15). Nevertheless, IFN-γ is routinely given to patients with chronic granulomatous disease who have particular difficulty in killing S. aureus (but not S. pneumoniae).
Because of the apparent difference in the role of IL-12 and IFN-γ during infection with S. aureus compared to other bacteria, we have now investigated the role of IL-12 during MRSA pneumonia in mice and, surprisingly, found that IL-12p35−/− mice were highly sensitive to pulmonary MRSA infection. Furthermore, treatment of wild-type (WT) mice with exogenous IL-12 protected against subsequent MRSA lung infection by stimulating NK cell IFN-γ production. Combination therapy with IL-12 and linezolid greatly increased protective efficacy compared to that of linezolid alone and rescued the majority of infected mice from death.
MATERIALS AND METHODS
Mice.
C57BL/6 IL-12p35−/− and IFN-γ−/− mice were originally obtained from Jackson Laboratories (Bar Harbor, ME). C57BL/6 mice with macrophages insensitive to IFN-γ (MIIG) were generated at Cincinnati Children's Hospital Medical Center (18). The mice were maintained and bred at Albany Medical College. All mice were 7 to 9 weeks of age at the time of infection. Age- and sex-matched WT controls were used for all experiments employing knockout mice. All experimental procedures were in compliance with the guidelines of the institutional animal care and use committee.
Bacteria and MRSA lung infection.
MRSA strain USA300 was used in these studies and was purchased from the ATCC (strain BAA-1556). The bacteria were grown overnight in brain-heart infusion broth (Becton Dickinson) and were frozen at −80°C.
For infection, mice were anesthetized by intraperitoneal (i.p.) injection with 100 μl of xylazine (20 mg/ml) and ketamine (1 mg/ml) in phosphate-buffered saline (PBS). The anesthetized mice were then i.n. inoculated with 40 μl PBS containing MRSA. The bacteria used for infection were plated on BBL Trypticase soy agar with 5% sheep blood (TSA II) to confirm the CFU counts.
IL-12 treatment.
Mice were inoculated i.n. with 1 μg of murine recombinant IL-12 in 25 μl phosphate-buffered saline (PBS) containing 1% normal mouse serum 24 h before MRSA challenge. For combination therapy with antibiotic, the animals were treated i.n. with either 1 μg of IL-12 or PBS vehicle and subcutaneously (s.c.) with 1 mg of linezolid (Pfizer) 4 and 24 h after bacterial challenge.
Bacterial burden and cytokine analysis.
At 4 and 24 h after bacterial infection, mice were sacrificed and their lungs, spleens, and blood were harvested. The organs were homogenized in 2 ml of PBS. Serial dilutions of the samples were inoculated on Trypticase soy agar plates and incubated for 24 h at 37°C to enumerate CFU. For cytokine analysis, organ homogenates and blood were centrifuged at 10,000 × g for 10 min. Supernatants were collected and stored at −80°C. IFN-γ, IL-6, and tumor necrosis factor alpha (TNF-α) levels were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available ELISA kits (BD Biosciences). ELISA was performed according to the manufacturer's instructions. All samples and standards were measured in duplicate.
Flow cytometric analysis of lung cells.
Mice were infected i.n. and sacrificed at 4 and 24 h postchallenge. Lungs were harvested, digested with collagenase D (Sigma) for 30 min at 37°C, and prepared as a single cell suspension by passing the cells through a 40-μm-pore-size nylon filter (BD Falcon). Cells were incubated with 1 μg/ml of anti-mouse FcγRII/III monoclonal antibody (MAb; clone 2.4G2) to block nonspecific staining and then incubated with 1 μg/ml of fluorescence-labeled antibodies. The MAbs used for flow cytometry included the following: fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (clone XMG1.2; eBioscience), FITC-anti-CD19 (clone CD6D5; Southern Biotech), phycoerythrin (PE)-conjugated anti-F4/80 (clone BM4; eBioscience), PE-anti-NK1.1 (clone NK136; BD Biosciences), PE-anti-CD3 (clone 17A2; BD Biosciences), PE-Cy7-anti-Ly6G (clone 1A8; eBioscience), and allophycocyanin (APC)-anti-CD11c (clone HL3; BD Biosciences). Data were collected using a FACSCanto flow cytometer (Becton Dickinson) and were analyzed with FlowJo software (Tree Star). Cells were gated according to forward scatter and side scatter, and cell types were identified as follows: neutrophils, Ly6G+ CD11b+; dendritic cells (DCs), F4/80− CD11c+ CD11b+; alveolar macrophages (AMs), F4/80+ CD11c+ CD11b−; interstitial macrophages (IMs), F4/80+ CD11c+ CD11b+; natural killer (NK) cells, NK1.1+ CD3−; T cells, CD3+ CD19−; and B cells, CD19+ CD3−.
IFN-γ intracellular staining.
MRSA-specific IFN-γ-producing cells were induced in the pulmonary tract of mice by i.n. administration of 1 × 108 CFU MRSA. The lungs were harvested 24 h postinfection, and isolated cells were restimulated ex vivo by culturing 5 × 105 cells/well in 96-well plates with or without equal numbers of MRSA for 2 h, followed by a 1-h incubation with 10 μg/ml brefeldin A (Sigma). The cells were washed in PBS containing 2% fetal calf serum, and Fc receptors were blocked by incubation with mouse 2.4G2 anti-FcγRII/III MAb for 20 min at 4°C. The cells were then stained with FITC-conjugated anti-NK1.1 MAb (clone PK136; BioLegend), APC-conjugated anti-CD4 MAb (clone RM4-5; BD Biosciences), and PE-Cy7-conjugated anti-CD8 MAb (clone 53-6.7; BD Biosciences) MAbs. Dead cells were labeled with 7-aminoactinomycin D (eBioscience). This was followed by a 20-min incubation with BD fixation/permeabilization solution. The cells were washed twice with BD Perm/Wash buffer and stained for 30 min with PE-conjugated anti-IFN-γ (clone XMG1.2; BioLegend). After the final wash, the cells were suspended in 200 μl of PBS containing 2% bovine serum albumin (BSA). Stained cells were quantitated using a FACSCanto flow cytometer.
In vivo NK cell depletion.
Mice were injected i.p. with 400 μg of PK136 anti-NK1.1 MAb in 0.2 ml PBS on days −4 and −2 before infection using a previously described protocol (19, 20). As a control, irrelevant IgG2a was used in the same manner. Depletion of lung NK cells was confirmed by examination of NK1.1 and DX5 expression by flow cytometry. The treated mice were inoculated i.n. with 1 μg of IL-12 in 25 μl PBS on day −1. On day 0, the mice were infected i.n. with 1 × 108 CFU MRSA in 40 μl PBS. Twenty-four hours postinfection, bronchoalveolar lavage fluid (BALF) was obtained by lavaging the lung with 1 ml PBS; the lung tissues were homogenized in 2 ml of PBS, and samples were plated on Trypticase soy agar containing 5% sheep blood.
Statistical analysis.
All data were analyzed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Survival curves were analyzed by using a log rank test. Statistical significance for all other assays was evaluated by the Mann-Whitney U test. A P value of <0.05 was considered to be statistically significant.
RESULTS
IL-12p35-deficient mice have enhanced susceptibility to pulmonary MRSA infection.
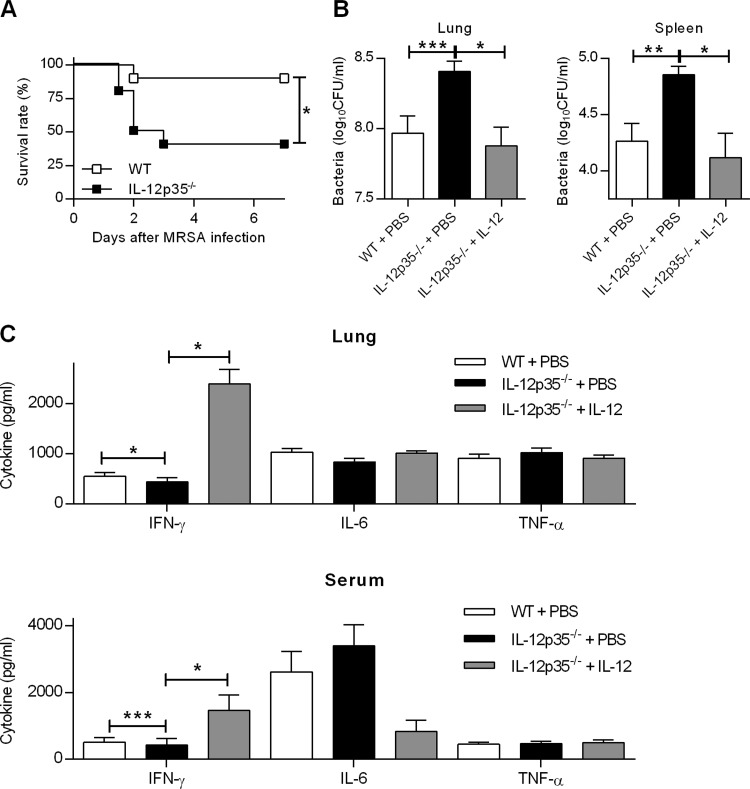
Little is understood about the protective or detrimental role of IL-12 during MRSA infection. To determine the contribution of IL-12 in pulmonary MRSA infection, we used an i.n. inoculation mouse model in which the 50% lethal dose (LD50) and the LD100 following i.n. infection of C57BL/6 WT mice were 1.5 × 108 and 2.5 × 108 CFU, respectively. WT and IL-12p35−/− mice were i.n. challenged with 108 bacteria and monitored for survival, bacterial burdens in lungs and spleens, and cytokine expression in lungs and sera. It was found that following MRSA infection, the survival rate of IL-12p35−/− mice was significantly lower than that of WT mice (Fig. 1A). Differences in survival were observed as early as 2 days following i.n. infection. In addition, 24 h after infection, bacterial loads in the lungs and spleens were approximately three times higher in IL-12p35−/− mice than those in WT mice (Fig. 1B). IL-12p35−/− mice exhibited lower levels of IFN-γ in their lungs and sera than WT mice (Fig. 1C). However, there were no significant differences in IL-6 or TNF-α levels between MRSA-infected IL-12p35−/− and WT mice. The defective bacterial clearance and cytokine expression in infected IL-12p35−/− mice was completely restored by i.n. administration of recombinant IL-12 (Fig. 1B and C). These results indicate that IL-12 plays a critical role in host defense against pulmonary MRSA infection.
FIG 1.
IL-12p35-deficient mice have increased susceptibility to respiratory MRSA infection. (A) WT and IL-12p35−/− mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle and then 24 h later were i.n. infected with 1 × 108 CFU of MRSA. Survival was monitored every 12 h for 7 days. Each group contained 10 mice, and the results of two independent experiments were pooled. *, P < 0.05 as determined by log rank test. Bacterial loads (B) and cytokine levels (C) in lung and spleen homogenates and in serum were determined 24 h after MRSA infection. Each bar represents the mean ± standard error of the mean (SEM) of the results from 4 to 19 mice/group. The data were pooled from three independent experiments. Statistical analysis was performed by using the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Exogenous IL-12 treatment protects against lethal MRSA infection.
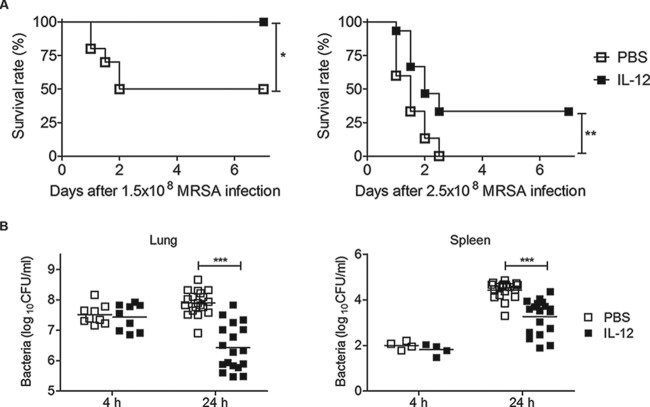
To determine the possible effect of exogenous IL-12 treatment on susceptibility to respiratory MRSA infection, IL-12 was inoculated i.n. into WT mice 24 h before i.n. bacterial challenge. Survival rates and bacterial loads were measured following inoculation of either 1.5 × 108 (the approximate LD50) or 2.5 × 108 CFU (LD100). Following inoculation with 1.5 × 108 CFU, all IL-12-treated mice survived, whereas control mice that instead received PBS vehicle had a 50% survival rate (Fig. 2A, left). Upon infection with 2.5 × 108 CFU, all vehicle-treated mice died within 3 days, whereas 33% of IL-12-treated mice survived (Fig. 2A, right). Preliminary experiments indicated that 1 μg of IL-12 given i.n. was optimal for protective efficacy with no observable toxicity (data not shown). In separate groups of mice, bacterial burdens in the lungs and spleens were determined 4 and 24 h postchallenge and found to be consistent with survival rates; i.e., bacterial burdens in the lungs and spleens of IL-12-treated mice were significantly lower than those in vehicle-treated mice (Fig. 2B). While no differences in CFU were observed at 4 h postinfection, pretreatment with IL-12 resulted in an approximately 50-fold reduction in lung CFU and a 7-fold reduction in spleen CFU at 24 h. Thus, administration of exogenous IL-12 resulted in greater bacterial clearance in MRSA-infected mice and significantly improved survival rates.
FIG 2.
IL-12 treatment improves protection against respiratory MRSA infection. WT mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle 24 h before i.n. MRSA infection. (A) Treated mice were challenged i.n. with either 1.5 × 108 (left) or 2.5 × 108 (right) CFU. Survival was monitored every 12 h for 7 days. Each group contained 10 to 15 mice, and the results of three independent experiments were pooled. *, P < 0.05; **, P < 0.01 as determined by a log rank test. (B) Lung and spleen bacterial levels 4 and 24 h after challenge i.n. with 1 × 108 CFU of MRSA. Each symbol represents an individual mouse, and the line indicates the mean. Data were pooled from three independent experiments. Statistical analysis was performed by using the Mann-Whitney U test. ***, P < 0.001.
IL-12 administration increases NK cell recruitment and IFN-γ levels in lungs of MRSA-infected mice.
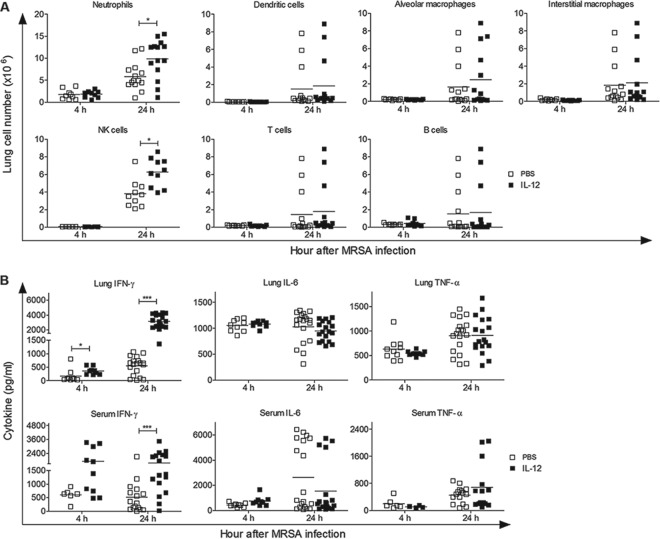
We next determined if pretreatment with IL-12 altered cell recruitment and/or cytokine production that may be required for effective antibacterial host defense. To address this, mice were administered either IL-12 or PBS vehicle i.n. 24 h prior to i.n. MRSA challenge, lungs and blood were harvested 4 and 24 h postinfection, and total cell populations and cytokine levels were quantitated. There were significantly greater numbers of NK cells and neutrophils in the lungs of IL-12-treated mice than in those of PBS-treated mice 24 h after bacterial challenge (Fig. 3A). However, there were no differences between PBS- and IL-12-treated mice in the numbers of dendritic cells, macrophages, or T and B cells recruited into the lungs 4 and 24 h after infection.
FIG 3.
Effect of IL-12 pretreatment on lung cell levels and cytokine production following MRSA challenge. WT mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle 24 h before i.n. infection with 1 × 108 CFU of MRSA. (A) Four hours and 24 h after infection, lungs were harvested and single cell suspensions were prepared. The cells were stained with fluorescent-conjugated antibodies and analyzed by flow cytometry as described in Materials and Methods. (B) Cytokine levels in lung homogenates and serum were determined by ELISA 4 and 24 h after infection. Each symbol represents an individual mouse, and the line indicates the mean. The data were pooled from three independent experiments. Statistical analysis was performed by using the Mann-Whitney U test. *, P < 0.05; ***, P < 0.001.
In addition, expression levels of IFN-γ in the lungs and sera were significantly higher in IL-12-treated mice than in PBS-treated mice, while the levels of IL-6 and TNF-α did not differ significantly between the two groups (Fig. 3B). Thus, the enhanced survival and bacterial clearance that are seen after MRSA infection of IL-12-treated mice are associated with greater NK cell and neutrophil recruitment and increased IFN-γ production.
NK cells are the major source of IFN-γ after IL-12 treatment.
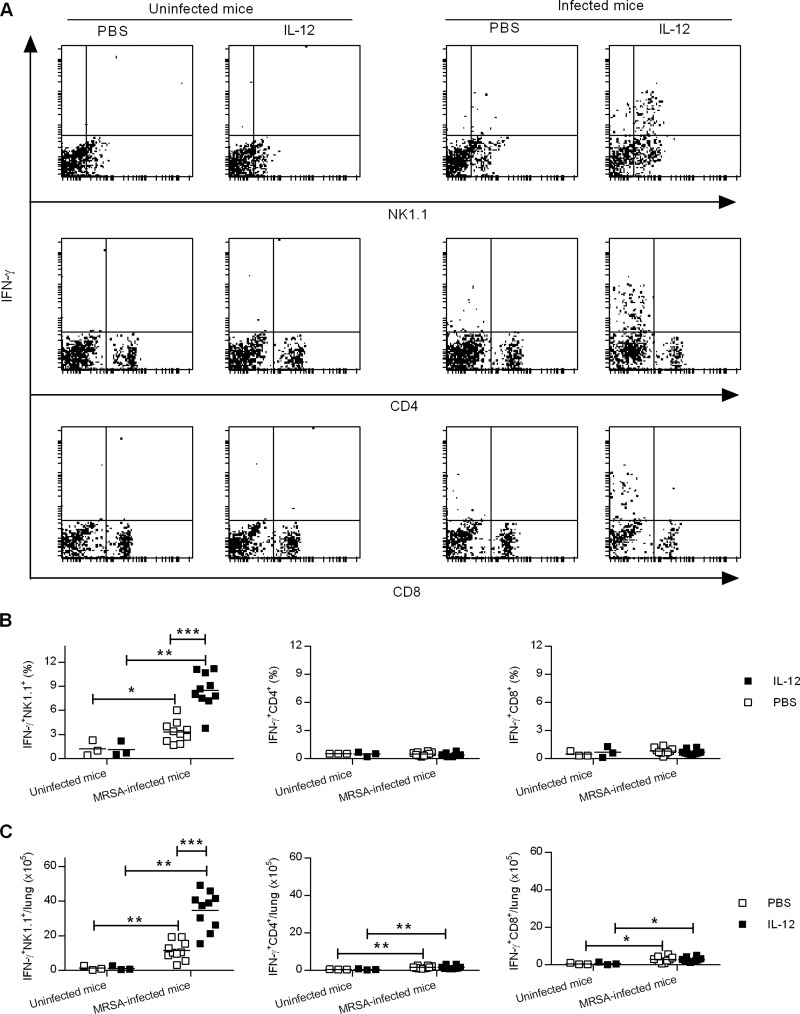
IFN-γ secretion by NK and T cells is critical for host defense against most bacterial infections (21). To elucidate the mechanism of IL-12-induced protection against pulmonary MRSA infection, we determined the lung cell population(s) responsible for IFN-γ production in response to i.n. IL-12 treatment. Single-cell suspensions were prepared before and 24 h after bacterial infection and analyzed by flow cytometry. Mice treated with IL-12 had significantly increased percentages and numbers of IFN-γ+ NK1.1+ cells, whereas IFN-γ+ CD4+ and IFN-γ+ CD8+ T cell populations were essentially comparable between IL-12- and PBS-treated mice (Fig. 4). These data suggest that IL-12 treatment provides protection through early recruitment of IFN-γ-producing NK cells.
FIG 4.
Intranasal IL-12 treatment induces IFN-γ production by NK cells. WT mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle 24 h before infection with 1 × 108 CFU of MRSA. Lung cells from uninfected mice and mice infected 24 h earlier were isolated and restimulated in vitro with MRSA for 2 h. They were then stained for surface NK1.1, CD4, and CD8 and for cytoplasmic IFN-γ, followed by flow cytometry analysis. (A) Representative dot plots from each experimental group. (B and C) Percentages (B) and numbers (C) of IFN-γ+ NK1.1+, IFN-γ+ CD4+, and IFN-γ+ CD8+ lung cells. Each symbol represents an individual mouse, and the line indicates the mean. The data were pooled from two independent experiments. Statistical analysis was performed by using the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-12-induced protection against MRSA infection is dependent upon NK cells and IFN-γ signaling in macrophages.
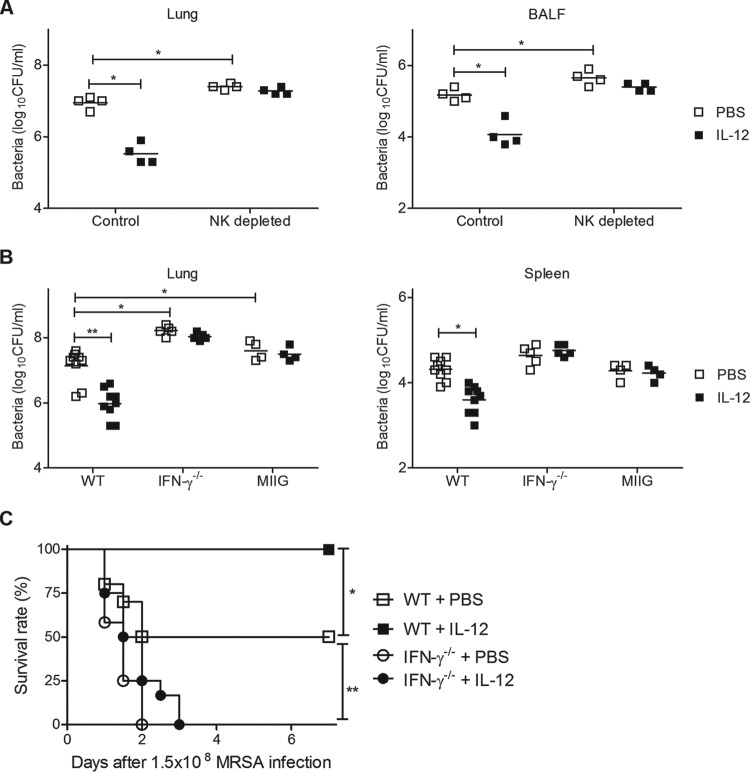
NK cells represent a major target cell type for IL-12 binding (10) and have been reported to play a critical protective role in host defense against pulmonary S. aureus infection (22). To elucidate the role of NK cells in IL-12-mediated protection, mice were pretreated i.n. with either IL-12 or PBS vehicle 24 h before MRSA challenge, and bacterial growth in NK cell-depleted mice was determined. IL-12 treatment again resulted in significantly decreased bacterial burdens in the lungs of isotype control-treated WT mice within 24 h postchallenge, but no decreases in CFU counts were observed following depletion of NK cells (Fig. 5A).
FIG 5.
The importance of NK cells and IFN-γ in IL-12-mediated protection. Mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle 24 h before infection with 1 × 108 CFU of MRSA. (A) Mice were treated with isotype control MAb or with anti-NK1.1 MAb to deplete NK cells and CFU in lungs, and BALF levels were determined 24 h after MRSA infection. (B) CFU were enumerated in the lungs and spleens of WT, IFN-γ−/−, and MIIG mice 24 h after infection. Each symbol represents the value for an individual mouse. The horizontal line is the mean for the group. Statistical analysis was performed by using the Mann-Whitney U test. (C) WT and IFN-γ−/− mice were treated i.n. with either 1 μg of IL-12 or PBS vehicle 24 h before infection with 1.5 × 108 CFU of MRSA. Survival was monitored every 12 h for 7 days. Each group contained 10 to 12 mice, and the results of two independent experiments were pooled. Statistical analysis was performed by using the log rank test.*, P < 0.05; **, P < 0.01.
We also examined the requirement for IFN-γ in IL-12-mediated protection using IFN-γ−/− mice as well as mice that express a truncated IFN-γ receptor in the CD68+ cell subset, with the result that IFN-γ signaling is specifically inhibited in macrophages; these mice are termed mice with macrophages insensitive to IFN-γ (MIIG) (18). The mice were inoculated i.n. with IL-12 or PBS vehicle 24 h before MRSA challenge. The decreases in bacterial burden observed in WT mice after IL-12 treatment were not seen in IFN-γ−/− or MIIG mice (Fig. 5B). In fact, lung bacterial burdens were significantly higher in IL-12-treated IFN-γ−/− mice than in PBS-treated WT mice. Furthermore, the protection from lethality seen in WT mice after IL-12 administration was not observed in IFN-γ−/− mice (Fig. 5C). Together, these results show that IFN-γ signaling in macrophages plays an essential role in mediating IL-12-induced resistance against pulmonary MRSA infection.
IL-12 and antibiotic combination treatment improves protection against MRSA infection in WT mice but not in IFN-γ−/− mice.
Linezolid is an oxazolidinone antibiotic that has broad activity against Gram-positive bacteria, including MRSA (23). In fact, recent studies have shown that linezolid is a better choice than other antibiotics for treatment of MRSA pneumonia (9). Nevertheless, even with appropriate antibiotic treatment, MRSA infection is still often associated with poor clinical outcome (3). To determine the effect of combined linezolid and IL-12 treatment on protection against respiratory MRSA infection, WT and IFN-γ−/− mice were challenged i.n. with a high dose of MRSA (5 × 108 CFU) and treated 4 and 24 h later with IL-12 or PBS vehicle with or without linezolid. All untreated mice died within 24 h of infection, and no protection was observed using IL-12 alone (Fig. 6), indicating that the efficacy of IL-12 seen in our previous experiments described above required prophylactic IL-12 treatment and was not effective even 4 h after initiation of bacterial infection. Antibiotic alone was similarly marginally protective, with a survival rate of only 14%. However, combined therapeutic administration of linezolid and IL-12 improved the survival rates of infected WT mice to 71%. No protection was observed in IFN-γ−/− mice. These results indicate that a combination of IL-12 and linezolid treatment following MRSA infection can significantly improve survival compared to treatment with either agent alone.
FIG 6.
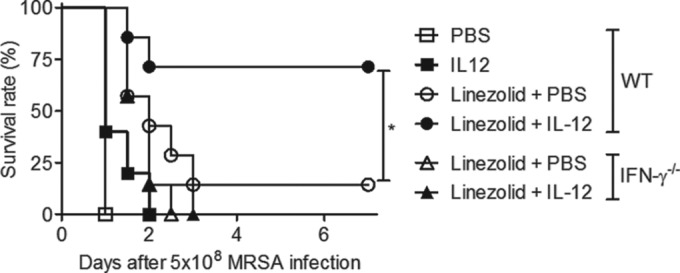
Combination therapy with IL-12 and linezolid improves survival following MRSA pneumonia. WT and IFN-γ−/− mice were challenged i.n. with 5 × 108 CFU of MRSA and were then treated i.n. 4 and 24 h later with either PBS vehicle, IL-12 alone, linezolid alone, or a combination of IL-12 and linezolid. Survival of infected mice was monitored every 12 h for 7 days. Each group contained 7 to 10 mice, and the results of two independent experiments were pooled. *, P < 0.05 as determined by a log rank test.
DISCUSSION
The importance of IL-12 during bacterial infections has been extensively investigated (11–13, 24), including in S. aureus bacterial colonization (16, 17) and S. aureus-induced brain abscesses (15). In the two cases, IL-12 was found to be either nonprotective or actually detrimental for resistance to S. aureus infection. However, the potential role of IL-12 in host defense against MRSA pneumonia has remained only poorly understood. In the present study, we used IL-12p35−/− mice and WT mice treated with exogenous IL-12 and surprisingly found that IL-12 was critical for survival from pulmonary MRSA infection. Protective efficacy was dependent upon IFN-γ production by NK cells and correlated with expression of functional IFN-γ receptor (IFN-γR) signaling on phagocytic cells. Finally, IL-12 given prophylactically was effective but lost efficacy when administered therapeutically, and antibiotic treatment of infected mice was similarly only minimally effective. However, combined therapeutic administration of IL-12 and antibiotic following bacterial infection allowed the majority of infected mice to survive. Thus, IL-12 can be a potential adjunctive treatment for prevention of pulmonary MRSA-induced mortality.
The conclusions that IL-12 is necessary for survival from respiratory infection with MRSA and that IL-12-mediated protection is dependent on IFN-γ expression are based on multiple lines of evidence. First, the absence of IL-12p35 resulted in significant increases in bacterial loads and reduced survival. IL-12p35−/− mice also had lower pulmonary IFN-γ levels than WT mice. The reduced bacterial clearance and IFN-γ production seen in infected IL-12p35−/− mice were completely restored by i.n. administration of exogenous IL-12. Second, administration of IL-12 to WT mice significantly decreased MRSA bacterial loads and prevented death of the infected mice. IL-12 treatment also significantly increased expression of IFN-γ-producing NK cells in the lungs. Protection by IL-12 was not observed in the absence of NK cells or IFN-γ. These results are consistent with previous investigations with other bacterial infectious agents, which similarly showed that exogenous IL-12 treatment can protect naive mice against pulmonary infection with S. pneumoniae, F. tularensis, and Mycobacterium tuberculosis (11–13, 24). Finally, combination treatment with IL-12 and the linezolid antibiotic improved protection against MRSA pneumonia.
We found that IL-12 increased pulmonary clearance of MRSA by promoting NK cell and neutrophil accumulation. Of note, we did not observe enhanced bacterial clearance in NK cell-depleted mice given IL-12. These results indicate that NK cells play an essential role in mediating IL-12-induced resistance against pulmonary MRSA infection. Several recent studies have shown that NK cells and neutrophils have major roles in protection against respiratory microbes such as S. pneumoniae and MRSA (12, 22, 25, 26). We previously reported that IL-12 promotes IFN-γ-dependent neutrophil recruitment into the lungs of mice after i.n. infection with S. pneumoniae (12); others have likewise found an involvement of NK cells after infection of mice with Bordetella pertussis (27).
Administration of IL-12 enhanced production of IFN-γ by NK cells but not by CD4+ or CD8+ T cells. This observation was consistent with our previous findings showing the presence of large numbers of IFN-γ-secreting NK cells in the lungs of IL-12-treated mice after i.n. infection with F. tularensis or S. pneumoniae (12, 28, 29). Our data also demonstrated the critical role of IFN-γ in IL-12-mediated protection against pulmonary MRSA. While the role of IFN-γ in protection against MRSA infection is poorly understood, it has been shown to be important in the clearance of pulmonary infections with S. pneumoniae, F. tularensis, and Pseudomonas aeruginosa (11, 30, 31). Finally, we showed that expression of functional IFN-γRs on phagocytic cells was critical for IL-12-induced protection, which is consistent with previous findings showing that IFN-γ treatment can increase killing of MRSA by human monocytes (32, 32). Indeed, our preliminary data have demonstrated that i.n. IL-12 treatment can enhance in vivo phagocytosis of MRSA in a mouse model (Q. T. Nguyen, Y. Furuya, and D. W. Metzger, unpublished data). Taken together, our results suggest that IL-12 induces a cascade of events beginning with NK cell recruitment and activation, followed by IFN-γ production. IFN-γ then binds to macrophages in the lungs and initiates effective killing of MRSA by these cells.
Although IL-12 was effective when administered prophylactically before initiation of infection, it was not protective when inoculated as early as 4 h after bacterial challenge. Nevertheless, the therapeutic use of IL-12 in combination with conventional antibiotics has shown promise against a variety of pathogens. Indeed, we found that combination therapy with IL-12 and linezolid improved protection against MRSA pneumonia. MRSA is a bacterium responsible for several difficult-to-treat infections in humans. Linezolid is broadly active against Gram-positive bacteria, including drug-resistant strains. This antibiotic inhibits bacterial protein synthesis by preventing formation of the 70S initiation complex and is now felt to be the drug of choice for treating patients with MRSA pneumonia (9). Nevertheless, MRSA infections in humans are associated with poor clinical outcomes even when appropriate antibiotic therapies are initiated (3). Previous investigators have reported that combination therapy with IL-12 and antibiotics, such as gentamicin, fluconazole, or clarithromycin-rifabutin, can promote the clearance of Mycobacterium avium (33), F. tularensis (34), and Cryptococcus neoformans (35) more effectively than therapy with any of the agents alone. In addition, combination therapy with IL-12 and ampicillin is a potentially useful modality for the treatment of open fracture-associated infections (36). Although the mechanisms underlying the efficacy of combined IL-12 and linezolid treatment remain to be elucidated, our findings suggest that this strategy is worthy of further investigation for improving the management of this important opportunistic pathogen in MRSA-infected patients.
In conclusion, our results show that exogenous IL-12 can increase protection prophylactically against respiratory MRSA infection. It is interesting that exogenous IL-12 can protect against S. aureus and S. pneumoniae pulmonary infections through activation of macrophages, since the type 1-associated pathway has been reported to be detrimental for resistance to staphylococcal infections at other sites and since staphylococcal but not pneumococcal infections are controlled primarily by IFN-γ-enhanced oxidative killing by neutrophils as shown by the clinical experience in chronic granulomatous disease patients. Treatment with exogenous IL-12 protected against pulmonary MRSA infection by enhancing NK cell-derived IFN-γ production, which by the use of MIIG mice was found for the first time to directly stimulate lung macrophage antibacterial activity. An additional novel finding in our study was that IL-12 can be used therapeutically in combination with antibiotics to aid in the treatment of MRSA pneumonia.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (grant R01 AI41715).
We thank the Center for Immunology and Microbial Disease Immunology Core laboratory for technical assistance.
We declare no conflicts of interest.
REFERENCES
- 1.Bradley SF. 2002. Staphylococcus aureus infections and antibiotic resistance in older adults. Clin Infect Dis 34:211–216. doi: 10.1086/338150. [DOI] [PubMed] [Google Scholar]
- 2.DeRyke CA, Lodise TP Jr, Rybak MJ, McKinnon PS. 2005. Epidemiology, treatment, and outcomes of nosocomial bacteremic Staphylococcus aureus pneumonia. Chest 128:1414–1422. doi: 10.1378/chest.128.3.1414. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 6.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, Talan DA. 2012. Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 54:1126–1133. doi: 10.1093/cid/cis022. [DOI] [PubMed] [Google Scholar]
- 7.Pletz MW, Burkhardt O, Welte T. 2010. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin? Comparison of pharmacology and clinical efficacy. Eur J Med Res 15:507–513. doi: 10.1186/2047-783X-15-12-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 34:1481–1490. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 9.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 11.Duckett NS, Olmos S, Durrant DM, Metzger DW. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun K, Salmon SL, Lotz SA, Metzger DW. 2007. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun 75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto N, Kawakami K, Kinjo Y, Miyagi K, Kinjo T, Uezu K, Nakasone C, Nakamatsu M, Saito A. 2004. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect 6:1241–1249. doi: 10.1016/j.micinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Fournier B, Philpott DJ. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 18:521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Held J, Preuße C, Döser A, Richter L, Heppner FL, Stenzel W. 2013. Enhanced acute immune response in IL-12p35−/− mice is followed by accelerated distinct repair mechanisms in Staphylococcus aureus-induced murine brain abscess. J Infect Dis 208:749–760. doi: 10.1093/infdis/jit126. [DOI] [PubMed] [Google Scholar]
- 16.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol 11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satorres SE, Alcaráz LE, Cargnelutti E, Di Genaro MS. 2009. IFN-gamma plays a detrimental role in murine defense against nasal colonization of Staphylococcus aureus. Immunol Lett 123:185–188. doi: 10.1016/j.imlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, Karp CL, Aliberti J, Flick MJ, Jordan MB. 2010. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol 184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul-Careem MF, Lee AJ, Pek EA, Gill N, Gillgrass AE, Chew MV, Reid S, Ashkar AA. 2012. Genital HSV-2 infection induces short-term NK cell memory. PLoS One 7:e32821. doi: 10.1371/journal.pone.0032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnbach C, Daws MR, Niemi EC, Nakamura MC. 2001. Immune rejection of a large sarcoma following cyclophosphamide and IL-12 treatment requires both NK and NK T cells and is associated with the induction of a novel NK T cell population. J Immunol 167:2569–2576. doi: 10.4049/jimmunol.167.5.2569. [DOI] [PubMed] [Google Scholar]
- 21.Schroder K, Hertzog PJ, Ravasi T, Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 22.Small CL, McCormick S, Gill N, Kugathasan K, Santosuosso M, Donaldson N, Heinrichs DE, Ashkar A, Xing Z. 2008. NK cells play a critical protective role in host defense against acute extracellular Staphylococcus aureus bacterial infection in the lung. J Immunol 180:5558–5568. doi: 10.4049/jimmunol.180.8.5558. [DOI] [PubMed] [Google Scholar]
- 23.Leach KL, Brickner SJ, Noe MC, Miller PF. 2011. Linezolid, the first oxazolidinone antibacterial agent. Ann N Y Acad Sci 1222:49–54. doi: 10.1111/j.1749-6632.2011.05962.x. [DOI] [PubMed] [Google Scholar]
- 24.Cooper AM, Magram J, Ferrante J, Orme IM. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvy BA, Harmsen AG. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499–512. doi: 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. 2004. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Byrne P, McGuirk P, Todryk S, Mills KH. 2004. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol 34:2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- 28.Furuya Y, Kirimanjeswara GS, Roberts S, Metzger DW. 2013. Increased susceptibility of IgA-deficient mice to pulmonary Francisella tularensis live vaccine strain infection. Infect Immun 81:3434–3441. doi: 10.1128/IAI.00408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez MC, Duckett NS, Baron SD, Metzger DW. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol 232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Rubins JB, Pomeroy C. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect Immun 65:2975–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol 159:2858–2866. [PubMed] [Google Scholar]
- 32.DeForge LE, Billeci KL, Kramer SM. 2000. Effect of IFN-gamma on the killing of S. aureus in human whole blood. Assessment of bacterial viability by CFU determination and by a new method using alamarBlue. J Immunol Methods 245:79–89. [DOI] [PubMed] [Google Scholar]
- 33.Doherty TM, Sher A. 1998. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol 160:5428–5435. [PubMed] [Google Scholar]
- 34.Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale JM, Arulanandam BP. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother 48:4513–4519. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemons KV, Brummer E, Stevens DA. 1994. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother 38:460–464. doi: 10.1128/AAC.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyce BM, Lindsey BA, Clovis NB, Smith ES, Hobbs GR, Hubbard DF, Emery SE, Barnett JB, Li B. 2012. Additive effects of exogenous IL-12 supplementation and antibiotic treatment in infection prophylaxis. J Orthop Res 30:196–202. doi: 10.1002/jor.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]