Abstract
The spread of antibiotic resistance among Gram-negative bacteria is a serious clinical threat, and infections with these organisms are a leading cause of mortality worldwide. Traditional novel drug development inevitably leads to the emergence of new resistant strains, rendering the new drugs ineffective. Therefore, reviving the therapeutic potentials of existing antibiotics represents an attractive novel strategy. Novicidin, a novel cationic antimicrobial peptide, is effective against Gram-negative bacteria. Here, we investigated novicidin as a possible antibiotic enhancer. The actions of novicidin in combination with rifampin, ceftriaxone, or ceftazidime were investigated against 94 antibiotic-resistant clinical Gram-negative isolates and 7 strains expressing New Delhi metallo-β-lactamase-1. Using the checkerboard method, novicidin combined with rifampin showed synergy with >70% of the strains, reducing the MICs significantly. The combination of novicidin with ceftriaxone or ceftazidime was synergistic against 89.7% of the ceftriaxone-resistant strains and 94.1% of the ceftazidime-resistant strains. Synergistic interactions were confirmed using time-kill studies with multiple strains. Furthermore, novicidin increased the postantibiotic effect when combined with rifampin or ceftriaxone. Membrane depolarization assays revealed that novicidin alters the cytoplasmic membrane potential of Gram-negative bacteria. In vitro toxicology tests showed novicidin to have low hemolytic activity and no detrimental effect on cell cultures. We demonstrated that novicidin strongly rejuvenates the therapeutic potencies of ceftriaxone or ceftazidime against resistant Gram-negative bacteria in vitro. In addition, novicidin boosted the activity of rifampin. This strategy can have major clinical implications in our fight against antibiotic-resistant bacterial infections.
INTRODUCTION
Bacterial infections remain one of the leading causes of death worldwide. The ever-escalating problem of antibiotic resistance leads to the redundancy of many antibiotics, resulting in increased morbidity and mortality in both developed and developing countries. In particular, the effectiveness of antimicrobial agents against Gram-negative pathogens, for example, Enterobacteriaceae, are being compromised at an alarming rate (1).
Bacteria in the Enterobacteriaceae family cause an arsenal of serious infections, including pneumonia, wound infections, meningitis, urinary tract infections, intra-abdominal infections (1), and nosocomial bacteremia (2). Extended-spectrum β-lactamase (ESBL)-producing strains now predominate in many areas, conferring resistance to cephalosporins and remaining sensitive only to carbapenems and the older, more toxic polymyxin antibiotics such as colistin (3). Furthermore, since 2007, infections with New Delhi metallo-β-lactamase 1 (NDM-1)-producing “superbugs'” have emerged. For these infections, virtually all antibiotics, including carbapenems, are ineffective. Most NDM-1 strains are usually susceptible only to “last line” drugs such as colistin, which exhibits nephro- and neurotoxicity (4), and the bacteriostatic glycylcycline tigecycline (5). The most optimal strategy to overcome resistant infections is to use novel antimicrobial agents. However, the traditional strategy of antibiotic discovery cannot maintain pace with the high rate of resistance emergence and resistance occurs just a few years after market release (6). In addition, the discovery of novel antibiotics is costly and arduous, which means producing large numbers of antibiotic classes within a short period of time is extremely challenging (7–9).
Reviving the potency of existing antibiotics by combining them with novel agents is an extremely desirable strategy to tackle resistance (10). Antimicrobial peptides, in particular those targeting the bacterial cell envelope, have been shown to synergize with conventional antibiotics (11). The dual action of weakening of the cell envelope and increasing permeability may allow the intracellular antibiotic concentration to reach a lethal level, which is unachievable by the antibiotic alone. Furthermore, the use of multiple agents in combination may reduce or retard the emergence of resistance to the individual antimicrobial components (10, 12).
It has been suggested that novicidin, a novel 18-residue cationic antimicrobial peptide, acts by inserting itself into the head group region of the selectively targeted bacterial membrane bilayer. This subsequently causes membrane perturbation and transient pore formation and is bactericidal via the resulting leakage of bacterial cell contents (13–15). Significant antimicrobial effects have been noted with several Gram-negative organisms, such as Escherichia coli and Salmonella enterica (15). Novicidin was developed from ovispirin, which in turn originated from an ovine cathelicidin known as sheep myeloid antimicrobial peptide 29 (SMAP-29) (13). This allowed for the construction of a peptide more suitable for use as a therapeutic agent (14).
In this study, we aimed to investigate the effects of novicidin in combination with conventional antibiotics, namely, rifampin and the extended-spectrum cephalosporins, ceftriaxone and ceftazidime, against 101 Gram-negative strains, including resistant E. coli and bacteria in the Klebsiella-Enterobacter-Serratia (KES) group. In addition, investigations were carried out to determine the mechanism of action, hemolytic activity, and cytotoxicity of novicidin.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used were 94 antibiotic-resistant Gram-negative clinical isolates, including 61 E. coli and 33 isolates in the KES group from St George's Hospital, London, England. In addition, 7 strains with the blaNDM-harboring plasmid were used: ATCC BAA-2468, BAA-2469, BAA-2470, BAA-2471, BAA-2472, and BAA-2473 and NCTC 13443. Strain ATCC BAA-2468 is identified as Enterobacter cloacae, strains ATCC BAA-2469 and BAA-2471 are identified as E. coli, and strains ATCC BAA-2470, BAA-2472, BAA-2473 and NCTC 13443 are identified as Klebsiella pneumoniae. Bacterial strains were grown in nutrient broth no. 2 (Oxoid, United Kingdom) and on tryptone soya agar plates (Oxoid). The antibiotics used were as follows: rifampin (Sanofi), ceftriaxone (Stravencon), ceftazidime (Wockhardt), cefixime (Suprax), and cefotaxime (Reig Jofre). Antibiotics were prepared in water or the provided solvent to an appropriate concentration. Novicidin was kindly provided by Novozymes A/S, Denmark.
In vitro susceptibility to novicidin and antibiotics.
The MICs of novicidin, rifampin, ceftriaxone, ceftazidime, cefixime, and cefotaxime for the 101 strains were calculated by using the broth microdilution method. The MIC for each agent was identified as the lowest concentration required to inhibit bacterial growth. The MIC50 and MIC90 values were calculated as the lowest concentrations required to inhibit growth in 50 and 90% of the strains, respectively.
Checkerboard assays to measure combination effects of novicidin and antibiotics.
The checkerboard assay method was used for the measurement of combination effects of novicidin with the antibiotics. Combinations of two drugs were prepared in 96-well plates (Fisher Scientific UK) using drug concentrations starting from 2-fold higher than their MIC values and then serially diluted in a 2-fold manner. After the addition of a log-phase bacterial inoculum of 1 × 105 to 5 × 105 CFU/ml, plates were incubated at 37°C for 24 h and then read using an ELx800 absorbance microplate reader (Bio-Tek). The effects of the combinations were examined by calculating the fractional inhibitory concentration index (FICI) of each combination as follows: [(MIC of drug A, tested in combination)/(MIC of drug A, tested alone)] + [(MIC of drug B, tested in combination)/(MIC of drug B, tested alone)]. The profile of the combination was defined as synergistic if the FICI was ≤0.5, indifferent if the FICI was >0.5 but ≤4.0 and antagonistic if the FICI was >4 (16).
Time-kill curves of antibiotics alone and in combination with novicidin.
Twofold serial drug dilutions were prepared, added to a 96-well plate alone and in combination, and incubated at 37°C with a log-phase bacterial inoculum of 1 × 107 to 5 × 107 CFU/ml. After 0, 1, 2, 4, 7, and 24 h of incubation, viability expressed as CFU/ml was determined by plating 100 μl of serial dilutions onto tryptone soy agar (Oxoid) plates, followed by incubation at 37°C for 24 h. The colonies were counted using an aCOLyte colony counter (Synbiosis) and analyzed using the accompanying software. Synergistic activity was defined as a ≥2-log10 decrease in CFU counts at 24 h of the combination compared to the most effective single agent, in addition to a ≥2-log10 decrease compared to the 0-h count. Indifference was defined as a ≤1-log10 change in CFU counts, and antagonism was defined as a ≥2-log10 increase in CFU at 24 h, of the combination compared to the most effective single agent (17).
Measurement of bacterial cytoplasmic membrane potential.
The permeability of the bacterial cytoplasmic membrane after drug treatment was assessed using a fluorescence assay as previously described (18, 19). Log-phase cultures were washed twice and resuspended in a rejuvenating buffer (5 mM HEPES [pH 7.2], 20 mM glucose) to an optical density (OD) at 600 nm of 0.05. Membrane potential sensitive dye DiSC3 (3′3-dipropylthiadicarbocyanine iodide; Sigma) (5) was added to the resuspended cells to a final concentration of 0.4 μM, followed by incubation until a stable reduction in fluorescence was achieved as a result of DiSC3 (5) uptake and cell quenching due to an intact membrane. Then, 100 mM KCl was added to equilibrate the K+ ion concentration intra- and extracellularly. The bacterial cell suspension was added to a 96-well microtiter plate, followed by the addition of drugs in triplicate. Fluorescence was measured by using a GloMax-Multi+ microplate reader (Promega) at an excitation wavelength of 622 nm and an emission wavelength of 670 nm. Any drug-induced disruption of the cytoplasmic membrane resulted in an increase in measurable florescence.
PAE of antibiotics alone and in combination with novicidin.
Bacteria were cultured overnight at 37°C in nutrient broth. One milliliter of the culture was transferred to fresh nutrient broth medium containing single or combinatory drugs. For the single drugs, concentrations 2, 5, or 10-fold higher than MIC values of the drug were utilized. For the combinations, concentrations 5-fold higher than the minimal enhancement concentrations of both drugs were selected according to the checkerboard results. After 1 h of drug exposure, the cultures were washed three times to remove the antimicrobial agents. The bacterial cells were resuspended into nutrient broth and grown at 37°C with continuous shaking at 100 rpm. Bacterial viability was determined by CFU counting at 0, 1, 2, 3, 4, 6, and 8 h. The postantibiotic effect (PAE) was calculated as follows: PAE = T − C, where T is the time taken for drug exposed culture to increase by 1-log CFU counts, and C is the time taken for control culture to increase by 1-log CFU counts (20).
Ex vivo hemolysis assay.
A venous blood sample from a male human donor was collected shortly before testing. Aliquots (10 μl) of the heparinized blood were added to 0.5 ml of saline solution (0.9% NaCl) containing different concentrations of novicidin in triplicate. After 1 h of incubation at 37°C, the mixtures were centrifuged for 5 min at 5,000 × g to sediment the intact cells. The supernatants were isolated, and the absorbance values were measured at a wavelength of 545 nm. Hemolysis of novicidin was analyzed against negative (0% lysis) and positive controls (100% lysis) to calculate the percentage of hemolyzed cells, using the formulae as follows: hemolysis = (ODtest − ODnegative control)/(ODpositive control − ODnegative control) × 100. An ethics approval (H-D-2007-0055) was obtained from Danish National Committee on Health Research Ethics for using human blood.
Assessment of cytotoxicity using neutral red uptake assay.
To assess the effects of cytotoxicity of novicidin, the L929 mouse fibroblast cell line was utilized. Cells were grown in Eagle minimum essential medium with 10% fetal bovine serum to 80% confluence. Adherent cells were harvested and seeded at a concentration 5 × 105 cells per well into a 96-well microtiter plate, which was incubated for 24 h at 37°C. Different concentrations of novicidin were added to the cells, followed by incubation at 37°C for 24 and 72 h. Neutral red (25 mg/liter) was added posttreatment for 3 h at 37°C and removed by washing the cells twice with phosphate-buffered saline containing CaCl2/MgCl2. Intracellular neutral red was extracted using neutral red removal solution (50% ethanol, 1% acetic acid, and 49% water) for 15 min. Neutral red uptake was measured at 540 nm, and cell viability was determined as a percentage of the untreated control. Sodium dodecyl sulfate (SDS) was used as a positive control.
RESULTS
In vitro susceptibility to novicidin and the antibiotics.
The MICs for novicidin, rifampin, ceftriaxone, ceftazidime, cefixime, and cefotaxime were assessed for the 94 Gram-negative clinical isolates and 7 NDM-1 strains. As shown in Table 1, the MIC for novicidin for the 101 strains ranged from 1 to 8 mg/liter with an MIC50 and MIC90 at 2 and 4 mg/liter, respectively. The MIC for rifampin varied between 4 and >1,024 mg/liter. The MIC50 and MIC90 were 16 and 32 mg/liter, respectively. The MIC for ceftriaxone, ceftazidime, cefixime and cefotaxime ranged between 0.03125 and 2,048 mg/liter. The MIC50 and MIC90 were 1,024 and 2,048 mg/liter for ceftriaxone, 256 and 2,048 mg/liter for cefixime, 128 and 1,024 mg/liter for ceftazidime, and 512 and 2,048 mg/liter for cefotaxime, respectively.
TABLE 1.
MIC values for novicidin and antibiotics used in this study
| Antibiotic | MIC (mg/liter) |
||
|---|---|---|---|
| Range | MIC50 | MIC90 | |
| Novicidin | 1 to 8 | 2 | 4 |
| Rifampin | 4 to >1,024 | 16 | 32 |
| Ceftriaxone | 0.03125 to >2,048 | 1,024 | 2,048 |
| Cefixime | 0.03125 to >2,048 | 256 | 2,048 |
| Ceftazidime | 0.03125 to >2,048 | 128 | 1,024 |
| Cefotaxime | 0.03125 to >2,048 | 512 | 2,048 |
Checkerboard analysis of combination effects.
The combination effects of novicidin combined with rifampin, ceftriaxone, and ceftazidime were determined using the broth microdilution checkerboard assay against 94 clinical isolates and 7 NDM-1 strains. The FIC indices for the combinations are shown in Table 2. The combination of novicidin with rifampin was shown to have synergistic activity with >70% of both E. coli and isolates in KES group, with FIC indices between 0.018 and 0.5. In addition, the combination was shown to have synergistic effects with all 7 NDM-1 strains. Novicidin reduced the MIC of rifampin between 2- and 512-fold, with the majority of strains exhibiting 4- or 8-fold reductions in MIC values (see Tables S1, S2, and S3 in the supplemental material). Novicidin combined with ceftriaxone showed synergy with 57.4% of the E. coli strains and 69.7% of isolates in KES group. The combination of novicidin with ceftazidime presented synergy with 63.9% of the E. coli strains and 78.8% of isolates in KES group. The FIC indices for the NDM-1 strains were unable to be determined since the MICs for ceftriaxone and ceftazidime were higher than the maximum achievable checkerboard concentration of 2,048 mg/liter. As shown in Table 3, synergistic activities were demonstrated in the majority (89.7%) of the ceftriaxone-resistant strains compared to a minority of the ceftriaxone-sensitive strains (16.7%). A similar pattern was observed with the novicidin and ceftazidime combination, whereby synergy was seen in 94.1% of resistant strains compared to 3.8% of sensitive strains. Novicidin reduced the MIC of ceftriaxone or ceftazidime from 2- to >2,048-fold (see Tables S1 and S2 in the supplemental material).
TABLE 2.
Combination activity of novicidin with rifampin, ceftriaxone, and ceftazidime against the 101 Gram-negative Enterobacteriaceae strains
| Strain or isolate type | Combination activity | FICI | Total no. (%) of strains with activity when novicidin was combined with |
||
|---|---|---|---|---|---|
| Rifampin | Ceftriaxone | Ceftazidime | |||
| E. coli strains | Synergy | ≤0.5 | 43 (70.5) | 35 (57.4) | 39 (63.9) |
| Indifferent | >0.5, <4 | 18 (29.5) | 26 (42.6) | 22 (36.1) | |
| Antagonism | ≥4 | 0 | 0 | 0 | |
| KES isolates | Synergy | ≤0.5 | 28 (84.8) | 23 (69.7) | 26 (78.8) |
| Indifferent | >0.5, <4 | 5 (15.2) | 10 (30.3) | 7 (21.2) | |
| Antagonism | ≥4 | 0 | 0 | 0 | |
| NDM-1 strains | Synergy | ≤0.5 | 7 (100) | ||
| Indifferent | >0.5, <4 | 0 | |||
| Antagonism | ≥4 | 0 | |||
TABLE 3.
Combination activity of novicidin with ceftriaxone and ceftazidime against the 94 Gram-negative clinical isolates
| Strain type (no. of strains) | Total no. (%) of strains with activity when novicidin was combined with ceftriaxone and ceftazidime |
||
|---|---|---|---|
| Synergy (FICI ≤ 0.5) | Indifferent (FICI > 0.5 and < 4) | Antagonism (FICI ≥ 4) | |
| Ceftriaxone-resistant strains (58) | 52 (89.7) | 6 (10.3) | 0 |
| Ceftriaxone-sensitive strains (36) | 6 (16.7) | 30 (83.3) | 0 |
| Ceftazidime-resistant strains (68) | 64 (94.1) | 4 (5.9) | 0 |
| Ceftazidime-sensitive strains (26) | 1 (3.8) | 25 (96.2) | 0 |
Time-kill assays confirmed synergy of novicidin combined with rifampin, ceftriaxone, or ceftazidime.
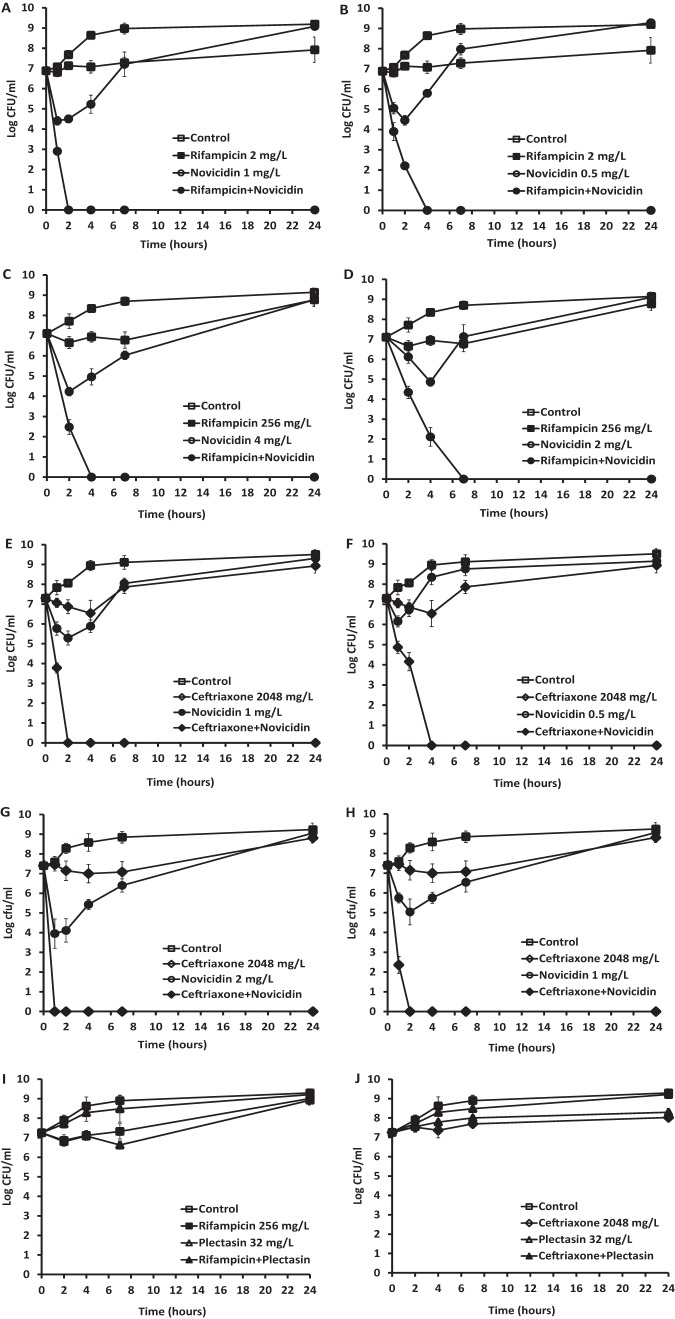
Time-kill assays were performed to examine the activities of novicidin in combination with rifampin, ceftriaxone, and ceftazidime against five strains of E. coli and KES group clinical strains, which represented an FICI of <0.5 for each drug combination. The combination of rifampin and novicidin was also tested against the seven NDM-1 strains. A range of different concentrations was tested according to checkerboard analysis, and the most effective and synergistic activities are shown. As shown in Fig. 1, rifampin at 2 mg/liter (Fig. 1A and B) and at 256 mg/liter (Fig. 1C and D) failed to reduce the viability of the clinical isolate and the NDM-1 E. coli, and novicidin at 1 or 0.5 mg/liter (Fig. 1A and B) and at 4 or 2 mg/liter (Fig. 1C and D) showed an initial killing of the bacteria, but regrowth was seen. However, when rifampin at 2 mg/liter was combined with novicidin at 1 (Fig. 1A) or 0.5 (Fig. 1B) mg/liter, 100% killing of the E. coli cells was achieved at 2 and 4 h posttreatment, respectively. Similarly, when rifampin at 256 mg/liter was combined with novicidin at 4 (Fig. 1C) and 2 (Fig. 1D) mg/liter, complete killing of the NDM-1 E. coli was achieved at 4 and 7 h posttreatment, respectively. There were significant differences in the reduction of CFU counts between the combination of novicidin with rifampin and each of the single-drug (rifampin or novicidin) treatments (P < 0.0001).
FIG 1.
Time-kill analysis showing the effects of novicidin in combination with rifampin and ceftriaxone against antibiotic-resistant E. coli strains. The peptide and antibiotics alone, or each combined with novicidin, were added to the bacterial cultures, and CFU counts were carried out at different time points. (A and B) Combination of rifampin at 2 mg/liter and novicidin at 1 mg/liter (A) or 0.5 mg/liter (B) against a clinical isolate of E. coli. (C and D) Combination of rifampin at 256 mg/liter and novicidin at 4 mg/liter (C) or 2 mg/liter (D) against an NDM-1 E. coli strain. (E and F) Combination of ceftriaxone at 2,048 mg/liter and novicidin at 1 mg/liter (E) or 0.5 mg/liter (F) against a clinical isolate of E. coli. (G and H) Combination of ceftriaxone at 2,048 mg/liter and novicidin at 2 mg/liter (G) or 1 mg/liter (H) against a clinical isolate of the KES group. (I and J) Negative controls were a combination of plectasin at 32 mg/liter with rifampin at 256 mg/liter against an NDM-1 E. coli isolate (I) and a combination of plectasin at 32 mg/liter with ceftriaxone at 2,048 mg/liter against a clinical isolate of the KES group (J). These results are means and standard deviations (SD) from two independent experiments.
Novicidin and ceftriaxone combinations were tested against ceftriaxone-resistant E. coli and KES group clinical isolates. As seen in Fig. 1E, F, G, and H, ceftriaxone at 2,048 mg/liter was unable to reduce the CFU counts of both strains. However, when novicidin was added in the culture at 1 or 0.5 mg/liter and at 2 or 1 mg/liter, the bacterial cells were rapidly killed, showing 100% reduction in the CFU count at 2 or 4 h posttreatment for the E. coli isolate, respectively (Fig. 1E and F), and at 1 or 2 h posttreatment for the KES group strain (Fig. 1G and H), respectively, demonstrating significant synergy. There were significant differences in the reduction of CFU counts between combination of novicidin with ceftriaxone and each of the single drug (ceftriaxone or novicidin) treatments (P < 0.0001). The ability of novicidin enhancement to rifampin or ceftriaxone was also compared to another defensin, plectasin which was neither bactericidal on its own nor did it boost the activity of rifampin (Fig. 1I) or ceftriaxone (Fig. 1J) against Gram-negative bacteria to validate the assay. Similar patterns of combination activities were observed for the strains tested when novicidin was combined with ceftazidime (data not shown).
Membrane-permeabilizing effects of novicidin against E. coli and KES group isolates.
The effects of novicidin at the cytoplasmic bacterial membrane with both E. coli and an isolate from the KES group were investigated with fluorescence assays. Immediately after novicidin exposure, a sharp concentration-dependent increase in fluorescence occurred with the E. coli strain (Fig. 2), indicating disruption of the bacterial membrane which led to the leakage of the fluorescent dye. A similar effect was observed when novicidin was used to treat the strain in the KES group despite only high concentrations of novicidin, such as 64 and 32 mg/liter, resulting in an increase in fluorescence (data not shown).
FIG 2.
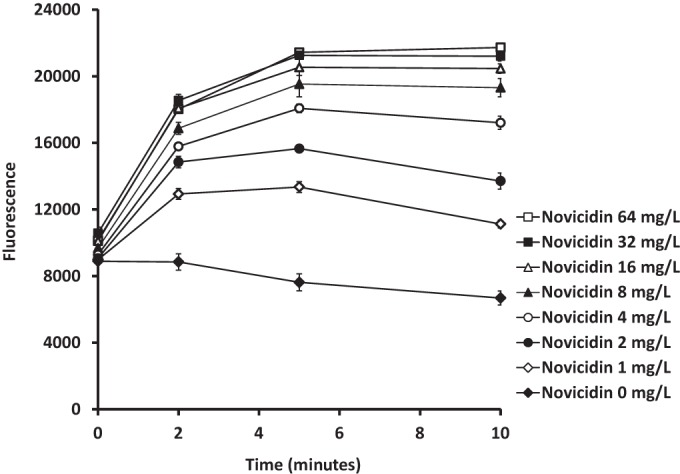
Determination of cytoplasmic membrane potential changes by novicidin against a clinical isolate of E. coli. A log-phase E. coli culture was incubated with DiSC3 (5) to a final concentration of 0.4 μM until no more quenching was detected, which was followed by the addition of 0.1 M KCl. Novicidin was incubated with the cultures at different concentrations. The changes in fluorescence were monitored at various time points. The data are means and SD from two independent experiments.
Postantibiotic effect of novicidin alone and novicidin-antibiotic combinations.
The PAEs of novicidin, rifampin, or ceftriaxone singly and in combinations were determined; rifampin was used at 5-fold higher than the MIC level and ceftriaxone at 10-fold higher than the MIC level. Since novicidin was rapidly bactericidal at a level 5-fold higher than its MIC, an MIC level that was 2-fold higher was used to induce the PAE. Due to their enhanced synergistic activities, the same concentrations for novicidin and rifampin or ceftriaxone used singly for PAE induction would completely kill all of the bacterial cells within 1 h if combined. Therefore, to induce PAEs with combination treatment, levels 5-fold higher than the minimal enhancement concentrations for novicidin and rifampin or ceftriaxone were used and chosen from the checkerboard results. As shown in Fig. 3A, the PAEs of both novicidin and rifampin were estimated at 52.8 min for the E. coli strain. The novicidin and rifampin combination doubled the PAE to 121.8 min despite substantially lower concentrations being used (P < 0.0001). As shown in Fig. 3B, the PAE of novicidin was 84 min, and ceftriaxone produced no PAE. The novicidin and ceftriaxone combination exhibited a prolonged PAE of 117 min (P < 0.0001).
FIG 3.
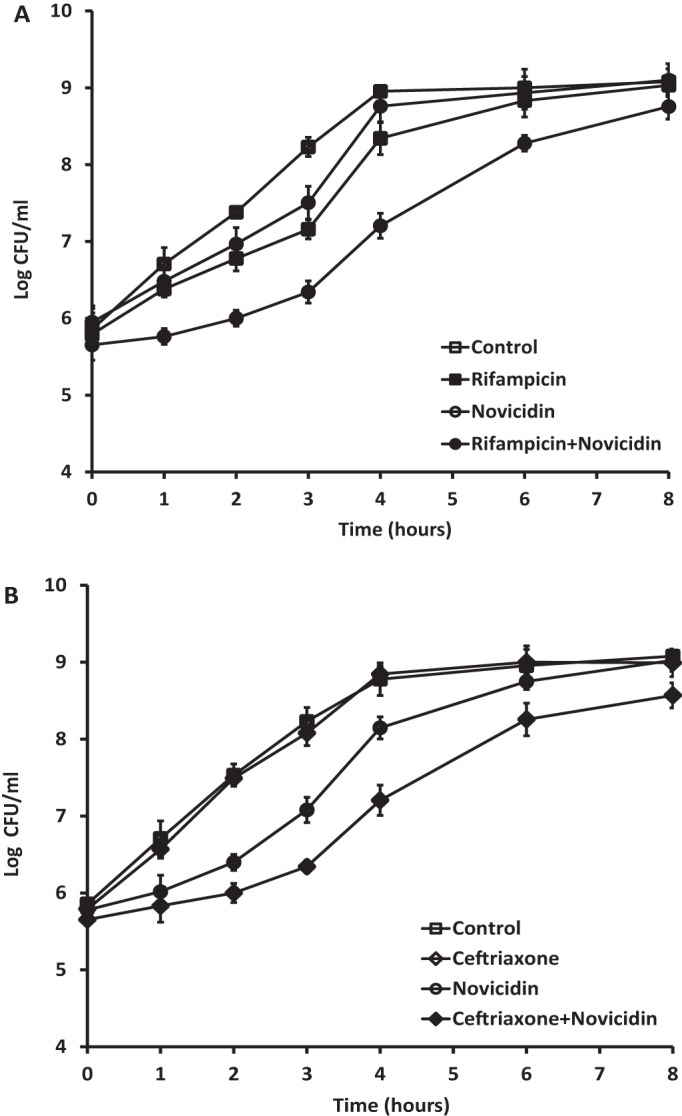
Induction of rifampin (A) and ceftriaxone (B) PAEs by novicidin against a clinical isolate of E. coli. The concentrations used for single-drug PAE induction were rifampin at 80 mg/liter, novicidin at 2 mg/liter, and ceftriaxone at 1,024 mg/liter. For combination PAE induction, rifampin at 20 mg/liter plus novicidin at 0.625 mg/liter or ceftriaxone at 640 mg/liter plus novicidin at 0.625 mg/liter were used. The data are means and SD from two independent experiments.
Hemolytic effects of novicidin.
Hemolysis due to novicidin was tested using human blood. As shown in Table 4, at the lowest tested novicidin concentration of 125 mg/liter, hemolysis occurred at a rate of 4.4% and it ranged up to 19.9% at the highest tested concentration of 1,000 mg/liter. A 50% hemolytic concentration could not be accurately predicted due to the nonlinear correlation with novicidin concentration; hemolysis, however, is shown to occur at >1,000 mg/liter based on the current data. Extrapolation provides an estimate of between 2,500 and 3,000 mg/liter. One-hundred percent hemolysis was seen when the blood was added into distilled water (Table 4). The experiments were repeated twice with reproducible results.
TABLE 4.
Hemolytic effects of novicidin at different concentrations
| Treatment groupa | Hemolysis (%) |
|---|---|
| Novicidin concn (mg/liter) | |
| 125 | 4.4 |
| 250 | 7.7 |
| 500 | 13.2 |
| 750 | 13.3 |
| 1,000 | 19.9 |
| Negative control | 0 |
| Positive control | 100 |
For the negative control, blood was mixed with saline solution. For the positive control, blood was mixed with distilled water.
Determination of cytotoxicity by neutral red uptake.
To assess the cytotoxicity of novicidin, neutral red uptake was measured after treatment of the murine fibroblasts with different concentrations of novicidin. As seen in Table 5, cell viability was well conserved and remained between 93 to 99% after 24 h of novicidin exposure, and 98 to 102% after 72 h of exposure for all tested concentrations. This indicates low levels of general cytotoxicity even with prolonged exposure. SDS was used as a positive control: concentrations of 80, 100, and 120 mg/liter reduced cell viability to 80, 9, and 0% at 24 h and 55, 0, and 0% at 72 h, respectively, confirming the validity of the assay. These experiments were repeated twice with reproducible results.
TABLE 5.
Cell viability following treatment with novicidin assessed via neutral red uptake
| Novicidin or SDS concn (mg/liter) | Viability (%) |
|
|---|---|---|
| 24 h | 72 h | |
| Novicidin | ||
| 0 | 100 | 100 |
| 25 | 95 | 102 |
| 50 | 93 | 102 |
| 100 | 99 | 98 |
| 200 | 99 | 101 |
| SDS | ||
| 80 | 80 | 55 |
| 100 | 9 | 0 |
| 120 | 0 | 0 |
DISCUSSION
Novicidin is a newly derived antimicrobial peptide. In this study, we demonstrated for the first time that novicidin synergized with rifampin and extended-spectrum cephalosporins (ceftriaxone and ceftazidime) against Gram-negative antibiotic-resistant bacterial strains in vitro. The 94 clinical isolates from the Enterobacteriaceae family covered a broad host distribution in the South London area, and the 7 NDM-1 strains represented the most resistant type of Gram-negative bacteria. Most of the ceftriaxone and ceftazidime resistant bacteria were also resistant to cefotaxime and cefixime, indicating that these were ESBL-producing strains.
Rifampin is an important component of the combination regimen used for the treatment of tuberculosis and many Gram-positive bacterial infections (21). Rifampin is not considered to be standard treatment for Enterobacteriaceae infections, and thus a breakpoint for resistance is not available. Our results showed that the MIC50 and MIC90 for rifampin were 16 and 32 mg/liter, respectively. Recently, rifampin has been introduced in combination therapy for the treatment of infections caused by multidrug resistant Gram-negative bacteria (22, 23). Our checkerboard analysis revealed that the combination of novicidin and rifampin showed synergistic effects with >70% of the tested strains with marginally higher effectiveness with the bacterial strains in KES group compared to E. coli. Novicidin was able to revive the activity of rifampin by reduction of rifampin MIC by 2- to 512-fold. The combination was also synergistic with all of the strains harboring NDM-1 plasmids. Synergistic activity of novicidin with rifampin was confirmed using time-kill assays, a method allowing for a more dynamic analysis of bactericidal and combinatorial effects. Time-kill assays were performed with multiple strains, repeatedly demonstrating that at concentrations at which both novicidin and rifampin were ineffective alone, in combination, rapid bactericidal activities were seen with 100% elimination of the bacterial cells within a few hours of drug exposure, which substantially decreased the treatment duration. Rifampin alone required higher concentrations such as 128 mg/liter to completely eradicate E. coli cells in culture (data not shown), and this concentration was only able to reduce the CFU counts of a KES group strain by 2 logs (data not shown). However, when combined with novicidin at 0.5 or 1 mg/liter, rifampin at concentrations of just 2 mg/liter killed 100% of the bacterial cells at 4 or 2 h posttreatment (Fig. 1A and B). The combination was also able to enhance the activities of rifampin against NDM-1 strains (Fig. 1C and D); however, high rifampin concentrations were required.
Novicidin also enhanced the activities of ceftriaxone and ceftazidime. Interestingly, the majority of synergy was observed with strains showing resistance to ceftriaxone or ceftazidime. This was also confirmed with time-kill assays testing multiple strains. Ceftriaxone has a long half-life and is used to treat septicemia, pneumonia, meningitis, and urinary tract infections. Clinical pharmacokinetic data revealed that after a single intravenous injection of a standard 2,000-mg dose the plasma Cmax was ∼257 mg/liter, and at 24 h postadministration the plasma concentration was ∼15 mg/liter. However, in urine, the Cmax of the ceftriaxone was ∼2,692 mg/liter within 2 h after intravenous administration of 2,000 mg (24). Ceftazidime, like ceftriaxone, has broad-spectrum activity and is one of the few agents in this class to be used clinically against Pseudomonas spp. Ceftazidime pharmacokinetic data show comparative serum Cmax, since a 1,000-mg intravenous dose produced a peak concentration of ∼140 mg/liter. Similarly, much higher concentrations are present in the urine. At up to 6 h postinfusion of a 50-mg/kg dose of ceftazidime, the concentration in collected urine samples ranged from 2,370 to 11,340 mg/liter, with ca. 75% of the drug being recovered unchanged (25). Based on these data, it may be argued that novicidin-cephalosporin combinations may not be clinically appropriate for the treatment of septicemia since 2,048 mg/liter appears to be an unattainable serum concentration. However, pharmacokinetic analysis of novicidin in combination with the antibiotics may give more realistic estimations of the concentrations required to achieve synergistic and bactericidal effects. Nevertheless, the extremely high concentrations of both ceftriaxone and ceftazidime in the urine indicate that either of these agents in combination with novicidin may be clinically applicable in treating urinary tract infections.
The combination of novicidin with rifampin or ceftriaxone was able to suppress bacterial growth against our tested bacterial strains after the drugs had been removed. Interestingly, although ceftriaxone alone was unable to produce a PAE (26), a prolonged PAE was generated in the combination with novicidin. Therefore, novicidin and the antibiotic combinations, possibly by prolonging the PAE, are able to reduce the likelihood of resistance development. A longer PAE also contributes a therapeutic advantage in devising dosing intervals for drug regimens. Generally, a longer PAE enables less frequent drug doses while maintaining therapeutic efficacy; this can reduce adverse effects and increase patient compliance (20).
The precise mechanism underlying the antibiotic enhancing activities of novicidin is unclear. Due to decreased cell envelope permeability and altered efflux pump systems, Gram-negative bacteria are intrinsically resistant to many antibiotics, such as rifampin. Rifampin inhibits bacterial DNA-dependent RNA polymerase (23), and its action on bacterial cells is concentration dependent. It has been shown that compounds that target the cell wall or cell membrane were found to potentiate the activities of other antibiotics (11, 18, 27, 28). Previous work on artificial membranes showed that low concentrations of novicidin result in transient pore formation and increased concentrations cause cell membrane disruption (13, 29). It has also been suggested that novicidin accumulates on the membrane surface until a detergent-like disintegration occurs (known as the carpet mechanism) (13). Consistent with this finding, we showed that novicidin disturbed the cytoplasmic membrane potential by depolarizing the membrane, and even at very low concentrations, significant fluorescence release was observed. It is likely that the enhanced activities of rifampin with novicidin were due to increased cell membrane permeability against the Gram-negative bacteria, leading to higher intracellular accumulations of rifampin (30, 31).
Cephalosporins are β-lactam antibiotics and interact with transpeptidases, which are also known as penicillin-binding proteins (32), blocking the terminal step in bacterial cell wall biosynthesis (33). Accordingly, the synergy between novicidin and ceftriaxone or ceftazidime may be attributed to a “double hit” mechanism: (i) the disruption of the membrane by novicidin and (ii) the inhibition of cell well biosynthesis by ceftriaxone or ceftazidime, which may be sufficient in reducing the integrity of the cell envelope, resulting in cell death. Our checkerboard analysis showed that synergy was more likely with ceftriaxone- or ceftazidime-resistant strains, and resistance to such agents is usually due to the acquisition of plasmids carrying ESBL genes, producing enzymes which hydrolyze the β-lactam ring of antibiotics. It is unclear how novicidin enhances the activities of these cephalosporins against resistant strains. We hypothesized that the enhanced antibiotic activities were likely due to the action of pore formation by novicidin, leading to the elimination of enzymes or plasmids, the resistance determinants. However, this notion needs to be further tested.
The findings from our study demonstrate proof of concept, displaying the potential of peptide-antibiotic combinations which undoubtedly contribute to important clinical applications. First, our demonstration of novicidin as a powerful antibiotic enhancer strongly illustrates that other similar peptides or compounds may potentially be beneficial above and beyond their direct antimicrobial properties. Second, the addition of novicidin reduced MICs and improved the rate of bactericidal activities of antibiotics; therefore, highly resistant Gram-negative bacteria which are extremely difficult to kill can be eliminated from the bacterial culture. Finally, novicidin exhibited a very low hemolytic activity, a finding which was in agreement with those of Dorosz et al. (14). In addition, novicidin was nontoxic, and cell viability was well conserved after treatment with different concentrations of novicidin. Combination therapy with novicidin shows promise for becoming a novel and much clinically desired therapeutic option to treat “superbug” infections. In vivo work is under way aiming to expose the therapeutic potential of novicidin in combination regimens to treat infections caused by antibiotic-resistant Gram-negative bacteria.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded with support from the European Commission under grant agreement 278998 (BacAttack).
This communication reflects the views only of its authors, and the Commission cannot be held responsible for any use which may be made of the information contained here.
We thank Julie Johnson from St George's Healthcare NHS Trust for kindly providing the clinical strains and Novozymes A/S Denmark for providing novicidin.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01245-15.
REFERENCES
- 1.Paterson DL. 2006. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control 34:S20–S28. doi: 10.1016/j.ajic.2006.05.238. [DOI] [PubMed] [Google Scholar]
- 2.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 4.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect 10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J Antimicrob Chemother 66:689–692. doi: 10.1093/jac/dkq520. [DOI] [PubMed] [Google Scholar]
- 6.Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ. 2010. Challenges of antibacterial discovery revisited. Ann N Y Acad Sci 1213:5–19. doi: 10.1111/j.1749-6632.2010.05828.x. [DOI] [PubMed] [Google Scholar]
- 7.Coates AR, Halls G, Hu Y. 2011. Novel classes of antibiotics or more of the same? Br J Pharmacol 163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson CJ, Power E, Ruebsamen-Waigmann H, Labischinski H. 2004. Antibacterial research and development in the 21st Century: an industry perspective of the challenges. Curr Opin Microbiol 7:445–450. doi: 10.1016/j.mib.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, British Society for Antimicrobial Chemotherapy Working Party. 2011. Discovery research: the scientific challenge of finding new antibiotics. J Antimicrob Chemother 66:1941–1944. doi: 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]
- 10.Kalan L, Wright GD. 2011. Antibiotic adjuvants: multicomponent anti-infective strategies. Expert Rev Mol Med 13:e5. doi: 10.1017/S1462399410001766. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Liu A, Vaudrey J, Vaiciunaite B, Moigboi C, McTavish SM, Kearns A, Coates A. 2015. Combinations of beta-lactam or aminoglycoside antibiotics with plectasin are synergistic against methicillin-sensitive and methicillin-resistant Staphylococcus aureus. PLoS One 10:e0117664. doi: 10.1371/journal.pone.0117664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soothill G, Hu Y, Coates A. 2013. Can we prevent antimicrobial resistance by using antimicrobials better? Pathogens 2:422–435. doi: 10.3390/pathogens2020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen SB, Otzen DE. 2010. Impact of the antimicrobial peptide novicidin on membrane structure and integrity. J Colloid Interface Sci 345:248–256. doi: 10.1016/j.jcis.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 14.Dorosz J, Gofman Y, Kolusheva S, Otzen D, Ben-Tal N, Nielsen NC, Jelinek R. 2010. Membrane interactions of novicidin, a novel antimicrobial peptide: phosphatidylglycerol promotes bilayer insertion. J Phys Chem B 114:11053–11060. doi: 10.1021/jp1052248. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan VS, Vad BS, Otzen DE. 2013. Novicidin's membrane permeabilizing activity is driven by membrane partitioning but not by helicity: a biophysical study of the impact of lipid charge and cholesterol. Biochim Biophys Acta 1834:996–1002. doi: 10.1016/j.bbapap.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 17.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Coates AR. 2012. Enhancement by novel anti-methicillin-resistant Staphylococcus aureus compound HT61 of the activity of neomycin, gentamicin, mupirocin, and chlorhexidine: in vitro and in vivo studies. J Antimicrob Chemother 68:374–384. doi: 10.1093/jac/dks384. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Hancock RE. 1999. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem 274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Spivey JM. 1992. The postantibiotic effect. Clin Pharm 11:865–875. [PubMed] [Google Scholar]
- 21.Rodriguez-Pardo D, Pigrau C, Corona PS, Almirante B. 2015. An update on surgical and antimicrobial therapy for acute periprosthetic joint infection: new challenges for the present and the future. Expert Rev Anti-Infect Ther 13:249–265. doi: 10.1586/14787210.2015.999669. [DOI] [PubMed] [Google Scholar]
- 22.Nastro M, Rodriguez CH, Monge R, Zintgraff J, Neira L, Rebollo M, Vay C, Famiglietti A. 2014. Activity of the colistin-rifampicin combination against colistin-resistant, carbapenemase-producing Gram-negative bacteria. J Chemother 26:211–216. doi: 10.1179/1973947813Y.0000000136. [DOI] [PubMed] [Google Scholar]
- 23.Drapeau CM, Grilli E, Petrosillo N. 2010. Rifampicin combined regimens for gram-negative infections: data from the literature. Int J Antimicrob Agents 35:39–44. doi: 10.1016/j.ijantimicag.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20:634–641. doi: 10.1128/AAC.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeder JS, Spino M, Tesoro AM, MacLeod SM. 1983. High-pressure liquid chromatographic analysis of ceftazidime in serum and urine. J Antimicrob Chemother 24:720–724. doi: 10.1128/AAC.24.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess DS. 2005. Use of pharmacokinetics and pharmacodynamics to optimize antimicrobial treatment of Pseudomonas aeruginosa infections. Clin Infect Dis 40(Suppl 2):S99–S104. doi: 10.1086/426189. [DOI] [PubMed] [Google Scholar]
- 27.Giacometti A, Cirioni O, Del Prete MS, Barchiesi F, Fortuna M, Drenaggi D, Scalise G. 2000. In vitro activities of membrane-active peptides alone and in combination with clinically used antimicrobial agents against Stenotrophomonas maltophilia. Antimicrob Agents Chemother 44:1716–1719. doi: 10.1128/AAC.44.6.1716-1719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anantharaman A, Rizvi MS, Sahal D. 2010. Synergy with rifampin and kanamycin enhances potency, kill kinetics, and selectivity of de novo-designed antimicrobial peptides. Antimicrob Agents Chemother 54:1693–1699. doi: 10.1128/AAC.01231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertelsen K, Dorosz J, Hansen SK, Nielsen NC, Vosegaard T. 2012. Mechanisms of peptide-induced pore formation in lipid bilayers investigated by oriented 31P solid-state NMR spectroscopy. PLoS One 7:e47745. doi: 10.1371/journal.pone.0047745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pages JM, Sandrine AF, Mahamoud A, Bolla JM, Davin-Regli A, Chevalier J, Garnotel E. 2010. Efflux pumps of gram-negative bacteria, a new target for new molecules. Curr Top Med Chem 10:1848–1857. doi: 10.2174/156802610793176620. [DOI] [PubMed] [Google Scholar]
- 31.Zechini B, Versace I. 2009. Inhibitors of multidrug resistant efflux systems in bacteria. Recent Pat Anti infect Drug Discov 4:37–50. doi: 10.2174/157489009787260061. [DOI] [PubMed] [Google Scholar]
- 32.Barbas JA, Diaz J, Rodriguez-Tebar A, Vazquez D. 1986. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol 165:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosowska-Shick K, McGhee PL, Appelbaum PC. 2010. Affinity of ceftaroline and other beta-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 54:1670–1677. doi: 10.1128/AAC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.