Abstract
There is increasing interest in the use of lipophilic copper (Cu)-containing complexes to combat bacterial infections. In this work, we showed that Cu complexes with bis(thiosemicarbazone) ligands [Cu(btsc)] exert antibacterial activity against a range of medically significant pathogens. Previous work using Neisseria gonorrhoeae showed that Cu(btsc) complexes may act as inhibitors of respiratory dehydrogenases in the electron transport chain. We now show that these complexes are also toxic against pathogens that lack a respiratory chain. Respiration in Escherichia coli was slightly affected by Cu(btsc) complexes, but our results indicate that, in this model bacterium, the complexes act primarily as agents that deliver toxic Cu ions efficiently into the cytoplasm. Although the chemistry of Cu(btsc) complexes may dictate their mechanism of action, their efficacy depends heavily on bacterial physiology. This is linked to the ability of the target bacterium to tolerate Cu and, additionally, the susceptibility of the respiratory chain to direct inhibition by Cu(btsc) complexes. The physiology of N. gonorrhoeae, including multidrug-resistant strains, makes it highly susceptible to damage by Cu ions and Cu(btsc) complexes, highlighting the potential of Cu(btsc) complexes (and Cu-based therapeutics) as a promising treatment against this important bacterial pathogen.
INTRODUCTION
Copper (Cu) is an essential trace micronutrient in bacteria but is bacteriotoxic in excess. Nutrient Cu inserts into specific high-affinity sites in proteins or forms complexes with low-molecular-weight thiols, such as glutathione. As a consequence, the concentration of “free” Cu ions in cells has been estimated to be vanishingly low (subfemtomolar) (1). Any excess Cu beyond this normal binding capacity may be mislocated to nonspecific and low-affinity metal ion binding sites, thereby inactivating crucial enzymes and interfering with normal metabolism (2). As the midpotential of the Cu(II)/Cu(I) redox couple is biologically accessible, these weakly bound Cu ions can also cause further toxicity by catalyzing gratuitous electron transfer and promoting redox stress (3). The widespread damage caused by excess Cu ions is termed Cu poisoning. Almost all bacterial species, including those that have no apparent use for Cu, possess homeostatic systems that protect them against an excess of this ion (4). These systems consist typically of a Cu ion efflux pump that operates under the control of a Cu-specific transcriptional regulator.
As a result of its chemistry, Cu is considered a promiscuous or broad-spectrum antimicrobial, but there is concern regarding its universal toxicity. In the preantibiotic era, simple ionic Cu salts were used to treat a variety of infections (5), but these salts are now associated with significant toxicity (6). The difficulty in delivering bactericidal Cu at doses that are nontoxic to human tissues may explain the failure to translate Cu-based therapeutics into the modern medical setting. The emergence of bacteria with resistance to classical antibiotics, combined with the paucity of new compounds in the pipeline, has renewed interest in the development of Cu as an antimicrobial (7–9). This research is motivated further by recent findings that physiological Cu may be harnessed as a direct antimicrobial in innate immune cells (10, 11).
Ionic Cu salts have a limited ability to cross membranes, as their translocation relies on specific transport proteins, and so they have little potential to be developed as anti-infective drugs. Cu ions generally show restricted penetration into target tissues and bacteria, and thus exceptionally high concentrations are needed to achieve a bactericidal effect in vitro and in vivo. To assist in the delivery of Cu ions across lipid membranes, several lipophilic ligands or proligands have been developed (9, 12). These molecules are termed Cu ionophores for their ability to act as carriers of Cu ions.
Of interest in this work are bis(thiosemicarbazone) ligands that bind Cu(II) as small, uncharged, lipophilic, and stable complexes [Cu(btsc)] (Fig. 1). We and others have shown that Cu(btsc) complexes exert antimicrobial activity against several human pathogens, including Gram-negative bacteria such as Neisseria gonorrhoeae (13) and Mycobacterium tuberculosis (14), as well as the Gram-positive bacterium Staphylococcus aureus (15). However, the mechanism of antibacterial action of Cu(btsc) complexes has not been examined fully. Although it is assumed that they operate as Cu ionophores (14, 15), we have shown that Cu(btsc) complexes can also act as direct respiratory inhibitors in N. gonorrhoeae (13). Here, we describe experiments using Escherichia coli that were aimed at determining whether Cu(btsc) complexes act primarily by inhibition of the respiratory chain or by delivery of bioavailable Cu ions. We also assessed the susceptibility of a range of bacterial pathogens to these complexes, including multidrug-resistant strains of N. gonorrhoeae.
FIG 1.
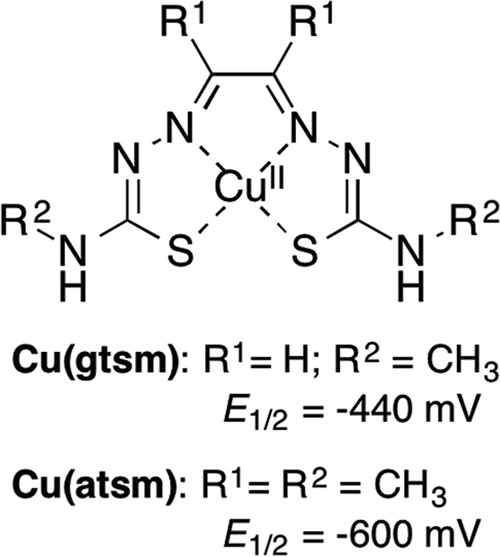
Structure of Cu(btsc) complexes. Midpoint reduction potentials are versus Ag/AgCl (21).
MATERIALS AND METHODS
Cu stocks.
Stocks of aqueous Cu salts, supplied as CuCl2 or Cu(NO3)2, were prepared in deionized water, and their concentrations were standardized using bathocuproine disulfonate as described elsewhere (16). The two salts were used interchangeably in experiments. Stocks of Cu-pyrithione, Cu-neocuproine, and Cu-disulfiram complexes were prepared in dimethyl sulfoxide (DMSO) by mixing standardized CuCl2 with 2.5 molar equivalents of the respective free ligands. Stocks of Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) [Cu(atsm)] and glyoxal-bis(N4-methylthiosemicarbazonato)Cu(II) [Cu(gtsm)] were prepared in neat DMSO and standardized using their solution absorbance in DMSO [for Cu(atsm)], λmax = 457 nm and ε = 7,200 M−1 cm−1; for Cu(gtsm), (λmax = 472 nm and ε = 8,700 M−1 cm−1). DMSO was used as a vehicle control in all experiments.
Strains and growth conditions.
N. gonorrhoeae strains (see Table 3) were grown on GC agar (Oxoid) supplemented with 1% (vol/vol) IsoVitaleX (Becton Dickinson). Liquid cultures were prepared in GC broth supplemented with IsoVitaleX and 0.042% (wt/vol) sodium bicarbonate. E. coli strains MG1655 (laboratory strain K-12) and EC958 (fluoroquinolone-resistant strain ST131) and Salmonella enterica serovar Typhimurium SL1344 were propagated on LB agar or broth. Haemophilus influenzae RdKw20 was propagated on BHI medium (Oxoid) containing 10 μg/ml NAD+ and 10 μg/ml hemin. Streptococcus pneumoniae D39 was propagated on THY base (Oxoid) containing 10 U/ml catalase. S. aureus strain ATCC 22913 (methicillin sensitive) and SR2852 (methicillin resistant) strains were grown on tryptic soy medium (Becton Dickinson). Lactobacillus acidophilus NCFM was grown on L-MRS agar (Oxoid) under anaerobic growth conditions. All bacteria were grown for 12 to 16 h at 37°C. N. gonorrhoeae, S. pneumoniae, and S. aureus were grown in the presence of 5% (vol/vol) atmospheric CO2. Anaerobic growth was performed in an anaerobic jar containing an AnaeroGen sachet (Oxoid).
TABLE 3.
Susceptibility of multidrug-resistant strains of N. gonorrhoeae to Cu salt, Cu(atsm), and Cu(gtsm)
| Straina | Reference | MIC (μM)b |
||
|---|---|---|---|---|
| Cu salt | Cu(atsm) | Cu(gtsm) | ||
| Antibiotic sensitive | ||||
| 1291 | 45 | 250 (0) | 0.8 (0.1) | 0.1 (0.0) |
| FA1090 | 46 | 250 (0) | 0.9 (0.1) | 0.1 (0.0) |
| F62 | 47 | 250 (0) | 0.7 (0.1) | 0.1 (0.0) |
| Antibiotic resistant | ||||
| MS11 | 48 | 250 (0) | 2.3 (0.2) | 0.1 (0.0) |
| FA6140 | 49 | 250 (0) | 3.0 (0.3) | 0.1 (0.2) |
| F89 | 29 | 250 (0) | 1.7 (0.3) | 0.1 (0.0) |
| H041 | 30 | 250 (0) | 1.9 (0.2) | 0.1 (0.0) |
| FA19 | 50 | 250 (0) | 0.9 (0.1) | 0.1 (0.0) |
| DW120 | 32 | 250 (0) | 2.2 (0.2) | 0.1 (0.0) |
| KH15 | 32 | 250 (0) | 2.7 (0.3) | 0.1 (0.0) |
| KH14 | 33 | 250 (0) | 0.7 (0.1) | 0.1 (0.0) |
Antibiotic resistance profiles of selected strains are shown in Table S1 in the supplemental material.
Data presented were averaged from three independent experiments. Standard deviations are in parentheses.
Determination of MICs.
MICs were determined by agar dilution method. Briefly, bacterial lawns from an overnight agar culture were resuspended in the appropriate broth to 107 to 108 CFU/ml, and 5 μl of each dilution was spotted onto new solid medium containing various concentrations of the desired Cu source. The amount of DMSO was maintained at 0.5% (vol/vol). The MIC was defined as the minimum concentration at which no growth was visible after 24 h.
Assays of gonococcal killing kinetics.
N. gonorrhoeae lawns from an overnight agar culture were resuspended in broth to an optical density at 600 nm (OD600) of 0.1 (ca. 107 CFU/ml). Cu complexes were added to the desired final concentration, and the mixtures were incubated at 37°C with gentle shaking. Survival was monitored for up to 7 h by conventional colony counting.
Measurement of intracellular Cu levels in E. coli.
E. coli was resuspended in broth to an OD600 of 0.1 (ca. 108 CFU/ml) and used to seed fresh solid medium containing the desired Cu source. After an overnight growth, bacteria were harvested, washed once in phosphate-buffered saline (PBS) containing 10% (vol/vol) DMSO, once in PBS containing 1 mM EDTA, and finally once in PBS without any additive. The final pellet was dissolved in concentrated nitric acid. Amounts of Cu and other transition metal ions were determined by inductively coupled plasma optical emission spectrometry (ICP OES). Results were standardized to total biomass, as represented by total protein content. The amount of protein was assayed using the Quanti BCA kit (Sigma).
To evaluate the antibacterial activity of the Cu complexes under the same conditions, the initial inoculum was diluted serially up to 10−7, and 5 μl of each dilution was plated on duplicate plates with solid medium containing the various Cu sources. Visible colonies were enumerated after 24 h to determine the efficiency of plating.
Assays of β-galactosidase activity in E. coli.
The promoter region of copA from E. coli MG1655 (150 bp upstream from the translation start codon ATG) was amplified using the primer pair PcopA-F (5′-CATCAGGGATTCAGATAAATGTCTAATCCTGA-3′) and PcopA-R (5′-CTGATGAAGCTTAAAACACTCCTTTAAGACAG-3′). The PCR product was cloned between the BamHI and HindIII sites of the low-copy-number vector pQF50 containing a promoterless lacZ gene (17). The resulting pQF50::PcopA plasmid was transformed into E. coli DH5α. Colonies that were blue on agar plates containing ampicillin (100 μg/ml), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg/ml), and CuCl2 (500 to 1000 μM) were selected and grown to the mid-exponential phase. Cultures were challenged with Cu complexes for 0 to 120 min without any shaking. β-Galactosidase activities were measured using ortho-nitrophenyl-β-galactoside following procedures for the standard Miller assay. Raw values of β-galactosidase activity were normalized to OD600. Results were expressed as induction (fold) over the uninduced control at the same time point.
Assays of respiration in E. coli.
E. coli was cultured to the mid-exponential phase, harvested, resuspended in LB to 10% of the original volume, and kept on ice. Respiration was measured at 35°C in 2 ml of broth containing 50 μM Cu salt, Cu(gtsm), or Cu(atsm). Consumption of O2 was initiated by adding 10 μl of the bacterial suspension. Respiration was monitored for 15 min using an S1/mini-Clark-type oxygen electrode (Hansatech Instruments) in conjunction with an Oxytherm control unit.
E. coli membrane vesicles were isolated as described previously for N. gonorrhoeae (13). Rates of NADH oxidation were measured spectrophotometrically at 340 nm following procedures described previously for submitochondrial particles from rat liver (18).
Assays of using cervical epithelial cells.
Immortalized and adherent ME-180 cervical epithelial cells (ATCC HTB33) were cultured routinely according to the manufacturer's instructions in McCoy's 5a modified medium (ATCC 30-2007) containing fetal bovine serum (10% [vol/vol]; ATCC 30-2020) and penicillin and streptomycin (100 IU/ml and 100 μg/ml, respectively; Cellgro 30-002-CI [Corning]). Cells were seeded at 1 × 106 cells/ml and allowed to adhere overnight. The resulting monolayers were transitioned into fresh medium containing Cu(gtsm), Cu(atsm), or Cu salt. The concentration of DMSO in all samples was 0.1% (vol/vol). After 24 h, the supernatant was collected and checked for cells that might have detached during treatment. The remaining adherent cells were allowed to recover for 1 to 2 h and subsequently brought into suspension using trypsin-EDTA. Trypan blue was added, and cells were enumerated in a hemocytometer.
RESULTS
Antibacterial activity of Cu(btsc) complexes for selected bacterial pathogens.
Two prototypes of the Cu(btsc) family, Cu(gtsm) and Cu(atsm) (Fig. 1), exerted dose-dependent antibacterial activity against N. gonorrhoeae strain 1291 (13). Both complexes were effective at concentrations where the unmetallated H2btsc ligands and the uncomplexed, or “free,” aqueous Cu2+ ions were ineffective (Table 1) (13). The MICs were 0.1 μM (0.03 μg/ml) for Cu(gtsm) and 0.8 μM (0.3 μg/ml) for Cu(atsm) (Table 1). We also evaluated the antigonococcal activities of additional Cu ionophores, including Cu-disulfiram, Cu-neocuproine, and Cu-pyrithione. The MIC of Cu-pyrithione (0.13 μM, ca. 0.04 μg/ml) was comparable to that of Cu(gtsm) (Table 1). For this study, we examined Cu(gtsm) in greater depth, as the availability of the structural analogue Cu(atsm) with a lower efficacy allowed us to probe the mode of action in more detail.
TABLE 1.
Antibacterial activity of several Cu complexes against N. gonorrhoeae
| Complexa | MIC (μM) |
|---|---|
| Cu(gtsm) | 0.10 |
| Cu-pyrithione | 0.13 |
| Cu(atsm) | 0.80 |
| Cu-neocuproine | 0.80 |
| Cu-disulfiram | 15–20 |
| Cu salt | 250 |
The complexes were prepared and their concentrations standardized as described in Materials and Methods.
The mode of action of Cu(gtsm) and Cu(atsm) against N. gonorrhoeae was bactericidal. Complete killing of N. gonorrhoeae (∼107 CFU/ml) by 1 μM Cu(gtsm) was achieved within 1.5 h, while an equal dose of Cu(atsm) required at least 5 h to elicit an equivalent effect (Fig. 2). In contrast, ionic Cu salts displayed no bactericidal activity within this time period (Fig. 2). The relative MICs and killing kinetics established that Cu(gtsm) and Cu(atsm) were more toxic than Cu salts and that Cu(gtsm) was more toxic than Cu(atsm).
FIG 2.
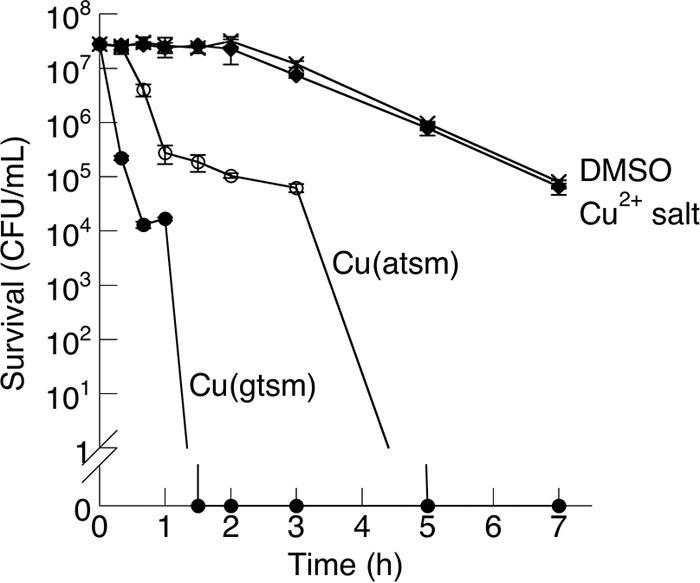
Time-dependent bactericidal effects of Cu(btsc) complexes (1 μM each) against N. gonorrhoeae 1291. Number of surviving CFU was plotted against time postchallenge. The effects of Cu salt (1 μM) and DMSO control are also shown. Each data point was averaged from three independent replicates. Error bars represent standard deviations from the means.
Cu(gtsm) and Cu(atsm) were also effective against several additional and unrelated bacterial pathogens, including S. aureus (methicillin-sensitive and resistant strains), S. pneumoniae, and H. influenzae, but at MICs that were appreciably higher (Table 2). E. coli (fluoroquinolone-sensitive and resistant strains) and S. Typhimurium were the most resistant. In the case of E. coli, bacterial growth was observed even at Cu(gtsm) concentrations as high as 25 μM (7.5 μg/ml), although there was a 1,000-fold reduction in the plating efficiency compared with the untreated control (see Fig. S1 in the supplemental material). In comparison, exposure to 25 μM Cu(atsm) had no effect (see Fig. S1 in the supplemental material). Due to limited solubility of Cu(btsc) complexes, higher concentrations were not tested.
TABLE 2.
Susceptibility of selected bacterial pathogens to Cu(atsm) and Cu(gtsm)
| Organisma | MIC (μM) |
|
|---|---|---|
| Cu(atsm) | Cu(gtsm) | |
| N. gonorrhoeae | 0.8 | 0.1 |
| H. influenzae | 10 | 1 |
| S. aureus | >10 | 1.5 |
| S. pneumoniae | >10 | 2 |
| L. acidophilus | >25 | 5 |
| S. Typhimurium | >25 | >25 |
| E. coli | >25 | >25 |
Strain information is available in Materials and Methods.
Since N. gonorrhoeae and E. coli represented the most susceptible and most resistant test organisms, respectively, they were examined further, the former because it may be a promising target for Cu- and Cu(btsc)-based therapeutics and the latter because its resistance properties and its amenability to molecular analysis might help in determining the mode of Cu(btsc) action.
Inhibition of respiration in E. coli by Cu(btsc) complexes and antibacterial activity under anaerobic growth conditions.
Recently, we showed that Cu(gtsm) and, to a lesser extent, Cu(atsm) suppressed aerobic respiration in N. gonorrhoeae (13) and mitochondria (18). Within the electron transport chain, NADH dehydrogenases (Nuo or complex I [H+ translocating] and Nqr [Na+ translocating]) were identified as the primary targets of inhibition. Inhibition occurred at or near the site of ubiquinone reduction, and it was independent of the release of free Cu ions (18). Instead, an intact Cu(gtsm) or Cu(atsm) molecule was determined to be the inhibitory species. Subsequently, we proposed that the action of Cu(btsc) complexes as respiratory inhibitors may be a major mechanism of their antibacterial activity (13). However, our present work has now shown that these complexes were also effective against bacteria that do not respire, such as S. pneumoniae (Table 2).
Cu(gtsm) (50 μM) also suppressed respiration in E. coli, as indicated by a decrease in the total amount of O2 consumed after 15 min, while equal concentrations of Cu salts or Cu(atsm) had no effect (Fig. 3A). E. coli possesses two respiratory NADH dehydrogenases, Nuo and Ndh-2 (a single-subunit flavoenzyme which does not translocate H+) (19), which may be targets of Cu(gtsm) inhibition. However, Cu(gtsm) only weakly suppressed the rates of NADH oxidation in isolated membrane vesicles containing both Nuo and Ndh-2 (Fig. 3B). The concentration for half-maximal inhibition (I50) value was extrapolated to be >130 μM (see Fig. S2 in the supplemental material), well beyond the solubility limit for Cu(gtsm), indicating that the NADH dehydrogenases in E. coli were not major targets of respiratory inhibition by Cu(gtsm). This finding was not altogether surprising, as Nuo and Ndh-2 in E. coli were also less sensitive to inhibition by the classical Nuo or complex I antagonist rotenone. This difference has been ascribed to subtle structural differences at or near the sites of ubiquinone reduction (20).
FIG 3.
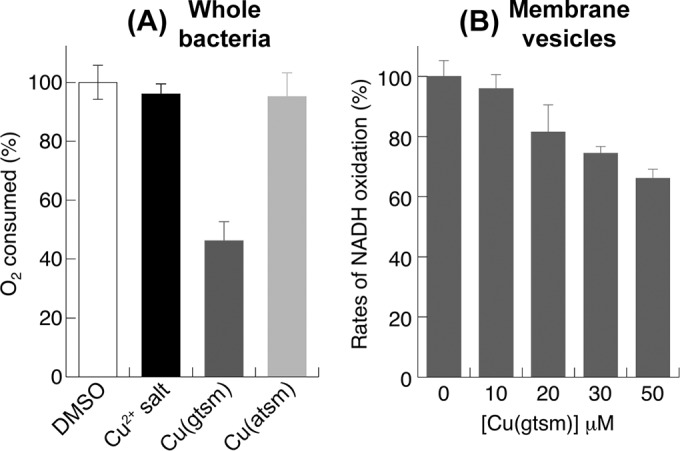
Inhibitory effects of Cu(btsc) complexes on aerobic respiration by E. coli. (A) Amounts of O2 consumed by whole bacteria over 15 min in the presence of various Cu sources (50 μM). (B) Rates of NADH oxidation by isolated membrane vesicles in the presence of Cu(gtsm) (0 to 50 μM). (A and B) Results were shown as a percentage of the unchallenged control. Each data point was averaged from three independent replicates. Error bars represent standard deviations from the means.
More importantly, Cu(gtsm) was also inhibitory to E. coli under anaerobic growth conditions, as evidenced by a curve of plating efficiency that was essentially indistinguishable to that obtained under aerobic growth conditions (see Fig. S1 in the supplemental material). Therefore, unlike the situation in N. gonorrhoeae (13), inhibition of aerobic respiration did not appear to contribute significantly to the antibacterial activity of Cu(gtsm) against E. coli.
Boosting of intracellular Cu levels by Cu(btsc) complexes.
The MICs for both Cu(gtsm) and Cu(atsm) were consistently lower than those for ionic Cu salts by >2 orders of magnitude (Table 2). Unlike charged Cu ions, neutral Cu(btsc) complexes are presumed to be readily membrane permeant. Thus, treatment with Cu(btsc) complexes would be expected to drive a greater accumulation in bacterial Cu contents than equal doses of Cu salts. However, previous analyses of total Cu by ICP OES detected no such effect in N. gonorrhoeae (13). As N. gonorrhoeae was killed by low nanomolar doses of Cu(btsc) complexes (Tables 1 and 2), we reasoned that any gain in intracellular Cu might remain below the detection limit of these measurements.
The analyses of Cu content were repeated here using E. coli because of its ability to tolerate micromolar concentrations of Cu(gtsm) and Cu(atsm) (Table 2). First, we established that treatment with 10 to 15 μM ionic Cu salts increased the total Cu content of E. coli 2- to 3-fold compared with the untreated control (Fig. 4A). These amounts of intracellular Cu were noninhibitory, and there was no decrease in plating efficiency (Fig. 4B). Treatment with similar doses of Cu(atsm) induced a comparable rise in Cu levels (Fig. 4A), again without any loss in plating efficiency (Fig. 4B). In contrast, exposure to equal doses of Cu(gtsm) led to a greater accumulation of Cu that was 5- to 8-fold higher than that in the unchallenged control (Fig. 4A). This modest boost in intracellular Cu levels was correlated with an antibacterial effect, and there was a 5- to 10-fold reduction in plating efficiency (Fig. 4B). There was no change in the levels of other transition metal ions (see Fig. S3 in the supplemental material), confirming that the antibacterial effect of Cu(gtsm) was Cu dependent.
FIG 4.
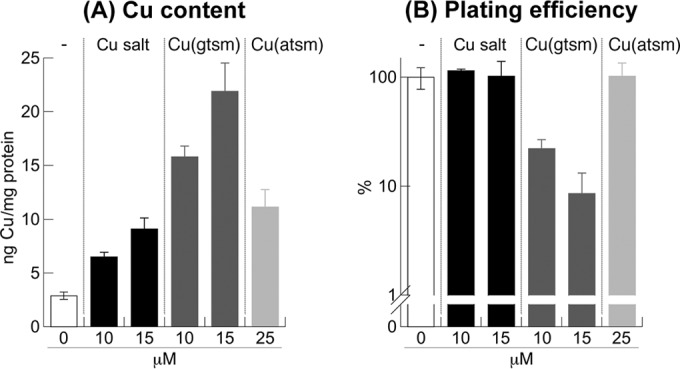
Effects of Cu(btsc) complexes on total intracellular Cu content (A) and plating efficiency of E. coli (B), presented as a percentage of the unchallenged control. Treatment time was 24 h. Each data point was averaged from three independent replicates. Error bars represent standard deviations from the means.
Dissociation of Cu from Cu(btsc) complexes as bioavailable ions.
The relative ability of Cu(gtsm) and Cu(atsm) to promote intracellular accumulation of Cu matched their relative antibacterial potency (Table 2). In fact, for all bacterial pathogens that we tested, the MICs of Cu(gtsm) were invariably lower than those of Cu(atsm) (Table 2). This result was in line with the proposed action of Cu(btsc) complexes as Cu carriers. The Cu(II) center in Cu(btsc) is bound strongly and is not thought to be dissociated as Cu(II) ions. Instead, Cu is released as Cu(I) (see Fig. 8). This occurs upon reduction of the Cu(II) center by cytoplasmic reductants, such as thiols (21). As a consequence of a higher Cu(II)/Cu(I) reduction midpoint potential for Cu(gtsm) (Fig. 1), dissociation of Cu(I) ions from Cu(gtsm) is ensured (21). In contrast, Cu(atsm) possesses a lower Cu(II)/Cu(I) midpoint potential, and thus dissociation of Cu(I) ions from Cu(atsm) is not thought to occur, except in hypoxic cells (22, 23).
FIG 8.
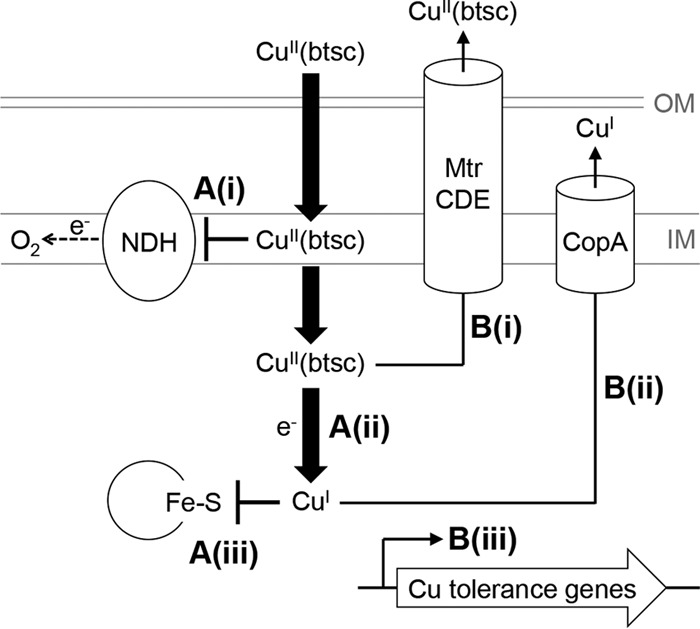
Schematic representation of the antibacterial action of Cu(btsc) complexes. (A) Mechanisms of action, showing (i) inhibition of membrane-bound NADH dehydrogenases of the electron transport chain by the intact Cu(btsc) complex, (ii) reduction of the Cu(II) center and dissociation of Cu(I) as bioavailable ions, and (iii) poisoning of enzymes by Cu(I) ions. (B) Mechanisms of tolerance, showing (i) efflux of the intact Cu(btsc) complex by the MtrCDE efflux pump or other promiscuous drug transporters, (ii) efflux of bioavailable Cu(I) ions, and (iii) activation of other dedicated Cu ion tolerance genes.
The final amounts of intracellular Cu delivered by Cu(gtsm) and Cu(atsm) (<25 ng Cu/mg protein) were well below the maximum tolerance capacity of E. coli. Exposure to higher doses of Cu salts (1,500 μM) led to the accumulation of Cu to 200 ng Cu/mg protein (see Fig. S3 in the supplemental material), but the plating efficiency of bacteria remained unaffected (see Fig. S1 in the supplemental material). This apparent disconnect between total Cu content and antibacterial potency has been observed previously (13, 15). Here, it must be noted that ICP OES measurements do not differentiate between Cu that is captured by the bacterium as bioavailable ions and Cu that remains coordinated as a Cu(btsc) complex.
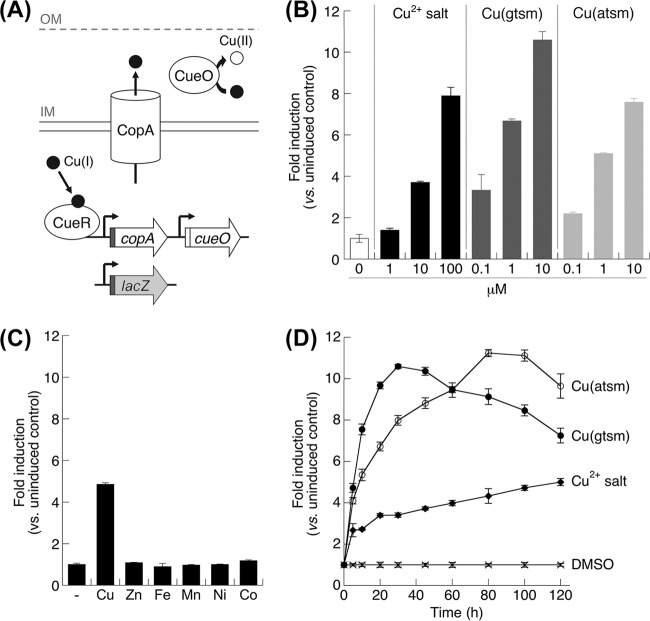
To examine the dissociation of Cu from Cu(btsc) complexes as bioavailable ions, we exploited the innate bacterial response to excess Cu. The system in E. coli is particularly well characterized (Fig. 5A) and amenable to analysis. In E. coli, increased intracellular Cu is sensed by the Cu(I)-specific transcriptional regulator CueR. In turn, CueR activates the expression of CopA, a membrane-bound P-type ATPase that exports Cu(I) out of the cytoplasm, and CueO, a periplasmic cuprous oxidase that oxidizes Cu(I) to the less toxic form Cu(II) (Fig. 5A) (24). In this work, we fused a plasmid-borne, promoterless lacZ transcriptional reporter gene with the promoter region of the copA gene (PcopA-lacZ) (Fig. 5A) and subsequently tested the ability of ionic Cu salts, Cu(gtsm), and Cu(atsm) to induce β-galactosidase activity in E. coli. This opportunity was not available with N. gonorrhoeae, as the CueR regulon and any other recognizable Cu detoxification system are absent, with the sole exception of the efflux pump CopA (25). Furthermore, unlike expression of the copA gene in E. coli, expression of gonococcal copA is controlled by an unidentified mechanism that does not appear to involve Cu (25).
FIG 5.
Dissociation of bioavailable Cu ions from Cu(btsc) complexes. (A) Cu detoxification system in E. coli. To determine the presence of bioavailable Cu(I) ions in the cytoplasm, the promoter region of copA (in red) was fused to a promoterless lacZ gene (in gray). IM, inner membrane; OM, outer membrane. (B) Dose-dependent induction of the copA promoter. β-Galactosidase activity was assayed at 2 h postexposure. (C) Response of the PcopA-lacZ fusion to divalent transition metal ions (supplied as chloride salt, 100 μM each) at 2 h postexposure. (D) Time-dependent induction of the copA promoter in response to Cu salt (100 μM), Cu(gtsm) (10 μM), or Cu(atsm) (25 μM). (B to D) Each data point was averaged from three replicates. Error bars represent standard deviations from the means. The results shown are representative of at least three independent experiments.
Addition of Cu salts to the growth medium led to a dose-dependent increase in β-galactosidase activity (Fig. 5B). No induction was observed in the presence of other transition metal ions (Fig. 5C), thus validating the Cu-specific response of the PcopA-lacZ fusion. More importantly, exposure to Cu(gtsm) also led to a robust induction of β-galactosidase activity (Fig. 5B), consistent with the intracellular release of bioavailable Cu ions from Cu(gtsm), presumably as Cu(I). While the minimum dose of Cu salts required for induction was 10 μM, activation by Cu(gtsm) was observed at concentrations as low as 0.1 μM (Fig. 5B). Moreover, activation of PcopA-lacZ by Cu(gtsm) was rapid and the maximum response was achieved as early as 20 min postexposure (Fig. 5D). There was a detectable decrease in this response after 40 min, presumably due to the toxic effects of Cu(gtsm) or the dissociated Cu(I) ion. By comparison, induction of PcopA-lacZ by ionic Cu salts occurred gradually over a period of at least 2 h (Fig. 5D). These findings further confirmed that Cu(gtsm) is a more efficient source of intracellular bioavailable Cu(I) ions than are uncomplexed Cu salts.
As mentioned earlier, the Cu center in Cu(atsm) is considered to display less intracellular dissociation than Cu(gtsm) (21–23). However, like Cu(gtsm), Cu(atsm) also activated the PcopA promoter, as detected by an increase in β-galactosidase activity (Fig. 5B). The maximum magnitudes of activation by Cu(atsm) and Cu(gtsm) were comparable (Fig. 5B and D). These results provided strong evidence that Cu was also released from Cu(atsm) as bioavailable Cu(I) ions. However, compared with Cu(gtsm), there was a reproducible lag in the response to Cu(atsm), and a maximum was achieved only after 80 min of exposure (Fig. 5D). Taken together, these data are consistent with the view that Cu(gtsm) is a more efficient Cu delivery agent than Cu(atsm).
Susceptibility of Cu tolerance mutants of E. coli to Cu(btsc) complexes and dissociation of Cu ions in the cytoplasm.
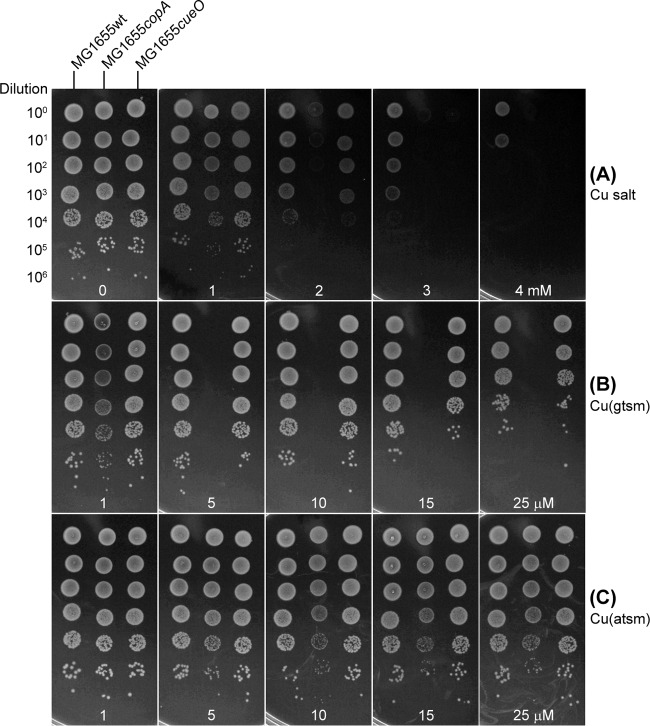
Activation of the PcopA promoter by Cu(gtsm) and Cu(atsm) implied that expression of copA and export of Cu(I) from the cytoplasm by CopA may mediate bacterial resistance to these Cu complexes. To test this proposal, we examined whether inactivation of copA in E. coli enhanced susceptibility to Cu(btsc) complexes. The effect of copA (and cueO) mutation on Cu tolerance in E. coli has been well characterized previously (26, 27). In this work, we confirmed that the copA mutant strain of E. coli was more sensitive to inhibition by Cu salts than the isogenic parent strain (Fig. 6A), consistent with the established role of CopA in tolerance to Cu ions. Importantly, the copA mutant was also sensitized to Cu(gtsm), and no bacterial growth was observed above 5 μM (Fig. 6B). This reduction in MIC compared with the wild type (MIC >25 μM) (Table 2) supports a role for Cu(I) ion efflux by CopA in the detoxification of Cu(gtsm).
FIG 6.
Susceptibility of E. coli mutant strains to Cu salt (A), Cu(gtsm) (B), and Cu(atsm) (C), as determined by efficiency of plating on solid medium. Serial dilutions of bacteria are shown on the left. Concentrations of the various Cu sources were indicated at the bottom. Treatment time was 24 h. The results shown are representative of at least three independent experiments.
A cueO mutant strain of E. coli that lacks the periplasmic cuprous oxidase (Fig. 5A) also displayed a Cu salt-sensitive phenotype (Fig. 6A). However, susceptibility of the cueO mutant to Cu(gtsm) was indistinguishable from that of the wild type (Fig. 6B). This result indicated that, although oxidation of toxic Cu(I) to Cu(II) by CueO was required for tolerance to free Cu ions and salts, it was not essential for resistance to Cu(gtsm). While CopA protects against cytoplasmic Cu toxicity, CueO operates in the periplasm (Fig. 5A). Thus, the apparent requirement for CopA but not CueO indicated that dissociation of bioavailable Cu ions from Cu(gtsm) occurred specifically in the cytoplasm and not in the periplasm.
In the case of Cu(atsm), there was no reduction in its MIC against the copA mutant (Fig. 6C). However, there was a noticeable decrease in colony size (Fig. 6C), which was consistent with a suppressed growth rate in liquid medium (see Fig. S4 in the supplemental material). These observations suggest that the CopA efflux pump may also confer tolerance to Cu(atsm), although the effect was subtle, presumably because generation of bioavailable Cu ions from this complex was inefficient (Fig. 5D). As expected, Cu(atsm) had no observable effect on the cueO mutant (Fig. 6C).
The toxic effects of Cu and other metal ions are known to be affected severely by chemical speciation or potential binding and buffering by components of the culture medium (28). Thus, it is often the case that the less complex the medium, the lower the MICs. The inhibitory effects of Cu(gtsm) and Cu(atsm) on the growth of the most sensitive mutant, the copA mutant, were similar when tested in LB or in M9 medium (see Fig. S4 in the supplemental material), suggesting that there was minimal release of free Cu ions from Cu(btsc) complexes in the extracellular medium.
Efficacy of Cu(btsc) complexes against MDR strains of N. gonorrhoeae.
The demonstrated action of Cu(gtsm) and Cu(atsm) as carriers of Cu ions and the established mechanism of Cu ion poisoning by mismetallation of enzymes and promotion of redox stress are distinct from the known modes of action of conventional antibiotics. Thus, we propose that these complexes may represent a promising new strategy for the treatment of antibiotic-resistant bacterial infections. Their low relative MICs against N. gonorrhoeae (Table 2) indicated that Cu(gtsm) and Cu(atsm) are highly potent against this bacterium. Therefore, we extended this work to test the potential for Cu(btsc) complexes to be used against multidrug-resistant (MDR) N. gonorrhoeae.
Cu(gtsm) showed robust activity against several antibiotic-resistant isolates of N. gonorrhoeae (Table 3), including the MDR strains F89 (29) and H041 (30), which are resistant to β-lactams (except carbapenems), fluoroquinolones, macrolides, tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, chloramphenicol, and nitrofurantoin. The MICs for these isolates were comparable to that for the drug-susceptible strain 1291 (Table 3), suggesting that the antibacterial activity of Cu(gtsm) was not diminished by enzymes and transporters that confer resistance to other antibiotics. In N. gonorrhoeae, these include the pilus secretin PilQ (31) and the MtrFCDE multidrug efflux pump system (32). To test this hypothesis, we determined the MICs of Cu(gtsm) for strains KH15 and DW120, which are isogenic mutants of the drug-susceptible strain FA19 that express higher basal levels of the MtrCDE pump (32). Although these strains showed increased resistance to multiple antibiotics and antimicrobial peptides compared with the parent strain (32), they were no less susceptible to Cu(gtsm) (Table 3).
Like Cu(gtsm), Cu(atsm) was also effective against MDR isolates and MtrFCDE-overexpressing strains of N. gonorrhoeae (Table 3). However, there were >2-fold increases in MICs compared to the antibiotic-susceptible strains (Table 3). This loss of efficacy indicated that Cu(atsm) may be a substrate for the MtrCDE efflux pump. Consistent with this proposal, inactivation of the mtrD gene (strain KH14) (33) led to a modest but reproducible decrease in the MIC of Cu(atsm) compared to the isogenic parent strain (FA19) (Table 3). The loss of mtrD did not have any effect on the MIC of Cu(gtsm) (Table 3).
Viability of host epithelial cells in the presence of antimicrobial doses of Cu(btsc) complexes.
To ascertain the potential of Cu and Cu(btsc) complexes as clinically useful antigonococcal agents, we examined whether Cu(gtsm) and Cu(atsm) exert an effect on the viability of cervical epithelial cells (ME-180 epithelial cells) in vitro. As shown in Fig. 7, incubation of ME-180 monolayers with up to 0.5 μM Cu(gtsm) and 5 μM Cu(atsm) (ca. 5× the MIC against N. gonorrhoeae) (Table 3) for 24 h did not result in loss of cell viability. There was no loss in cell numbers (Fig. 7), and more than 95% of these cells retained the ability to exclude trypan blue (Fig. 7). Under our experimental conditions, the ME-180 cell line did not withstand treatment with DMSO beyond 0.1% (vol/vol). Thus, higher concentrations of the Cu complexes were not tested because of poor solubility in the culture medium without DMSO. Nevertheless, previous work with human prostate epithelial cells had used up to 100 μM without any observable loss of viability (34), indicating a potential therapeutic index of 1,000 for N. gonorrhoeae. These results indicate that at antimicrobial doses, the test compounds exert minimal toxicity toward host cells.
FIG 7.
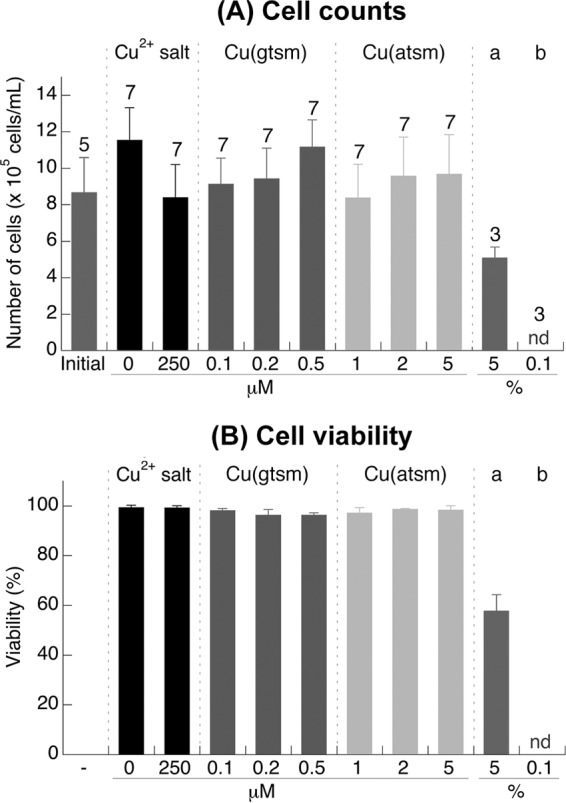
Effects of Cu(btsc) complexes on total cell counts (A) and cell viability (B) of ME-180 cervical epithelial cells. The treatment time was 24 h. The viability of cells in panel B was determined by using the set of cells used for counting in panel A. The number of replicates is shown above each column. Error bars represent standard deviations from the means. There was no statistically significant difference in the cell counts of viability from the different treatments. The results shown are representative of at least three independent experiments. DMSO (5% [vol/vol]) (columns a) and Triton X-100 (0.1% [vol/vol]) (columns b) were also included as positive controls for cell death.
DISCUSSION
Insight into the mode of antibacterial action of Cu(btsc) complexes. (i) Dynamics of Cu boosting by Cu(btsc) complexes.
The use of lipophilic ligands to deliver metal ions into cells is an established concept, particularly in the detection and intervention of cancers (35). In terms of antimicrobial applications, this concept has been demonstrated by the use of zinc pyrithione (Zn-PYT) as an antifungal in soaps and shampoos. The mode of action of Zn-PYT depends on transchelation with free Cu ions, presumably from the extracellular environment, and subsequent delivery of these Cu ions into the target organism (36). Coordination complexes that act as carriers for Cu ions are now being increasingly investigated as a novel approach to combat bacterial infections (9, 13–15).
The bis(thiosemicarbazone) family of Cu carriers displays anticancer activities (37). There is also interest in their potential as neurotherapeutics and as imaging agents for hypoxia. Our group and others have now demonstrated that Cu(btsc) complexes are also promising antimicrobials (13–15). However, while studies with mammalian systems have established the action of these complexes as agents that alter the bioavailability of Cu, studies with bacteria have not been equally conclusive, as the final Cu content of treated bacteria did not always correlate with survival or viability (13, 15). These studies have focused on the total amounts of Cu at the end point (e.g., the ICP OES measurements in Fig. 4A), but the present work suggests that the kinetics of Cu influx and potential efflux by detoxification systems must also be considered (Fig. 8). This model is likely to be universal to all lipophilic Cu carriers and not limited to those containing btsc ligands.
Our results with E. coli showed that both Cu(gtsm) and Cu(atsm) enter the bacterial cytoplasm more rapidly than do ionic Cu salts. These complexes are membrane permeant, probably via passive diffusion, as an uptake system has not been identified (38). As these complexes are uncharged, they would equilibrate rapidly across bacterial membranes and would not accumulate as intact molecules to a high intracellular concentration. However, reduction of the Cu(II) center and subsequent dissociation as bioavailable Cu(I) ion would generate a powerful mass action effect (Fig. 8). This thermodynamically driven influx of Cu ions may overwhelm basal Cu tolerance and cause Cu poisoning. By comparison, the more restricted entry of ionic Cu salts may allow activation of dedicated Cu detoxification mechanisms, which would enable the bacterial cell to amass and, more importantly, survive larger final amounts of total Cu.
The above model extends to the observed difference between the antibacterial activities of Cu(gtsm) and Cu(atsm). While studies with mammalian systems suggest little intracellular dissociation of bioavailable Cu ions from Cu(atsm) (21–23), our work with E. coli indicated that it does occur, although it is less efficient than the equivalent process from Cu(gtsm). We cannot discount possible variations in the rates of membrane penetration by the two complexes as a result of subtle differences in lipophilicity (39). Nevertheless, it is more likely that, as a consequence of a lower Cu(II)/Cu(I) midpoint potential for Cu(atsm) (Fig. 1), the rate of reduction (and, subsequently, dissociation) of Cu from Cu(atsm) is also lower. The bacterial cell thus has more time to respond and detoxify the excess Cu, resulting in a lower antibacterial potency of Cu(atsm).
Our results with MtrCDE-overexpressing strains of N. gonorrhoeae (Table 3) also indicate that potential efflux of Cu(gtsm) and Cu(atsm) as intact molecules out of the cytoplasm must not be overlooked. Cu(gtsm) rapidly and efficiently dissociates within the cytoplasm, and thus this complex may evade active export by promiscuous efflux transporters. By comparison, Cu(atsm) may linger as an intact molecule and be exported prior to dissociation and subsequent release of bioavailable Cu(I) ions (Fig. 8). This removal of Cu(atsm) from the cytoplasm, by either MtrCDE or other efflux systems, would further reduce its antibacterial efficacy. Although we have not tested this idea directly, it is likely that the AcrAB-TolC multidrug efflux pump system will contribute to the observed resistance of this bacterium to Cu(atsm) (40).
(ii) Correlation between bacterial physiology and susceptibility to Cu(btsc) complexes.
The antibacterial activity of Cu(btsc) complexes has now been tested against several important human pathogens, including S. pneumoniae, H. influenzae, uropathogenic E. coli, Salmonella (Table 2), M. tuberculosis (14), and S. aureus (15). Not all of these showed equal promise as targets for Cu(btsc) therapeutics, but all showed less susceptibility than N. gonorrhoeae. The explanation for these differences may relate to bacterial physiology.
Compared with most other bacterial pathogens, N. gonorrhoeae possesses a Cu detoxification system that is unusually underdeveloped. It consists of a single Cu efflux pump, CopA, and no additional cytoplasmic or periplasmic accessories (25). Importantly, Cu does not induce the expression of the copA gene. Thus, while gonococcal CopA may participate in general maintenance of Cu levels during regular metabolism (25, 41), it may be unable to confer resistance to severe Cu stress. This absence of an inducible resistance system coincides with the availability of targets of poisoning by Cu or Cu(btsc) complexes (Fig. 8). These targets include iron-sulfur (Fe-S) cluster-containing enzymes, such as coproporphyrinogen(III) oxidase in the pathway for heme biosynthesis (41). In addition, N. gonorrhoeae depends on two NADH dehydrogenases that are both susceptible to inhibition by Cu(btsc) complexes (13). As a consequence, N. gonorrhoeae displays hypersensitivity to inhibition by Cu salts and Cu(btsc) complexes, particularly Cu(gtsm) (Tables 1 to 3).
In E. coli, major targets of Cu poisoning are available, such as the Fe-S cluster enzymes fumarase in the tricarboxylic acid (TCA) cycle and isopropylmalate dehydratase in the pathway for branched-chain amino acid synthesis (42). However, this bacterium also possesses sophisticated and robust, inducible defenses against Cu toxicity (24). In addition, E. coli uses a versatile respiratory electron transport system and fermentative systems that are less sensitive to Cu(btsc) complexes. These arguments correlate well with our finding that E. coli is more resistant to inhibition by Cu(btsc) complexes, even Cu(gtsm) (Table 2). Similarly, the Cu detoxification system in S. pneumoniae is relatively well developed compared with the system in N. gonorrhoeae. However, S. pneumoniae has a relatively low dependence on Fe-S cluster enzymes and it does not contain a respiratory chain. This may explain the limited sensitivity of this bacterium to Cu(atsm) and Cu(gtsm) (Table 2). For these Cu-tolerant bacteria, the antimicrobial efficacy of Cu delivery agents might be enhanced if they are used in conjunction with a CopA antagonist to trap excess Cu ions.
Cu delivery agents as a novel concept for the topical treatment of N. gonorrhoeae.
Gonorrhea is the second most prevalent sexually transmissible infection worldwide, and management of this disease represents a significant challenge to public health. There is no vaccine, and thus, antibiotic treatment remains the only method to control the spread of infection. However, MDR strains have developed resistance to virtually all first-line antibiotics (43). Our work suggests that delivery of bioavailable Cu ions may represent a new approach to combat gonococcal infections. The application of copper in the cervix and vagina is an established concept, and intrauterine devices containing elemental copper are one of the most common and most effective nonhormonal contraceptives worldwide (44). Here, we showed that Cu salts and Cu(btsc) complexes did not affect the viability of cervical epithelial cells in vitro at concentrations that were inhibitory to the gonococcus (Fig. 7). Moreover, Cu salts and Cu(btsc) complexes were ineffective against lactic acid bacteria, as exemplified by Lactobacillus acidophilus (Table 2), suggesting that Cu delivery agents can be used to target gonococci without significantly affecting the commensal flora. Crucially, unlike other bacterial pathogens that have been identified as potential targets for treatments by Cu delivery agents, including M. tuberculosis (14), S. aureus (15), and Cryptococcus neoformans (9), N. gonorrhoeae is primarily an extracellular mucosal pathogen that colonizes surfaces of the genitourinary epithelium. Gonococcal infections are thus amenable to topical drug formulations and would bypass many of the challenges of a systemic route for the delivery of Cu.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge National Health and Medical Research Council (Australia) Program Grant 565526 to A.G.M., as well as NIH grant R21 AI 103270-02 and VA Merit Award 510 1BX000112-07 from the Biomedical Laboratory Research and Development Service of the Department of Veterans Affairs to W.M.S. K.Y.D. is supported by an Endeavor Research Fellowship (Department of Education and Training, Australia). W.M.S. is the recipient of a Senior Research Career Scientist Award from the Biomedical Laboratory Research and Development Service of the Department of Veterans Affairs. M.A.S. is supported by an Australian Research Council Future Fellowship (FT100100662).
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States government.
We have no competing financial interest to declare.
We thank B. Paterson (The University of Melbourne, Australia) for synthesis of Cu(btsc) compounds and M. Mostert (The University of Queensland, Australia) for technical assistance with ICP OES measurements. We are also grateful to M. Achard (The University of Queensland, Australia) for construction of E. coli mutant strains, M. Turner (The University of Queensland, Australia) for providing L. acidophilus, R. Nicholas (University of North Carolina—Chapel Hill) for N. gonorrhoeae strains F89 and HO41, and S. Satola (Emory University) for S. aureus strains.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01289-15.
REFERENCES
- 1.Xiao Z, Loughlin F, George GN, Howlett GJ, Wedd AG. 2004. C-terminal domain of the membrane copper transporter Ctr1 from Saccharomyces cerevisiae binds four Cu(I) ions as a cuprous-thiolate polynuclear cluster: sub-femtomolar Cu(I) affinity of three proteins involved in copper trafficking. J Am Chem Soc 126:3081–3090. doi: 10.1021/ja0390350. [DOI] [PubMed] [Google Scholar]
- 2.Foster AW, Osman D, Robinson NJ. 2014. Metal preferences and metallation. J Biol Chem 289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M, Morris H, Cronin MTD. 2005. Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 4.Osman D, Cavet JS. 2008. Copper homeostasis in bacteria. Adv Appl Microbiol 65:217–247. doi: 10.1016/S0065-2164(08)00608-4. [DOI] [PubMed] [Google Scholar]
- 5.Dollwet HHA, Sorenson JRJ. 1985. Historic uses of copper-compounds in medicine. Trace Elem Med 2:80–87. [Google Scholar]
- 6.Galhardi CM, Diniz YS, Faine LA, Rodrigues HG, Burneiko RC, Ribas BO, Novelli EL. 2004. Toxicity of copper intake: lipid profile, oxidative stress and susceptibility to renal dysfunction. Food Chem Toxicol 42:2053–2060. doi: 10.1016/j.fct.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 7.EspíritoSanto C, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, Grass G. 2011. Bacterial killing by dry metallic copper surfaces. Appl Environ Microbiol 77:794–802. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Festa RA, Helsel ME, Franz KJ, Thiele DJ. 2014. Exploiting innate immune cell activation of a copper-dependent antimicrobial agent during infection. Chem Biol 21:977–987. doi: 10.1016/j.chembiol.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem 284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HLT. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helsel ME, Franz KJ. 2015. Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Trans 44:8760–8770. doi: 10.1039/C5DT00634A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djoko KY, Paterson BM, Donnelly PS, McEwan AG. 2014. Antimicrobial effects of copper(II) bis(thiosemicarbazonato) complexes provide new insight into their biochemical mode of action. Metallomics 6:854–863. doi: 10.1039/c3mt00348e. [DOI] [PubMed] [Google Scholar]
- 14.Speer A, Shrestha TB, Bossmann SH, Basaraba RJ, Harber GJ, Michalek SM, Niederweis M, Kutsch O, Wolschendorf F. 2013. Copper-boosting compounds: a novel concept for antimycobacterial drug discovery. Antimicrob Agents Chemother 57:1089–1091. doi: 10.1128/AAC.01781-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haeili M, Moore C, Davis CJ, Cochran JB, Shah S, Shrestha TB, Zhang Y, Bossmann SH, Benjamin WH, Kutsch O, Wolschendorf F. 2014. Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:3727–3736. doi: 10.1128/AAC.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. 2011. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J Biol Chem 286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farinha MA, Kropinski AM. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol 172:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djoko KY, Donnelly PS, McEwan AG. 2014. Inhibition of respiratory complex I by copper(II)-bis(thiosemicarbazonato) complexes. Metallomics 6:2250–2259. doi: 10.1039/C4MT00226A. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun MW, Gennis RB. 1993. Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J Bacteriol 175:3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno H, Miyoshi H, Inoue M, Niidome Y, Iwamura H. 1996. Structural factors of rotenone required for inhibition of various NADH-ubiquinone oxidoreductases. Biochim Biophys Acta 1276:195–202. doi: 10.1016/0005-2728(96)00078-3. [DOI] [PubMed] [Google Scholar]
- 21.Xiao ZG, Donnelly PS, Zimmermann M, Wedd AG. 2008. Transfer of copper between bis(thiosemicarbazone) ligands and intracellular copper-binding proteins. Insights into mechanisms of copper uptake and hypoxia selectivity. Inorg Chem 47:4338–4347. doi: 10.1021/ic702440e. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly PS, Caragounis A, Du T, Laughton KM, Volitakis I, Cherny RA, Sharples RA, Hill AF, Li QX, Masters CL, Barnham KJ, White AR. 2008. Selective intracellular release of copper and zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide. J Biol Chem 283:4568–4577. doi: 10.1074/jbc.M705957200. [DOI] [PubMed] [Google Scholar]
- 23.Obata A, Yoshimi E, Waki A, Lewis JS, Oyama N, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. 2001. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann Nucl Med 15:499–504. doi: 10.1007/BF02988502. [DOI] [PubMed] [Google Scholar]
- 24.Rensing C, Grass G. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 25.Djoko KY, Franiek JA, Edwards JL, Falsetta ML, Kidd SP, Potter AJ, Chen NH, Apicella MA, Jennings MP, McEwan AG. 2012. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infect Immun 80:1065–1071. doi: 10.1128/IAI.06163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grass G, Rensing C. 2001. Genes involved in copper homeostasis in Escherichia coli. J Bacteriol 183:2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci U S A 97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haase H, Hebel S, Engelhardt G, Rink L. 2015. The biochemical effects of extracellular Zn(2+) and other metal ions are severely affected by their speciation in cell culture media. Metallomics 7:102–111. doi: 10.1039/C4MT00206G. [DOI] [PubMed] [Google Scholar]
- 29.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimuta K, Unemo M, Nakayama S, Morita-Ishihara T, Dorin M, Kawahata T, Ohnishi M. 2013. Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob Agents Chemother 57:5225–5232. doi: 10.1128/AAC.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiley DM, Jacobsson S, Tapsall JW, Nissen MD, Sloots TP, Unemo M. 2010. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J Antimicrob Chemother 65:2543–2547. doi: 10.1093/jac/dkq377. [DOI] [PubMed] [Google Scholar]
- 32.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 33.Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC, Shafer WM. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 34.Cater MA, Pearson HB, Wolyniec K, Klaver P, Bilandzic M, Paterson BM, Bush AI, Humbert PO, La Fontaine S, Donnelly PS, Haupt Y. 2013. Increasing intracellular bioavailable copper selectively targets prostate cancer cells. ACS Chem Biol 8:1621–1631. doi: 10.1021/cb400198p. [DOI] [PubMed] [Google Scholar]
- 35.Tisato F, Marzano C, Porchia M, Pellei M, Santini C. 2010. Copper in diseases and treatments, and copper-based anticancer strategies. Med Res Rev 30:708–749. doi: 10.1002/med.20174. [DOI] [PubMed] [Google Scholar]
- 36.Reeder NL, Kaplan J, Xu J, Youngquist RS, Wallace J, Hu P, Juhlin KD, Schwartz JR, Grant RA, Fieno A, Nemeth S, Reichling T, Tiesman JP, Mills T, Steinke M, Wang SL, Saunders CW. 2011. Zinc pyrithione inhibits yeast growth through copper influx and inactivation of iron-sulfur proteins. Antimicrob Agents Chemother 55:5753–5760. doi: 10.1128/AAC.00724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson BM, Donnelly PS. 2011. Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem Soc Rev 40:3005–3018. doi: 10.1039/c0cs00215a. [DOI] [PubMed] [Google Scholar]
- 38.Price KA, Crouch PJ, Volitakis I, Paterson BM, Lim S, Donnelly PS, White AR. 2011. Mechanisms controlling the cellular accumulation of copper bis(thiosemicarbazonato) complexes. Inorg Chem 50:9594–9605. doi: 10.1021/ic201334q. [DOI] [PubMed] [Google Scholar]
- 39.Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ. 2002. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem 7:249–259. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 40.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djoko KY, McEwan AG. 2013. Antimicrobial action of copper is amplified via inhibition of heme biosynthesis. ACS Chem Biol 8:2217–2223. doi: 10.1021/cb4002443. [DOI] [PubMed] [Google Scholar]
- 42.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.d'Arcangues C. 2007. Worldwide use of intrauterine devices for contraception. Contraception 75:S2–S7. doi: 10.1016/j.contraception.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Apicella MA. 1974. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis 130:619–625. doi: 10.1093/infdis/130.6.619. [DOI] [PubMed] [Google Scholar]
- 46.Nachamkin I, Cannon JG, Mittler RS. 1981. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect Immun 32:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider H, Griffiss JM, Williams GD, Pier GB. 1982. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol 128:13–22. [DOI] [PubMed] [Google Scholar]
- 48.Swanson J, Barrera O, Sola J, Boslego J. 1988. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med 168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danielsson D, Faruki H, Dyer D, Sparling PF. 1986. Recombination near the antibiotic resistance locus penB results in antigenic variation of gonococcal outer membrane protein I. Infect Immun 52:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maness MJ, Sparling PF. 1973. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis 128:321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.