Abstract
Iron acquisition is crucial for the growth of Aspergillus fumigatus. A. fumigatus biofilm formation occurs in vitro and in vivo and is associated with physiological changes. In this study, we assessed the effects of Fe chelators on biofilm formation and development. Deferiprone (DFP), deferasirox (DFS), and deferoxamine (DFM) were tested for MIC against a reference isolate via a broth macrodilution method. The metabolic effects (assessed by XTT [2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt]) on biofilm formation by conidia were studied upon exposure to DFP, DFM, DFP plus FeCl3, or FeCl3 alone. A preformed biofilm was exposed to DFP with or without FeCl3. The DFP and DFS MIC50 against planktonic A. fumigatus was 1,250 μM, and XTT gave the same result. DFM showed no planktonic inhibition at concentrations of ≤2,500 μM. By XTT testing, DFM concentrations of <1,250 μM had no effect, whereas 2,500 μM increased biofilms forming in A. fumigatus or preformed biofilms (P < 0.01). DFP at 156 to 2,500 μM inhibited biofilm formation (P < 0.01 to 0.001) in a dose-responsive manner. Biofilm formation with 625 μM DFP plus any concentration of FeCl3 was lower than that in the controls (P < 0.05 to 0.001). FeCl3 at ≥625 μM reversed the DFP inhibitory effect (P < 0.05 to 0.01), but the reversal was incomplete compared to the controls (P < 0.05 to 0.01). For preformed biofilms, DFP in the range of ≥625 to 1,250 μM was inhibitory compared to the controls (P < 0.01 to 0.001). FeCl3 at ≥625 μM overcame inhibition by 625 μM DFP (P < 0.001). FeCl3 alone at ≥156 μM stimulated biofilm formation (P < 0.05 to 0.001). Preformed A. fumigatus biofilm increased with 2,500 μM FeCl3 only (P < 0.05). In a strain survey, various susceptibilities of biofilms of A. fumigatus clinical isolates to DFP were noted. In conclusion, iron stimulates biofilm formation and preformed biofilms. Chelators can inhibit or enhance biofilms. Chelation may be a potential therapy for A. fumigatus, but we show here that chelators must be chosen carefully. Individual isolate susceptibility assessments may be needed.
INTRODUCTION
Aspergillus fumigatus, the ubiquitous saprophytic mold, frequently causes respiratory tract infections, including invasive pulmonary aspergillosis and allergic bronchopulmonary aspergillosis (1). A. fumigatus disease occurs most frequently in immunocompromised individuals, such as bone marrow transplant and other neutropenic patients, solid organ transplant recipients (2), and in those with cystic fibrosis (1, 3), chronic granulomatous disease, or chronic obstructive pulmonary disease (1, 4). Despite therapeutic advances in the development and administration of antifungals, mortality from A. fumigatus infection remains high (5).
A. fumigatus has shown, in vivo and in vitro, the ability to form biofilms, or complex aggregates of organisms embedded within a polymer-rich extracellular matrix, which demonstrate increased antimicrobial resistance (32). Thus, there is a need for other therapeutic methods with mechanisms that differ from those of the common antifungal targets of ergosterol or cell wall biosynthesis. Given the clinical prevalence of A. fumigatus biofilms (6), new therapies should demonstrate efficacy against A. fumigatus biofilms.
One potential mechanistic target lies in iron (Fe) acquisition, as it represents a key factor in A. fumigatus pathogenicity (7, 8). Previous data indicate that iron chelators can inhibit the planktonic conidial growth of A. fumigatus in vitro (9, 10). This has also been shown in murine models to serve as a treatment for aspergillosis or mucormycosis in combination with other antifungals (11). However, some iron chelators have been shown to enhance conidial growth (10), which makes the identification of therapeutic chelators especially important. Because of the lack of information on the effects of Fe chelators on A. fumigatus biofilms, we assessed the effects of different Fe chelators on the establishment and development of A. fumigatus biofilms in vitro.
(This study was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2014, under abstract no. M-436 [12].)
MATERIALS AND METHODS
Iron chelators and FeCl3.
Three iron chelators were studied. These were deferasirox (DFS) (Novartis Pharmaceuticals, East Hanover, NJ), deferoxamine (DFM) (APP Pharmaceuticals, Schaumburg, IL), and deferiprone (DFP) (Sigma-Aldrich Co., St. Louis, MO). Ferric chloride (FeCl3) was obtained from Sigma-Aldrich Co.
A. fumigatus.
One patient isolate that is known to be virulent, A. fumigatus 10AF, was used as a reference isolate (13, 14). Nine other clinical isolates were studied in comparison (see Results); all 10 were confirmed by molecular methods to be A. fumigatus sensu stricto (15). Long-term storage was performed as described previously (16). Four-day-old conidia were harvested and stored in 0.05% Tween 80 (J. T. Baker Chemical Co., Phillipsburg, NJ) in saline (0.9% NaCl) (Baxter Healthcare Corp., Deerfield, IL), as described previously (14).
Assessment of iron chelator effects on planktonic A. fumigatus growth.
MIC testing of iron chelators against planktonic A. fumigatus growth was performed using the CLSI M38-A2 protocol for broth macrodilution (17). In brief, an inoculum of 3 × 105 conidia was added to fresh RPMI 1640 medium in 5-ml polystyrene round-bottom tubes (BD Biosciences, Durham, NC). A 2-fold dilution series of DFP, DFS, DFM, or sterile H2O only (controls) in the medium was tested. The tubes were incubated at 37°C with shaking (100 rpm) for 24 h, and growth was determined visually. Measurement of metabolic activity by XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt) assay was also performed.
In vitro model of examining iron chelator and/or FeCl3 effects on A. fumigatus biofilm formation.
We chose to first test the effects of DFP and DFM on the development of A. fumigatus biofilms. Our laboratory first utilized and reported on a 12-well assay for studies of A. fumigatus biofilm development (18). In later studies, an assay using 96-well plates was explored. We found that our 96-well assay gives the same results if the conditions in both systems are closely controlled, as described in the studies. The 12-well assay is useful for the recovery of sufficient materials from the wells in various analyses at the conclusion of the experiments. The 96-well assay is both sparing of reagents and allows more replicates to be studied concurrently, which increases the chances of statistical significance occurring when differences between groups are small, e.g., at the inflection point of a dose-response curve. An analogous 96-well assay used to assay antifungal drugs, and differing in specifics from ours, was previously described (19). The interpretations made here are based on 7 experiments on biofilm formation in the 12-well format and 9 in the 96-well format, which included 10AF as a reference control.
For the 12-well assay, to form biofilms, sterile polystyrene disks (BioSurface Technologies, Bozeman, MT) were placed in 12-well tissue culture plates (Corning, Inc., Corning, NY). Each well contained 2.7 ml of fresh RPMI 1640 medium plus 0.3 ml of DFP or DFM in phosphate-buffered saline (PBS) (pH 7.3 to 7.5) (Lonza) or PBS only (controls), and 105 conidia/ml. The final chelator concentrations were 2-fold dilutions ranging from 2,500 μM to 39 μM. The disks were incubated for 16 h at 37°C to allow A. fumigatus cells to form biofilm in the presence of chelators. After this initial 16 h, nonadherent cells were removed through washing with PBS, and the disks were transferred to 3 ml of fresh RPMI 1640 medium with or without 10% fetal bovine serum (FBS) without iron chelators for an additional 24 h of growth at 37°C before quantification of fungal growth by the XTT assay. Experiments with FeCl3 supplementation involved growth of A. fumigatus for 16 h in the presence of 625 μM DFP alone, 625 μM DFP plus FeCl3 (2,500 μM to 156 μM), or FeCl3 alone (2,500 μM to 156 μM) and removal of the disks to fresh medium alone, followed by 24 h of additional growth at 37°C with shaking (70 rpm).
For the 96-well assay, after removing the Tween from the conidia by two washes in PBS, biofilms were formed on the flat-bottom wells (Costar 3596; Corning), and the same sequences, time intervals (with washing of the wells with PBS before the introduction of fresh medium at 16 h), chelator concentrations, media (FBS studies not done), and shaking protocols were used. The well volume was 0.2 ml, and the inoculum contained 2 × 103 conidia/well.
Preformed (i.e., established) biofilm experimental design.
In the 12-well assay, to allow the A. fumigatus to form biofilms, 105 conidia/ml in 3 ml of fresh RPMI 1640 medium were grown on flat polystyrene disks in 12-well tissue culture plates for 16 h. Preformed A. fumigatus biofilms were washed and then transferred to new wells containing 2.7 ml of RPMI 1640 medium and 0.3 ml of various concentrations of DFP (2,500 μM to 39 μM), DFM (2,500 μM to 156 μM), or PBS (controls) for an additional 24 h at 37°C with shaking (70 rpm). After incubation, an XTT assay was performed to measure biofilm metabolic activity. The experiments with FeCl3 were performed by exposing the preformed biofilms to 625 μM DFP, 625 μM DFP plus FeCl3 (2,500 μM to 39 μM), or FeCl3 alone (2,500 μM to 39 μM).
In the 96-well assay, the formation of preformed biofilm was performed as described for the biofilm formation assay. The formed biofilm was then washed in fresh medium and exposed to the same chelator concentrations, times, temperatures, and shaking conditions as described above. The interpretations made here are based on 15 experiments on preformed biofilm in the 12-well format and 20 in the 96-well format, including 10AF as a reference control.
XTT assay.
An assessment of the effects of iron chelators alone or in the presence of FeCl3 on the growth of A. fumigatus biofilm was done using an XTT assay. The tetrazolium salt XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt) (Sigma, St. Louis, MO) was used to measure the metabolic activity of A. fumigatus. Whereas XTT is a direct measure of the metabolic activity of cells, previous studies of A. fumigatus have indicated that XTT results are linear with mass and equated the XTT result with dry weight (19–21). Our confocal microscopy studies also address this point.
In the 12-well assay, in brief, disks, as described above, were rinsed 3 times in sterile saline and transferred to wells containing 3 ml of sterile PBS. Menadione (Sigma) (0.85 g) was added to 5 ml of acetone, mixed in a 1:11 ratio of menadione to XTT (1 mg/ml). The XTT-menadione solution (180 μl) was added to each well, and the plates were incubated in the dark for 2 h at 37°C. Following incubation, the contents of the wells were collected and centrifuged at 13,300 × g for 10 min, and the absorbance at 490 nm of the supernatant was determined with a spectrophotometer (Genesys 20; Thermo Scientific, Waltham, MA). For the XTT assay of planktonic A. fumigatus growth, 180 μl of the XTT-menadione solution was added directly to the 3 ml of RPMI 1640 in which the A. fumigatus was growing, and the tubes were incubated for 2 h at 37°C in the dark. For the 96-well biofilm assays, after washing, the concentrations used for the assay were 200 μg/ml XTT with 40 μM menadione (0.2 ml/well). After the 2 h incubation, the plates were centrifuged, and 0.15 ml of supernatant/well was transferred to a microtiter plate and the A490 determined with a microplate reader (Dynex, Chantilly, VA).
Confocal laser scanning microscopy.
To determine the effects on the morphology and architecture of A. fumigatus biofilms, biofilms were formed on disks as described above. The biofilms were challenged with chelators. After incubation at 37°C, the disks were washed three times in sterile PBS and stained using a fluorescent stain (FUN 1; Invitrogen Molecular Probes, Eugene, OR), prepared according to the manufacturer's instructions. One microliter of FUN 1 from a 10 mM stock was mixed in 1 ml of PBS. Staining was performed as previously described (22, 23). Three drops of the mixture was added on the top of the biofilm, which was then mounted on a glass slide and covered with a glass coverslip (22 by 22 mm). The disks were incubated for 45 min at 37°C in the dark. The FUN 1 stain was used to visualize the morphology of A. fumigatus biofilm, a bright green cytoplasmic stain produced after passive diffusion; viability by FUN 1 staining was not assessed.
Sections on the xy plane were taken at 1-μm intervals along the z axis to determine the depth of the biofilms. Microscopic visualization and acquisition of biofilm images were conducted at the Stanford Biofilm Research Center using an upright Leica TCDSP2 confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) equipped with an argon/krypton laser and detectors and filter sets for monitoring green fluorescence (excitation, 480 nm; emission, 517 nm). Images were obtained using 63× 1.4 Plan-Apochromat differential interference contrast (DIC) (Leica, Heidelberg, Germany) objectives. Multichannel simulated fluorescence projection (SFP) (a shadow projection) images and vertical cross sections of the biofilm were generated using the IMARIS software package (Bitplane AG, Zürich, Switzerland). The images were processed for display using the Photoshop software (Adobe, Mt. View, CA). Representative images were taken.
Serum effect.
Our studies presented here were, as were previously published studies of chelators on planktonic growth (10), done in serum-free medium to avoid the varied amounts of iron that would be present in various sera and the presence of competing endogenous iron-binding moieties that might be present in serum. However, preliminary experiments using medium supplemented with 10% FBS (Gibco, Grand Island, NY) (data not shown) indicated that although the presence of serum sharply reduced the chelation effect, there was some residual activity on the formation or further development of A. fumigatus biofilm only at the upper end of the range tested, 2,500 μM DFP (P < 0.01, conidia forming biofilm; P < 0.05, preformed biofilm). This preliminary result can be compared with the studies of biofilm formation or the growth of preformed biofilm in RPMI 1640 medium without added serum, detailed in Results.
Statistical analysis.
The 12-well assays included triplicate wells for each study group for analysis, and the 96-well assays included 7 replicate wells per group. Statistical differences were evaluated with one-way analysis of variance (ANOVA), followed by a Tukey post hoc test. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) was used for calculations. Statistical significance was considered at a P value of <0.05.
RESULTS
Chelator effects on planktonic A. fumigatus growth.
MIC tests of DFP and DFS against planktonic A. fumigatus showed 50% and 100% inhibition of growth by visual assessment at 1,250 μM and >2,500 μM, respectively. An evaluation of the inhibition of growth by the XTT assay showed identical results. MIC testing and the XTT assay with DFM showed no inhibition or stimulation of planktonic growth at any concentration tested (2-fold dilutions from 2,500 μM to 4.8 μM) (data not shown).
DFM effects on A. fumigatus biofilm.
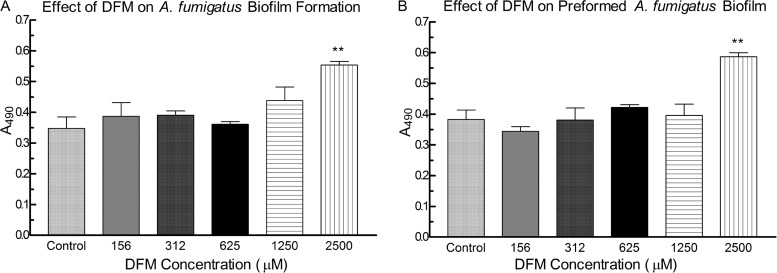
Fig. 1A and B show the effects of DFM on the metabolic activity of A. fumigatus forming biofilm and preformed A. fumigatus biofilm, respectively. Compared to untreated controls, DFM at concentrations of <1,250 μM did not have a positive or negative impact, whereas 2,500 μM significantly increased the metabolic activity of A. fumigatus forming biofilm or preformed biofilm (P < 0.01, both groups). At a concentration of 2,500 μM, DFM augmented A. fumigatus biofilm formation significantly compared to that with concentrations ranging from 156 μM to 625 μM (P < 0.05, 156 μM or 312 μM; P < 0.01, 625 μM) (Fig. 1A) and showed greater stimulation of preformed A. fumigatus biofilm than that with all other concentrations tested (P < 0.001, 156 μM; P < 0.01, 312 μM to 1,250 μM) (Fig. 1B).
FIG 1.
Effect of deferoxamine (DFM) on A. fumigatus biofilm formation and preformed A. fumigatus biofilm. (A) A. fumigatus conidia were exposed to DFM for 16 h, the disk was moved to new medium and incubated for an additional 24 h, and growth was assayed using XTT reduction, which was assessed by measuring the absorbance at 490 nm. (B) Preformed A. fumigatus biofilms were exposed to DFM for 24 h, and the resulting growth was assessed by XTT reduction by absorbance at 490 nm. Twelve-well assays were used. Each condition was performed in triplicate, and the results are pooled from two separate experiments on different days. Two asterisks indicate a significant P value (<0.01) compared to A. fumigatus control. (A) A DFM concentration of 2,500 μM enhanced A. fumigatus biofilm formation significantly more than 156 μM (P < 0.05), 312 μM (P < 0.05), and 625 μM (P < 0.01). (B) For preformed biofilms, 2,500 μM DFM was significantly more stimulatory than all other concentrations tested (P < 0.001, 156 μM; P < 0.01, 312 μM to 2,500 μM). The values shown are the means ± the standard deviations.
Effects of DFP on A. fumigatus biofilm.
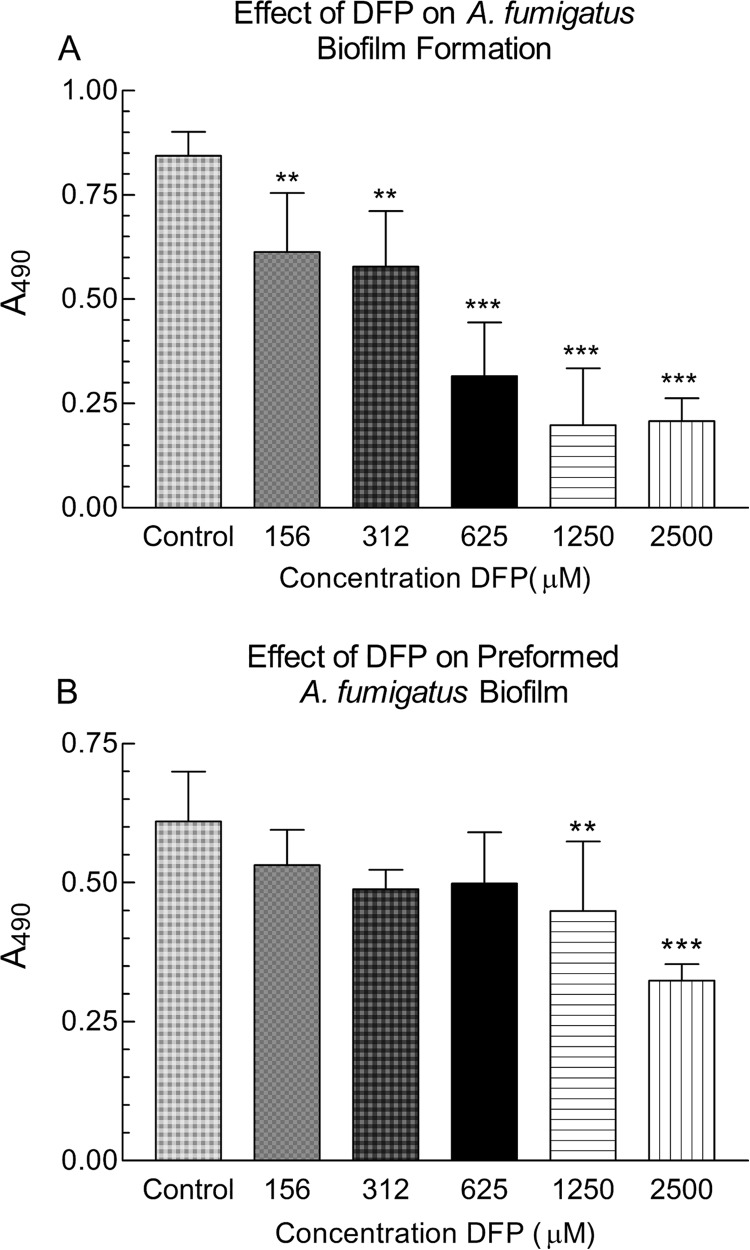
In these studies, we examined the effects of DFP on the formation of biofilm by A. fumigatus and its effect on preformed A. fumigatus biofilm. In representative experiments, DFP at concentrations from 156 μM to 2,500 μM significantly inhibited the metabolic activity of A. fumigatus forming biofilm in a dose-responsive manner (P < 0.01, 156 μM and 312 μM; P < 0.001, 625 to 2,500 μM) (Fig. 2A). Concentrations of <156 μM were rarely inhibitory in repeated experiments. Like the effect on formation of A. fumigatus biofilm, DFP at 1,250 μM and 2,500 μM significantly inhibited preformed A. fumigatus biofilms (P < 0.01 and 0.001, respectively) (Fig. 2B). In repeats of this experiment with this isolate, significant inhibition by 625 μM was commonly seen.
FIG 2.
Effect of deferiprone (DFP) on the formation or further development of A. fumigatus biofilm. (A) A. fumigatus conidia were exposed to DFP (or not exposed [control]) for 16 h, before 24 h of further growth in fresh DFP-free medium. (B) Preformed A. fumigatus biofilms were exposed to DFP (concentrations shown) for 24 h or not exposed (control). Ninety-six-well assays were used. Two asterisks denote a significant P value of <0.01, and three asterisks denote a significant P value of <0.001, compared to the experimental condition containing A. fumigatus alone. The values shown are the means ± the standard deviations.
Supplemental FeCl3 and DFP inhibition of A. fumigatus biofilm.
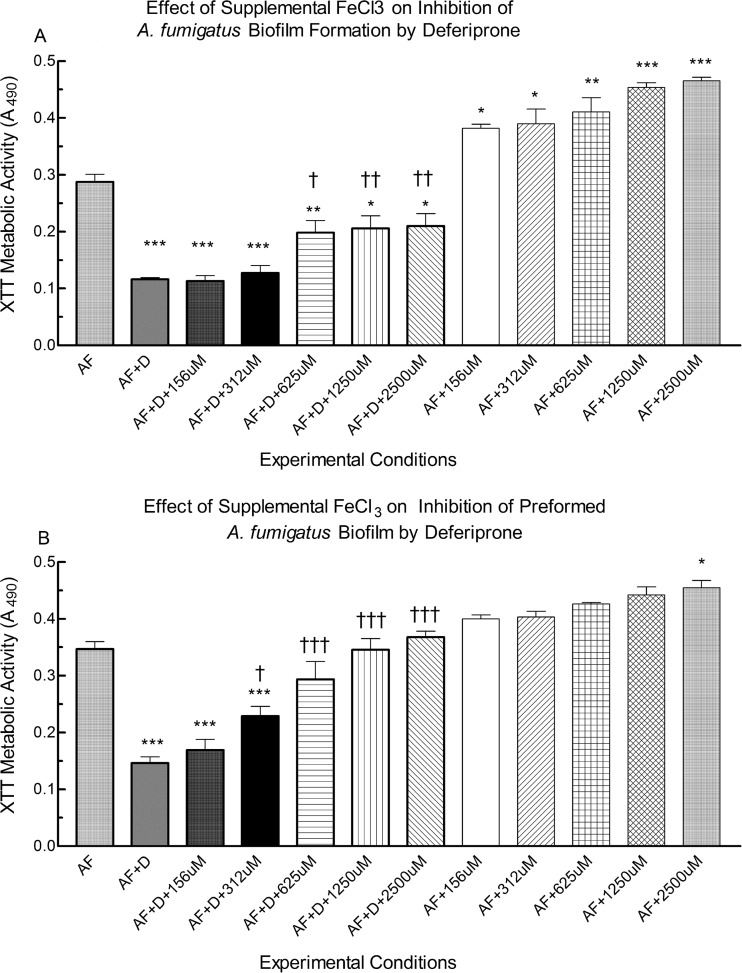
To confirm that the inhibition of biofilm by DFP was a result of the chelation of iron, we sought to overcome inhibition by adding supplemental iron. Figure 3A illustrates the effects of FeCl3 supplementation on the inhibition of A. fumigatus biofilm formation by a DFP concentration we had found to be inhibitory. When exposed to FeCl3 alone at any concentration tested, the metabolic activity of A. fumigatus forming biofilm significantly increased compared to that with A. fumigatus controls growing without supplemental iron (P < 0.05, 156 μM and 312 μM; P < 0.01, 625 μM; P < 0.001, 1,250 μM and 2,500 μM FeCl3) (Fig. 3A). In the presence of 625 μM DFP plus any concentration of FeCl3, A. fumigatus biofilm formation was significantly lower than that in the A. fumigatus controls (i.e., absence of DFP or FeCl3) (P < 0.001, 156 μM and 312 μM FeCl3; P < 0.01, 625 μM; P < 0.05, 1,250 μM or 2,500 μM). However, FeCl3 concentrations of ≥625 μM partially overcame inhibition of biofilm formation by DFP compared to A. fumigatus plus 625 μM DFP alone (P < 0.05, 625 μM FeCl3; P < 0.01, 1,250 μM and 2,500 μM FeCl3).
FIG 3.
Effect of iron on the DFP inhibition of formation or further development of A. fumigatus biofilm. (A) A. fumigatus conidia were exposed to 625 μM DFP (D) alone, 625 μM DFP plus various concentrations of FeCl3 (156 to 2,500 μM, shown as μM), or FeCl3 alone for 16 h or not exposed (AF), before 24 h of further growth in fresh medium without added DFP or FeCl3. (B) Preformed (16 h) A. fumigatus biofilms were exposed to 625 μM DFP alone, 625 μM DFP plus various concentrations of FeCl3 (assays with 39 to 78 μM FeCl3 not different than those with 156 μM; not shown), or FeCl3 alone for 24 h or not exposed. Biofilms were quantified via XTT assay with absorbance at 490 nm. Twelve-well assays were used. Each condition was performed in triplicate, and the results were pooled from two to five separate experiments on different days. A single asterisk or dagger denotes a significant P value of <0.05, two asterisks or daggers denote a significant P value of <0.01, and three asterisks or daggers denote a significant P value of <0.001, compared to the experimental condition containing A. fumigatus alone (AF) (asterisks) or A. fumigatus plus DFP (daggers). The values shown are the means ± the standard deviations.
Figure 3B shows the effects of various concentrations of FeCl3 (2,500 μM to 39 μM) on preformed A. fumigatus biofilms growing with or without 625 μM DFP. In the presence of DFP plus ≤312 μM FeCl3, preformed biofilm had lower metabolic activity than that of the untreated controls (P < 0.001, all comparisons). However, FeCl3 at ≥312 μM partially or fully overcame DFP inhibition of preformed biofilm (P < 0.05, 312 μM FeCl3; P < 0.001, ≥625 μM FeCl3, compared to A. fumigatus plus DFP). Although 312 μM FeCl3 only partially overcame DFP inhibition, FeCl3 at concentrations of ≥625 μM completely reversed inhibition, restoring preformed biofilms to levels similar to those of the A. fumigatus controls. In the presence of FeCl3 alone, only the 2,500 μM concentration significantly augmented further development of preformed A. fumigatus biofilm (P < 0.05).
Confocal microscopy analysis.
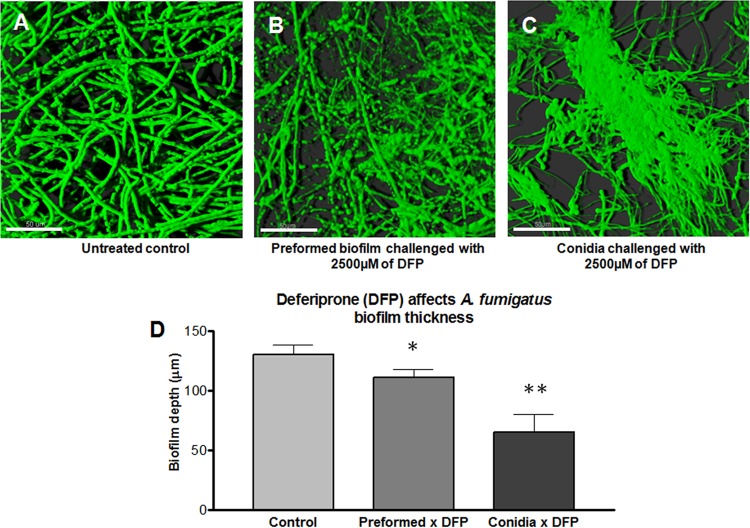
The effect of the chelator DFP on A. fumigatus biofilm thickness and morphology was assessed using confocal microscopy. The thickness results for the A. fumigatus biofilm after exposure of the conidia to DFP for 16 h is shown (Fig. 4). DFP resulted in a significant reduction in the fungal biofilm thickness compared to the untreated control (Fig. 4A, C, and D). Untreated biofilms showed an architecture formed by a dense filamentous multicellular structure with acute-angle dichotomous branching (Fig. 4A). Figure 4C shows the effect of DFP on A. fumigatus biofilm formation. Treatment with DFP resulted in a decreased number of hyphae, the presence of some “bulging” structures distributed throughout the disperse filaments, resulting in a reduction in filamentation, and the presence of some “glued” hyphae without a clear separation of the filamentous elements (Fig. 4C).
FIG 4.
Confocal laser scanning microscope (CLSM) images of A. fumigatus biofilm grown on polycarbonate disk surface. (A) Horizontal (xy) view of a reconstructed three-dimensional (3D) image of A. fumigatus biofilm (untreated control). (B) Horizontal (xy) view of a reconstructed 3D image of A. fumigatus preformed biofilm challenged with 2,500 μg/ml DFP. (C) Horizontal (xy) view of a reconstructed 3D image of A. fumigatus biofilm formation challenged with 2,500 μg/ml DFP. (D) Effect of DFP on A. fumigatus biofilm thickness. Assays were performed in triplicate, and images were taken from three different fields from each sample stained with FUN 1. The results are representative of two different experiments for each condition tested. The values shown are the means ± the standard deviations. The control, preformed × DFP, and conidia × DFP correspond to the conditions shown in panels A to C, respectively. One asterisk indicates a P value of <0.01, and two asterisks indicate a P value of <0.001 for the biofilm thickness compared to the untreated control. Magnification, ×63. Scale bar, 50 μm.
As was indicated by the biofilm thickness data (Fig. 4D), treatment of preformed A. fumigatus biofilm with DFP resulted in thinning of the hyphal mat. Many round structures, many of which occurred at the tips, were noted (Fig. 4B).
Survey of A. fumigatus isolates.
The effects of chelators on several A. fumigatus isolates, with respect to biofilm inhibition, were studied (Table 1). This revealed a polymorphism in susceptibility to DFP over a broad range of DFP concentrations. Of note, biofilm formation was almost always more susceptible than preformed biofilm.
TABLE 1.
Survey of chelator effect on A. fumigatus isolates
| Isolate | Lowest concn of DFP (μM) causing significant inhibition of: |
|
|---|---|---|
| A. fumigatus biofilm formation | Preformed A. fumigatus biofilm | |
| 10AF | 156 | 625–1,250 |
| 2 | 625 | 1,250 |
| 3 | ≤156 | 625 |
| 4 | 1,250 | >2,500 |
| 5 | ≤156 | 625 |
| 6 | 1,250 | 1,250 |
| 7 | 156 | 1,250 |
| 8 | 156 | 312 |
| 9 | 312 | 1,250 |
| 10 | 312 | 2,500 |
The results are based on 1 or 2 experiments for each isolate (except for 10AF, with ≥7 experiments).
DISCUSSION
The availability of iron and the ability to take up and utilize available iron are essential for the viability of A. fumigatus. Iron homeostasis is related to ≥24 genes in A. fumigatus, and iron uptake is done through a low-affinity ferrous iron system or one of two high-affinity systems, reductive assimilation or siderophores (24). Although essential for growth, iron also plays a critical role in the virulence of the organism, particularly in its resistance to reactive oxygen species produced by phagocytic cells, and in its capacity to adapt to hypoxic conditions during infection (24). The host response to infection includes mechanisms for the limitation of available iron. A. fumigatus can remove iron from serum transferrin (25), and lactoferrin produced in polymorphonuclear leukocytes (PMNs) has been shown to be statically inhibitory to A. fumigatus (9).
The critical role of iron to the viability and virulence of A. fumigatus makes it an attractive target for the control of infection, particularly through the use of iron chelators (26). In vivo studies have shown that the choice of chelator is crucial. DFM therapy has proven to be detrimental in models of mucormycosis and, to a lesser degree, of aspergillosis (8, 11, 27, 28). However, the use of DFS or DFP has been shown to limit fungal growth in vivo (8, 29).
Because of the potential importance of biofilms of A. fumigatus during infection (6, 30), particularly in airways, we have examined how iron depletion via a chelator affects biofilm formation and development in vitro. We show that iron chelators have differential effects on the establishment and further development of A. fumigatus biofilms, a finding that is consistent with previous reports of A. fumigatus growing planktonically (10, 11). We found that DFM at high concentrations stimulated biofilm formation and development in a manner similar to that with the supplementation of the medium with FeCl3. In contrast, we found that DFP inhibited both biofilm formation and the continued development of preformed biofilm. Furthermore, our results demonstrate that this inhibition is the result of iron chelation by the DFP, in that supplementation of the medium with FeCl3 reversed the inhibition. Serum, likely due to the presence of iron-containing molecules, such as transferrin, blunts the inhibitory effects of iron chelation by DFP at DFP concentrations of <2,500 μM. Last, excess Fe3+ enhanced biofilm, and concentrations sufficient to augment biofilm establishment were lower than those necessary to further stimulate preformed biofilm.
Overall, we found that iron chelators can either inhibit or enhance the formation and growth of biofilm, and the magnitude of these effects differs between preformed biofilm of and biofilm formation by A. fumigatus. However, the inhibition of biofilm formation and growth by an iron chelator can be overcome by supplementing the medium with Fe3+. We believe that the enhancement of biofilm by DFM is due to an ability of A. fumigatus to acquire the Fe-chelator complex via siderophore-like mechanisms, allowing the organism to access greater amounts of iron.
A range of susceptibilities of biofilm to DFP was discovered. This is similar to the polymorphism seen when surveying random clinical A. fumigatus isolates for susceptibility to antifungal drugs (2, 17). The greater susceptibility of biofilm formation than that of preformed biofilm to a chelator is consistent with the differential effects of Pseudomonas aeruginosa cells (or culture filtrates) on A. fumigatus biofilm demonstrated in our previous studies (18).
Iron chelation is a potential beneficial therapy for aspergillosis infections, including those involving biofilms, as in chronic pulmonary infections, a situation in which local, (i.e., nonsystemic) use may be a possibility. An attractive advantage is the clinical experience already shown with chelators in the therapy of iron overload disorders, including their pharmacology and toxicology (31). That favorable systemic chelator experience has been accumulated even in the presence of the complicated in vivo iron-containing and iron-denying environments. Use in lung infections may have the prospect of fewer host iron-denying competitors locally and an ancillary therapeutic effect on other concurrent pathogens. However, the specific chelators must be carefully chosen, as shown in this study, so as to mitigate fungal pathogenicity. Moreover, susceptibility testing in vitro may be required to predict which isolates are the best candidates for chelation therapy. A study of the interaction of chelators with antifungals against A. fumigatus biofilm is of considerable interest.
ACKNOWLEDGMENTS
These studies were partially supported by a grant from the Child Health Research Institute, Stanford Transdisciplinary Initiatives Program, and a gift from John Flatley. J.A.F. was partially supported by a grant from the Brazilian National Council for Scientific and Technological Development (CNPq). H.N. was supported in part by a grant from the Scientific and Technological Research Council of Turkey (TUBITAK).
REFERENCES
- 1.Kousha M, Tadi R, Soubani AO. 2011. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF, Infectious Diseases Society of America. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 3.Amin R, Dupuis A, Aaron SD, Ratjen F. 2010. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 137:171–176. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
- 4.Hsu JL, Ruoss SJ, Bower ND, Lin M, Holodniy M, Stevens DA. 2011. Diagnosing invasive fungal disease in critically ill patients. Crit Rev Microbiol 37:277–312. doi: 10.3109/1040841X.2011.581223. [DOI] [PubMed] [Google Scholar]
- 5.Lin SJ, Schranz J, Teutsch SM. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 6.Müller FM, Seidler M, Beauvais A. 2011. Aspergillus fumigatus biofilms in the clinical setting. Med Mycol 49(Suppl 1):S96–S100. doi: 10.3109/13693786.2010.502190. [DOI] [PubMed] [Google Scholar]
- 7.Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. 2005. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding l-ornithine N5-oxygenase, is required for virulence. Infect Immun 73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal SM Jr, Roy S, Vareechon C, Carrion S, Clark H, Lopez-Berges MS, Di Pietro A, Schrettl M, Beckmann N, Redl B, Haas H, Pearlman E. 2013. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog 9:e1003436. doi: 10.1371/journal.ppat.1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 178:6367–6373. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
- 10.Zarember KA, Cruz AR, Huang CY, Gallin JI. 2009. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus. Antimicrob Agents Chemother 53:2654–2656. doi: 10.1128/AAC.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim AS, Gebremariam T, Luo G, Fu Y, French SW, Edwards JE Jr, Spellberg B. 2011. Combination therapy of murine mucormycosis or aspergillosis with iron chelation, polyenes, and echinocandins. Antimicrob Agents Chemother 55:1768–1770. doi: 10.1128/AAC.01577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penner JC, Ferreira JA, Martinez M, Chen V, Hsu JL, Clemons KV, Stevens DA. 2014. Effect of iron (Fe) chelators on the formation and development of Aspergillus fumigatus (AF) biofilm (BF), abstr M-436 Abstr 54th Intersci Conf Antimicrob Agents Chemother, 5 to 9 September 2014, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning DW, Clemons KV, Hanson LH, Stevens DA. 1990. Restriction endonuclease analysis of total cellular DNA of Aspergillus fumigatus isolates of geographically and epidemiologically diverse origin. J Infect Dis 162:1151–1158. doi: 10.1093/infdis/162.5.1151. [DOI] [PubMed] [Google Scholar]
- 14.Denning DW, Stevens DA. 1991. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother 35:1329–1333. doi: 10.1128/AAC.35.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabino R, Ferreira JAG, Moss R, Valente J, Verissimo C, Carolino E, Clemons KV, Everson C, Banaei N, Penner J, Stevens DA. 2015. Molecular epidemiology of Aspergillus from cystic fibrosis patients. J Cyst Fibros 14:474–481. doi: 10.1016/j.jcf.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Denning DW, Clemons KV, Stevens DA. 1992. Quantitative preservation of viability of Aspergillus fumigatus. J Med Vet Mycol 30:485–488. doi: 10.1080/02681219280000661. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Ferreira JAG, Penner JC, Moss RB, Haagensen JAJ, Clemons KV, Spormann AM, Nazik H, Cohen K, Banaei N, Carolino E, Stevens DA. 2015. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One 10:e0134692. doi: 10.1371/journal.pone.0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96 well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meletiadis J, Mouton JW, Meis JFGM, Bouman BA, Donnelly JP, Verweij PE, EUROFUNG Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 39:3402–3408. doi: 10.1128/JCM.39.9.3402-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss BJ, Kim Y, Nandakumar MP, Marten MR. 2008. Quantifying metabolic activity of filamentous fungi using a colorimetric XTT assay. Biotechnol Prog 24:780–783. doi: 10.1021/bp070334t. [DOI] [PubMed] [Google Scholar]
- 22.Mowat E, Butcher J, Lang S, Williams C, Ramage G. 2007. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol 56:1205–1212. doi: 10.1099/jmm.0.47247-0. [DOI] [PubMed] [Google Scholar]
- 23.Seidler MJ, Salvenmoser S, Muller FMC. 2008. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother 52:4130–4136. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas H. 2012. Iron—a key nexus in the virulence of Aspergillus fumigatus. Front Microbiol 3:28. doi: 10.3389/fmicb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hissen AH, Chow JM, Pinto LJ, Moore MM. 2004. Survival of Aspergillus fumigatus in serum involves removal of iron from transferrin: the role of siderophores. Infect Immun 72:1402–1408. doi: 10.1128/IAI.72.3.1402-1408.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrettl M, Haas H. 2011. Iron homeostasis—Achilles' heel of Aspergillus fumigatus? Curr Opin Microbiol 14:400–405. doi: 10.1016/j.mib.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. 1993. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest 91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, Halder G, Kontoyiannis DP. 2008. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A 105:9367–9372. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim AS, Gebremariam T, French SW, Edwards JE Jr, Spellberg B. 2010. The iron chelator deferasirox enhances liposomal amphotericin B efficacy in treating murine invasive pulmonary aspergillosis. J Antimicrob Chemother 65:289–292. doi: 10.1093/jac/dkp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur S, Singh S. 2014. Biofilm formation by Aspergillus fumigatus. Med Mycol 52:2–9. doi: 10.3109/13693786.2013.819592. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski JL. 2011. Real-world use of iron chelators. Hematology Am Soc Hematol Educ Program 2011:451–458. doi: 10.1182/asheducation-2011.1.451. [DOI] [PubMed] [Google Scholar]
- 32.Reichhardt C, Ferreira JA, Joubert LM, Clemons KV, Stevens DA, Cegelski L. 10 July 2015. Analysis of the Aspergillus fumigatus biofilm extracellular matrix by solid-state nuclear magnetic resonance spectroscopy. Eukaryot Cell doi: 10.1128/EC.00050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]