Abstract
Two CYP51 inhibitors, posaconazole and the ravuconazole prodrug E1224, were recently tested in clinical trials for efficacy in indeterminate Chagas disease. The results from these studies show that both drugs cleared parasites from the blood of infected patients at the end of the treatment but that parasitemia rebounded over the following months. In the current study, we sought to identify a dosing regimen of posaconazole that could permanently clear Trypanosoma cruzi from mice with experimental Chagas disease. Infected mice were treated with posaconazole or benznidazole, an established Chagas disease drug, and parasitological cure was defined as an absence of parasitemia recrudescence after immunosuppression. Twenty-day therapy with benznidazole (10 to 100 mg/kg of body weight/day) resulted in a dose-dependent increase in antiparasitic activity, and the 100-mg/kg regimen effected parasitological cure in all treated mice. In contrast, all mice remained infected after a 25-day treatment with posaconazole at all tested doses (10 to 100 mg/kg/day). Further extension of posaconazole therapy to 40 days resulted in only a marginal improvement of treatment outcome. We also observed similar differences in antiparasitic activity between benznidazole and posaconazole in acute T. cruzi heart infections. While benznidazole induced rapid, dose-dependent reductions in heart parasite burdens, the antiparasitic activity of posaconazole plateaued at low doses (3 to 10 mg/kg/day) despite increasing drug exposure in plasma. These observations are in good agreement with the outcomes of recent phase 2 trials with posaconazole and suggest that the efficacy models combined with the pharmacokinetic analysis employed here will be useful in predicting clinical outcomes of new drug candidates.
INTRODUCTION
Approximately 10 million people are infected with Trypanosoma cruzi, the causative agent for Chagas disease, with approximately 40,000 new cases added annually (1). After the symptoms of acute infection wane, the parasite persists during a symptom-free indeterminate phase of the disease, which can last for several decades. About 20 to 40% of infected individuals develop clinical symptoms of cardiac injury, and 15% eventually progress to overt heart failure caused by left ventricular dysfunction (2). Treatment of Chagas disease relies on two antiparasitic drugs, nifurtimox and benznidazole, which were introduced into clinical use in the 1970s (3, 4). Both drugs suffer from multiple shortcomings, including toxicity, long treatment time, and uncertain efficacy in the chronic phase of Chagas disease (5, 6). Consequently, new drugs with a more favorable profile are needed.
Recent drug development efforts for Chagas disease have focused on repurposing antifungal drugs that inhibit lanosterol demethylase (CYP51), an enzyme in the ergosterol biosynthesis pathway (7). Antifungal CYP51 inhibitors, such as posaconazole and ravuconazole, inhibit T. cruzi growth in vitro with nanomolar 50% effective concentrations (EC50s), and both drugs effected cure in mouse models of Chagas disease in several independent studies (7). For example, a 20-day posaconazole treatment of mice infected with the T. cruzi Y strain yielded a cure rate slightly superior to that of a benznidazole regimen (80% versus 70%, respectively) (8). In another report, a 40-day posaconazole treatment of mice infected with the T. cruzi CL strain effected a 90% cure rate, compared to the 100% cure rate achieved with a 40-day regimen of benznidazole (9). In both of these studies, cure was defined as an absence of parasitemia recrudescence after prolonged immunosuppression.
Despite these promising preclinical studies, recent phase 2 clinical trials in patients with intermediate-phase Chagas showed that neither posaconazole nor E1224 (a prodrug of ravuconazole) effected lasting parasitemia suppression, as determined by quantitative PCR, in a majority of patients (10, 11). In the same trials, treatment with benznidazole translated into durable clearance of parasitemia/PCR negativity in most patients. In one clinical trial (CHAGASAZOL, ClinicalTrials.gov registration no. NCT01162967), 94% of patients treated with benznidazole according to protocol remained PCR negative during 40 weeks of posttreatment follow-up. Treatment with posaconazole resulted in parasitemia clearance at the end of the treatment, but 80 to 90% of these patients experienced parasitemia recrudescence during the follow-up phase. Very similar outcomes were observed in another trial (E1224, ClinicalTrials.gov registration no. NCT01489228) that compared the antiparasitic efficacies of benznidazole and E1224 (81% versus 31% of patients with sustained parasitemia suppression/PCR negativity according to the protocol for analysis). While these studies clearly demonstrated that benznidazole is a better anti-T. cruzi drug than posaconazole or E1224, it is important to emphasize that PCR negativity in these trials should not be equated with parasitological cure even in patients who experienced sustained parasitemia suppression. The failure of CYP51 inhibitors in these clinical trials suggests that the preclinical in vivo models used to evaluate anti-T. cruzi compounds have not predicted the performance of Chagas drug candidates in clinical settings.
In this study, we reevaluated the activity of posaconazole and benznidazole in a modified preclinical mouse efficacy model that closely resembles one described previously (9). The key features of this model include initiation of drug treatment after parasite replication is restrained by the adaptive immune system during the late acute phase of infection, long-term immunosuppression of mice after the end of treatment to allow for the expansion and highly sensitive detection of any surviving parasites, and a definition of cure as an absence of parasitemia (PCR negativity) throughout the course of immunosuppression. Mice infected with T. cruzi for 35 days were treated for 20 days with benznidazole and remained free of parasites after 1 month of immunosuppression. However, parasitemia in posaconazole-treated mice rebounded after immunosuppression even when posaconazole treatment was prolonged to 40 days. These observations were corroborated by treatment outcomes observed in an acute model of Chagas disease. During an acute infection, the efficacy of benznidazole improved with escalating dose (10 to 100 mg/kg of body weight per day). In contrast, the efficacy of posaconazole plateaued at low doses (3 to 10 mg/kg per day) and was inferior to that of benznidazole. These data are in good agreement with the outcomes of the posaconazole trial and suggest that the efficacy models described here will be useful in predicting the clinical performance of new Chagas drug candidates and dosing regimens.
MATERIALS AND METHODS
Ethics statement.
All procedures involving mice were performed in accordance with AAALAC standards and were reviewed and approved by the institutional animal care and use committee of the Novartis Institute for Biomedical Research.
Study drugs and formulations.
All chemicals were ordered from Sigma-Aldrich unless stated otherwise. Benznidazole, posaconazole (APAC Pharmaceutical), and cyclophosphamide were formulated in distilled water containing 0.5% methylcellulose and 0.5% Tween 80. During a treatment course, each mouse received 0.2 ml of drug suspension per dose by oral gavage (per os [p.o.]).
Trypanosoma cruzi culture.
NIH 3T3 fibroblast cells (ATCC) were passaged biweekly and were maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (HyClone) and 100 IU penicillin–100 μg/ml streptomycin (HyClone) at 37°C and 5% CO2. T. cruzi CL and Y strains were maintained in tissue culture as an infection in NIH 3T3 fibroblast cells. Briefly, 3 × 107 T. cruzi trypomastigotes were used to infect NIH 3T3 cells (6.25 × 105), and the infected 3T3 cells were then cultured until proliferating intracellular amastigotes transformed back into trypomastigotes and were released into the culture medium. During the infection, the tissue culture medium was changed biweekly and a day prior to collection of T. cruzi trypomastigotes for mouse efficacy studies. For mouse infections, tissue culture-derived trypomastigotes were washed with phosphate-buffered saline containing 0.5 mM CaCl2 and 0.5 mM MgCl2 (PBS) and resuspended in RPMI 1640 medium lacking heat-inactivated fetal bovine serum at a concentration of 106 trypomastigotes per ml.
Trypanosoma cruzi proliferation assay.
Briefly, NIH 3T3 cells (resuspended in RPMI 1640 medium containing 5% fetal bovine serum supplemented with 100 IU penicillin–100 μg streptomycin per ml) were seeded at 1,000 cells/well in microscopy-grade, clear bottom, 384-well plates (Greiner) and incubated overnight at 37°C and 5% CO2. The next day, NIH 3T3 cells were infected for 6 h with CL T. cruzi trypomastigotes at a multiplicity of infection (MOI) of 10 at 37°C and 5% CO2. Following infection, extracellular parasites were removed with RPMI 1640 medium washes. After an overnight incubation (37°C and 5% CO2), compounds (0.2% dimethyl sulfoxide [DMSO], final concentration) were added to plate wells containing infected NIH 3T3 cells. Forty-eight hours later, infected NIH 3T3 cells were fixed (4% paraformaldehyde in PBS), permeabilized (0.1% Triton X-100 in PBS), and stained using a 1:125,000 dilution (prepared in PBS) of SYBR green (Life Technologies). The plates were then scanned using the Evotec Opera high-content screening system (PerkinElmer), and amastigote proliferation was assessed by counting parasites within the 3T3 cells using CellProfiler version 2.1.0 cell image analysis software (12).
In vivo efficacy studies. (i) Mouse model of acute Chagas disease.
To measure antiparasitic activity during acute T. cruzi infection, C57BL/6 mice (Jackson Laboratories) were infected by intraperitoneal (i.p.) injection with 106 tissue culture-derived T. cruzi CL trypomastigotes. Treatment was initiated on day 7 postinfection, and five mice per treatment group were dosed p.o. with 10-, 30-, and 100-mg/kg once-per-day (q.d.) regimens of benznidazole and posaconazole for 5 days. Both drugs were formulated as described above. At the end of treatment, the mice were euthanized, and T. cruzi parasites present in the hearts of treated mice were quantified by qPCR. P values for the between-groups differences in efficacies were calculated with a Student's paired t test with a two-tailed distribution.
(ii) Mouse model of late acute Chagas disease.
To determine whether a drug regimen can effect parasitological cure in a model of late acute Chagas disease, C57BL/6 mice were infected by i.p. injection with 103 tissue culture-derived T. cruzi CL or Y trypomastigotes. Thirty-five days or more after infection, animals were dosed p.o. with various regimens of benznidazole (10, 30, and 100 mg/kg q.d.) and posaconazole (10, 20, 30, and 100 mg/kg q.d.). In experiments that included regimens of various durations, the longest treatment regimen was initiated on day 35 postinfection. The regimens employing shorter durations were initiated later, so that dosing of all treatment groups would end on the same day. Benznidazole regimens were administered for 10, 15, or 20 days, and posaconazole regimens were administered for 20, 25, or 40 days. Both drugs were formulated as described above. After completion of a treatment course, blood from treated mice was collected from the orbital venous sinus, and T. cruzi parasitemia was quantified by qPCR. Ten days following the end of drug treatment, mice underwent four cycles of cyclophosphamide immunosuppression, with each cycle lasting 1 week. During each immunosuppression cycle, mice were dosed with 200 mg/kg of cyclophosphamide p.o. on days 1 and 4, and blood samples were collected on day 5 from the orbital venous sinus. T. cruzi parasitemia in blood samples was quantified by qPCR. Cyclophosphamide was formulated as described above. P values for the between-group differences were calculated with Fisher's exact test for categorical variables.
Pharmacokinetic studies.
The pharmacokinetic (PK) properties of posaconazole and benznidazole were determined in naive mice (n = 3) after dosing these drugs at 5 mg/kg by the intravenous (i.v.) route and 20 mg/kg p.o. Blood samples were collected at 0.03, 0.33, 0.5, 1, 3, 10, and 24 h postdose. Plasma samples from mice treated with posaconazole and benznidazole during acute T. cruzi infection were collected on the first and fifth day of dosing (five plasma samples per day and per regimen, at 0.5, 1, 3, 7, 10, and 24 h postdose). Plasma drug quantification was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Liquid chromatography was performed using an Agilent 1200 high-performance liquid chromatography (HPLC) system (Agilent Technologies, Inc.) with a Waters Atlantis T3 (2.1 by 30 mm, 3-μm particle size) column. The HPLC system was coupled to an API 4000 Qtrap mass spectrometer (Applied Biosystems) for analyte detection and quantification.
Pharmacokinetic parameters were calculated with Phoenix, version 6.2 (Certara Pharsight), using a noncompartmental model as shown below. Two sets of pharmacokinetic parameters were calculated. The first set (Cmax, total and AUC0–24, total) refers to the total compound concentration present in the plasma, while the second set (Cmax, free and AUC0–24, free) refers to the compound concentration in the plasma that is not bound to the plasma proteins. The latter values were calculated from Cmax, total and AUC0–24, total using the plasma protein binding values measured here for benznidazole and posaconazole.
The total body clearance (CL) and the apparent volume of distribution at steady state (Vss) for intravenous data were calculated with the following equations: CL = dosei.v./AUC0–∞ and Vss = (dosei.v. · AUMC0–∞)/(AUC0–∞)2, where AUC0–∞ and AUMC0–∞ are the area under the concentration-time curve from time zero to infinity and the area under the first-moment concentration-time curve from time zero to infinity, respectively. The terminal elimination rate constant (kel) was derived from the slope of the log-linear line from at least the last three data points, and the half-life (t1/2) was calculated as follows: t1/2 = −ln(2)/kel.
The absolute oral bioavailability (F) was calculated using the following equation, assuming a linear, proportional relationship between AUC0–∞ and the dose: F = (AUC0–∞ p.o./AUC0–∞ i.v.) × (dosei.v./dosep.o.).
Single-dose plasma concentration-time data from infected mice were also fitted to a one-compartment model and used to predict drug levels after multiple days of dosing.
Measurement of plasma protein binding.
Protein binding in plasma with 5 μM posaconazole or benznidazole was assayed using the Rapid Equilibrium Device (RED) from Pierce. To 990 μl of blank plasma in a 96-well plate (Nalgene Nunc 96 DeepWell), 10 μl of 0.5 mM compound stock solution in acetonitrile/DMSO (19:1) was added to create plasma containing 5 μM compound. To each of the buffer wells of the RED plate, 350 μl of buffer and 200 μl of plasma containing 5 μM compound were added. The RED plate was sealed with a transparent adhesive seal (PerkinElmer Top Seal) and incubated at 37°C for 4 h while being vortexed at 120 rpm. Six hundred microliters of quench solution containing 500 nM Labetalol in acetonitrile/methanol (70:30) was placed in a destination plate. After incubation of the RED plate, 100 μl of plasma or buffer from the assay plate was added to specific wells in the destination plate, and 100 μl of either buffer or plasma was also added to those destination wells, in order to create final solutions with matching matrices. Samples were mixed well and stored at −20°C overnight. Prior to LC-MS/MS analysis, the sample plate was mixed well and centrifuged, and the supernatant transferred to a clean 96-well analysis plate.
Parasitemia quantification by real-time qPCR.
Blood collected from treated mice was used for extraction of total DNA using the High Pure PCR template preparation kit (Roche). The amounts of T. cruzi satellite DNA (195-bp fragment) in extracted DNA samples were quantified by real-time qPCR TaqMan assay (Life Technologies) with the following set of primers: AATTATGAATGGCGGGAGTCA (forward primer), CCAGTGTGTGAACACGCAAAC (reverse primer), and AGACACTCTCTTTCAATGTA (TaqMan MGB probe, 5′-FAM [6-carboxyfluorescein]-labeled reporter dye, nonfluorescent quencher). The amounts of mouse chromosomal DNA in extracted samples were quantified in parallel qPCRs using a GADPH (glyceraldehyde-3-phosphate dehydrogenase) TaqMan assay with the following set of primers: GCCGCCATGTTGCAAAC (forward primer), CGAGAGGAATGAGGTTAGTCACAA (reverse primer), and ATGAATGAACCGCCGTTAT (TaqMan MGB probe, 5′-FAM-labeled reporter dye, nonfluorescent quencher). Each qPCR mixture (10 μl) included 5 μl of TaqMan Gene Expression master mix (Life Technologies), 0.5 μl of a 20× primer/probe mix (Life Technologies), and 4.5 μl (50 ng) of total DNA extracted from blood samples. PCRs were run on the Applied Biosystems 7900HT instrument. T. cruzi parasitemia was expressed as the abundance of T. cruzi microsatellite DNA relative to the abundance of mouse GAPDH DNA.
RESULTS
In vivo efficacy of benznidazole—effects of treatment duration and dose escalation.
To model T. cruzi infection during Chagas disease, we employed a previously described mouse model with minor modifications (9). C57BL/6 mice were infected with T. cruzi trypomastigotes, and the infection was allowed to develop for 35 days or longer. After 35 days of infection, T. cruzi parasitemia was controlled by the host immune system and remained stable afterwards. Treatment failure was defined as a rebound in parasitemia following immunosuppression of the mice (13, 14).
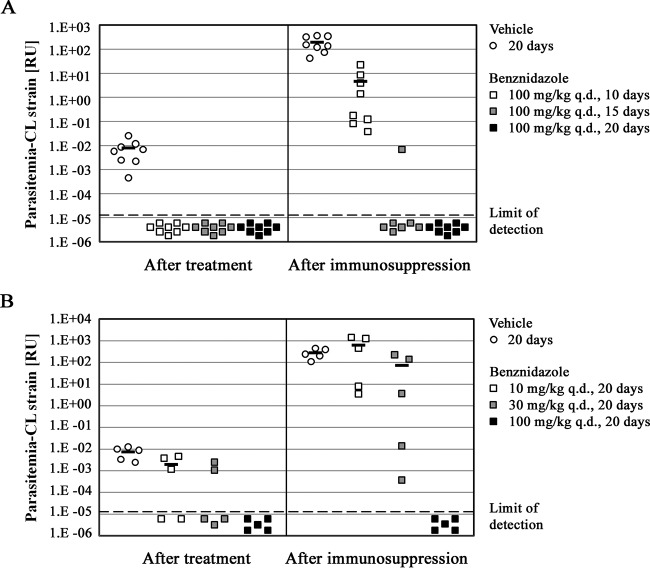
First, we set out to define the minimum duration of benznidazole treatment required to achieve parasitological cure after infection with the T. cruzi CL strain. We used a regimen of 100 mg/kg q.d., which has previously been identified as a curative regimen in a mouse Chagas disease model (15). Mice were treated for 10, 15, or 20 days, and parasitemia was quantified via qPCR. As shown by the results in Fig. 1A, all three therapy durations suppressed parasitemia below the limit of detection at the end of treatment. However, after 4 weeks of cyclophosphamide immunosuppression, significant differences in the antiparasitic activities of the three regimens became apparent. There was a rebound of parasitemia in all mice in the 10-day treatment group, but six of seven of mice in the 15-day group showed sustained parasitemia suppression with no detectable T. cruzi present at the end of the immunosuppression protocol. Further extension of benznidazole treatment to 20 days effected lasting parasitemia suppression in all eight treated mice (Fig. 1A). For comparison, all mice in the control group, which were dosed with the vehicle only, had parasitemia well above the limit of detection by qPCR at the end of treatment. In the course of 4 weeks of immunosuppression, the parasitemia increased approximately 10,000-fold, thus confirming unconstrained T. cruzi proliferation during immunosuppression and the high sensitivity of the protocol to detect parasites that survived a treatment regimen (Fig. 1A). We concluded that 20 days of treatment with benznidazole is both sufficient and close to the minimum duration required to achieve parasitological cure for all mice in this model.
FIG 1.
Effects of benznidazole treatment duration and dose escalation on achievement of parasitological cure. (A) Mice (n = 8 per treatment group) infected with T. cruzi CL strain trypomastigotes for 35 to 50 days were administered drug vehicle or 100 mg/kg q.d. benznidazole for durations that ranged from 10 to 20 days, as indicated in the key. Parasitemia in individual mice was measured immediately at the end of treatment (60 days after the infection for all treatment groups) and then again after 4 weeks of immunosuppression. The results for mice with undetectable levels of parasitemia are shown in the plot as below the limit of detection. For treatment groups in which the fraction of parasite-positive mice exceeded 50%, the mean parasitemia value is also shown (horizontal bars). One mouse in the group that received 100 mg/kg q.d. benznidazole for 15 days was euthanized after the start of immunosuppression due to an apparently coincidental bacterial infection that developed in this immunosuppressed mouse, and no result for this mouse is shown in the right-hand panel of the graph. (B) Mice (n = 5 per treatment group) infected with T. cruzi CL strain trypomastigotes for 35 days were treated for 20 days with drug vehicle or 10, 30, or 100 mg/kg q.d. benznidazole. The parasitemia levels in individual mice at the end of treatment (55 days after the infection for all treatment groups) and after 4 weeks of immunosuppression are shown, together with the average parasitemia values (horizontal bars; when more than 50% of mice in a group were parasite positive). RU, relative units.
We further investigated the minimal dose of benznidazole needed to effect parasitological cure with a 20-day dosing regimen. CL-infected C57BL/6 mice were treated with 10-mg/kg q.d., 30-mg/kg q.d., and 100-mg/kg q.d. regimens of benznidazole (Fig. 1B). Our results indicated that only treatment with the 100-mg/kg q.d. regimen translated into parasitological cure following immunosuppression. Neither the 10-mg/kg nor 30-mg/kg benznidazole dose effected lasting parasitemia suppression in a single mouse within the respective treatment groups (Fig. 1B). With this knowledge, the regimen of 100 mg/kg q.d. for 20 days was used as a point of reference for comparison of the compound's efficacy to that of benznidazole.
In vivo efficacy of posaconazole—effects of treatment duration and dose escalation.
Posaconazole was originally discovered as an antifungal agent. The first report of posaconazole anti-T. cruzi activity in a mouse model of Chagas disease explored dosing regimens that ranged from 5 mg/kg q.d. to 25 mg/kg q.d. and were administered for 43 days. The highest dose regimen produced a 100% cure rate in a mouse model of acute Chagas disease. Parallel testing of posaconazole at 15 mg/kg/day translated into a 50% cure rate in a chronic mouse model (16). In subsequent studies, a dose of 20 mg/kg q.d. was typically used to characterize the in vivo anti-T. cruzi activity of posaconazole (9, 17). In our experiments, such a regimen translated into a maximum plasma concentration (Cmax, total) of 5.9 μM and an AUC0–24, total of 97 h · μM at steady state. For comparison, the posaconazole regimen used in the corresponding Chagas trial (400 mg twice per day [18]) was reported to translate into a Cmax, total of 2.3 μM and an AUC0–24, total of 115 h · μM in patients (19).
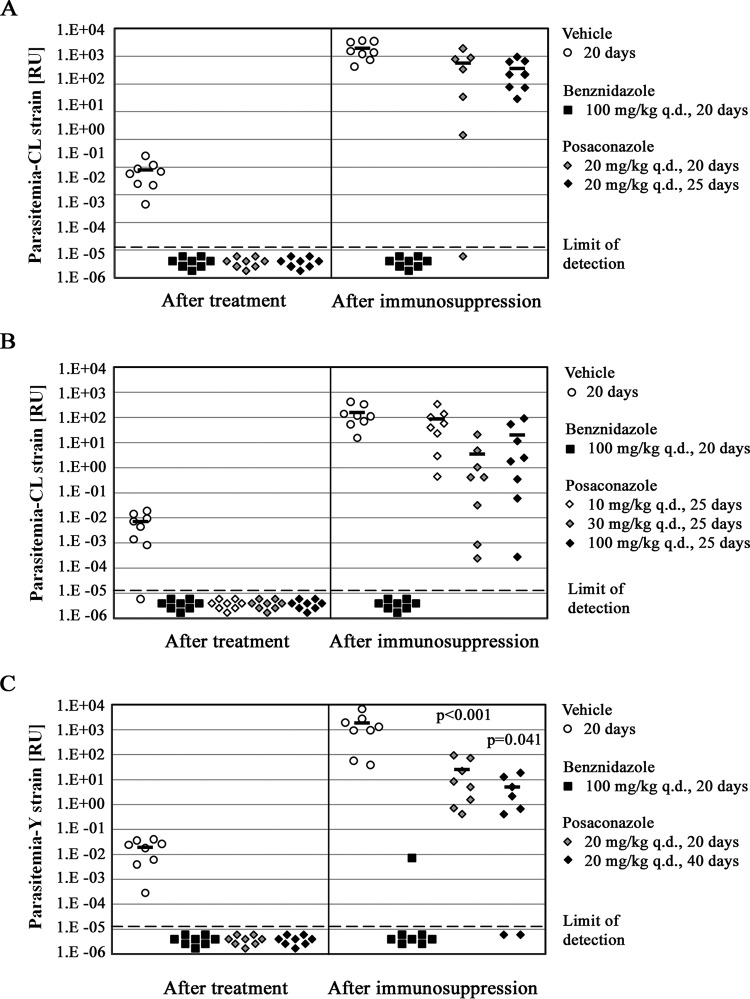
Posaconazole treatment at 20 mg/kg q.d. was tested for 20 or 25 days, and all mice in both treated groups had undetectable parasitemia at the end of the treatment (Fig. 2A). However, almost all treated mice, regardless of therapy duration, experienced parasitemia rebound after immunosuppression (Fig. 2A). Based on this outcome, we conclude that the minimal duration of treatment with the 20-mg/kg q.d. posaconazole regimen to achieve parasitological cure of CL infection is greater than 25 days. To determine whether posaconazole dose escalation could lead to parasitological cure, we treated CL-infected C57BL/6 mice for 25 days with 10 mg/kg q.d., 30 mg/kg q.d., and 100 mg/kg q.d. of posaconazole. We hypothesized that higher doses of posaconazole could lead to greater inhibition of parasite CYP51 and improved rates of cure of infected mice. If so, this result would provide an encouraging rationale for the development of improved CYP51 inhibitors that are currently being optimized for Chagas disease treatment (7, 20–26). As observed earlier, all posaconazole regimens suppressed T. cruzi parasitemia below the detection limit at the end of treatment. However, at the end of 4 weeks of posttreatment immunosuppression, all of the mice in all three treatment groups experienced parasitemia recrudescence (Fig. 2B). Even taking into account interim measures of parasitemia during the immunosuppression (data not shown), there was no indication that an increase in posaconazole dose led to improved antiparasitic activity within this dose range. In conclusion, escalation of the posaconazole dose up to 100 mg/kg q.d. did not have a noticeable effect on antiparasitic activity.
FIG 2.
Effects of posaconazole treatment duration and dose escalation on achievement of parasitological cure. (A) Mice (n = 8 per treatment group) infected with T. cruzi CL strain trypomastigotes for 35 or 40 days were administered drug vehicle or 100 mg/kg q.d. benznidazole for 20 days or 20 mg/kg q.d. posaconazole for 20 or 25 days, as indicated in the key. Parasitemia in individual mice was measured as described in the legend to Fig. 1. (B) Mice (n = 8 per treatment group) infected with T. cruzi CL strain trypomastigotes for 35 or 40 days were treated for 20 days with drug vehicle or 100 mg/kg q.d. benznidazole or for 25 days with 10, 30, and 100 mg/kg q.d. posaconazole. Parasitemia levels in individual mice are shown. The results for mice with undetectable levels of parasitemia are depicted in the plot as below the limit of detection. (C) Mice (n = 8 per treatment group) infected with T. cruzi Y strain trypomastigotes for 35 or 55 days were treated for 20 days with drug vehicle or 100 mg/kg q.d. benznidazole or for 20 or 40 days with 20 mg/kg q.d. posaconazole. Parasitemia in individual mice was measured at the end of treatment (75 days postinfection for all groups) and after 4 weeks of immunosuppression. Average parasitemia values (when more than 50% of mice in a group were parasite positive) are indicated by horizontal bars in all panels. The P values shown relate the treatment outcomes (cure versus no cure) obtained with the two posaconazole regimens to that effected by the benznidazole regimen.
We then asked whether the difference in in vivo efficacy between benznidazole and posaconazole observed with CL strain infections would extend to another T. cruzi isolate, the Y strain (Fig. 2C). This strain was previously described as partially benznidazole resistant and could provide a different setting for comparison of in vivo efficacy of the two drugs (27, 28). Mice with established T. cruzi Y strain infection (35 to 55 days, depending on the duration of therapy) were treated with benznidazole at 100 mg/kg q.d. for 20 days. Similar to the results for the T. cruzi CL infection, all benznidazole-treated mice had undetectable parasitemia at the end of treatment. After 4 weeks of immunosuppression, seven of eight benznidazole-treated mice still remained parasitemia free (Fig. 2C).
Two treatment durations were tested with the posaconazole 20-mg/kg q.d. regimen, 20 and 40 days. Both treatment durations produced outcomes similar to those observed with T. cruzi CL infections. At the end of treatment, all mice had parasitemia suppressed below the limit of detection. However, all mice in the 20-day treatment group experienced parasitemia rebound after immunosuppression (P < 0.001 for the comparison of the results for the benznidazole and posaconazole groups). When posaconazole therapy was extended to 40 days, only two of eight mice had sustained parasitemia clearance (P = 0.041) (Fig. 2C). Additionally, as observed for T. cruzi CL infections, the T. cruzi Y strain proliferated uncontrollably after immunosuppression of vehicle-treated mice (approximately 100,000-fold increase in the parasitemia signal) (Fig. 2C).
In summary, we observed clear differences between the antiparasitic activities of benznidazole and posaconazole in both T. cruzi CL and Y strains. Benznidazole treatment for 20 days at 100 mg/kg q.d. routinely resulted in no treatment failures. In contrast, despite escalation of either dose or duration, all of the dosing regimens that we tested for posaconazole led to treatment failure in most or all mice.
Antiparasitic activities of benznidazole and posaconazole in acutely infected hearts.
We next asked if there was evidence for a lower antiparasitic activity of posaconazole than of benznidazole when using an experimental model that has a simpler readout than whole-body parasitemia. For these experiments, we measured the T. cruzi CL strain burdens in the hearts of mice. To further simplify the interpretation of drug efficacy data, we started treatment only 7 days after infection and concluded the experiment on day 11 postinfection. Within this time span, both T. cruzi parasitemia and the heart burden increased at steady rates in the absence of antiparasitic drug treatment (see Fig. S1 in the supplemental material).
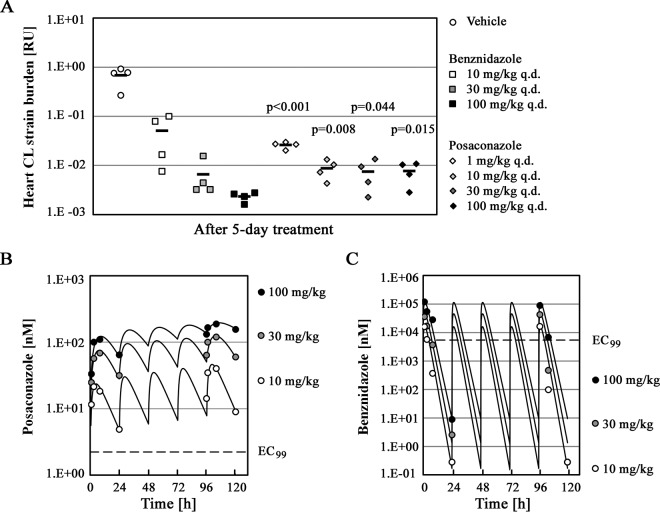
Five days of treatment with benznidazole at different doses (10 mg/kg q.d., 30 mg/kg q.d., and 100 mg/kg q.d.) effected rapid, dose-dependent reductions in heart parasite burdens (Fig. 3A). At the highest benznidazole dose of 100 mg/kg, mice had approximately 300-fold lower heart parasite burdens than mice treated with vehicle. Posaconazole treatment effectively reduced heart parasite loads by 95% to 99% at low doses (1 mg/kg q.d. and 10 mg/kg q.d.). However, dose escalation to 30 mg/kg q.d. or 100 mg/kg q.d. did not lead to any further reduction in parasite levels (Fig. 3A). Notably, the mean residual heart parasite burdens in mice treated with high posaconazole doses were significantly higher than that observed with the 100-mg/kg q.d. benznidazole regimen (approximately 3-fold higher; P < 0.05). We concluded that while benznidazole can reduce the parasite burden in the heart by 2 to 3 log in 5 days, posaconazole is less effective, and its efficacy is not improved with higher doses. These data suggest either saturable pharmacokinetics of posaconazole with an increasing dose or saturable parasite killing at higher exposures of posaconazole.
FIG 3.
PK/PD relationship for benznidazole and posaconazole in hearts of acutely infected mice. (A) Mice (n = 4 per treatment group) infected with T. cruzi CL strain trypomastigotes for 7 days were subsequently treated for 5 days with the following: drug vehicle, 10 to 100 mg/kg q.d. benznidazole, or 1 to 100 mg/kg q.d. posaconazole. Parasite burdens (following dose-response treatment) in hearts of infected mice are shown. The average parasitemia values (when more than 50% of mice in a group were parasite positive) are indicated by horizontal bars. The P values shown relate heart parasite burden values obtained with individual posaconazole regimens to that effected by 100 mg/kg q.d. benznidazole. (B and C) Plasma concentrations for posaconazole (10, 30, and 100 mg/kg) and benznidazole (10, 30, and 100 mg/kg) are shown, along with the respective EC99 values as determined in the T. cruzi proliferation assay.
Pharmacokinetic/pharmacodynamic (PD) profiling of benznidazole and posaconazole during acute infection.
The pharmacokinetic properties of benznidazole and posaconazole in naive mice after the administration of a single intravenous and oral dose were determined (Table 1). Benznidazole exhibited low to moderate clearance, a moderate volume of distribution, short half-life, and complete bioavailability of the oral dose. In contrast, posaconazole demonstrated very low clearance, moderate Vss, long half-life, and poor bioavailability. These pharmacokinetic properties yielded largely similar overall exposures (AUC0–24, total) of both drugs and 10-fold differences in maximum plasma concentrations (Cmax, total) for benznidazole and posaconazole at the same oral dose (AUC0–24, total, 78 versus 58 h · μM, and Cmax, total, 33.8 versus 3.4 μM). However, because of a large difference in plasma protein binding between the two drugs (27.2% for benznidazole versus 98.9% for posaconazole), the corresponding exposures of free drug in plasma differed radically between the drugs for AUC and Cmax. After an oral dose of 20 mg/kg of each drug, the free plasma exposure (AUC0–24, free) and free maximum plasma concentration (Cmax, free) of benznidazole were 95-fold and 625-fold higher than those of posaconazole (AUC0–24, free, 57 versus 0.6 h · μM, and Cmax, free, 25 versus 0.04 μM).
TABLE 1.
Pharmacokinetic parameters of benznidazole and posaconazole in naive mice after administration of a single intravenous or oral dose
| Dose and route of administration | PK parameter | Value for: |
|
|---|---|---|---|
| Benznidazole | Posaconazole | ||
| 5 mg/kg i.v. | CL (ml/min/kg) | 17 | 1.4 |
| Vss (liter/kg) | 0.9 | 0.9 | |
| t1/2 (h) | 0.7 | 8.2 | |
| 20 mg/kg p.o. | AUC0–24, total (h · μM) | 78 | 58 |
| AUC0–24, free (h · μM) | 57 | 0.6 | |
| Cmax, total (μM) | 34 | 3.2 | |
| Cmax, free (μM) | 25 | 0.04 | |
| Bioavailability (%) | 100 | 17 | |
To address the possibility that, in the course of the efficacy experiments, posaconazole exposure did not increase with increasing dose, we measured a time course of plasma drug concentration during the 5-day dosing regimen in the acute infection model described above. In the case of benznidazole, we observed changes in AUC0–24, total and Cmax, total parameters that tracked very closely with changes in the dose administered (Table 2). A similar relationship was also observed for posaconazole. Increasing the posaconazole dose from 10 mg/kg to 100 mg/kg resulted in an approximately 6-fold increase in both AUC0–24, total and Cmax, total (Table 2). Therefore, we concluded that the plateau in heart parasite burden reduction was not caused by a lack of increase of posaconazole exposure with increasing dose.
TABLE 2.
Pharmacokinetic parameters associated with 5 days of dosing of benznidazole and posaconazole in mice with acute T. cruzi infection
| Drug and dose (mg/kg q.d.) | Multiple of dose | AUC0–24, total (h · μM)a | AUC0–24, free (h · μM) | Cmax, total (μM)a | Cmax, free (μM) | Time above EC99 (h) |
|---|---|---|---|---|---|---|
| Benznidazole | ||||||
| 10 | 1× | 51 (1×) | 20 | 23 (1×) | 16 | 3 |
| 30 | 3× | 140 (2.8×) | 55 | 59 (2.6×) | 43 | 4 |
| 100 | 10× | 400 (7.9×) | 160 | 120 (5.4×) | 89 | 8 |
| Posaconazole | ||||||
| 10 | 1× | 68 (1×) | 0.75 | 4.4 (1×) | 0.05 | 24 |
| 30 | 3× | 230 (3.4×) | 2.5 | 12 (2.7×) | 0.13 | 24 |
| 100 | 10× | 420 (6.1×) | 4.6 | 19 (4.4×) | 0.21 | 24 |
AUC0–24 and Cmax parameters listed were derived from fitted pharmacokinetic functions using total and unbound plasma concentrations on the fifth day of dosing. Numbers in parentheses indicate multiples of the corresponding exposure parameter associated with the lowest dose.
We also examined the relationship between posaconazole's in vivo pharmacokinetic profile and its potency against intracellular T. cruzi in vitro (EC99 = 2.2 nM). Posaconazole is highly protein bound in mouse plasma (98.9% of posaconazole is bound to mouse plasma proteins, as determined in this study). The unbound posaconazole concentration is thus dramatically lower than the total measured plasma concentration. Adjustment for plasma protein binding revealed that the posaconazole unbound plasma concentration remained above the EC99 throughout the dosing period for the 30- and 100-mg/kg regimens (Fig. 3B). Benznidazole binds to plasma proteins to a much smaller extent (27%) than does posaconazole. However, it is also much less potent in vitro (EC99 = 5.4 μM). Plotting the benznidazole unbound plasma concentration versus its EC99 revealed that the relationship between benznidazole's pharmacokinetic profile and its in vitro antiparasitic potency is less favorable than that observed for posaconazole (Fig. 3C).
In summary, it is unlikely that the limited posaconazole antiparasitic activity observed in vivo is the result of an inferior pharmacokinetic profile or high plasma protein binding. Overall, we were unable to identify an additional factor that could account for the failure of posaconazole to clear parasites from T. cruzi-infected mice with prolonged treatments or high doses. Instead, our data are consistent with a saturable-killing phenomenon, whereby a population of parasites that are relatively resistant to posaconazole remains in infected mice treated with high doses of the drug or for extended durations.
DISCUSSION
Indeterminate and chronic forms of Chagas disease are characterized by low numbers of parasites that persist in specific tissues, including the heart and digestive tract. The progression of the disease into organ-specific syndromes (cardiomyopathy, megacolon, and megaesophagus) correlates with the presence of parasites in the affected organs (29, 30). According to the prevailing hypothesis, parasitological cure could translate into clinical benefit in patients suffering from intermediate-phase or symptomatic chronic Chagas disease (29, 30). While verification of this hypothesis is still ongoing (31), new antiparasitic drugs with activity against T. cruzi are being pursued and evaluated for clinical efficacy (10, 11). Because radical parasitological cure in patients is difficult to demonstrate, sustained suppression of parasitemia as measured by quantitative PCR (PCR negativity) is currently used as a surrogate for successful therapy or, more strictly, lack of treatment failure (32). However, this readout does not allow conclusive determination of radical parasitological cure in patients.
Recent clinical testing of posaconazole and the ravuconazole prodrug E1224 in Chagas disease patients did not yield favorable treatment outcomes despite predictions from preclinical in vivo data (8, 9, 33–35). In both trials, the fraction of treated patients who experienced long-term parasitemia suppression/PCR negativity (as determined by qPCR) was low and inferior to that achieved with benznidazole. Any disconnect between the outcomes of clinical trials and the performance of drugs in preclinical in vivo Chagas disease models represents a significant hurdle for the progression of future Chagas disease drugs into clinical testing. In this context, several suggestions have been made on how to improve the performance of CYP51 inhibitors in the clinic. These include increasing the dose, extending treatment time beyond 60 days, and treating patients with a benznidazole-CYP51 drug combination (8, 9, 11).
In this study, we characterized and compared the antiparasitic activities of benznidazole and posaconazole in a mouse model of Chagas disease that incorporates two key features. First, drug treatment is initiated only after allowing parasites to disseminate for 5 weeks, thus approximating the late acute stage of disease. At this point after infection, the rate of parasitemia increase is limited by the adaptive immune system, as is the case in human indeterminate-stage disease. Second, the model employs a highly sensitive parasite detection method that relies on exponential parasite proliferation during 4 weeks of immunosuppression. We routinely observed 10,000- to 100,000-fold increases in parasite PCR signals during the immunosuppression phase in control animals. It is important to note that neither of these two features is novel and that the mouse model described in this report closely resembles in most of its aspects a mouse model reported previously (13).
Our results demonstrate that the in vivo antiparasitic activity of posaconazole is inferior to that of benznidazole. A regimen of 100 mg/kg of benznidazole once a day for 20 days in a late acute infection model consistently effected parasitological cure, even after immunosuppression. A 20-day treatment with 20 mg/kg of posaconazole once a day was effective in reducing parasitemia below the detection level but failed to induce parasitological cure, as revealed after immunosuppression. We also explored two common strategies that can lead to improved drug efficacy: extension of treatment duration and increase in dose. Both of these failed to induce parasitological cure in a majority of posaconazole-treated mice. We did not observe any improvement in posaconazole antiparasitic activity within a 10-, 30-, and 100-mg/kg q.d. dose range, despite dose-dependent increases in drug exposure (AUC and Cmax). In a separate experiment utilizing the T. cruzi Y strain, an extension of posaconazole treatment up to 40 days cured only 25% of infected mice. These treatment outcomes point to the presence of a subpopulation of T. cruzi parasites in infected mice that do not respond to posaconazole treatment, even though a majority of T. cruzi parasites are killed by the drug. If true, T. cruzi infection could resemble some bacterial infections, such as tuberculosis, in which infected patients are known to harbor bacterial subpopulations that do not respond to treatment with growth inhibitor drugs (36). In the alternative scenario, the treatment-refractory T. cruzi cells would not have reduced sensitivity to posaconazole but would reside in a tissue(s) poorly penetrated by posaconazole.
It is not clear what mechanism underlies the limited in vivo efficacy of posaconazole. The maximal antiparasitic activity of posaconazole in the heart muscle of acutely infected mice is lower than that of benznidazole (Fig. 3A), which might indicate a lower rate of parasite killing by the CYP51 inhibitor or the presence of parasites unresponsive to posaconazole. Interestingly, the inferior in vivo efficacy of posaconazole observed in the acute model in this study resembles the limited in vitro efficacy of CYP51 inhibitors that was recently reported elsewhere (28). It remains to be determined whether a common mechanism underlies the in vitro (28) and in vivo (this study) observations.
Similarly, it is not clear why the experiments reported here produced outcomes that are different from those in previously published experiments. Several factors could be in play. A majority of studies that previously reported successful clearance of murine T. cruzi infections by posaconazole employed mice in early acute phase, with treatment starting as early as 1 day after the infection (8, 9, 33, 34, 37, 38). It is possible that T. cruzi parasites in longer-term infections respond differently to treatment with posaconazole than parasites in early acute infections. We are aware of only one previous study that employed longer-term murine T. cruzi infection for testing the efficacy of posaconazole in vivo (16). In that study, treatment failure was not evaluated by using an immunosuppression protocol, and it is possible that the sensitivity of parasite detection in that study was lower than in the current study. Failure to perform immunosuppression after the treatment also limits the sensitivity of three other studies from this list (33, 34, 38).
The mouse model of Chagas disease, together with the PK/PD analysis described in this report, can significantly improve the prioritization of preclinical drug candidates before advancing them into clinical development. One limitation of the current experimental design is that it cannot be extended to T. cruzi isolates that do not produce detectable parasitemia during immunosuppression. In such cases, a more thorough analysis of tissues of treated animals by PCR will be needed to demonstrate parasitological cure. Our evaluation of benznidazole and posaconazole clearly indicates that posaconazole is unable to effect parasitological cure in a mouse model as a single agent, which is in line with the clinical results. A trial with a combination arm of posaconazole and benznidazole is currently ongoing and might provide some hope that posaconazole can contribute to the treatment of Chagas disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rick Tarleton (University of Georgia) for providing the T. cruzi CL strain and Jair Lage de Siqueira-Neto (University of California San Diego) for providing the T. cruzi Y strain. An additional acknowledgment goes to Jaime Ballard for conducting the in vitro T. cruzi proliferation assays with benznidazole and posaconazole.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00689-15.
REFERENCES
- 1.WHO. 2015. Chagas disease (American trypanosomiasis). Fact sheet no. 340. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs340/en/. [Google Scholar]
- 2.Bern C, Kjos S, Yabsley MJ, Montgomery SP. 2011. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev 24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahia-Oliveira LM, Gomes JA, Cancado JR, Ferrari TC, Lemos EM, Luz ZM, Moreira MC, Gazzinelli G, Correa-Oliveira R. 2000. Immunological and clinical evaluation of chagasic patients subjected to chemotherapy during the acute phase of Trypanosoma cruzi infection 14-30 years ago. J Infect Dis 182:634–638. doi: 10.1086/315743. [DOI] [PubMed] [Google Scholar]
- 4.Cancado JR. 2002. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo 44:29–37. doi: 10.1590/S0036-46652002000100006. [DOI] [PubMed] [Google Scholar]
- 5.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 6.Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Buckner FS, Urbina JA. 2012. Recent developments in sterol 14-demethylase inhibitors for Chagas disease. Int J Parasitol Drugs Drug Resist 2:236–242. doi: 10.1016/j.ijpddr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diniz Lde F, Urbina JA, de Andrade IM, Mazzeti AL, Martins TA, Caldas IS, Talvani A, Ribeiro I, Bahia MT. 2013. Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis 7:e2367. doi: 10.1371/journal.pntd.0002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL. 2014. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 209:150–162. doi: 10.1093/infdis/jit420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatelain E. 2015. Chagas disease drug discovery: toward a new era. J Biomol Screen. 20:22–35. doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- 11.Urbina JA. 2015. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol 62:149–156. doi: 10.1111/jeu.12184. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. 2006. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustamante JM, Bixby LM, Tarleton RL. 2008. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med 14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldas S, Santos FM, de Lana M, Diniz LF, Machado-Coelho GL, Veloso VM, Bahia MT. 2008. Trypanosoma cruzi: acute and long-term infection in the vertebrate host can modify the response to benznidazole. Exp Parasitol 118:315–323. doi: 10.1016/j.exppara.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Richle RW, Raaflaub J. 1980. Difference of effective antitrypanosomal dosages of benznidazole in mice and man. Chemotherapeutic and pharmacokinetic results. Acta Trop 37:257–261. [PubMed] [Google Scholar]
- 16.Urbina JA, Payares G, Contreras LM, Liendo A, Sanoja C, Molina J, Piras M, Piras R, Perez N, Wincker P, Loebenberg D. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother 42:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivieri BP, Molina JT, de Castro SL, Pereira MC, Calvet CM, Urbina JA, Araujo-Jorge TC. 2010. A comparative study of posaconazole and benznidazole in the prevention of heart damage and promotion of trypanocidal immune response in a murine model of Chagas disease. Int J Antimicrob Agents 36:79–83. doi: 10.1016/j.ijantimicag.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Molina I, Gomez i Prat J, Salvador F, Trevino B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sanchez-Montalva A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 19.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 53:958–966. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andriani G, Amata E, Beatty J, Clements Z, Coffey BJ, Courtemanche G, Devine W, Erath J, Juda CE, Wawrzak Z, Wood JT, Lepesheva GI, Rodriguez A, Pollastri MP. 2013. Antitrypanosomal lead discovery: identification of a ligand-efficient inhibitor of Trypanosoma cruzi CYP51 and parasite growth. J Med Chem 56:2556–2567. doi: 10.1021/jm400012e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvet CM, Vieira DF, Choi JY, Kellar D, Cameron MD, Siqueira-Neto JL, Gut J, Johnston JB, Lin L, Khan S, McKerrow JH, Roush WR, Podust LM. 2014. 4-Aminopyridyl-based CYP51 inhibitors as anti-Trypanosoma cruzi drug leads with improved pharmacokinetic profile and in vivo potency. J Med Chem 57:6989–7005. doi: 10.1021/jm500448u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JY, Calvet CM, Gunatilleke SS, Ruiz C, Cameron MD, McKerrow JH, Podust LM, Roush WR. 2013. Rational development of 4-aminopyridyl-based inhibitors targeting Trypanosoma cruzi CYP51 as anti-Chagas agents. J Med Chem 56:7651–7668. doi: 10.1021/jm401067s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friggeri L, Hargrove TY, Rachakonda G, Williams AD, Wawrzak Z, Di Santo R, De Vita D, Waterman MR, Tortorella S, Villalta F, Lepesheva GI. 2014. Structural basis for rational design of inhibitors targeting Trypanosoma cruzi sterol 14alpha-demethylase: two regions of the enzyme molecule potentiate its inhibition. J Med Chem 57:6704–6717. doi: 10.1021/jm500739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunatilleke SS, Calvet CM, Johnston JB, Chen CK, Erenburg G, Gut J, Engel JC, Ang KK, Mulvaney J, Chen S, Arkin MR, McKerrow JH, Podust LM. 2012. Diverse inhibitor chemotypes targeting Trypanosoma cruzi CYP51. PLoS Negl Trop Dis 6:e1736. doi: 10.1371/journal.pntd.0001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus JM, Tatipaka HB, McGuffin SA, Chennamaneni NK, Karimi M, Arif J, Verlinde CL, Buckner FS, Gelb MH. 2010. Second generation analogues of the cancer drug clinical candidate tipifarnib for anti-Chagas disease drug discovery. J Med Chem 53:3887–3898. doi: 10.1021/jm9013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suryadevara PK, Racherla KK, Olepu S, Norcross NR, Tatipaka HB, Arif JA, Planer JD, Lepesheva GI, Verlinde CL, Buckner FS, Gelb MH. 2013. Dialkylimidazole inhibitors of Trypanosoma cruzi sterol 14alpha-demethylase as anti-Chagas disease agents. Bioorg Med Chem Lett 23:6492–6499. doi: 10.1016/j.bmcl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Diaz RA, Escario JA, Nogal-Ruiz JJ, Gomez-Barrio A. 2001. Biological characterization of Trypanosoma cruzi strains. Mem Inst Oswaldo Cruz 96:53–59. doi: 10.1590/S0074-02762001000100006. [DOI] [PubMed] [Google Scholar]
- 28.Moraes CB, Giardini MA, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, Chatelain E, Freitas-Junior LH. 2014. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep 4:4703. doi: 10.1038/srep04703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarleton RL, Zhang L. 1999. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol Today 15:94–99. doi: 10.1016/S0169-4758(99)01398-8. [DOI] [PubMed] [Google Scholar]
- 30.Urbina JA. 1999. Chemotherapy of Chagas' disease: the how and the why. J Mol Medicine 77:332–338. doi: 10.1007/s001090050359. [DOI] [PubMed] [Google Scholar]
- 31.Marin-Neto JA, Rassi A Jr, Avezum A Jr, Mattos AC, Rassi A, Morillo CA, Sosa-Estani S, Yusuf S, BENEFIT Investigators. 2009. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz 104(Suppl 1):319–324. doi: 10.1590/S0074-02762009000900042. [DOI] [PubMed] [Google Scholar]
- 32.Britto CC. 2009. Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations. Mem Inst Oswaldo Cruz 104(Suppl 1):122–135. doi: 10.1590/S0074-02762009000900018. [DOI] [PubMed] [Google Scholar]
- 33.Ferraz ML, Gazzinelli RT, Alves RO, Urbina JA, Romanha AJ. 2007. The anti-Trypanosoma cruzi activity of posaconazole in a murine model of acute Chagas' disease is less dependent on gamma interferon than that of benznidazole. Antimicrob Agents Chemother 51:1359–1364. doi: 10.1128/AAC.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. 2000. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother 44:150–155. doi: 10.1128/AAC.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbina JA, Payares G, Sanoja C, Lira R, Romanha AJ. 2003. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int J Antimicrob Agents 21:27–38. doi: 10.1016/S0924-8579(02)00273-X. [DOI] [PubMed] [Google Scholar]
- 36.Cohen NR, Lobritz MA, Collins JJ. 2013. Microbial persistence and the road to drug resistance. Cell Host Microbe 13:632–642. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner FS, Bahia MT, Suryadevara PK, White KL, Shackleford DM, Chennamaneni NK, Hulverson MA, Laydbak JU, Chatelain E, Scandale I, Verlinde CL, Charman SA, Lepesheva GI, Gelb MH. 2012. Pharmacological characterization, structural studies, and in vivo activities of anti-Chagas disease lead compounds derived from tipifarnib. Antimicrob Agents Chemother 56:4914–4921. doi: 10.1128/AAC.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferraz ML, Gazzinelli RT, Alves RO, Urbina JA, Romanha AJ. 2009. Absence of CD4+ T lymphocytes, CD8+ T lymphocytes, or B lymphocytes has different effects on the efficacy of posaconazole and benznidazole in treatment of experimental acute Trypanosoma cruzi infection. Antimicrob Agents Chemother 53:174–179. doi: 10.1128/AAC.00779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.