Abstract
Doxycycline is an antibiotic commonly used to treat Lyme disease and other bacterial infections. The MIC and minimum bactericidal concentration (MBC) for Borrelia burgdorferi have been investigated by different groups but were experimentally established in this study as a function of input cell density. We demonstrated that B. burgdorferi treated in the stationary phase has a higher probability of regrowth following removal of antibiotic. In addition, we determined experimentally and mathematically that the spirochetes which persist posttreatment do not have a longer lag phase but exhibit a lower growth rate than untreated spirochetes. Finally, we found that treating the spirochetes by pulse-dosing did not eliminate growth or reduce the persister population in vitro. From these data, we propose that B. burgdorferi persister development is stochastic and driven by slowed growth.
INTRODUCTION
Bacteria and humans have been in a constant “arms race” for survival. The advent of antibiotics has only provided another weapon in the arsenal that we use to protect ourselves, and shortly after penicillin's discovery, it was found that bacteria could become tolerant and resistant to this new class of weapons (1, 2). Antibiotic tolerance, or the capability of bacteria to enter a nondividing, dormant state in response to antibiotics, is not a new phenomenon, and it has been well studied for Escherichia coli and Pseudomonas aeruginosa (3–9). Similar studies have shown that the spirochete Borrelia burgdorferi can persist under low-nutrient stressful conditions (10, 11), and a similar mechanism may allow persistence in the presence of the bacteriostatic antibiotic doxycycline (12).
Recent work has suggested that a genetically homogeneous bacterial population can dynamically alter gene expression to adapt to changing environmental conditions, resulting in a phenotypically heterogeneous population (13). These dynamic changes in gene expression can result both from different individual bacterial responses to external stimuli and from preexisting variations within individual bacteria causing differing responses (6, 14). The term stochastic is used to describe these changes to reflect the randomness in which the changes can occur (6). As a concrete example, if an external environmental stimulus affects a bacterial population, the resulting changes may not be uniform, since bacterial subpopulations of the larger population will respond to the environmental triggers at different rates (13, 15). A unique challenge, therefore, is reconciling changes in gene expression with the emergence of unique subpopulations with phenotypic differences from the larger population.
If external stimuli on a bacterial subpopulation randomly trigger expression of genes that induce a state of dormancy, then a persister subpopulation can emerge. If the number of surviving subpopulations is known, then these data can be applied to predict the capability of a subpopulation of bacteria to survive an antibiotic treatment and, therefore, of the population as a whole to persist after antibiotic therapy. The ability of subpopulations to transition from a persister to a nonpersister state can be used to indirectly model the chance of finding persister subpopulations within a larger population. B. burgdorferi has been shown to persist when treated with tetracycline antibiotics (16, 17), but as of yet, the mechanism of B. burgdorferi persistence has not been described.
To establish parameters for antibiotic efficacy, the MIC and the minimum bactericidal concentration (MBC) of an antibiotic are two quantification values of antibiotics that are used. The MIC of a particular antibiotic is the minimum concentration that is necessary to inhibit growth. In the context of resistant mutants, the resistant population would grow readily in the presence of the MIC, whereas tolerant populations would stagnate in an established MIC but regrow after the removal of an antibiotic (6). The MBC is the lowest concentration of an antibiotic that is necessary to either eradicate bacteria or prevent the recovery of a bacterial population. Growth and recovery of the culture can be reliably indicated by the presence of motile spirochetes (18).
Importantly, determinations of MIC that are used to establish dosages in humans and animals are determined by in vitro culture of bacteria, unlikely to mimic the growth characteristics of the pathogen in vivo. Alterations in nutrients, oxygen, and cell density affect growth, which, in turn, will affect the efficacy of antibiotics, such as doxycycline, which target actively dividing cells.
Two of the most commonly prescribed antibiotics that are used to treat Lyme disease have different metabolic activities. Doxycycline acts on the bacterial 30S ribosomal subunit (19), while ceftriaxone can generally be described as having beta-lactam activity (20). Although previous clinical studies showed that doxycycline can be as effective as third-generation cephalosporin antibiotics like ceftriaxone (21, 22), recent in vitro work suggests that doxycycline may not be as effective as ceftriaxone against stationary-phase bacteria (23). Importantly, the role of immune responses to infection is integral to the treatment mode for microbiostatic antibiotics like doxycycline, such that results from in vitro studies should not be overinterpreted to the in vivo situation.
Different values have been obtained by several labs for the MICs and MBCs of antibiotics used against B. burgdorferi (16, 24–27), likely as a result of differences in methodology, strain variation, and cell density. Therefore, we performed MIC and MBC assays for doxycycline using a well-characterized strain of B. burgdorferi with various input cell densities. We then followed these assays with a probability assay specifically designed to quantify the emergence of persister populations after treatment with doxycycline. Our results indicate that B. burgdorferi growth dynamics affect the response to doxycycline treatment and prompt consideration of this factor when determining effective concentrations.
MATERIALS AND METHODS
Borrelia burgdorferi.
Low-passage (p4 or p5) Borrelia burgdorferi sensu stricto strain B31 clonal isolate 5A19 (28) was grown at 34°C in BSK-II medium (29), as described previously (30). Because the spirochetes are microaerophilic and gene expression is affected by oxygen levels (31), they were grown in a trigas incubator set at 5% CO2, 3% O2, and the rest N2. B. burgdorferi was seeded at a low concentration from a frozen glycerol stock and then grown to the necessary cell density.
MIC assays.
To determine the effect of growth phase on the MIC of doxycycline, the following experiments were performed. B. burgdorferi spirochetes were grown to early log phase at 5 × 105 cells/ml and 4 × 106 to 7 × 106 cells/ml, mid-log phase at 4 × 107 to 9 × 107 cells/ml, and late log phase at 1.25 × 108 cells/ml. The groups of cells were treated with increasing concentrations of doxycycline (0, 0.1, 0.25, 0.5, 1.0, and 2.5 μg/ml) for 5 days. Previous studies have used not less than 72 h of treatment for MIC determination (27, 32), but based on the time-dependent mechanism of doxycycline, we extended it to 5 days, as has also been reported previously (23, 33). The MIC was quantified as the lowest concentration necessary to inhibit growth over a 5-day period.
MBC assays.
To determine the effect of growth phase on the MBC of doxycycline, the assay was performed as follows. Low-passage strain B31 isolate 5A19 was grown in BSK-II medium in either 15-ml or 5-ml tubes to 108 cells/ml and then diluted to either 1 × 107 or 1 × 106 cells/ml. Similarly, B31 isolate 5A19 was grown to 107 cells/ml and diluted to either 2 × 105 or 1 × 106 cells/ml. All cultures were then treated with doxycycline hyclate for 5 days at concentrations of 0, 0.1, 1.0, 2.5, 5.0, 10.0, 25.0, and 50.0 μg/ml. After 5 days, the number of motile spirochetes in cultures was determined as an indicator of viability (18). A subculture of B. burgdorferi was then taken by removing half of the culture on day 5, gently pelleting it at 2,700 × g for 20 min at room temperature, and resuspending it in doxycycline-free medium. The cultures were checked to verify that the pellet was not lost after spinning. At day 5 after resuspension in doxycycline-free medium, the culture in which no motile spirochetes were detected, which came from the lowest concentration of antibiotic, was indicated as reflecting the MBC for doxycycline with B. burgdorferi. The assay was performed with 4 tubes per doxycycline concentration, and the MBC, assessed at 5 days posttreatment, was found to be 50 μg/ml or lower, depending on the bacterial cell density. This concentration was therefore used in the probability assays (see below).
Probability assays.
To quantify the amount of B. burgdorferi spirochetes that would persist in vitro after treatment with the MBC of doxycycline, an assay was set up as follows. For the first part of the assay, cells were diluted to one of the following test concentrations. B. burgdorferi cells were grown to 2 × 108 to 3 × 108 cells/ml and then diluted to concentrations of early-log-phase (2 × 106 cells/ml), mid-log-phase (2 × 107 cells/ml), and late-log-phase (1 × 108 cells/ml) densities in 15-ml Eppendorf tubes. The B. burgdorferi cultures were then treated with the MBC of doxycycline (50 μg/ml) for 5 days. On day 5, the B. burgdorferi cultures were pelleted and resuspended in doxycycline-free medium and allowed to regrow for 11 days. The end time point was established at 11 days after performing a test assay, which demonstrated that when regrowth occurred, it was most frequently seen within the first 11 days. Multiple tubes per treatment group were grown (see Table 2). At day 11, the cultures were checked, and the presence or absence of motile spirochetes was documented. If motile B. burgdorferi spirochetes were present, the sample was marked as “+” for growth; otherwise, it was scored as “−” for growth and a count of the tubes with motile spirochetes/total was obtained.
TABLE 2.
Probability assay I
| Parameter | Value for indicated portion of log phase |
||
|---|---|---|---|
| Early | Mid | Late | |
| Cell concn (cells/ml)a | 2 × 106 | 2 × 107 | 1 × 108 |
| No. of tubes with motile spirochetes | 23 | 36 | 36 |
| No. of tubes with nonmotile spirochetes | 37 | 14 | 0 |
| No. of tubes with motile spirochetes/total no. of tubes | 23/60 | 36/50 | 36/36 |
| % of tubes with motile spirochetes | 38 | 72 | 100 |
| Statistical significance (chi-squared with Yates's continuity correction) | Early vs mid, P = 0.000857 | Mid vs late, P = 0.00154 | Early vs late, P = 6.89 × 109 |
Concentration to which cells were diluted.
For the second part of the assay, B. burgdorferi cells were seeded from a glycerol stock, grown in BSK-II medium in 15-ml conical tubes to concentrations of 2 × 106 to 3 × 106 cells/ml, 2 × 107 to 3 × 107 cells/ml, and 1 × 108 cells/ml, and then treated with doxycycline for 5 days. The cultures were then resuspended in doxycycline-free medium as described previously. The B. burgdorferi cultures were allowed to regrow for 11 days and marked as “+” for growth and “−” for no growth as described above.
Pulse-dose assay.
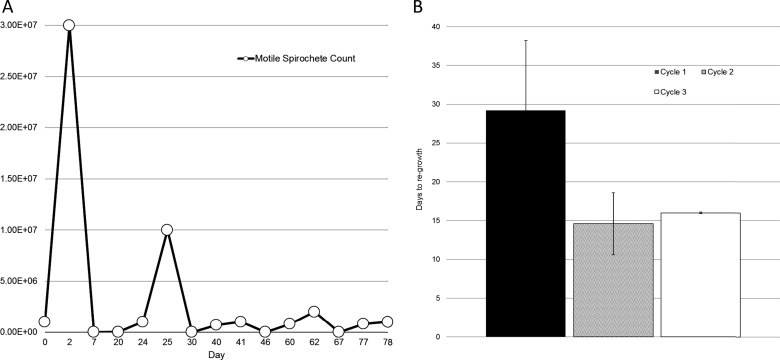
Cultures of B. burgdorferi were grown to 3 × 107 cells/ml in 5-ml snap-cap tubes, treated with 50 μg/ml of doxycycline for 5 days, and then pelleted and resuspended in doxycycline-free medium. The culture tubes were incubated and monitored each day for growth. When the growth resulting from surviving bacteria reached early log phase (≃5 × 106 cells/ml), 50 μg/ml of doxycycline was added before the B. burgdorferi cell density could grow to the initial assay concentration (3 × 107cells/ml). After 5 days of doxycycline treatment, the doxycycline was removed, and the B. burgdorferi cultures were monitored again. The cycle of treating and removing the doxycycline was repeated 3 times, similar to that described by Lewis (6). Thirty-four samples were used in the pulse-dose assays, and approximately 30% of the total number of samples regrew after the first treatment. Successive treatments had a regrowth rate of approximately 50%. The assay was fully completed (three treatments and regrowth phases) one time. At the pulse-dose assay endpoint, a glycerol stock was made from the B. burgdorferi culture for analysis and comparison with the probability assay. See Fig. 3A for a representative diagram.
FIG 3.
Pulse-dose assay. (A) Representative graph of the pulse-dose assay; (B) time to regrowth for each consecutive cycle of the assay. B. burgdorferi cultures (n = 34) were seeded initially at 7 × 105 cells/ml, treated with 50 μg/ml of doxycycline, and then switched to doxycycline-free medium. Cultures were monitored for regrowth, and then those that regrew (n = 5) were treated again before they reached their initial concentration. The process was repeated 2 more times for those that regrew (n = 2). The time to regrowth after each treatment cycle is displayed. Error bars indicate SD. No significant difference was observed in the time to regrowth between cycles 2 and 3.
Mathematical modeling.
To determine a mathematical model for the growth of persister subpopulations in B. burgdorferi, data from the probability assay and pulse-dose assay were analyzed. In a given assay with B. burgdorferi, for calculation purposes, a tube was considered a population. Logarithmic growth in bacterial cultures can be defined by the equation P = P0ekt, where P is final population, P0 is initial population, k is growth constant, and t is time. To create a model that could ascertain the quantity of persisters in any given population, the time to regrowth using both the probability assay and the pulse-dose assay was used to determine t (time to regrowth for the subpopulation P0 that regrows after doxycycline treatment). The final population P is simply defined as the quantity of a population that regrew after treatment, and the growth constant k can be derived from an average growth rate of populations over a defined period. While a logistic curve model is commonly used for bacterial batch cultures (34), applying this to our data did not result in a good fit; the k value derived was much higher than what was observed over several experiments (data not shown).
A given population of B. burgdorferi organisms is also composed of subpopulations p1, p2,…pn, described as P = ∑pn. During antibiotic treatment, a bacterial subpopulation that is not killed by antibiotic therapy and does not grow in the presence of antibiotics can be described as a subpopulation of dormant persisters. As observed in the pulse-dose assay and the probability assay, this subpopulation will grow and form a new population after the removal of doxycycline, meaning that the initial subpopulation will be P0 and the regrown population after removal of the antibiotic at time t will be P.
Limiting-dilution analysis.
A mid-log-phase culture of untreated B. burgdorferi spirochetes was diluted to 10 spirochetes per ml, mixed thoroughly, and plated by adding 100 μl (average of 1 spirochete per well) of the culture to each well of a 96-well plate. An additional 80 μl of medium was added to each well to prevent drying. After growth was visible by a change in medium color and a small pellet at bottom of well, four random wells were resuspended and counted.
Statistical analysis.
Analysis of variance (ANOVA), Student's t test, and Fisher's exact test were performed with GraphPad Prism and with R (http://www.R-project.org).
RESULTS
Effect of cell density on MIC.
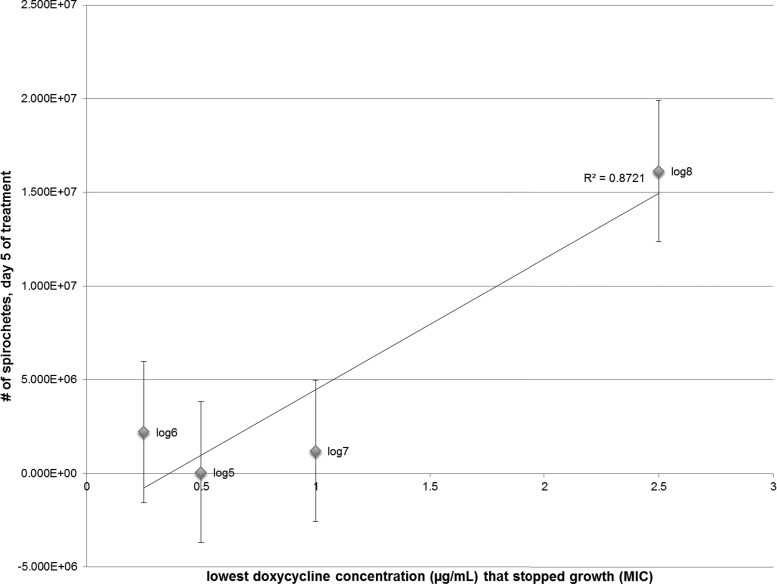
Several papers have been published on the MIC for doxycycline. The concentrations of B. burgdorferi that were used in the assays were either log 5 or log 6, and the MIC ranged from 0.25 μg/ml to 0.5 μg/ml, based on the criteria of inhibiting growth over a 3- to 5-day period (16, 23, 35). The results from the doxycycline MIC assays suggested that at early log phase, 0.25 μg/ml is a sufficient MIC (Table 1; see also Fig. S1 in the supplemental material). At mid-log phase, 1.0 μg/ml is required, while at late log phase, 2.5 μg/ml is the necessary MIC. Furthermore, if the number of motile spirochetes on day 5 is plotted against the lowest concentration that stopped growth, there is a linear correlation between decreasing cell density and doxycycline concentration (Fig. 1).
TABLE 1.
Results of MIC assay
| Input cell density (cells/ml) | Concn (μg/ml) of doxycycline that inhibited growth, day 0–day 5 |
|---|---|
| 2 × 105 | 0.25 |
| 7 × 106 | 0.25 |
| 8 × 107 | 1.0 |
| 1.25 × 108 | 2.5 |
FIG 1.
Relationship between B. burgdorferi population density and MIC of doxycycline. The largest cell density with a population decrease from day 0 to day 5 is plotted against the lowest concentration of doxycycline required to cause the decrease. The relationship suggests a linear correlation, with an R2 of 0.8721. For each data point, corresponding to input cell density (log 5 to log 8), 4 tubes are represented; error bars indicate standard errors of the means.
Effect of cell density on MBC.
We reasoned that although doxycycline is bacteriostatic, it acts on the 30S ribosomal subunit (36) to inhibit protein synthesis, so it could effectively eliminate a bacterial population if the concentration and duration were sufficient. This has been shown by other groups as well (26, 27). Therefore, an MBC assay was set up to determine what concentration of doxycycline would prevent growth and motility after 5 days. After 5 days, the MBC for the subcultures was determined by the number of motile spirochetes (Fig. 2). There was no motility observed on day 5 for any culture or cell density that was treated with 50 μg/ml, suggesting a doxycycline MBC of 50 μg/ml for B. burgdorferi.
FIG 2.
Number of motile B. burgdorferi spirochetes per concentration of doxycycline on day 5 posttreatment in the MBC assay. A growth inhibition assay was performed whereby cultures of differing initial concentrations and seed concentrations were treated with multiple concentrations of doxycycline. The number of motile spirochetes per culture was determined 5 days after the cessation of treatment. No growth was seen in any culture at this time point with treatments of 25 and 50 μg/ml. Error bars indicate standard errors of the means.
Next, we conducted a probability assay to determine whether regrowth would occur after a set time in early-log-, mid-log-, and stationary-phase populations. Following a 5-day treatment with 50 μg/ml of doxycycline, the cultures were examined after 11 days. If motile B. burgdorferi spirochetes were not observed by day 11, they were not positive after two more weeks, so we used day 11 as the time point. The assay was first performed by growing B. burgdorferi from a glycerol stock to stationary phase and then diluting the cultures to lower concentrations of early-log-phase, mid-log-phase, and early-stationary-phase growth. There was a significant difference in the number of cultures with motile B. burgdorferi in the early-log-, mid-log-, and stationary-phase groups (Table 2). When the assay was repeated by growing the cultures from a glycerol stock to the desired concentrations, there was no regrowth or motility in any group except the stationary-phase group (Table 3). A significant difference (P < 0.01) between the assays where spirochetes were diluted to the desired concentration versus those grown to the desired concentration was observed, which further suggested that population density and growth phase differentially affect the ability of B. burgdorferi to form persister cells in the presence of doxycycline.
TABLE 3.
Probability assay II
| Parameter | Value for indicated portion of log phase |
||
|---|---|---|---|
| Early | Mid | Late | |
| Cell density (cells/ml)a | 2 × 106–3 × 106 | 2 × 107–3 × 107 | 1 × 108 |
| No. of tubes with motile spirochetes | 0 | 0 | 12 |
| No. of tubes with nonmotile spirochetes | 22 | 22 | 31 |
| No. of tubes with motile spirochetes/total no. of tubes | 0/22 | 0/22 | 12/43 |
| % of tubes with motile spirochetes | 0 | 0 | 28 |
| Statistical significance (chi-squared with Yates's continuity correction) | Early vs mid, null | Mid vs late (approximation), P = 0.0162 | Early vs late (approximation), P = 0.0162 |
Density to which cells were grown.
Effect of pulse-dosing on B. burgdorferi regrowth.
To explore the possibility that a small number of persister cells survive posttreatment, a pulse-dose assay similar to the model used by Kim Lewis (6, 7, 37) with E. coli and P. aeruginosa was established. We expected that the persister population would be reduced by each subsequent treatment, and this would be evidenced by lack of regrowth or by slower regrowth. Our results show that the treatment was unable to kill the persisters, which regrew after each treatment (Fig. 3). Furthermore, the rate of regrowth did not significantly decline after the first treatment cycle (Fig. 3B). Interestingly, while the first treatment was applied to a dense culture, subsequent treatments were applied to early-log-phase spirochetes, which would not have been expected to produce persisters.
By examining growth after each pulse, we determined that the time to regrowth did not increase (Fig. 3) with subsequent pulses (and after the 2nd and 4th pulses actually decreased). The calculated initial cell density increased slightly as well. This indicates that the persister population was not reduced in proportion with multiple treatments.
Exploring the mechanism of persister development.
Given these results, we hypothesized that repeated administration of antibiotics (or pulses) could produce a population selected for B. burgdorferi persisters. A glycerol stock was made from the culture of B. burgdorferi that regrew after 3 treatments in the pulse-dose assay. This was designated the “persister regrowth (PR)” stock and was used to examine the mechanism of persister development. We reasoned that if the PR stock consisted of a population that has been selected for the persister phenotype, then a higher proportion of this population has the potential to form persisters. Thus, if we subjected it to our probability assay, treatment at all growth phases should result in persister development. Conversely, if the PR stock consisted of bacteria which all have the same potential for persister development, the results should be the same as those shown in Tables 2 and 3. The results are shown in Table 4. The PR stock of B. burgdorferi did regrow after treatment, albeit at a lower frequency than for the untreated stock for each growth phase. These data indicate that the PR population is not selected for persister development and the process is likely stochastic.
TABLE 4.
Comparison of posttreatment growth between persister isolate and untreated isolate
| Parameter | Value for: |
|||||
|---|---|---|---|---|---|---|
| Early-log-phase B31 5A19 | Early-log-phase persister isolate | Mid-log-phase B31 5A19 | Mid-log-phase persister isolate | Late-log-phase B31 5A19 | Late-log-phase persister isolate | |
| No. of tubes with motile spirochetes | 23 | 5 | 36 | 10 | 36 | 9 |
| No. of tubes with nonmotile spirochetes | 37 | 26 | 14 | 20 | 0 | 17 |
| No. of tubes with motile spirochetes/total no. of tubes | 23/60 | 5/31 | 36/50 | 10/30 | 36/36 | 9/26 |
| % of tubes with motile spirochetes | 38 | 16 | 72 | 30 | 100 | 34 |
| Statistical significance (Fisher's exact test) | P = 0.0355 | P = 0.001 | P = 4.224 × 10−9 | |||
Mathematical model of persister growth.
If the results of the probability assay and the pulse-dose assay are analyzed together, several useful data points can be obtained. As already described, if a population of B. burgdorferi in stationary phase is treated with doxycycline, it is more likely to adopt a persister phenotype. To describe it another way: as cell density increases, so does the tendency for each bacterial cell to transition into a survival state upon the introduction of the antibiotic doxycycline. At earlier growth phases, a persister population may exist, but the time t to regrowth is much greater, and the chance of regrowth is much less than 50% (Table 2). To create a predictive model which could ascertain in any given population the likelihood of such an occurrence, we must first assume that growth rate is constant within the cycle of regrowth, and if growth occurs, it will be exponential and follow the exponential growth rate P = P0ekt.
Bacterial populations do not follow simple logarithmic growth but proceed through lag, log, and stationary phases. In our analysis of growth following doxycycline treatment, we had difficulty applying the equation P = P0ekt because k was variable and t was a large enough number that P0 was calculated to be ≪1. In essence, we assumed that the lag phase was longer than for untreated cells and determined that the growth constant varied depending on the cell density and period of growth. For example, cell population densities of <105 cells/ml do not appear to be in logarithmic growth (see Fig. S1 in the supplemental material), so to determine the growth rate constant k, the values used were 2 for time t, 4 × 105 cells/ml for P0, and 7.2 × 107 cells/ml for P (see Fig. S1), which provide a k value of 2.596. However, if this constant is used for the equation P = P0ekt to determine the initial population of persisters following cessation of treatment in pulse I of our pulse-dose assay, the culture reached 1 × 107 (P) after 18 days (t), so solving for P0 gives 0.345 (less than 1 bacterial cell).
In order to estimate the regrowth mathematically, we therefore attempted to calculate the time it would take 1 persister cell to begin regrowth, in essence the lag phase, which is prolonged following treatment. We determined the growth rate (which is not constant) for periods of growth between log 4 and log 6, log 5 and log 6, and log 6 and log 7 and determined the average to be 1.335 (Table 5), which was used for k. In the pulse-dose assay, the longest period to regrowth (after pulse I) was 18 days. To determine the lag period, we set P0 at 1, calculated t, and subtracted the calculated t from the actual t. This resulted in an extrapolated lag phase of 6 days.
TABLE 5.
Persister growth calculations
| Growth period | Calculation of k | Pulse | P | P0 | t (days) |
|---|---|---|---|---|---|
| log 4–log 6 | 1.23289 | I | 1.00E + 07 | 0.67 | 18 |
| log 5–log 6 | 0.75165 | II | 1.00E + 06 | 0.94 | 11 |
| log 6–log 7 | 2.02125 | III | 4.17E + 05 | 0.692 | 14 |
| Avg log 4–7 | 1.3352633 | IV | 8.00E + 05 | 1.018 | 10 |
To experimentally determine the time to regrowth of a single bacterial cell, we performed a limiting-dilution analysis. A mid-log-phase culture of untreated B. burgdorferi spirochetes was plated at an average of 1 spirochete per well in a 96-well plate. After growth was visible, four random wells were resuspended and counted, giving 1.08 × 108, 1.153 × 108, 1.88 × 108, and 1.28 × 108 after 12 days plus 21 h. The average of 1.351 × 108 was used to calculate t using the untreated cell growth rate of 2.596, with a result of 7.211 days. Subtracting this from the actual growth duration also provided a lag phase of 6 days. Thus, it appears that the lag phase for a single cell is about 6 days, identical to the extrapolated lag phase for persister cells, and it is the growth rate that is lower for antibiotic-treated B. burgdorferi.
DISCUSSION
The addition of any substance which causes alterations in the individual bacterium or small subpopulations of bacteria can have profound effects on the bacterial population as a whole. These subpopulations, although genetically identical to the bulk population, will uniquely respond to external stimuli. The data suggest that one such external stimulus is the antibiotic doxycycline. These different responses can confer an advantage to survival of the bacterial population as a whole when the population is subjected to adverse conditions (6, 38–40). Since the subpopulations are genetically identical and have no specific mechanism to inactivate antibiotics, this is fundamentally different from resistance. The bacterial subpopulations which correctly induce the genes that activate dormancy, while the larger population may get killed by an antibiotic, are termed persisters, a term coined by Joseph Bigger (1).
Mechanisms of persistence in other bacterial species have been well studied. Escherichia coli can be induced into a persisting state using toxin/antitoxin (TA) modules such as tisB (7, 41, 42). The tisB gene induces a decrease in proton motive force and ATP but is normally repressed. Subpopulations which induce it under normal growth conditions do not survive (7), such as if it is accidentally induced during SOS repair. In cases where a DNA topoisomerase inhibitor, such as ciprofloxacin, is present, bacterial subpopulations which induce tisB have a better chance of survival, while other subpopulations which attempt SOS repair will most likely be killed off (7).
The regrowth of persisters after antibiotic treatment may also be influenced by environmental conditions and not by a predetermined subpopulation number (43). Stationary-phase E. coli cultures were diluted and added to minimal or LB medium, with norfloxacin and ampicillin. When CFU were plotted against time for all cultures, minimal medium produced a delay in the bacteria exiting from a dormant, persister state (43). Interestingly, a different experiment whose purpose was to look for quorum sensing in E. coli showed that if old medium from stationary-phase cells was added to growing cultures, there was no effect on persister formation in growing cell populations (6). This result could suggest that multiple pathways can trigger dormancy and persistence, as has been well described (7, 44). Additionally, the study (43) proposed that instead of populations being rigidly described as “persister” and “nonpersister,” populations and subpopulations of bacteria should be more dynamically defined. Subpopulations of persisters can change depending on the environment, and the activation of the correct metabolic pathway can prompt individual cells to risk growth and awake from dormancy (45), similar to stochastic responses. As bacteria respond to stimuli, the responses can be modeled as a transition from one state to another during a time interval (15, 38). Although numerous different genes and pathways in a bacterium may be expressed in response to a stimulus, a simplified biphasic model can be used to examine specific criteria, such as a growth or death response to a given stimulus. In addition, predictions about the probability of changes in state can be made (13, 46).
While persister formation is not unique to B. burgdorferi, certain characteristics of this pathogen may influence or affect its entrance into dormancy. These spirochetes traverse between commensal inhabitance of ticks, survival and proliferation in reservoir hosts, and pathogenic persistence in incidental hosts. Entry into slow growth or dormancy is necessitated by prolonged periods of nutrient deprivation within the unfed tick. In addition, the spirochetes may occupy niches in the mammalian host that receive lower levels of oxygen and blood flow and nutrients (47). Therefore, the ability to form persisters, while yet stochastic in nature, may be more advantageous for B. burgdorferi than for other bacterial pathogens. Indeed, the frequency of B. burgdorferi persisters was observed to be higher than that of E. coli in a recent study (48).
In addition, the MICs and MBCs for multiple antibiotics have been shown to vary with different strains and clinical isolates of B. burgdorferi, though no direct resistance mechanism has been identified (27, 32). In particular, a study of erythromycin resistance showed dramatic differences in susceptibility among several strains, along with increased tolerance induced with preexposure (49). While we used only one strain in our study, our pulse-dose assay combined with the probability assay indicated that preexposure did not enhance tolerance, indicating that different mechanisms may govern the responses to antibiotics. Importantly, the impact of host adaptation may be significant. Notably, clinical isolates were shown to be more tolerant of erythromycin, possibly due to the adoption of a slow-growing phenotype in vivo.
This work was performed entirely in vitro and therefore did not take into account the influence of host adaptation, immune responses, or the tissue penetration of antibiotic on doxycycline treatment of a B. burgdorferi infection. However, multiple studies with animals have shown that B. burgdorferi is not fully eradicated with antibiotic treatment (16, 17, 26, 50–52). The development of slow-growing or dormant persisters in the presence of doxycycline (23, 33), a microbiostatic antibiotic, would indicate that reliance on immune control coincident with treatment would be necessary for efficacy of antimicrobial therapy. Given the multiple strategies that B. burgdorferi utilizes to evade the immune response (53), the survival of persisters after treatment with doxycycline is a reasonable possibility. If persisters do develop in vivo, then prolonged antibiotic therapy may not offer significant improvement of clinical outcome (54), should this be the result of dormant persisters (perhaps also attenuated [51]) continuing to elicit inflammatory responses. Our pulse-dose assay results also indicated that pulsing with doxycycline may not be effective. This is in contrast to a recent report, but it is important to note that different antibiotics with different mechanisms were used in those studies (33).
These studies imply that a portion of B. burgdorferi organisms may be tolerant to doxycycline following their entry into a state of dormancy. The results presented here further indicate that the development of B. burgdorferi persisters is governed by stochasticity and that dormancy may be related to both growth phase and cell density. It has been shown with other bacteria (55–57) that oxidative stress or nutrient deprivation can induce a persister state. It is possible that a lack of readily available nutrients for some B. burgdorferi subpopulations, similar to what would occur at stationary phase or in the unfed tick (58), can induce persister development and dormancy and enable survival after the introduction of an antibiotic like doxycycline. Follow-up studies using transcriptome sequencing (RNA-Seq) will aim to identify the specific genes induced among B. burgdorferi survivors of antibiotic treatment that are associated with a persister state.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant 2P20-RR020159-08 for The Louisiana Center of Biomedical Research Excellence (CoBRE) in Experimental Infectious Disease Research.
We thank Dale Embers (University of Illinois at Chicago) for mathematics consultation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00883-15.
REFERENCES
- 1.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin. Lancet 244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 2.Demerec M. 1948. Origin of bacterial resistance to antibiotics. J Bacteriol 56:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomanen E, Tomasz A. 1990. Mechanism of phenotypic tolerance of nongrowing pneumococci to beta-lactam antibiotics. Scand J Infect Dis Suppl 74:102–112. [PubMed] [Google Scholar]
- 4.Tuomanen E, Durack DT, Tomasz A. 1986. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob Agents Chemother 30:521–527. doi: 10.1128/AAC.30.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomanen E. 1986. Phenotypic tolerance: the search for beta-lactam antibiotics that kill nongrowing bacteria. Rev Infect Dis 8(Suppl 3):S279–S291. [DOI] [PubMed] [Google Scholar]
- 6.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 7.Lewis K. 2012. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 211:121–133. doi: 10.1007/978-3-642-28951-4_8. [DOI] [PubMed] [Google Scholar]
- 8.Tuomanen E, Schwartz J, Sande S. 1990. The vir locus affects the response of Bordetella pertussis to antibiotics: phenotypic tolerance and control of autolysis. J Infect Dis 162:560–563. doi: 10.1093/infdis/162.2.560. [DOI] [PubMed] [Google Scholar]
- 9.Wiuff C, Zappala RM, Regoes RR, Garner KN, Baquero F, Levin BR. 2005. Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob Agents Chemother 49:1483–1494. doi: 10.1128/AAC.49.4.1483-1494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pal U, Dai J, Li X, Neelakanta G, Luo P, Kumar M, Wang P, Yang X, Anderson JF, Fikrig E. 2008. A differential role for BB0365 in the persistence of Borrelia burgdorferi in mice and ticks. J Infect Dis 197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, Eggers CH, Anderson JF, Radolf JD, Fikrig E. 2007. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol 63:694–710. [DOI] [PubMed] [Google Scholar]
- 12.Embers ME, Barthold SW. 2012. Borrelia burgdorferi persistence post-antibiotic treatment, p 229–257. In Embers ME. (ed), The pathogenic spirochetes: strategies for evasion of host immunity and persistence. Springer, New York, NY. [Google Scholar]
- 13.Thattai M, van Oudenaarden A. 2004. Stochastic gene expression in fluctuating environments. Genetics 167:523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elowitz MB, Levine AJ, Siggia ED, Swain PS. 2002. Stochastic gene expression in a single cell. Science 297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- 15.Avery SV. 2006. Microbial cell individuality and the underlying sources of heterogeneity. Nat Rev Microbiol 4:577–587. doi: 10.1038/nrmicro1460. [DOI] [PubMed] [Google Scholar]
- 16.Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. 2010. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother 54:643–651. doi: 10.1128/AAC.00788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. 2012. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Nguyen A, Qiu D, Luft BJ. 2009. In vitro activity of tigecycline against multiple strains of Borrelia burgdorferi. J Antimicrob Chemother 63:709–712. doi: 10.1093/jac/dkn551. [DOI] [PubMed] [Google Scholar]
- 19.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yotsuji A, Mitsuyama J, Hori R, Yasuda T, Saikawa I, Inoue M, Mitsuhashi S. 1988. Mechanism of action of cephalosporins and resistance caused by decreased affinity for penicillin-binding proteins in Bacteroides fragilis. Antimicrob Agents Chemother 32:1848–1853. doi: 10.1128/AAC.32.12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dattwyler RJ, Luft BJ, Kunkel MJ, Finkel MF, Wormser GP, Rush TJ, Grunwaldt E, Agger WA, Franklin M, Oswald D, Cockey L, Maladorno D. 1997. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med 337:289–294. doi: 10.1056/NEJM199707313370501. [DOI] [PubMed] [Google Scholar]
- 22.Ogrinc K, Logar M, Lotric-Furlan S, Cerar D, Ruzic-Sabljic E, Strle F. 2006. Doxycycline versus ceftriaxone for the treatment of patients with chronic Lyme borreliosis. Wien Klin Wochenschr 118:696–701. doi: 10.1007/s00508-006-0698-7. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Wang T, Shi W, Zhang S, Sullivan D, Auwaerter PG, Zhang Y. 2014. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbe Infect 3:e49. doi: 10.1038/emi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. 2013. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ates L, Hanssen-Hubner C, Norris DE, Richter D, Kraiczy P, Hunfeld KP. 2010. Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi. Ticks Tick Borne Dis 1:30–34. doi: 10.1016/j.ttbdis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. 2008. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother 52:1728–1736. doi: 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicklinger M, Wienecke R, Neubert U. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J Clin Microbiol 41:1791–1793. doi: 10.1128/JCM.41.4.1791-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zückert WR. 2005. Laboratory maintenance of Borrelia burgdorferi. Curr Protoc Microbiol Chapter 12:Unit 12C.1. doi: 10.1002/9780471729259.mc12c01s4. [DOI] [PubMed] [Google Scholar]
- 30.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 31.Seshu J, Boylan JA, Gherardini FC, Skare JT. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect Immun 72:1580–1586. doi: 10.1128/IAI.72.3.1580-1586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. 2005. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob Agents Chemother 49:1294–1301. doi: 10.1128/AAC.49.4.1294-1301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. 26 May 2015. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother doi: 10.1128/aac.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kargi F. 2009. Re-interpretation of the logistic equation for batch microbial growth in relation to Monod kinetics. Lett Appl Microbiol 48:398–401. doi: 10.1111/j.1472-765X.2008.02537.x. [DOI] [PubMed] [Google Scholar]
- 35.Stanek G, Baradaran-Dilmaghani R. 1996. In vitro susceptibility of thirty Borrelia strains from various sources against eight antimicrobial chemotherapeutics. Infection 24:60–63. doi: 10.1007/BF01780660. [DOI] [PubMed] [Google Scholar]
- 36.Knowles DJ, Foloppe N, Matassova NB, Murchie AI. 2002. The bacterial ribosome, a promising focus for structure-based drug design. Curr Opin Pharmacol 2:501–506. doi: 10.1016/S1471-4892(02)00205-9. [DOI] [PubMed] [Google Scholar]
- 37.Lewis K. 2008. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol 322:107–131. [DOI] [PubMed] [Google Scholar]
- 38.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 39.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 40.Codling EA, Plank MJ, Benhamou S. 2008. Random walk models in biology. J R Soc Interface 5:813–834. doi: 10.1098/rsif.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dörr T, Vulic M, Lewis K. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol 8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips I, Culebras E, Moreno F, Baquero F. 1987. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother 20:631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- 43.Jõers A, Kaldalu N, Tenson T. 2010. The frequency of persisters in Escherichia coli reflects the kinetics of awakening from dormancy. J Bacteriol 192:3379–3384. doi: 10.1128/JB.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen S, Lewis K, Vulic M. 2008. Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli. Antimicrob Agents Chemother 52:2718–2726. doi: 10.1128/AAC.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epstein SS. 2009. Microbial awakenings. Nature 457:1083. doi: 10.1038/4571083a. [DOI] [PubMed] [Google Scholar]
- 46.Rainey PB, Beaumont HJ, Ferguson GC, Gallie J, Kost C, Libby E, Zhang XX. 2011. The evolutionary emergence of stochastic phenotype switching in bacteria. Microb Cell Fact 10(Suppl 1):S14. doi: 10.1186/1475-2859-10-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barthold SW, Persing DH, Armstrong AL, Peeples RA. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol 139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 48.Feng J, Auwaerter PG, Zhang Y. 2015. Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS One 10:e0117207. doi: 10.1371/journal.pone.0117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terekhova D, Sartakova ML, Wormser GP, Schwartz I, Cabello FC. 2002. Erythromycin resistance in Borrelia burgdorferi. Antimicrob Agents Chemother 46:3637–3640. doi: 10.1128/AAC.46.11.3637-3640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodzic E, Imai D, Feng S, Barthold SW. 2014. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bockenstedt LK, Mao J, Hodzic E, Barthold SW, Fish D. 2002. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis 186:1430–1437. doi: 10.1086/345284. [DOI] [PubMed] [Google Scholar]
- 52.Straubinger RK, Summers BA, Chang YF, Appel MJ. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 35:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Embers ME, Ramamoorthy R, Philipp MT. 2004. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect 6:312–318. [DOI] [PubMed] [Google Scholar]
- 54.Klempner MS, Hu LT, Evans J, Schmid CH, Johnson GM, Trevino RP, Norton D, Levy L, Wall D, McCall J, Kosinski M, Weinstein A. 2001. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y, Vulic M, Keren I, Lewis K. 2012. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56:4922–4926. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balaban NQ, Gerdes K, Lewis K, McKinney JD. 2013. A problem of persistence: still more questions than answers? Nat Rev Microbiol 11:587–591. doi: 10.1038/nrmicro3076. [DOI] [PubMed] [Google Scholar]
- 58.Piesman J, Oliver JR, Sinsky RJ. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia Burgdorferi) in vector ticks (Ixodes Dammini). Am J Trop Med Hyg 42:352–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.