Abstract
Leishmaniases comprise a spectrum of diseases caused by protozoan parasites of the Leishmania genus. Treatments available have limited safety and efficacy, high costs, and difficult administration. Thus, there is an urgent need for safer and more-effective therapies. Most trypanosomatids have an essential requirement for ergosterol and other 24-alkyl sterols, which are absent in mammalian cells. In previous studies, we showed that Leishmania amazonensis is highly susceptible to aryl-quinuclidines, such as E5700, which inhibit squalene synthase, and to the azoles itraconazole (ITZ) and posaconazole (POSA), which inhibit C-14α-demethylase. Herein, we investigated the antiproliferative, ultrastructural, and biochemical effects of combinations of E5700 with ITZ and POSA against L. amazonensis. Potent synergistic antiproliferative effects were observed against promastigotes, with fractional inhibitory concentration (FIC) ratios of 0.0525 and 0.0162 for combinations of E5700 plus ITZ and of E5700 plus POSA, respectively. Against intracellular amastigotes, FIC values were 0.175 and 0.1125 for combinations of E5700 plus ITZ and E5700 plus POSA, respectively. Marked alterations of the ultrastructure of promastigotes treated with the combinations were observed, in particular mitochondrial swelling, which was consistent with a reduction of the mitochondrial transmembrane potential, and an increase in the production of reactive oxygen species. We also observed the presence of vacuoles similar to autophagosomes in close association with mitochondria and an increase in the number of lipid bodies. Both growth arrest and ultrastructural/biochemical alterations were strictly associated with the depletion of the 14-desmethyl endogenous sterol pool. These results suggest the possibility of a novel combination therapy for the treatment of leishmaniasis.
INTRODUCTION
Leishmaniasis is caused by protozoan parasites of the Leishmania genus and is transmitted by female phlebotomine sandflies. The disease is spread around the world and is endemic in 98 countries (1, 2). Its diverse clinical manifestations depend mainly on the Leishmania species and the host's immune status (3, 4). One of the most prevalent clinical manifestations of leishmaniasis is the presence of localized lesions in the skin (cutaneous leishmaniasis [CL]), which are characterized by superficial ulcers. Certain species establish infection in the oropharyngeal and nasal mucosae (mucocutaneous leishmaniasis [MCL]), causing disfiguring lesions (4). CL and MCL have severe social and economic impacts. One of the main etiological agents of leishmaniasis in the New World is Leishmania amazonensis (1, 3); this parasite can escape to multiple cutaneous sites and cause an unhealed form of diffuse cutaneous leishmaniasis (DCL), or to the liver, spleen, bone marrow, or distant lymph nodes and cause visceral leishmaniasis (VL). DCL is characterized by the appearance of multiple lesions, both acneiform and ulcerated (4), while VL can lead to death if untreated (5). PKDL (post-kala-azar dermal leishmaniasis) is a clinical manifestation that can appear after etiological treatment of VL patients (1); patients with chronic PKDL can serve as reservoir hosts of infection (5). Owing to the fact that these diseases have traditionally received very limited funding for control and research, they are included among the list of neglected tropical diseases (NTDs) (6).
For almost 7 decades, pentavalent antimonials (SB[v]) have remained the first-line etiological treatment against leishmaniasis in most areas where it is endemic (7), with the exception of India (8), where acquired drug resistance has rendered these drugs useless. Second-line treatments are based on the use of amphotericin B formulations (deoxycholate or liposomal) and pentamidine isethionate, which are toxic and expensive. Miltefosine (Impavido) is an alkyl-lysophospholipid analogue originally developed as an anticancer agent that has selective anti-Leishmania activity in vitro and in vivo; it is currently the first-line treatment in some countries in Asia, Africa, and Europe. Despite the fact that miltefosine is orally available, it is teratogenic and thus contraindicated for women of fertile ages (8, 9). Thus, due to the toxicity, cost, and high rate of resistance to the current drugs used for the treatment of leishmaniasis, there is an urgent need to identify new therapeutic alternatives.
Trypanosomatids and fungi have an essential requirement for ergosterol and other 24-alkyl sterols that are absent in mammalian cells (10). Several studies have demonstrated the potent effects of different ergosterol biosynthesis inhibitors (EBIs) on these microorganisms, as these agents interfere with some essential steps in the ergosterol biosynthesis pathway (10). Itraconazole (ITZ) and posaconazole (POSA), two well-known azoles that inhibit the enzyme sterol C-14α-demethylase (CYP51) and are frequently used as antifungal agents, also have effects in vitro and in vivo against trypanosomatids, including organisms from the Leishmania and Trypanosoma genera (10–19). Recently, we demonstrated that ITZ and POSA have a potent effect against L. amazonensis, inhibiting its growth, disrupting mitochondrial function, and affecting the ultrastructure of several organelles (17). Aryl-quinuclidine derivatives, which inhibit squalene synthase (SQS), the first committed step of the pathway, and were originally developed as cholesterol-lowering agents, also have potent antiproliferative effects against L. amazonensis and Trypanosoma cruzi (20–23); in particular, the bis-aryl-quinuclidine E5700 is an extremely potent inhibitor of both T. cruzi and L. amazonensis SQS, with 50% inhibitory concentrations (IC50s) in the single-digit nanomolar to subnanomolar range, and induces complete growth arrest and loss of cell viability of both parasites in vitro, in association with the complete depletion of their endogenous sterol pools (21, 23). More recently, it was demonstrated that E5700 is capable of blocking the proliferation of a Candida tropicalis strain resistant to fluconazole, itraconazole, and amphotericin B (24). Thus, SQS and CYP51 are essential enzymes for ergosterol biosynthesis and have been described as promising targets for the development of new chemotherapeutic agents against trypanosomatids and fungi.
The concept of combination therapies for the specific treatment of diseases caused by trypanosomatid parasites has received increasing attention in recent years, as they allow a reduction of the drugs' doses and/or the duration of the treatment, thus reducing concomitant toxicities, and at the same time they stave off the development of drug resistance by the pathogens (1, 3, 25). In particular, the use of ergosterol biosynthesis inhibitors as potential anti-Leishmania and anti-T. cruzi agents lends naturally to the consideration of combination therapies, because drugs acting at different steps of the pathway are expected and have been shown to have synergistic effects, in vitro and in vivo (25). Thus, the aim of this work was to investigate the effects of E5700 in combination with itraconazole (ITZ) and posaconazole (POSA) on the proliferation and viability of promastigotes and intracellular amastigotes of L. amazonensis and evaluate their effects in the sterol composition and in the physiological and ultrastructural aspects of these cell types.
MATERIALS AND METHODS
Parasites.
The MHOM/BR/75/Josefa strain of L. amazonensis used in this study was isolated in 1975 from a patient with diffuse cutaneous leishmaniasis by Cesar A. Cuba-Cuba (Brasilia University, Brazil) and kindly provided by the Leishmania Collection of the Instituto Oswaldo Cruz (code IOCL 0071-FIOCRUZ). It has been maintained via inoculation into the base of BALB/c mouse tails. First, amastigote forms were obtained from these mice and transformed into promastigotes that were axenically cultured in Warren's medium (brain heart infusion plus hemin and folic acid) (26) supplemented with 10% fetal bovine serum at 25°C. To obtain intracellular amastigotes for the antiproliferative studies, metacyclic infective promastigotes were used to infect macrophage cultures. To this end, peritoneal macrophages from CF1 mice were harvested by washing them with Hanks' solution and plated in 24-well tissue culture chamber slides, allowing them to adhere to the slides for 24 h at 37°C in 5% CO2 in RPMI medium (Gibco) supplemented with 10% fetal bovine serum. Adherent macrophages were infected with metacyclic promastigotes at a macrophage-to-parasite ratio of 1:10 at 35°C for 2 h and then washed twice with RPMI medium to remove noninternalized parasites. Infected cultures were incubated for 24 h in RPMI medium supplemented with 10% fetal bovine serum.
Drugs.
Posaconazole was provided by Schering Plough Research Institute, Kenilworth, NJ. Itraconazole was purchased from Janssen Pharmaceutica. E5700 {(3R)-3-[[2-benzyl-6-[3R,4S)-3-hydroxy-4-methoxypyrrolidin-1-yl]pyridine-3-yl]ethynyl]quinuclidin-3-ol monohydrate} was provided by Tsukuba Research Laboratories, Eisai Co. The compounds were dissolved in dimethyl sulfoxide (DMSO) at 10 mM (for ITZ and POSA) or 1 mM (for E5700) stock solutions and stored at −20°C. For the experiments, new dilutions were prepared in culture medium to ensure that the final DMSO concentration in the cultures did not exceed 0.1%.
In vitro antiproliferative effects of monotherapy and combination therapy.
The susceptibility of L. amazonensis to ITZ, POSA, and E5700 was evaluated by using parasite proliferation curves in the absence or the presence of drugs alone or in combination. Promastigote cultures were initiated at a cell density of 1.0 × 106 cells/ml, and ITZ, POSA, and/or E5700 was added at different concentrations and combinations from concentrated stock solutions after 24 h of growth. Cells densities were evaluated daily in a Neubauer chamber during 96 h of growth. To evaluate the effects of compounds on L. amazonensis intracellular amastigotes, macrophages were infected as described previously and incubated with different concentrations and combinations of E5700, POSA, and ITZ after 24 h of infection. Fresh medium with drugs was added daily for 3 days (72 h of treatment). After this time, cultures were fixed in Bouin's solution (70% picric acid, 5% acetic acid, and 25% formaldehyde in aqueous solution), washed with 70% ethanol, followed by washing in distilled water and then stained with Giemsa solution for 1 h. The number of intracellular amastigotes and macrophages (infected or not) were counted via light microscopy. Association indices (the mean number of parasites internalized multiplied by the percentage of infected macrophages divided by the total number of macrophages) were determined and used as a parameter to calculate the percentage of infection for each condition used in this study. The concentration that inhibited 50% of growth (IC50s) was calculated. At least three independent experiments were performed for each condition.
Determination of FIC indices and isobologram construction.
To determine if the combinations between the inhibitors are synergic, additive, or antagonist against promastigotes and intracellular amastigotes, classical isobolograms were used, and fractional inhibitory concentrations (FIC index) were calculated as described by Hallander et al. (27). For promastigotes, MIC values was used to calculate the FIC index, using the follow equation: ∑FIC = [(MIC of combination A)/(MIC of A alone)] + [(MIC of combination B)/(MIC of B alone)], where the MIC is the minimum concentration of a drug, used alone or in combination, required to induce complete growth arrest and loss of cell viability, as verified by the subsequent reduction of cell densities and microscopic examination. For intracellular amastigotes, FICs were calculated using the IC50s, where ∑FIC = [(IC50 of combination A)/(IC50 of A alone)] + [(IC50 of combination B)/(IC50 of B alone)]. The IC50s were used in this situation, because it was not possible to determine via light microscopy the drug concentrations that led to loss of cell viability of the intracellular parasites.
Extraction and separation of neutral lipids.
These procedures have been described previously (21–23, 28–30). Briefly, L. amazonensis was cultured as described above, in the presence of E5700, POSA, and ITZ, alone or in combination, at concentrations that led to complete growth arrest after 72 h. Lipids were extracted with chloroform-methanol (2:1, vol/vol). The extract was dried and suspended in a minimum volume of chloroform. The chloroform suspension was applied to a silicic acid column (1.5 by 4 cm) and washed with 4 column volumes of chloroform to separate neutral lipids from other lipid fractions.
Free sterols analysis.
For quantitative analysis and structural assignment, neutral lipids were separated in a capillary high-resolution column (30 m by 0.25 mm inner diameter [i.d.] HP-5MS column; 5% diphenyl, 95% dimethylpolysilxane; 0.25-μm film thickness) in an Agilent Technologies 7890A gas chromatograph equipped with an Agilent 5975C MSD/DS Performance Turbo EI detection system. Lipids were dissolved in ethyl acetate and injected into the column at an initial temperature of 50°C (1 min), followed by a temperature increase to 270°C at a rate of 20°C/min and a further rise to 300°C at a rate of 1°C/min. The carrier gas (He) flow was kept constant at 1 ml/min. The injector temperature was 250°C; the detector was kept at 280°C. To estimate the level of endogenous sterols/cell in control and drug-treated cultures, the total areas of the corresponding chromatographic peaks were divided by the cell densities of the cultures.
Estimation of the mitochondrial transmembrane electric potential.
The mitochrondrial transmembrane electric potential (ΔΨm) was determined using JC-1 fluorochrome, which is a lipophilic cationic mitochondrial vital dye that becomes concentrated in the mitochondrion. The dye exists as a monomer at low concentrations, where the emission is at 530 nm (green fluorescence), but at higher concentrations it forms J-aggregates after accumulation in the mitochondrion and emission is at 590 nm (red fluorescence). Thus, the fluorescence of JC-1 is considered an indicator of an energized mitochondrial state, and it has been used to measure the ΔΨm in Leishmania (16, 17, 31). Control (untreated) promastigotes and cells treated with E5700, ITZ, and POSA, alone or in combination, were harvested, washed in phosphate-buffered saline (PBS; pH 7.2), added to a mitochondrial reaction medium containing 125 mM sucrose, 65 mM KCl, 10 mM HEPES/K+ (pH 7.2), 2 mM Pi, 1 mM MgCl2, and 500 μM EGTA, and counted in a Neubauer chamber. For the analysis, 1.0 × 107 parasites were incubated in 10 μg/ml JC-1 in a black 96-well plate during 23 min with readings made every 1 min using a microplate reader, the SpectraMax M2/M2e spectrofluorometer (Molecular Devices, USA). After this time, 2 μM carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) was added to abolish the ΔΨm. This allowed comparison of the magnitude of the ΔΨm under the different experimental conditions. FCCP at 2 μM was also used as a positive control. The ratio between the reading at 590 nm and the reading at 530 nm (the 590:530 ratio) was obtained for calculating the relative ΔΨm value. The experiments were repeated at least three times in triplicate.
Determination of ROS and superoxide radicals.
Intracellular reactive oxygen species (ROS) levels were measured as described previously (16, 32). A total of 3 × 107 promastigotes were harvested, washed in PBS (pH 7.2), resuspended in PBS (500 μl), and incubated with H2DCFDA (10 μg/ml for 1 h at 25°C), a cell-permeable green probe. After 1 h, cells were washed and resuspended in 600 μl PBS, added in a black 96-well plate, and then analyzed in a microplate reader, the SpectraMax M2/M2e spectrofluorometer (Molecular Devices, USA), using the pair of 507-nm and 530-nm wavelengths as emission and excitation wavelengths, respectively. For the analysis of mitochondrial superoxide, MitoSOX Red indicator (Molecular Probes, USA) was used. Mitochondrial superoxide is generated as a by-product of oxidative phosphorylation and can be measured using the MitoSOX Red indicator, which is a fluorogenic dye highly specific for mitochondria of live cells (33). Thus, 3 × 107 cells were harvested, washed in PBS (pH 7.2), resuspended in 4 μM MitoSOX Red diluted in Hanks' solution, and incubated for 20 min at 25°C. Then, cells were washed and resuspended in 600 μl of mitochondrial reaction medium, containing 125 mM sucrose, 65 mM KCl, 10 mM HEPES/K+ (pH 7.2), 2 mM Pi, 1 mM MgCl2, and 500 μM EGTA. After that, the cell suspension was transferred to a black 96-well plate, in a final volume of 200 μl/well. The analyses were performed in triplicate at 510 nm and 580 nm as the emission and excitation wavelengths, respectively.
Electron microscopy.
Control and treated promastigotes and intracellular amastigotes were fixed for 24 h at 4°C in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2). After fixation, cells were washed with 0.1 M cacodylate buffer (pH 7.2) and postfixed in a solution containing 1% OsO4, 1.25% potassium ferrocyanide, 5 mM CaCl2, and 0.1 M cacodylate buffer (pH 7.2) for 30 min. Afterward, cells were washed in the same buffer. For transmission electron microscopy (TEM), cells were dehydrated in an acetone series and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Zeiss 900 electron microscope. For scanning electron microscopy (SEM), promastigotes were dehydrated in an ethanol series, critical point dried in CO2, mounted on stubs, sputtered with a thin gold layer, and observed under a Jeol 5310 electron microscope.
Neutral lipid accumulation.
For quantification of the presence of lipid bodies induced by the different treatments, 1.0 × 107 cells were harvested, washed in PBS (pH 7.2), and incubated with 10 μg/ml Nile Red (Sigma, Brazil) for 20 min. After that, cells were washed in PBS twice before analysis, and the final volume in each well was 200 μl of cell suspension in PBS. The experiment was performed in triplicate using a black 96-well plate. Readings were taken in a microplate reader, the SpectraMax M2/M2e spectrofluorometer (Molecular Devices, USA), using the wavelengths 485 and 538 nm for excitation and emission, respectively.
Statistical analysis.
All the graphics in the figures were created using the means of three independent experiments, and the bars represent the standard deviations of the means. The statistical significance of differences among the groups was assessed using the one-way analysis of variance (ANOVA) test followed by Bonferroni's multiple-comparison test in the GraphPad Prisma 5 software. Results were considered statistically significant when P was <0.05 (*), <0.01 (**), and <0.001 (***).
RESULTS
In vitro combined drug treatment against extracellular promastigotes and intracellular amastigotes in peritoneal macrophages.
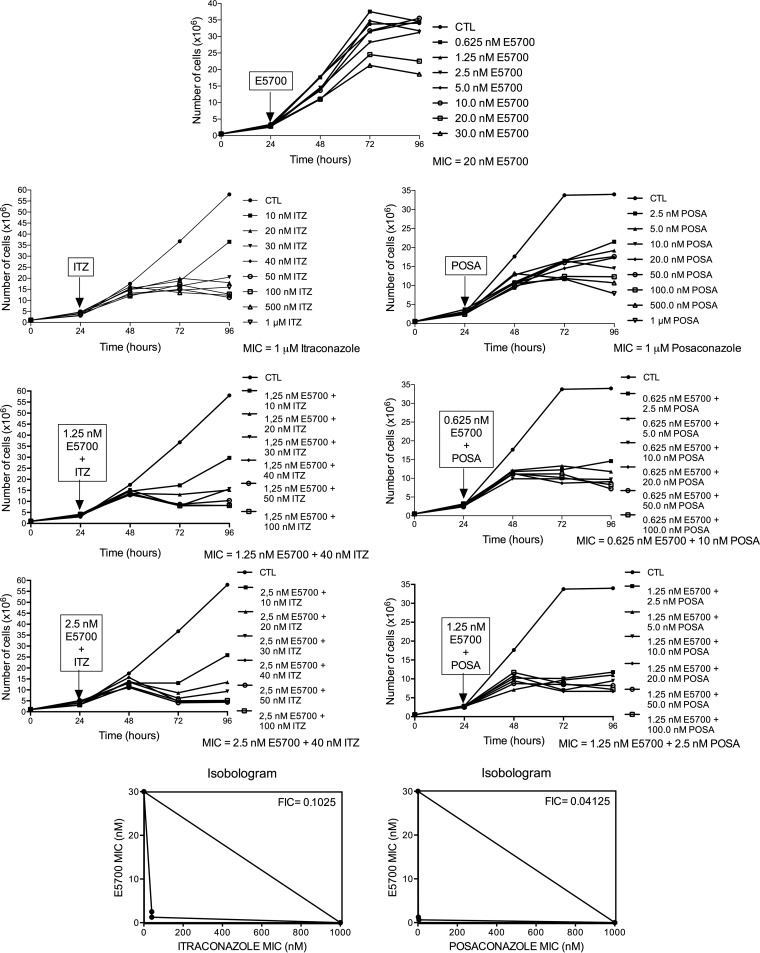
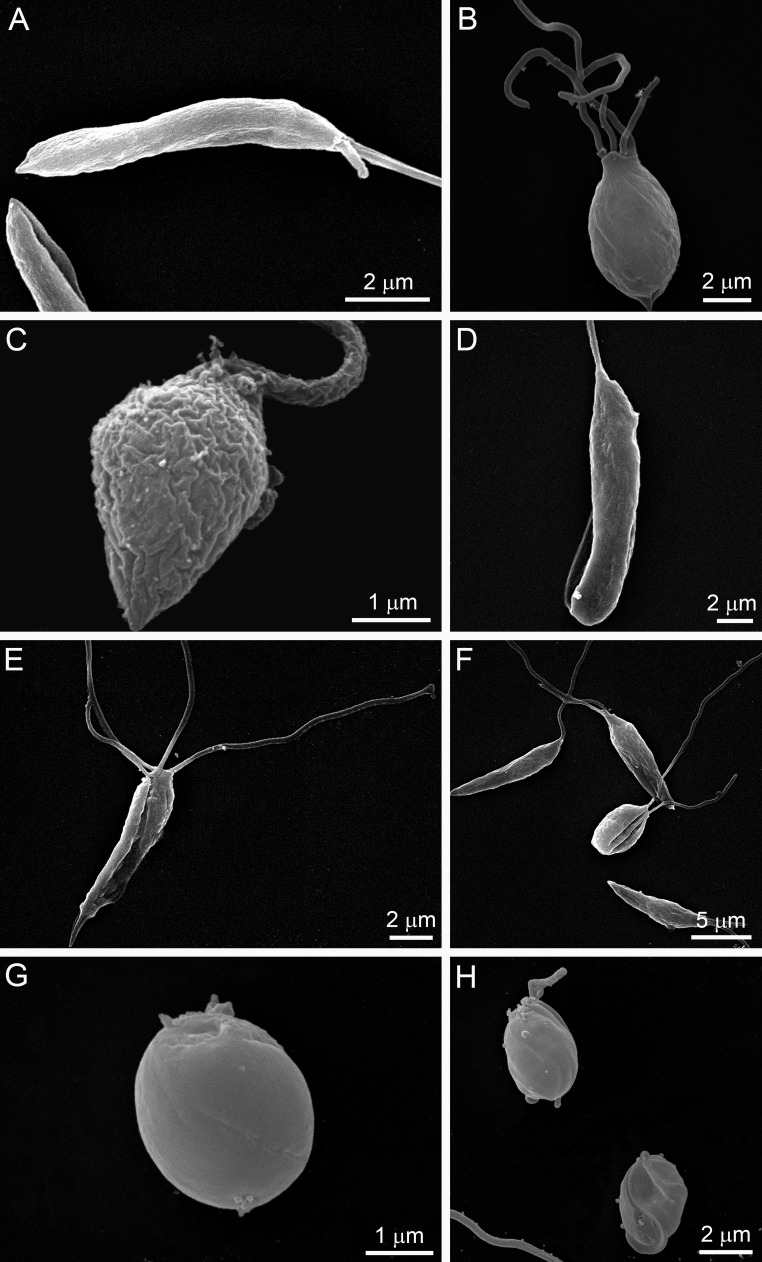
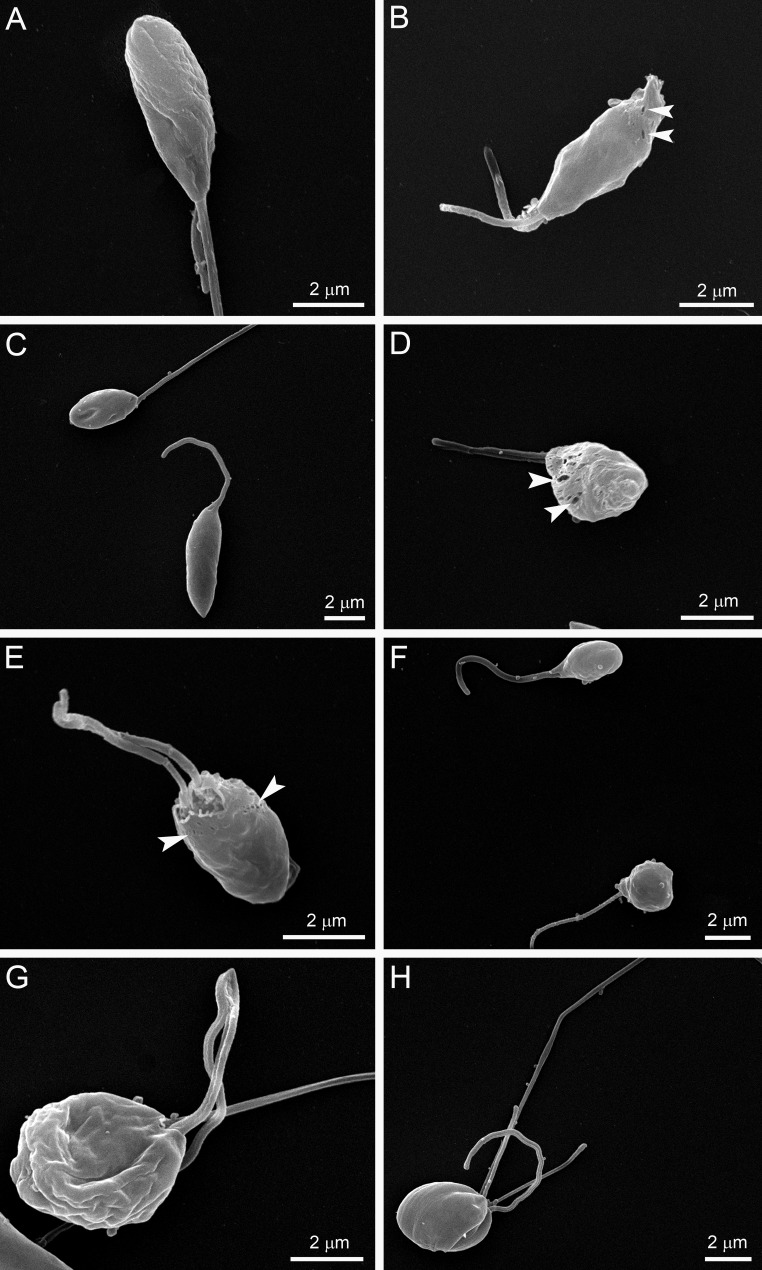
Figure 1 shows the antiproliferative effects of POSA, ITZ, and E5700, alone or in combination, on the proliferation of L. amazonensis promastigotes. After 48 h of treatment, ITZ and POSA had MICs of 1 μM, while E5700 was 10-fold more effective, with a MIC of 100 nM; these values agree within an order of magnitude with the results of our previous studies on the activities of the drugs used alone against this organism (17, 21). When POSA or ITZ was combined with E5700, potent antiproliferative effects were observed, with MIC values of 1.25 nM E5700 plus 40 nM ITZ, of 0.625 nM E5700 plus 10 nM POSA, of 2.5 nM E5700 plus 20 nM ITZ, and of 1.25 nM E5700 plus 5 nM POSA. Using the method proposed by Hallander et al. (27), FICs were calculated (see Materials and Methods). The FIC values were 0.0525 and 0.0162 for combinations of E5700 plus ITZ and of E5700 plus POSA, respectively. These results demonstrated a remarkable synergism for the combined actions of the drugs, leading to a 25-fold reduction of the MIC of ITZ in the presence of a concentration of E5700 80-fold lower than its MIC when acting alone and a 100-fold reduction of the MIC of POSA in the presence of a concentration of E5700 160-fold lower than its MIC when acting alone. Scanning electron microscopy revealed several alterations on the cell surface (Fig. 2 and 3) and the shapes of promastigotes after treatment with the drugs, acting alone or in combination. When promastigotes were treated with 1 μM ITZ, 1 μM POSA, 30 nM E5700, or 150 nM E5700, they appeared rounded (Fig. 2B, C, and F to H), swollen (Fig. 2B to D), or had more than one flagellum (Fig. 2B and E). After treatment with the drug combinations at the concentrations that led to complete growth arrest (see above), the alterations were very similar to those observed with the drugs acting alone at their respective MIC (Fig. 3). Promastigotes treated with the drug combinations appeared generally rounded and swollen (Fig. 3A to H), and in cells exposed to 1.25 nM E5700 plus 2.5 nM POSA (Fig. 3B and D, arrowheads) or 1.25 nM E5700 plus 40 nM ITZ (Fig. 3E, arrowheads), large pores were observed in the plasma membrane. Cells with more than one flagellum were also frequently observed (Fig. 3H).
FIG 1.
Antiproliferative effects of E5700, ITZ, and POSA, alone and in combination, against L. amazonensis promastigotes. Parasites were treated with different concentrations for 72 h to evaluate growth. Several combinations between E5700, ITZ, and POSA were tested. The arrows indicate the time of addition of the drugs at the indicated concentrations. The MIC values represent the MIC that produced total death of the parasite population in culture. Isobolograms illustrate the combined effects between E5700 and ITZ or POSA on L. amazonensis promastigotes. The FIC was calculated according to the method described by Hallander et al. (27). FIC values less than 0.5 indicate a synergistic effect.
FIG 2.
SEM images of L. amazonsensis promastigotes treated with E5700, ITZ, or POSA for 48 h. (A) Control; (B) 1 μM POSA; (C) 1 μM ITZ; (D, E, and F) 30 nM E5700; (G and H) 150 nM E5700. The images show significant alterations in the shapes of promastigotes after treatment with the MICs of the drugs. Some cells appeared rounded (B, C, and F to H), swollen (B to D), and/or had more than one flagellum (B and E). After treatment with 150 nM E5700, in many fields all cells appeared rounded (G and H).
FIG 3.
SEM images of L. amazonsensis promastigotes treated with different combinations of E5700 with ITZ or POSA for 48 h. (A) Result with 0.625 nM E5700 plus 10 nM POSA; (B to D) 1.25 nM E5700 plus 2.5 nM POSA; (E and F) 1.25 nM E5700 plus 40 nM ITZ; (G and H) 2.5 nM E5700 plus 40 nM ITZ. The much lower concentrations in the drug combinations produced the same effects as with the drugs alone at their MICs. Promastigotes appeared completely altered, being rounded and swollen (A to H). The treatments with 1.25 nM E5700 plus 2.5 nM POSA (B and D) or 1.25 nM E5700 plus 40 nM ITZ (E) resulted in the appearance of large pores in the membranes of the parasites (arrowheads). The images also include a promastigote with more than one flagellum (H).
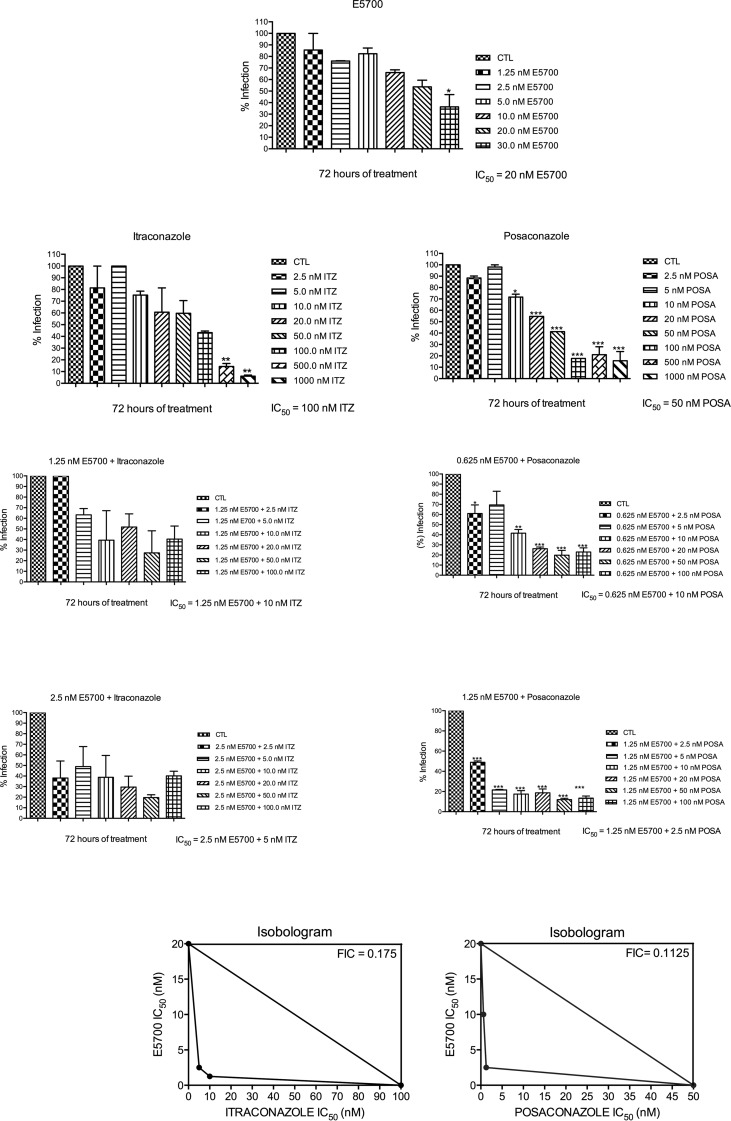
Against the clinically relevant intracellular amastigotes, the antiproliferative effects of the drugs acting alone were more potent than against the extracellular promastigotes, with IC50s for ITZ, POSA, and E5700 of 100, 50, and 20 nM, respectively; as with the promastigotes, these values agreed within an order of magnitude with the results of our previous studies on the activities of the drugs when used alone against this organism (17, 21). Again, strong synergistic effects were observed for the drug combinations, with IC50s of 2.5 nM E5700 plus 5 nM ITZ and 1.25 nM E5700 plus 2.5 nM POSA, which correspond to FICs of 0.175 and 0.1125 for E5700 plus itraconazole and E5700 plus posaconazole, respectively (Fig. 4). Again, these results indicated a potent synergism for the combined actions of the drugs, leading to a 20-fold reduction of the IC50 of ITZ in the presence of a concentration of E5700 8-fold lower than its IC50 when acting alone and a 20-fold reduction of the IC50 of POSA in the presence of a concentration of E5700 16-fold lower than its IC50 when acting alone.
FIG 4.
Antiproliferative effects of E5700, ITZ, and POSA, alone and in combination, against L. amazonensis intracellular amastigotes after 72 h of treatment. The graphs show the percentages of infection, which were determined as described in Materials and Methods. The concentrations that inhibited 50% of the growth (IC50s) were calculated. At least three independent experiments were performed for each condition. Isobolograms illustrate the combined effects between E5700 and ITZ or POSA on L. amazonensis intracellular amastigotes. The FICs were calculated according to the method of Hallander et al. (27). FIC values less than 0.5 indicate a synergistic effect.
For miltefosine, a standard drug to treat leishmaniasis, the IC50s obtained in our model of infection were 25 μM and 20 μM for promastigotes and intracellular amastigotes, respectively (data not shown). Thus, sterol biosynthesis inhibitors, used as monotherapies or in combinations, were markedly more potent that miltefosine against both proliferative stages of this parasite.
Effects of the combinations between E5700 and posaconazole or itraconazole on the fine structure of L. amazonensis.
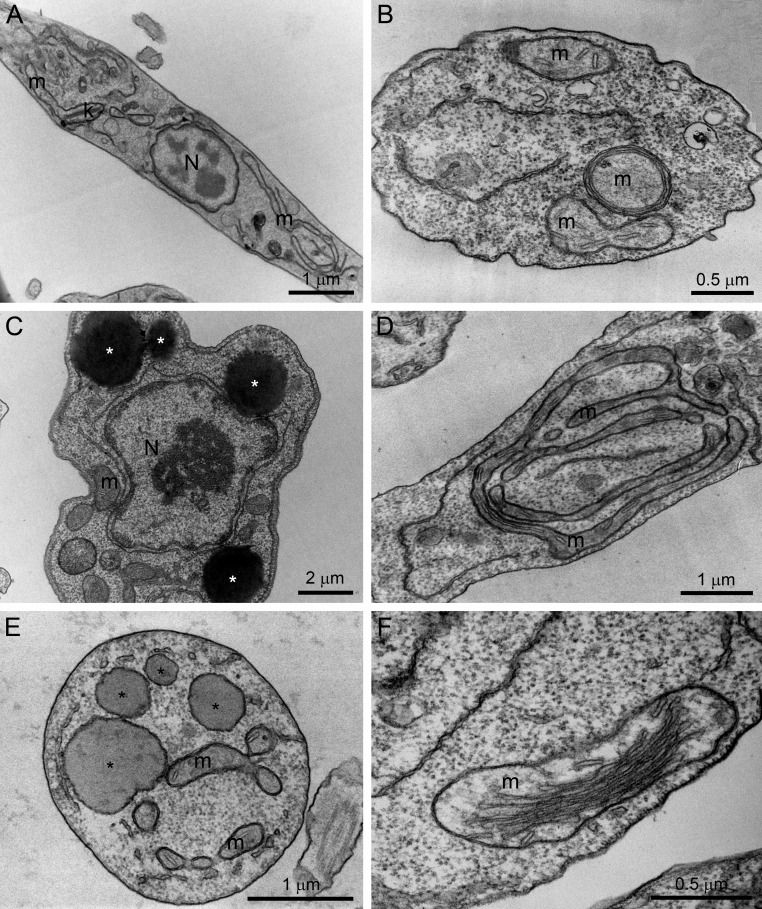
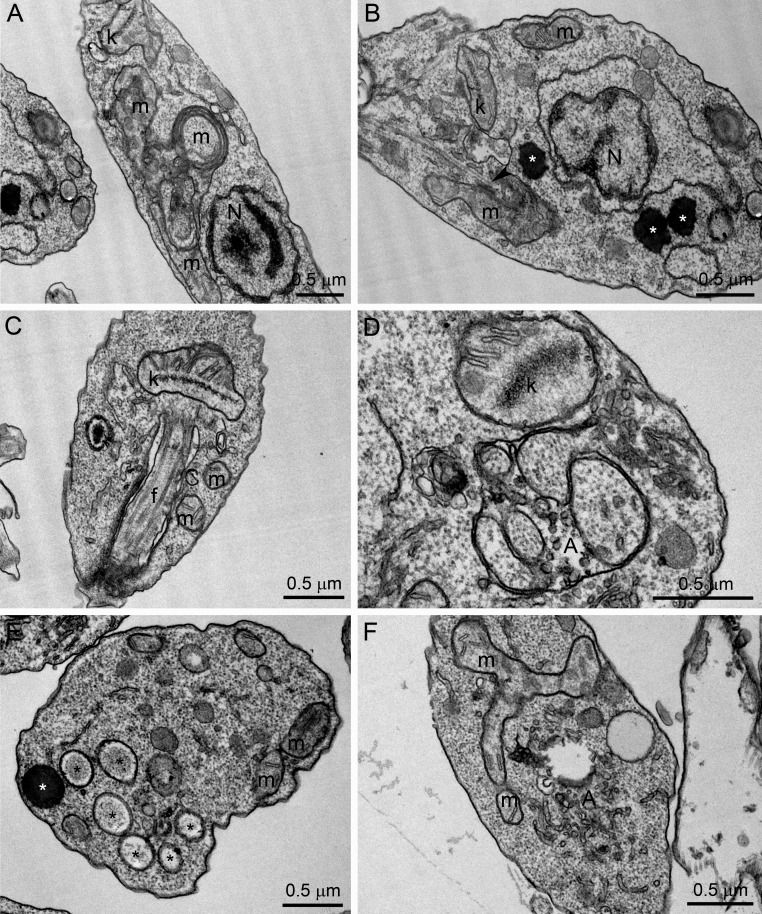
The effects of POSA, ITZ, and E5700 alone on the ultrastructure of promastigotes have been described previously (17, 21). Transmission electron microscopy was carried out with cells treated with the drug combinations in order to compare the effects with the drugs alone. As found before (17, 21), the main alterations caused by the treatment of promastigotes with 1 μM ITZ, 1 μM POSA, or 30 nM E5700 were an intense mitochondrial swelling followed by its disorganization (Fig. 5B, D, and F), with a significant increase of the cristae, as suggested by the image in Fig. 5F, and the presence of several lipid bodies, which sometimes appear near the endoplasmic reticulum and mitochondrion profiles (Fig. 5C and E). These alterations were also observed with the combinations of low drug concentrations that led to complete growth arrest; thus, treatment with 0.625 nM E5700 plus 5 nM POSA and of 1.25 nM E5700 plus 40 nM ITZ caused several alterations on the fine structure of the parasite, comparable or more severe than those observed with the MICs of the drugs acting alone, including (i) mitochondrial swelling and disorganization (Fig. 6A, B, and F), (ii) appearance of circular cristae (Fig. 6A), (iii) changes in the structure of the kinetoplast (Fig. 6C and D), (iv) presence of several lipid bodies (Fig. 6B and E), and (v) presence of autophagosome-like structures close to organelles such as mitochondria (Fig. 6D and F).
FIG 5.
Ultrathin sections of L. amazonensis promastigotes in the control group (A) or in groups treated with E5700, ITZ, or POSA (B to F) for 48 h. (B) Result with 1 μM POSA; (C) 1 μM ITZ; (D to F) 30 nM E5700. Several alterations were observed, such as the presence of several lipid bodies (asterisks), which sometimes appeared near the endoplasmic reticulum and mitochondrion profiles (C and E), and intense disorganization and swelling of the mitochondrion (B, D, and F). N, nucleus; m, mitochondrion; k, kinetoplast.
FIG 6.
Ultrathin sections of L. amazonensis promastigotes treated with different combinations of E5700 and POSA or ITZ. (A to C) The 0.625 nM E5700 plus 5 nM POSA group; (D to F) 1.25 nM E5700 plus 40 nM ITZ. Several alterations were observed, such as mitochondrial swelling and disorganization (A, B, and F), appearance of circular cristae (A), changes in the structure of the kinetoplast (C and D), presence of several lipid bodies (B and E; asterisks), and the appearance of autophagosome-like structures close to organelles such as the mitochondria (D and F). N, nucleus; m, mitochondrion; k, kinetoplast; f, flagellum; A, autophagosome.
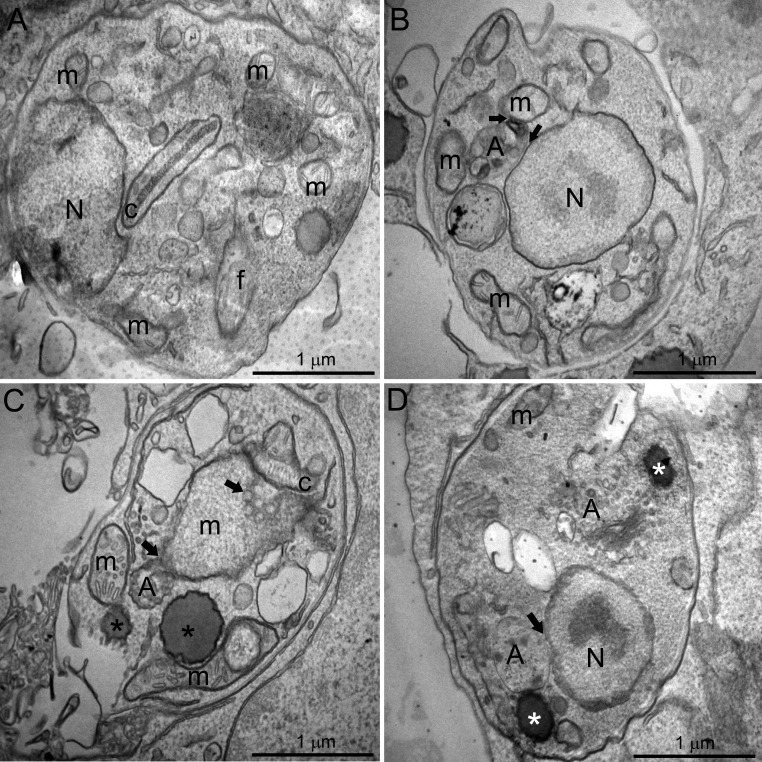
Ultrastructural alterations were also observed in L. amazonensis intracellular amastigotes after treatment with the inhibitors (Fig. 7). After just 48 h of treatment using the much lower concentrations of the inhibitors in combinations, 1.25 nM E5700 plus 40 nM ITZ (Fig. 7B and C) or 0.625 nM E5700 plus 5 nM POSA, it was possible to observe the presence of some vacuoles similar to autophagosomes containing many small vesicles and membrane profiles (Fig. 7D). Sometimes, these structures appear in close association with organelles, such as the mitochondrion (Fig. 7B and C, arrows) and nucleus (Fig. 7B and D, arrows). Figure 7B also shows a close association between the endoplasmic reticulum and glycosomes (arrowhead). In addition, alterations in the mitochondrion and kinetoplast were observed (Fig. 7C), including swelling and appearance of circular cristae. Furthermore, treatments induced the appearance of lipid bodies (Fig. 7C and D, asterisks), some of them in close association with the mitochondrion (Fig. 7C, arrowhead).
FIG 7.
Ultrathin sections of L. amazonensis intracellular amastigotes. (A) Control intracellular amastigotes. (B to D) Amastigotes treated with different combinations of E5700 and ITZ or POSA for 48 h: 1.25 nM E5700 plus 40 nM ITZ (B and C); 0.625 nM E5700 plus 5 nM POSA (D). Different ultrastructural alterations were observed: (i) presence of some vacuoles containing many small vesicles and membrane profiles similar to autophagosomes, sometimes close to organelles such as the mitochondrion and nucleus (B to D, arrows); (ii) mitochondrial swelling and appearance of circular cristae (C); (iii) presence of lipid bodies (C and D; asterisks). N, nucleus; m, mitochondrion; k, kinetoplast; f, flagellum; A, autophagosome.
Effects of POSA and ITZ in combination with E5700 on the free sterol composition of promastigotes.
We next analyzed the effects of POSA and ITZ in combination with E5700 on the free sterol composition of promastigotes by using high-resolution capillary gas chromatography coupled to mass spectrometry (21–23, 28–30) (see Materials and Methods). The results are presented in Tables 1 to 3. The free sterols of control (untreated) promastigotes are ergosta-5,7,24(24′)-trien-β-ol (5-dehydro episterol) and ergosta-5,7,24(24′)-dien-β-ol (episterol), both synthesized de novo, which account for 75% and 16%, respectively, of the total sterols, and cholesterol, taken passively from the growth medium and accounting for 9%. Parasites grown in the presence of increasing levels of E5700 displayed a dose-dependent reduction of the percentage of endogenous sterols with a corresponding increase in the proportion of cholesterol, which accounts for ca. 56% in cells treated with 100 nM E5700 for 72 h (Table 1) and a concomitant 12-fold reduction (from 12 μg/108 cells to 1 μg/108 cells; 92%) of the content of endogenous sterols compared with untreated cells. Such results are consistent with those of our previous study on the effects of E5700 and ER-119884 on this parasite (21) and agree qualitatively and quantitatively with the notion that the primary mechanism of action of these compounds is a blockade of endogenous sterol biosynthesis at the level of SQS, the first committed step of the pathway.
TABLE 1.
Free sterols present in L. amazonensis promastigotes grown in the absence or presence of E5700
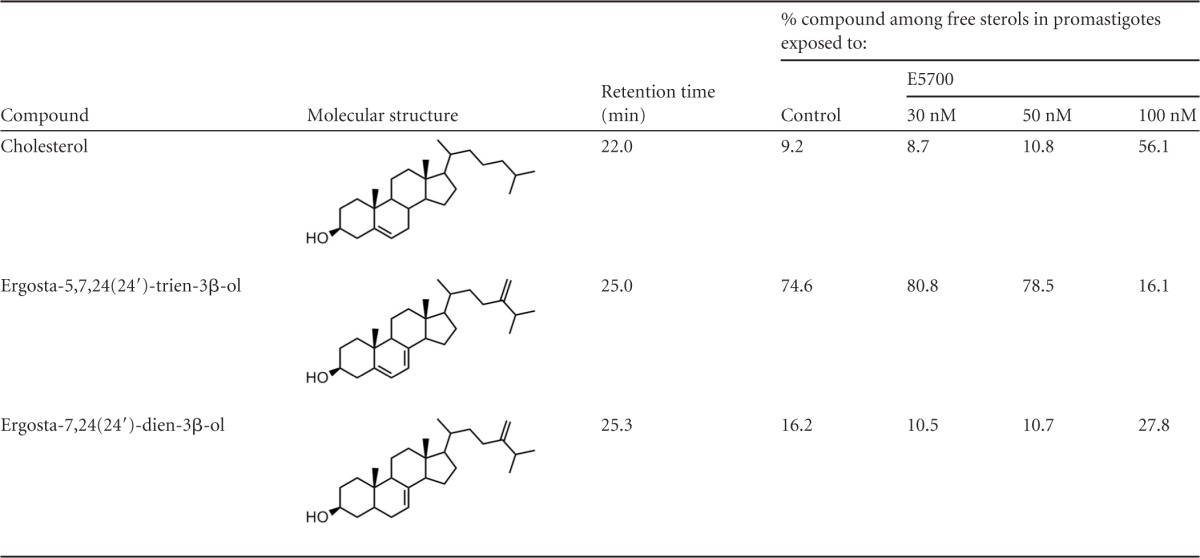
TABLE 3.
Free sterols present in L. amazonensis promastigotes grown in the absence or presence of POSA and/or E5700
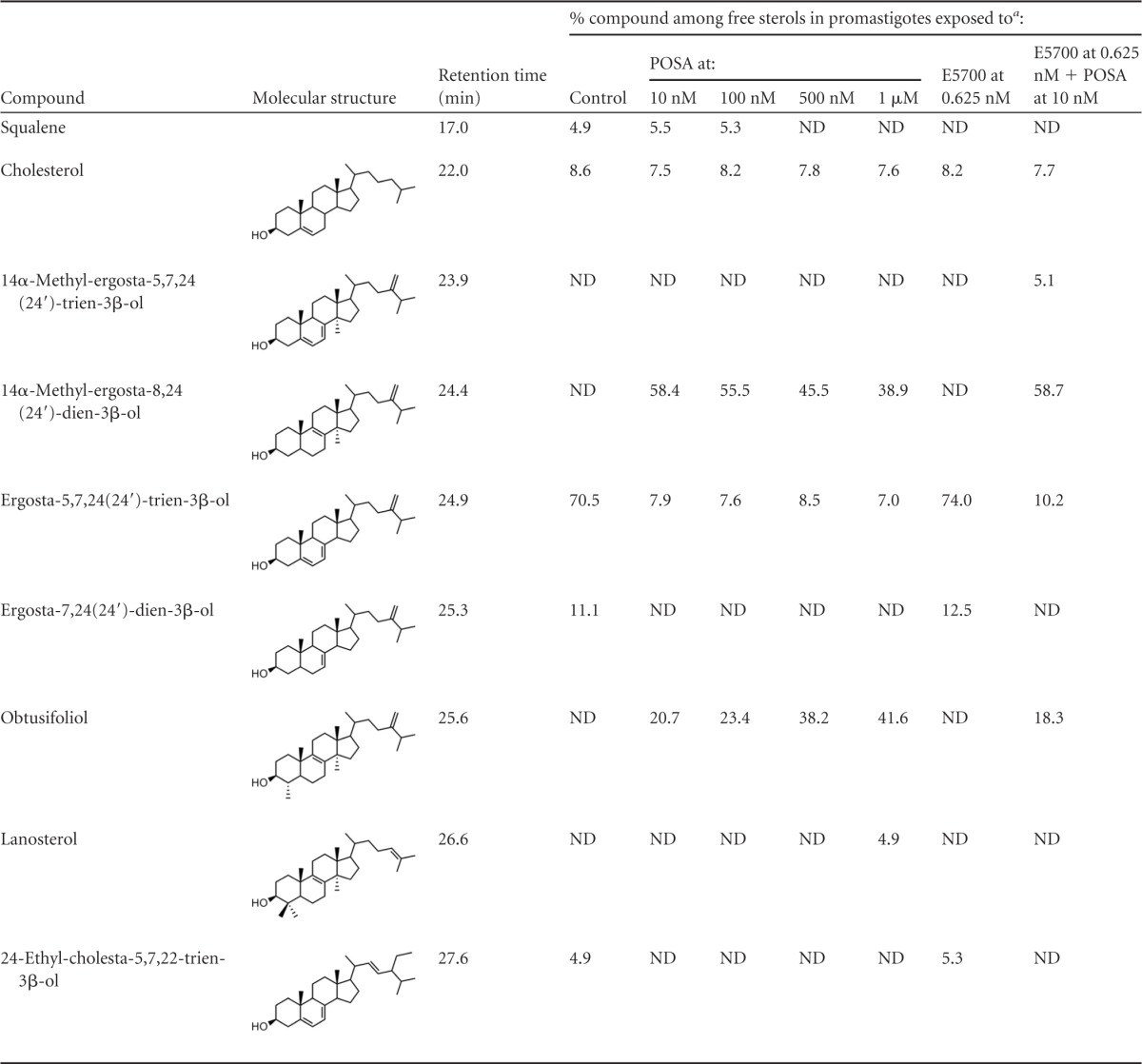
ND, not detected.
Tables 2 and 3 show the effects of ITZ and POSA, alone and in combination with E5700, on free sterols of the promastigotes. When ITZ and POSA acted alone, there was a dramatic reduction of the endogenous 14-desmethyl sterols (episterol and 5-dehydro-episterol), with a concomitant accumulation of 14-methyl-sterols, particularly 14α-methyl-ergosta-8,24(24′)-dien-β-ol, obtusifoliol [4,14-dimethyl-ergosta-8,24(24′)-dien-β-ol] and, to a lesser extent, lanosterol [4,4′,14-trimethyl-cholesta-8,24(25)-dien-β-ol]; these 14-methyl sterols account for 85 to 88% of total free sterols at the MIC (1 μM), while cholesterol levels in treated and untreated cells remained constant, at 7 to 9%. It must be noted that, in contrast with the results obtained with E5700, there were no significant differences in the levels of endogenous sterols per cell among the different treatment groups (data not shown), as expected. Although there are no previous reports on the effects of ITZ or POSA on the sterol composition of L. amazonensis promastigotes, these results agree completely with those of previous studies on the effects of the CYP51 inhibitors ketoconazole and terbinafine, an inhibitor of squalene epoxidase, on L. braziliensis, L. mexicana (30), and combinations of ketoconazole with 22,26-azasterol, an inhibitor of sterol 24(25)-methenyltransferase, on L. amazonensis (34) promastigotes. Finally, in cells treated with low levels of E5700 (0.625 and 1.25 nM) (Tables 2 and 3), there were no significant differences in the sterol composition compared with untreated cells, except for the total disappearance of squalene, as expected. However, in cells treated with these low levels of the SQS inhibitors in combination with low levels of ITZ or POSA, which led to complete growth inhibition and loss of cell viability, the sterol composition resembled that of cells treated with the MICs of the latter drugs, plus an additional reduction of the levels of 14-desmethyl sterols due to the effect of E5700 and the accumulation of 14-α-methyl-5,7,24(24′)-ergosta-trien-3-β-ol, a compound not detected in cells treated with single drugs.
TABLE 2.
Free sterols present in L. amazonensis promastigotes grown in the absence or presence of ITZ and/or E5700

ND, not detected.
Taken together, these findings are consistent with the presumed mechanism of action of the different drugs and confirm that the drug combinations that led to complete growth arrest and loss of cell viability had a free sterol composition comparable to that of cells incubated with ITZ or POSA alone at their respective MICs.
Effects of POSA and ITZ in combination with E5700 on the mitochondrial physiology of promastigotes.
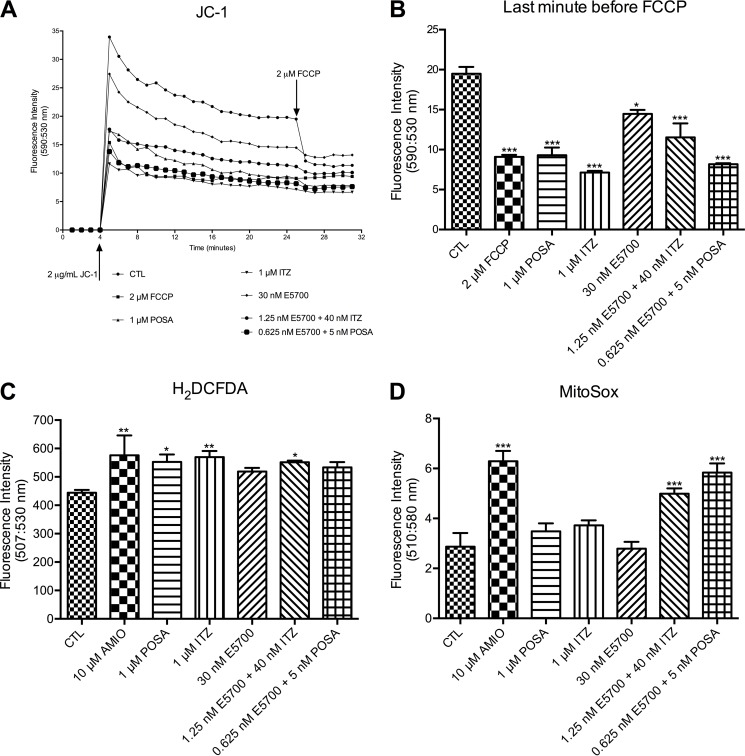
To investigate the effect of sterol biosynthesis inhibitors on the mitochondrial function, L. amazonensis promastigotes were treated for 48 h with inhibitors alone or in combination, prior to analysis (Fig. 8). For that, three criteria were used: (i) the transmembrane electric potential of the inner mitochondrial membrane, obtained using JC-1 fluorochrome; (ii) production of ROS, detected by using a green H2DCFDA probe; (iii) detection of the mitochondrial superoxide using MitoSOX Red, a mitochondrial superoxide indicator. The classic protonophore uncoupler FCCP was used as a positive control to dissipate the mitochondrial electrochemical H+ gradient in analysis with JC-1. To determine the Δψm, the ratio of fluorescence intensity obtained at 590 nm (which represents the J-aggregates [red fluorescence] that accumulate in intact and energized mitochondria) and 530 nm (which represent the J-monomers [green fluorescence], which are a marker for deenergized mitochondria) were used. The decrease of this value indicates a collapse in the mitochondrial transmembrane potential. Treatments with the drugs alone or in combination resulted in a marked reduction in the Δψm (Fig. 8A and B). The treatment with 1.25 nM E5700 plus 40 nM ITZ or 0.625 nM E5700 plus 5 nM POSA caused a strong effect on the Δψm, similar to the effect caused by FCCP. Comparing the effects on the Δψm in cells subjected to monotherapy versus combination therapy, the effects on the mitochondrial potential were similar but with much lower concentrations required in the combination treatments (25-fold lower for ITZ and 200-fold lower for POSA).
FIG 8.
Analysis of the mitochondrial transmembrane electric potential (A and B) and intracellular ROS (C) and superoxide production (D) in L. amazonensis promastigotes from the control group or treated with E5700, POSA, or ITZ, alone or in combination, for 48 h of treatment. (A) The Δψm values were evaluated over 26 min, before addition of 2 μM FCCP to abolish the mitochondrial membrane potential. The decrease of the Δψm value indicates a collapse in the mitochondrial transmembrane potential. (B) Results at the last minute before addition of 2 μM FCCP, showing the Δψm. It is possible to observe that the treatment with 1.25 nM E5700 plus 40 nM ITZ or of 0.625 nM E5700 plus 5 nM POSA for 48 h caused a strong effect on the Δψm, similar to those caused by FCCP. (C) Production of ROS was measured in the cells. Control and treated cells were incubated with H2DCFDA, and the fluorescence intensity was quantified via a microplate reader. The treatment was able to increase ROS production. (D) Analysis of superoxide production in the treated cells for 48 h. A significant increase was observed just after the treatment with two combinations, 1.25 nM E5700 plus 40 nM ITZ versus 0.625 nM E5700 plus 5 nM POSA. These data suggest a potent synergistic effect of the inhibitors on the mitochondrial physiology. The experiments were performed three times, each time in triplicate, and the results shown are representative of these experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The analyses of ROS and superoxide production were performed at 48 h after treatment, under the same conditions used for the analysis of the mitochondrial transmembrane potential with JC-1 (Fig. 8). Amiodarone (AMIO) was used as a positive control, since it is able to inhibit the oxidative phosphorylation by inducing a dissipation of the Δψm and an increase in ROS production in L. amazonensis (16). All treatments, alone or in combination, induced a significant increase in ROS production (Fig. 8C); however, only positive controls and the combinations were able to induce an increase in mitochondrial superoxide production (Fig. 8D). These data confirmed potent synergic effects of the combinations also on the mitochondrial physiology of promastigotes.
Analysis of lipid bodies accumulation and effects of the EBIs on L. amazonensis free sterol composition.
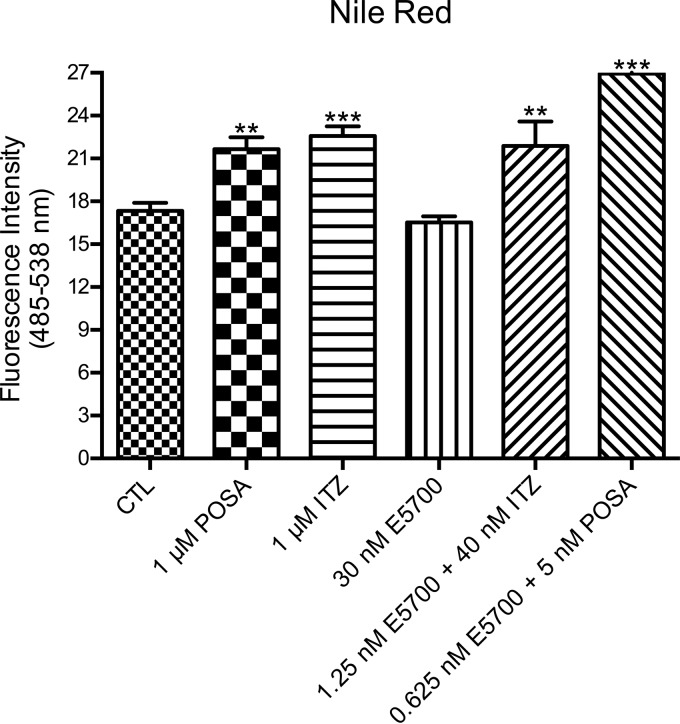
Quantitative fluorimetric analyses using Nile Red were performed to evalaute a possible accumulation of neutral lipids inside the lipid bodies (LBs). Nile Red is a phenoxazone dye that fluoresces intensely when bound to hydrophobic particles, such as lipid bodies (35). Quantitative fluorimetry indicated that treatments with 1 μM POSA or 1 μM ITZ were able to induce significant increases in the accumulation of lipid bodies (Fig. 9), in agreement with previous results published by our group (17). On the other hand, the treatment with 30 nM E5700 was not able to induce this increase compared with the control parasites. However, when cells were incubated with the low-dose combinations of E5700 with ITZ or POSA, the increase of lipid bodies was very significant and similar to that induced by the drugs acting alone at higher concentrations (Fig. 9), again indicating synergic effects on this metabolic alteration.
FIG 9.
Analysis of lipid body accumulation in L. amazonensis promastigotes in the control group and groups treated with E5700, POSA, or ITZ, alone or in combination. The results indicated that treatment with 1 μM POSA and 1 μM ITZ induced a significant increase in the accumulation of lipid bodies, different from those observed after treatment with 30 nM E5700. On the other hand, treatment with the combinations of drugs caused a strong increase of Nile Red accumulation. Fluorescence intensity is expressed in arbitrary units. The experiments were performed three times, each time in triplicate, and the data shown are representative of these experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Ergosterol biosynthesis inhibitor compounds have marked in vitro activities against protozoan parasites of the Trypanosomatidae family, and several of them have been shown to be highly efficacious in in vitro or in vivo models of acute and chronic Chagas' disease and leishmaniasis (11–17, 20–23, 36–44). Azole derivatives, selective inhibitors of sterol C-14 demethylase (CYP51) from fungi and protozoan parasites, are currently the mainstay for the treatment of fungal infections. Among them, itraconazole has several advantages in the treatment of invasive fungal infections, due to its low toxicity and consistent oral bioavailability (45). Posaconazole is structurally similar to itraconazole, has an extended range of antifungal activity, and superior pharmacokinetic properties, but its oral bioavailability is extremely variable (46). Both posaconazole and itraconazole have been demonstrated effective against cutaneous leishmaniasis in several human clinical trials (12–14). Recently, our group showed the potent in vitro effects of itraconazole and posaconazole against Leishmania amazonensis (17). Benaim et al. (47) demonstrated the specific antiparasitic effects of amiodarone and the synergistic effects of combinations with posaconazole against Trypanosoma cruzi, in vitro and in vivo; similar effects were more recently reported for Leishmania mexicana and miltefosine (48).
There is a growing recognition of the relevance of combination therapies to address several limitations of currently available anti-Leishmania drugs, including toxicity as well as natural and acquired drug resistance (1, 3, 49–52). Indeed, several studies have reported the superior efficacies of combination therapies against leishmaniasis, and some of them demonstrated the synergic effects of combinations of amphotericin B with other available drugs, such as miltefosine, paromomycin, meglumine antimoniate, or azythromycin (49–52). The remarkable in vitro antiproliferative synergism against both proliferative stages of L. amazonensis of combinations of ITZ and POSA with E5700 observed in this work, and its correlate with the effects of the drugs on promastigote free sterol composition, confirms the notion that drugs acting at sequential steps of a metabolic pathway should have synergistic effects (25). We have recently reported similar synergistic effects of combinations of POSA and E5700 against T. cruzi intracellular amastigotes (53), but the FIC values obtained in the current study are, to the best of our knowledge, the lowest ever reported for the effects of drug combinations against any trypanosomatid parasite. Comparing the MIC and IC50s with those of miltefosine, a standard drug used to treat leishmaniasis, the combinations were very potent, as they reduced significantly the growth of L. amazonensis at subnanomolar concentrations. Thus, our results confirmed that combinations of sterol biosynthesis inhibitors are a promising therapeutic intervention for the treatment of leishmaniasis and Chagas' disease.
By using several techniques, such as electron microscopy and fluorimetry, the different drug combinations were shown to have also potent synergistic deleterious effects on the morphology, ultrastructure, and mitochondrial function of the promastigotes, as they dramatically altered the fine structures of the single mitochondrion of the parasite cells and collapsed the mitochondrial inner transmembrane electric potential (Δψm), resulting in a significant increase in the production of ROS and mitochondrial superoxide. These alterations in the mitochondrial structure and function corroborated the results of previous studies that indicated that the trypanosomatids' mitochondrion has a special requirement for sterols and is one of the most important targets for the sterol biosynthesis inhibitors (17, 54–57). While we recognize that alterations in mitochondrial structure and function are reported as common effects of cell injury in general, we hypothesize that the effects reported here are due specifically to the drugs tested. In addition to the intense ultrastructural alterations observed in the mitochondria, several vacuoles similar to autophagosomes were also seen in parasites treated with the drugs at their MIC, and the drug combinations, some of them in close association with the mitochondrion and in both promastigotes and intracellular amastigotes. Thus, it is possible that treatment with the combinations potentiates autophagy, particularly mitophagy. Finally, several studies have demonstrated the presence of lipid bodies, lipid-rich organelles that function in cell metabolism and signaling (58, 59), in parasites treated with sterol biosynthesis inhibitors, and it has been shown in several cases that these alterations sometimes are related with an abnormal accumulation of endogenous intermediates produced by the inhibition of ergosterol biosynthesis (16, 17, 21). In this work, we found that combination treatments with E5700 and ITZ or POSA caused a significant increase of lipid bodies, similar to the effects of azoles alone at their MICs.
In conclusion, combinations of POSA and ITZ with E5700 have remarkably potent antiproliferative, ultrastructural, and physiological effects against both proliferative stages of L. amazonensis, strictly in association with inhibition of de novo sterol biosynthesis, indicating that such combinations are a promising approach to safer and more potent specific treatments of leishmaniasis. These results support in vivo studies in murine models of cutaneous and mucocutaneous leishmaniasis after treatment with combinations of CYP51 and SQS inhibitors, aimed at establishing the potential usefulness of such combination therapies for the treatment of human disease.
ACKNOWLEDGMENTS
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Conselho Nacional de Desenvolvimento Científico e Tecnológica, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. J.A.U. is an Emeritus Investigator of Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela.
REFERENCES
- 1.Barrett MP, Croft SL. 2012. Management of trypanosomiasis and leishmaniasis. Br Med Bull 104:175–196. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto H, Lauletta Lindoso JA. 2012. Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin North Am 26:293–307. doi: 10.1016/j.idc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 5.Herwaldt BL. 1999. Leishmaniasis. Lancet 354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 6.Caffrey CR, Steverding D. 2008. Recent initiatives and strategies to developing new drugs for tropical parasitic diseases. Expert Opin Drug Discov 3:173–186. doi: 10.1517/17460441.3.2.173. [DOI] [PubMed] [Google Scholar]
- 7.Cruz AK, de Toledo JS, Falade M, Terrão MC, Kamchonwonqpaisan S, Kyle DE, Uthaipibull C. 2009. Current treatment and drug discovery against Leishmania spp. and Plasmodium spp.: a review. Curr Drug Targets 10:178–192. doi: 10.2174/138945009787581177. [DOI] [PubMed] [Google Scholar]
- 8.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J. 2002. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med 347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 10.de Macedo-Silva ST, de Souza W, Rodrigues JCF. 2015. Sterol biosynthesis pathway as an alternative for the anti-protozoan parasite chemotherapy. Curr Med Chem 22:2186–2198. doi: 10.2174/0929867322666150319120337. [DOI] [PubMed] [Google Scholar]
- 11.Al-Abdely HM, Graybill JR, Loebenberg D, Melby PC. 1999. Efficacy of the triazole SCH 56592 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous and visceral leishmaniases. Antimicrob Agents Chemother 43:2910–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paniz-Mondolfi AE, Stavropoulos C, Gelanew T, Loucas E, Alvarez AMP, Benaim G, Polsky B, Schoenian G, Sordillo EM. 2011. Successful treatment of Old World cutaneous leishmaniasis caused by Leishmania amazonensis with posaconazole. Antimicrob Agents Chemother 55:1774–1776. doi: 10.1128/AAC.01498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassiri-Kashani M, Firooz A, Khamesipour A, Mojtahed F, Nilforoushzadeh M, Hejazi H, Bouzari N, Dowlati Y. 2005. A randomized, double-blind, placebo-controlled clinical trial of itraconazole in the treatment of cutaneous leishmaniasis. Eur Acad Dermatol Venereol 19:80–83. doi: 10.1111/j.1468-3083.2004.01133.x. [DOI] [PubMed] [Google Scholar]
- 14.Consigli J, Danielo C, Gallerano V, Papa M, Guidi A. 2006. Cutaneous leishmaniasis: successful treatment with itraconazole. Int J Dermatol 45:46–49. doi: 10.1111/j.1365-4632.2004.02429.x. [DOI] [PubMed] [Google Scholar]
- 15.de Souza W, Rodrigues JCF. 2009. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip Perspect Infect Dis 2009:642502. doi: 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Macedo-Silva ST, de Oliveira Silva TL, Urbina JA, de Souza W, Rodrigues JC. 2011. Antiproliferative, ultrastructural, and physiological effects of amiodarone on promastigote and amastigote forms of Leishmania amazonensis. Mol Biol Int 2011:876021. doi: 10.4061/2011/876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Macedo-Silva ST, Urbina JA, de Souza W, Rodrigues JCF. 2013. In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS One 8:e83247. doi: 10.1371/journal.pone.0083247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbina JA, Payares G, Contreras LM, Liendo A, Sanoja C, Molina J, Piras M, Piras R, Perez N, Wincker P, Loebenberg D. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother 42:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbina JA. 2009. Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz 104:311–318. doi: 10.1590/S0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues JCF, Urbina JA, de Souza W. 2005. Antiproliferative and ultrastructural effects of BPQ-OH, a specific inhibitor of squalene synthase, on Leishmania amazonensis. Exp Parasitol 111:230–238. doi: 10.1016/j.exppara.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues JCF, Concepcion JL, Rodrigues C, Caldera A, Urbina JA, de Souza W. 2008. In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob Agents Chemother 52:4098–4114. doi: 10.1128/AAC.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R. 2002. Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana. Mol Biochem Parasitol 125:35–45. doi: 10.1016/S0166-6851(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 23.Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. 2004. In vitro and in vivo activities of E5700 and ER-119884, two novel orally active squalene synthase inhibitors, against Trypanosoma cruzi. Antimicrob Agents Chemother 48:2379–2387. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida K, Visbal G, Rodrigues JCF, Urbina JA, de Souza W, Rozental S. 2011. Two squalene synthase inhibitors, E5700 and ER-119884, interfere with cellular proliferation and induce ultrastructural and lipid profile alterations in a Candida tropicalis strain resistant to fluconazole, itraconazole, and amphotericin B. J Infect Chemother 17:563–570. doi: 10.1007/s10156-010-0190-1. [DOI] [PubMed] [Google Scholar]
- 25.Urbina JA. 2010. New insights in Chagas' disease treatment. Drugs Future 35:409–419. doi: 10.1358/dof.2010.035.05.1484391. [DOI] [Google Scholar]
- 26.Warren LG. 1960. Metabolism of Schizotrypanum cruzi Chagas. I. Effect of culture age and substrate concentration on respiratory rate. J Parasitol 46:529–539. [PubMed] [Google Scholar]
- 27.Hallander HO, Dornbush K, Gezelius L, Jacobson K, Karlsson I. 1982. Synergism between aminoglycosides and cephalosporin with antipseudomonal activity: interaction index and killing curve method. Antimicrob Agents Chemother 22:743–752. doi: 10.1128/AAC.22.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbina JA, Visbal G, Contreras LM, McLaughlin G, Docampo R. 1997. Inhibitors of Δ24-sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob Agents Chemother 41:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urbina JA, Vivas J, Visbal G, Contreras LM. 1995. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Δ(24,25) sterol methyltransferase inhibitors and their combination with ketoconazole. Mol Biochem Parasitol 73:199–210. doi: 10.1016/0166-6851(95)00117-J. [DOI] [PubMed] [Google Scholar]
- 30.Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA. 1996. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine. A comparative study with azole-susceptible Leishmania mexicana. Antimicrob Agents Chemother 40:2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, Jaisankar P, Majunder HK. 2008. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolymethane through inhibition of FoF1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol 74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- 32.Sen N, Das BB, Ganguly A, Mukherjee T, Bandyopadhyay S, Majumder HK. 2004. Camptothecin-induced imbalance in intracellular cation homeostasis regulates programmed cell death in unicellular hemoflagellate Leishmania donovani. J Biol Chem 279:52366–52375. doi: 10.1074/jbc.M406705200. [DOI] [PubMed] [Google Scholar]
- 33.Batandier C, Fontaine E, Kériel C, Leverve XM. 2002. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med 6:175–187. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues JCF, Attias M, Rodriguez C, Urbina JA, de Souza W. 2002. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a Δ(24,25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob Agents Chemother 46:487–499. doi: 10.1128/AAC.46.2.487-499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo RCN, D'Avila H, Wan H, Bozza PT, Dvorak AM, Weller PF. 2011. Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem 59:540–556. doi: 10.1369/0022155411404073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Beach DH, Goad LJ, Holz GG Jr. 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol Biochem Parasitol 31:149–162. doi: 10.1016/0166-6851(88)90166-1. [DOI] [PubMed] [Google Scholar]
- 38.Berman JD. 1981. Activity of imidazoles against Leishmania tropica in human macrophage cultures. Am J Trop Med Hyg 30:566–569. [DOI] [PubMed] [Google Scholar]
- 39.Berman JD, Holz GG Jr, Beach DH. 1984. Effects of ketoconazole on growth and sterol biosynthesis of Leishmania mexicana promastigotes in culture. Mol Biochem Parasitol 12:1–13. doi: 10.1016/0166-6851(84)90039-2. [DOI] [PubMed] [Google Scholar]
- 40.Berman JD, Goad LJ, Beach DH, Holz GG Jr. 1986. Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol Biochem Parasitol 20:85–92. doi: 10.1016/0166-6851(86)90145-3. [DOI] [PubMed] [Google Scholar]
- 41.Planer JD, Hulverson MA, Arif JA, Ranade RM, Don R, Buckner FS. 2014. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl Trop Dis 8:e2977. doi: 10.1371/journal.pntd.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandharkar T, Zhu X, Mathur R, Jiang J, Schmittgen TD, Shaha C, Webovetz KA. 2014. Studies on the antileishmanial mechanisms of action of the arylimidamide DB766: azole interactions and role of CYP51122A1. Antimicrob Agents Chemother 58:4682–4689. doi: 10.1128/AAC.02405-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCall LI, Aroussi AE, Choi JY, Vieira DF, Muylder G, Johnston JB, Chen S, Kellar D, Siqueira-Neto JL, Roush WR, Podust LM, McKerrow JH. 2015. Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of sterol 14α-demethylase. PLoS Negl Trop Dis 9:e0003588. doi: 10.1371/journal.pntd.0003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepesheva GI, Hargrove TY, Rachakonda G, Wawrzak Z, Pomel S, Cojean S, Nde PN, Nes WD, Locuson CW, Calcutt W, Waterman MR, Daniels JS, Loiseau PM, Villalta F. 2015. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. J Infect Dis doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaller MA, Messer SA, Hollis RJ, Jones RN. 2001. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob Agents Chemother 45:2862–2564. doi: 10.1128/AAC.45.10.2862-2864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolton MJ, Ray JE, Marriott D, McLachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob Agents Chemother 56:2806–2813. doi: 10.1128/AAC.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benaim G, Sanders JM, Garcia-Marchán Y, Colina C, Lira R, Caldera AR, Payares G, Sanoja C, Burgos JM, Leon-Rossel A, Conception JL, Schiman AG, Levin M, Oldfield E, Urbina JA. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J Med Chem 49:892–899. doi: 10.1021/jm050691f. [DOI] [PubMed] [Google Scholar]
- 48.Serrano-Martin X, Payares G, De Lucca M, Martinez JC, Mendoza-Leon A, Benaim G. 2009. Amiodarone and miltefosine act synergistically against Leishmania mexicana and can induce parasitological cure in a murine model of cutaneous leishmaniasis. Antimicrob Agents Chemother 53:5108–5113. doi: 10.1128/AAC.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundar S, Rai M, Chakravarty J, Agarwal D, Agrawal N, Vaillant M, Olliaro P, Murray HW. 2008. New treatment approach in Indian visceral leishmaniasis: single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin Infect Dis 47:1000–1006. doi: 10.1086/591972. [DOI] [PubMed] [Google Scholar]
- 50.Sundar S, Sinha PK, Rai M, Verma DK, Alan S, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumary P, Lal CS, Arora R, Sharma B, Ellis S, Strub-Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 51.Morais-Teixeira E, Gallupo MK, Rodrigues LF, Romanha AJ, Rabello A. 2013. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three New World Leishmania species. J Antimicrob Agents 69:150–154. doi: 10.1093/jac/dkt318. [DOI] [PubMed] [Google Scholar]
- 52.Singh N, Kumar M, Singh RK. 2012. Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med 5:485–497. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 53.Shang N, Li Q, Ko TP, Chan HC, Li J, Zheng Y, Huang CH, Ren F, Chen CC, Zhu Z, Galizzi M, Li ZH, Rodrigues-Poveda CA, Gonzalez-Pacanowska D, Veiga-Santos P, de Carvalho TMU, de Souza W, Urbina JA, Wang AH, Docampo R, Li K, Liu YL, Oldfield E, Guo RT. 2014. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog 10:e1004114. doi: 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessler RL, Soares MJ, Probst CM, Krieger MA. 2013. Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death. PLoS One 8:e55497. doi: 10.1371/journal.pone.0055497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues JCF, Bernardes CF, Visbal G, Urbina JA, Vercesi AE, de Souza W. 2007. Sterol methenyl transferase inhibitors alter the ultrastructure and function of the Leishmania amazonensis mitochondrion leading to potent growth inhibition. Protist 158:447–456. doi: 10.1016/j.protis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues CO, Catisti R, Uyemura SA, Vercesi AE, Lira R, Rodriguez C, Urbina JA, Docampo R. 2001. The sterol composition of Trypanosoma cruzi changes after growth in different culture media and results in different sensitivity to digitonin-permeabilization. J Eukaryot Microbiol 48:588–594. doi: 10.1111/j.1550-7408.2001.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 57.Palmié-Peixoto IV, Rocha MR, Urbina JA, de Souza W, Einicker-Lamas M, Motta MCM. 2006. Effects of sterol biosynthesis inhibitors on endosymbiont-bearing trypanosomatids. FEMS Microbiol Lett 255:33–42. doi: 10.1111/j.1574-6968.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 58.D'Avila H, Freire-de-Lima CG, Rogue NR, Teixeira L, Barja-Fidalgo C, Silva AR, Melo RC, Dosreis GA, Castro-Faria-Neto HC, Bozza PT. 2011. Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by uptake of apoptotic cells are associated with prostalglandin E2 generation and increased parasite growth. J Infect Dis 204:951–961. doi: 10.1093/infdis/jir432. [DOI] [PubMed] [Google Scholar]
- 59.Walther TC, Farese RV Jr. 2009. The life of lipid droplets. Biochim Biophys Acta 1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]