Abstract
The aim of this study was to determine the biopharmaceutical characteristics of tobramycin (TOB) after nebulization in rats. TOB was administered by intravenous (i.v.) bolus or intratracheal nebulization (3 mg · kg−1), and concentrations were determined in plasma and epithelial lining fluid (ELF) by liquid chromatography-tandem mass spectrometry. The ratio of the TOB concentration in ELF to the plasma area under the curve (AUC) was more than 200 times as high after NEB as after i.v. bolus administration, indicating that TOB nebulization offers a biopharmaceutical advantage over i.v. administration.
TEXT
Much higher intrapulmonary antibiotic concentrations may be achieved after nebulization (NEB) than after systemic administration, which may be of value for the treatment of pulmonary infections (1). However, this potential advantage may vary between compounds and could be much greater for antibiotics with a weak rather than a strong ability to permeate membranes, as recently shown for colistin (2) in comparison with ciprofloxacin or moxifloxacin (3). The aim of this study was to confirm this hypothesis by investigating the pulmonary pharmacokinetics (PK) of tobramycin (TOB), another antibiotic with a weak ability to permeate membranes that is clinically available for pulmonary administration, by using the same standardized protocol as before (2).
Transepithelial transport across Calu-3 monolayers was tested to determine the apparent ability of TOB to permeate membranes (Papp) (4, 5). Animal experiments were approved by the Poitou Charentes Ethic Committee (COMETHEA) and registered by the French Ministry of Higher Education and Research (01733.01). Male Sprague-Dawley rats (n = 50, 300 to 350 g; Janvier Laboratories, Le Genest-St-Isle, France) were used and housed as previously described (2). A first group of anesthetized rats received a 3-mg · kg−1 bolus dose of TOB (TOB sulfate solution, 50 mg · ml−1, Nebcine; Erempharma) by the tail vein. A second group of anesthetized rats received the same dose of TOB (100 μl) by intratracheal NEB under anesthesia (2, 3). In the two groups, bronchoalveolar lavage (BAL) fluid and blood samples were collected 0.25, 0.5, 1, 2.5, and 4 h after TOB administration (four or five rats per sampling time) (2, 3). The TOB assay used was adapted from a previously described method (6). A Waters Alliance 2695 separation module coupled with a Waters Micromass Quattro micro API tandem mass spectrometer was used. Chromatographic separation was done with an X bridge C18 column (5.0 μm, 150 by 2.1 mm [inside diameter]; Waters, St-Quentin en Yvelines, France) with a mobile phase composed of 0.1% (vol/vol) formic acid in water and 0.1% formic acid in acetonitrile (75:25, vol/vol) at a flow rate of 0.2 ml · min−1. Quantification was performed in the positive-ion mode with multiple-reaction monitoring of m/z transitions 468.2 → 163.1 for TOB and 448.2 → 160.2 for sisomycin, the internal standard. The intraday and interday variabilities in plasma and BAL fluid were determined at three concentrations with a precision and accuracy of <15%. Concentrations of urea in plasma and BAL fluid were measured as previously described (7). TOB concentrations in epithelial lining fluid (CELF) were derived from measured TOB concentrations in BAL fluid (CBAL) after correction by urea dilution (7). TOB concentrations in plasma and ELF versus time were simultaneously analyzed by a nonlinear mixed-effects method with S-ADAPT software (v 1.52), and the final structural PK model was derived from previous studies (2, 3). One or two compartments were assessed to describe TOB PK in plasma, but the one-compartment model was kept. TOB PK in ELF were first tested with one ELF compartment with a fixed physiological volume (VELF = 30 μl · kg−1) as previously described (2, 3) and with the addition of a depot compartment (2), but two compartments with distinct volumes (VELF1, VELF2) were necessary to reflect the previously described complexity of ELF PK (8). In the final model, compartments were connected by two-direction equilibrium distribution clearances, but the addition of an influx clearance from the ELF1 compartment to the central compartment was necessary for satisfactory data fitting. Systemic bioavailability after NEB was fixed at its maximum value (100%). Areas under the plasma and ELF concentration-versus-time curves from time zero to infinity (AUCplasma, AUCELF) were calculated from the model (Berkeley Madonna, version 8.3.18; University of California at Berkeley). Elimination half-lives (t1/2,plasma and t1/2,ELF) after intravenous (i.v.) administration and NEB were derived from the model. Prism5 (GraphPad, La Jolla, CA) was used for statistical comparisons.
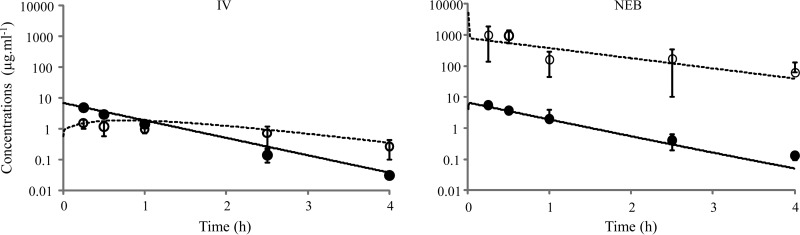
Using Calu-3, the highest TOB concentrations (250 and 2,500 μg · ml−1) induced a loss of cell integrity. With the lowest concentrations (25 and 2.5 μg · ml−1) applied to the apical side of monolayers, the TOB concentrations on the basal side were below the limit of quantification (2.5 ng · ml−1), meaning that the Papp of TOB should be <0.05.10−6 cm · s−1. In vivo, rapid appearance of TOB in plasma was observed after NEB with an early concentration peak at 0.25 h, corresponding to the first sampling time (Fig. 1). These initial plasma drug concentrations were not significantly different (P > 0.05; Mann-Whitney test) after NEB and i.v. administrations (5.45 ± 0.90 versus 4.89 ± 0.70 μg · ml−1). Elimination half-lives in plasma were virtually identical after NEB and i.v. administrations (t1/2,plasma,NEB = 0.57 h; t1/2,plasma,i.v. = 0.53 h). Notably, the t1/2 in ELF after NEB (t1/2,ELF,NEB = 0.92 h) was slightly longer than that in plasma (Fig. 1) for unexplained reasons that required a model with two ELF compartments. Interestingly, the TOB concentrations at distribution equilibrium (2.5 and 4 h) were approximately 250 times as high in ELF after NEB than after i.v. administration (Fig. 1). Accordingly, the AUC in ELF was 242 times as high after NEB as after i.v. administration of TOB (AUCELF,NEB = 1,212 μg · h · ml−1 versus AUCELF,i.v. = 5.1 μg.h.ml−1). This effect of the route of administration may also be assessed by comparing the ELF-to-plasma AUC ratio after NEB (AUCELF,NEB/AUCplasma,NEB = 222) with that after i.v. administration (AUCELF,i.v./AUCplasma,i.v. = 0.97). Together, these data show that, at least under these experimental conditions, the route of administration has a major effect on TOB concentrations within ELF, as was previously observed with colistin (2). TOB is a smaller molecule than colistin (respective molecular masses, 467.5 and 1,166 g · mol−1) but has a log P value close to that of colistin (respectively, −6.5 and −8.1) (ChemaAxon, www.drugbank.ca) and a weak apparent ability to permeate membranes (<0.05.10−6 cm · s−1), like that of colistin (2) but much weaker than those of fluoroquinolones (0.8 ± 0.03.10−6 cm · s−1 for ciprofloxacin and 8.3 ± 0.12.10−6 cm · s−1 for moxifloxacin) (3).
FIG 1.
Predicted concentration-versus-time profiles of TOB in plasma (solid line) and ELF (dashed line) after the administration of 3 mg · kg−1 i.v. or by NEB from simultaneous modeling of plasma and ELF PK. Closed and open symbols, respectively, represent experimental mean TOB concentrations in plasma and ELF ± standard deviations.
In conclusion, this study has confirmed that, as for colistin, TOB NEB offers a biopharmaceutical advantage by achieving high ELF and low systemic concentrations, consistent with its limited ability to permeate membranes.
ACKNOWLEDGMENT
We thank Hélène Fayard for her technical assistance in this study.
REFERENCES
- 1.Falagas ME, Trigkidis KK, Vardakas KZ. 2015. Inhaled antibiotics beyond aminoglycosides, polymyxins and aztreonam: a systematic review. Int J Antimicrob Agents 45:221–233. doi: 10.1016/j.ijantimicag.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Gontijo AV, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob Agents Chemother 58:3950–3956. doi: 10.1128/AAC.02819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gontijo AV, Brillault J, Gregoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, moxifloxacin, and grepafloxacin. Antimicrob Agents Chemother 58:3942–3949. doi: 10.1128/AAC.02818-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brillault J, De Castro WV, Couet W. 2010. Relative contributions of active mediated transport and passive diffusion of fluoroquinolones with various lipophilicities in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother 54:543–545. doi: 10.1128/AAC.00733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother 53:1457–1462. doi: 10.1128/AAC.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keevil BG, Lockhart SJ, Cooper DP. 2003. Determination of tobramycin in serum using liquid chromatography-tandem mass spectrometry and comparison with a fluorescence polarisation assay. J Chromatogr B Analyt Technol Biomed Life Sci 794:329–335. doi: 10.1016/S1570-0232(03)00492-6. [DOI] [PubMed] [Google Scholar]
- 7.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother 54:3702–3707. doi: 10.1128/AAC.00411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yapa SWS, Li J, Porter CJH, Nation RL, Patel K, McIntosh MP. 2013. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother 57:5087–5095. doi: 10.1128/AAC.01127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]