Abstract
Macrolides are the antimicrobials of choice for treating human campylobacteriosis. The recent emergence of erm(B) in Campylobacter bacteria threatens the utility of this class of antibiotics. Here we report the constitutive and inducible expression of erm(B) in Campylobacter isolates derived from diarrheal patients and food-producing animals. Constitutive expression of erm(B) was associated with insertion and deletion in the regulatory region of the gene, providing the first documentation of the differential expression of erm(B) in Campylobacter bacteria.
TEXT
Campylobacter bacteria are among the most common causes of bacterium-mediated diarrheal disease worldwide (1). Additionally, Campylobacter infection can lead to extraintestinal complications such as polyarthralgia (i.e., reactive arthritis) and Guillain-Barré syndrome (2). In general, the occurrence of human Campylobacter infection has been attributed largely to the consumption of contaminated food animal products, resulting from the high prevalence of Campylobacter in these animals (3).
Erythromycin, a 14-membered macrolide antibiotic, is recommended for use as the first-line treatment of campylobacteriosis (4), while 16-membered macrolides (e.g., tylosin and spiramycin) are some of the most common growth-promoting agents in food animal production worldwide (5). Macrolide resistance among Campylobacter jejuni and Campylobacter coli strains is generally much more severe in developing countries than in developed countries (5–9). In addition, there is a much higher frequency of macrolide resistance in C. coli than that in C. jejuni, both in isolates derived from humans and in those from food animals (6, 10, 11). Very recently, the rRNA methylase Erm(B) has emerged as an important mechanism of acquired macrolide-lincosamide (ML) resistance in C. jejuni and C. coli (8, 12, 13), the two most common human disease-causing Campylobacter species. In Gram-positive bacteria, the expression of erm genes can be either constitutive or inducible (14). The mechanism of erm(B) induction has not been thoroughly investigated (14); however, the induction of erm(C) has been well studied and it is believed to be due to structural alterations, including sequence deletions, duplications/insertions, and point mutations in the erm(C) regulatory region, which acts as a translational attenuator (15). To our knowledge, no information is available concerning the mechanism of erm(B) induction in Campylobacter bacteria.
This study included 29 Campylobacter strains with a chromosome-borne erm(B) gene (28 of C. coli, 1 of C. jejuni), 27 of which were collected during 2007 to 2012 from food-producing animals (chickens, swine, and ducks) and diarrheal patients in different provinces and cities in China (8) and 2 of which (DZB40 and 86c) were collected in 2013 from swine slaughterhouses in Shandong and Guangdong, respectively. Details of the origins and identification of the above-mentioned 27 Campylobacter strains have been described previously (8).
Campylobacter isolates were evaluated for susceptibility to 14-, 15-, and 16-membered macrolides, as well as clindamycin, by the agar dilution method with incubation at 42°C for 24 h under microaerophilic conditions according to CLSI document VET01-A4 (16). C. jejuni ATCC 33560 was used as a quality control strain. To determine MICs following induction, isolates were grown on Mueller-Hinton agar containing either erythromycin at 0.5 mg/liter or clindamycin at 0.25 mg/liter. Three independent experiments were conducted to confirm the reproducibility of the MIC patterns.
The erm(B)-positive Campylobacter strains were classified as having a constitutive ML antibiotic resistance phenotype if resistance was independent of preincubation with the inducer, while inducible resistance was dependent on preincubation (Table 1). Inducible-erm(B)-positive C. coli strains DZB40 and 86c were susceptible to all of the antibiotics tested, except for tylosin, prior to induction. Following induction with erythromycin and clindamycin independently, the two strains attained resistance to erythromycin and azithromycin and also showed increased MICs of tylosin (Table 1). This observation is consistent with previous findings by other investigators (17, 18) and further suggests that some bacteria with inducible erm are susceptible to macrolides and lincosamides before induction, although isolates carrying erm(B) usually exhibit ML resistance. Notably, the two inducible-erm(B)-carrying strains did not show increased clindamycin MICs after induction. This is similar to previous findings on erm(C) induction (19, 20), which showed that a strain expressing inducible erm(C) developed resistance only to 14- and 15-membered macrolides and remained susceptible to lincosamides. In this study, erythromycin and clindamycin were able to act as inducers of erm(B) gene expression in Campylobacter to various degrees. The MICs for the other 27 Campylobacter strains were almost identical; hence, the MICs for 7 epidemiologically distinct Campylobacter strains, as representatives of the 27 Campylobacter strains, are shown in Table 1. These 27 strains, including the only C. jejuni strain (C179b), were highly resistant to ML antibiotics without prior induction, indicating constitutive expression of erm(B).
TABLE 1.
MICs of ML antibiotics for Campylobacter strains determined with and without induction
| Straina | Hostb | Regionc | Typed | MIC (mg/liter)e |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythromycin |
Azithromycin |
Tylosin |
MIC (mg/liter) Clindamycin |
||||||||||||
| N | I-e | I-c | N | I-e | I-c | N | I-e | I-c | N | I-e | I-c | ||||
| C179b | C | GD | C | 512 | 512 | 512 | 128 | 128 | 128 | >1,024 | >1,024 | >1,024 | 512 | 512 | 512 |
| ZP-GX-1 | C | SD | C | 256 | 256 | 256 | 256 | 256 | 256 | >1,024 | >1,024 | >1,024 | 512 | 512 | 512 |
| ZC113 | S | SD | C | 512 | 512 | 512 | 256 | 256 | 256 | >1,024 | >1,024 | >1,024 | 1,024 | 1,024 | 1,024 |
| DZB4 | S | SD | C | 256 | 256 | 256 | 128 | 128 | 128 | >1,024 | >1,024 | >1,024 | 1,024 | 1,024 | 1,024 |
| DH67 | D | SD | C | 512 | 512 | 512 | 128 | 128 | 128 | >1,024 | >1,024 | >1,024 | 1,024 | 1,024 | 1,024 |
| SH-CCD11C073 | H | SH | C | 512 | 512 | 512 | 128 | 128 | 128 | >1,024 | >1,024 | >1,024 | 1,024 | 1,024 | 1,024 |
| HN-CCD07046 | H | HN | C | 256 | 256 | 256 | 256 | 256 | 256 | >1,024 | >1,024 | >1,024 | 1,024 | 1,024 | 1,024 |
| DZB40 | S | SD | I | 2 | 32 | 16 | <0.25 | 8 | 4 | 8 | 128 | 32 | 1 | 1 | 1 |
| 86c | S | GD | I | 4 | 32 | 16 | <0.25 | 8 | 4 | 16 | 128 | 16 | 1 | 1 | 1 |
All strains are C. coli, except C179b (C. jejuni). The first seven strains (except DZB40 and 86c) are representatives of the 27 Campylobacter strains collected from 2007 to 2012, which have been described previously (8). The other 20 Campylobacter strains were 158, LH-GX-14, ZP-GX-12, ZP-GX-14, ZP-GX-20, ZP-GX-26, ZP-GX-28, ZP-GX-35, ZP-GX-36, ZP-GX-37, ZP-GX-5, ZP-GX-8, SH-CCD11C226, SH-CCD11C287, SH-CCD11C365, SH-CCD11C416, SH-CCD11C419, SH-CCD11C490, SH-CCD11C518, and SH-CCD11C682.
C, chicken; S, swine; D, duck; H, human.
GD, Guangdong Province; SD, Shandong Province; SH, Shanghai Municipality; HN, Henan Province.
C, constitutive; I, inducible.
The erythromycin, azithromycin, tylosin, and clindamycin MIC breakpoints were all ≥8 mg/liter. N, not induced; I-e, induced with erythromycin; I-c, induced with clindamycin.
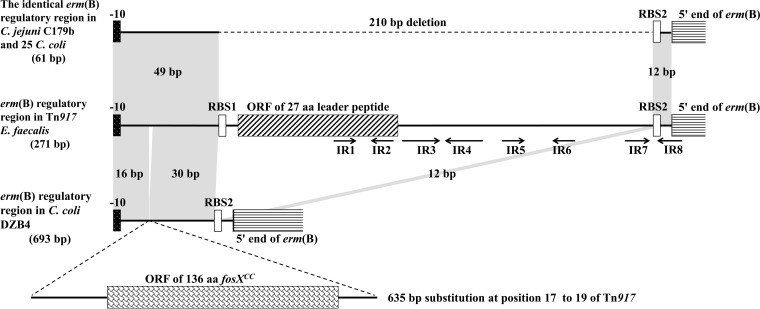
Potential attenuators located within the regulatory region of constitutively expressed erm(B) in Campylobacter isolates were amplified by PCR as described previously and then sequenced three times in both directions from independent replicons (21). The inducibly expressed erm(B) regulatory region in Tn917 of Enterococcus faecalis (GenBank accession no. M11180) was used as the reference sequence. PCR amplification of the erm(B) regulatory regions, from the −10 signal site to the 5′ end of erm(B), of all 27 Campylobacter strains revealed the presence of two different amplification products. The erm(B) regulatory region of DZB4 yielded an amplification product of 693 bp (GenBank accession number KC876749), whereas the erm(B) regulatory regions of the remaining 26 Campylobacter strains produced a smaller 61-bp product (e.g., C179b, accession number KF864551) (Fig. 1). A comparison with the equivalent region of the inducibly expressed erm(B) gene of Tn917 is presented in Fig. 1. On the basis of this comparison, the only difference was a 210-bp deletion in the regulatory region of C179b, compared with that of Tn917, that removed the entire erm(B) attenuator. Several open reading frame (ORFs) are present within the 210-bp deleted region, including an ORF corresponding to a 27-amino-acid (aa) leader peptide that is known to play a crucial role in the induction process as a partner in mRNA secondary-structure formation (15). Moreover, the deletion of IR3 to IR7 renders IR8 accessible to ribosomes, independent of the presence or absence of an inducer. The simultaneous absence of the leader peptide and several inverted repeats (IRs) from these 26 Campylobacter strains explains the observed constitutive ML resistance.
FIG 1.
Comparison of the regulatory regions of constitutively expressed erm(B) from C. coli DZB4, C. jejuni C179b, and 25 other C. coli strains (shown in Table 1) with the corresponding region of inducibly expressed erm(B) of Tn917. RBS1 and RBS2 represent the ribosome binding sites of the leader peptide and erm(B), respectively. The ORFs corresponding to the 27-aa leader peptide, the 5′ terminus of erm(B), and the fosfomycin resistance gene fosXCC are shown as boxes. The arrows indicate IR1 to IR8, which are responsible for the stem-loop structures of mRNA in Tn917. Regions of 100% homology are marked by gray shading. The 210-bp deletion in C179b and the 635-bp substitution in DZB4 are indicated.
C. coli isolate DZB4 contained a 635-bp substitution of the three bases (GTT) at position 17 that are present in Tn917 (Fig. 1). Sequence analysis revealed the presence of a novel 136-aa ORF, fosXCC, in the 635-bp substitution, which was recently reported to mediate resistance to fosfomycin in Campylobacter (22). As both the leader peptide and IRs found in Tn917 were missing from the erm(B) regulatory region of DZB4, the large substitution likely had the same effect on erm(B) expression as the 210-bp deletion that was detected in the other 26 Campylobacter strains. This kind of large insertion in the erm regulatory region, causing a shift from inducible to constitutive erm gene expression, was also observed in the erm(A) regulatory region of Streptococcus agalactiae (17). The possibility of the insertion of another antibiotic resistance gene into the regulatory region, ensuring the transmission of the multiple-drug resistance element into the genome of Campylobacter, is a cause for concern. However, the 635-bp substitution carrying the fosfomycin resistance gene fosXCC in DZB4 has not yet been identified in any other Campylobacter strains.
In the two inducible erm(B)-positive C. coli strains DZB40 and 86c, a 27-aa leader peptide and four pairs of IRs (IR1 to IR8) were detected in the erm(B) regulatory region, which could form stable RNA secondary structures and modulate the expression of erm(B). It has been reported that inducible erm expression was a consequence of differences in mRNA base composition (14, 15). Additionally, induction could cause erm expression by destabilizing mRNA secondary structures in the inducible erm strains (14, 15). The molecular and structural bases for the induction of erm(B) in Campylobacter are unknown and remain to be determined in future studies. Interestingly, both DZB40 and 86c were susceptible to most of the antibiotics tested prior to induction. After induction, the MICs of macrolides substantially increased but did not reach the levels of strains with constitutively expressed erm(B) (Table 1). The coding sequence (CDS) of erm(B) in the two isolates was intact and did not show any frame shift, substitution, or sequence deletion that might affect the MICs. Thus, the lower MICs could not be explained by alteration of the CDS. It is possible that inducible erm(B) is not expressed at a level as high as that of constitutive erm(B) and thus confers only an intermediate level of resistance.
In conclusion, constitutive erm(B) gene expression in Campylobacter was more prevalent, probably because of the use of erythromycin and tylosin for therapeutic purposes and growth promotion in food-producing animals. The data obtained in this study suggest that constitutively expressed erm(B) of Campylobacter might have evolved from inducibly expressed erm(B) (e.g., Tn917) following deletion or recombination in the regulatory region. Moreover, the 210-bp deletion in the constitutively expressed erm(B) regulatory region was consistently detected in the chromosomal DNA of 26 epidemiologically distinct C. coli and C. jejuni strains isolated from humans and food-producing animals, suggesting the presence of a common mechanism for constitutive expression. Additionally, erm(B)-positive Campylobacter strains that are susceptible to ML antibiotics, such as the inducible erm(B)-positive Campylobacter strain investigated in this study, pose a hidden risk to public health, as they would not be detected by diagnostic methods that rely only on the resistance phenotype. Furthermore, inducible erm(B)-positive Campylobacter bacteria in the intestines of humans and food-producing animals treated for extensive periods with the appropriate inducer antibiotics might increase the reservoir of resistant Campylobacter bacteria.
ACKNOWLEDGMENTS
This study was funded by grants from the National Basic Research Program of China (2013CB127200) and the National Natural Science Foundation of China (U1031004).
REFERENCES
- 1.Ruiz-Palacios GM. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Engberg J. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections, p 99–121. In Nachamkin I, Szymanski CM, Blaser MJ (ed), Campylobacter, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 3.Humphrey T, O'Brien S, Madsen M. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol 4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibreel A, Taylor DE. 2006. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 58:243–255. doi: 10.1093/jac/dkl210. [DOI] [PubMed] [Google Scholar]
- 6.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis 7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterlund A, Hermann M, Kahlmeter G. 2003. Antibiotic resistance among Campylobacter jejuni/coli strains acquired in Sweden and abroad: a longitudinal study. Scand J Infect Dis 35:478–481. doi: 10.1080/00365540310010949. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q, Shen J. 2014. Emergence of multidrug-resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrob Agents Chemother 58:5405–5412. doi: 10.1128/AAC.03039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, Wu C, Wang Y, Jeon B, Shen Z, Wang Y, Zhang Q, Shen J. 2011. Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int J Food Microbiol 146:94–98. doi: 10.1016/j.ijfoodmicro.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Belanger AE, Shryock TR. 2007. Macrolide-resistant Campylobacter: the meat of the matter. J Antimicrob Chemother 60:715–723. doi: 10.1093/jac/dkm300. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Naren G, Wu C, Wang Y, Dai L, Xia L, Luo P, Zhang Q, Shen J. 2010. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol 144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Deng F, Wang Y, Zhang Y, Shen Z. 2015. Characterization of the genetic environment of the ribosomal RNA methylase gene erm(B) in Campylobacter jejuni. J Antimicrob Chemother 70:613–615. doi: 10.1093/jac/dku418. [DOI] [PubMed] [Google Scholar]
- 13.Qin S, Wang Y, Zhang Q, Deng F, Shen Z, Wu C, Wang S, Zhang J, Shen J. 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug-resistant Campylobacter coli. J Antimicrob Chemother 69:964–968. doi: 10.1093/jac/dkt492. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq R, Courvalin P. 2002. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob Agents Chemother 46:2727–2734. doi: 10.1128/AAC.46.9.2727-2734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisblum B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob Agents Chemother 39:797–805. doi: 10.1128/AAC.39.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial disk and dilution susceptibility tests for bacterial isolated from animals; approved standard—fourth edition. CLSI document VET01-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Culebras E, Rodríguez-Avial I, Betriu C, Picazo JJ. 2005. Differences in the DNA sequence of the translational attenuator of several constitutively expressed erm(A) genes from clinical isolates of Streptococcus agalactiae. J Antimicrob Chemother 56:836–840. doi: 10.1093/jac/dki337. [DOI] [PubMed] [Google Scholar]
- 18.Douthwaite S, Jalava J, Jakobsen L. 2005. Ketolide resistance in Streptococcus pyogenes correlates with the degree of rRNA dimethylation by Erm. Mol Microbiol 58:613–622. doi: 10.1111/j.1365-2958.2005.04863.x. [DOI] [PubMed] [Google Scholar]
- 19.Allen NE. 1977. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother 11:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq R, Courvalin P. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother 35:1267–1272. doi: 10.1128/AAC.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosato A, Vicarini H, Leclercq R. 1999. Inducible or constitutive expression of resistance in clinical isolates of streptococci and enterococci cross-resistant to erythromycin and lincomycin. J Antimicrob Chemother 43:559–562. doi: 10.1093/jac/43.4.559. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Yao H, Deng F, Liu D, Zhang Y, Shen Z. 2015. Identification of a novel fosXCC gene conferring fosfomycin resistance in Campylobacter. J Antimicrob Chemother 70:1261–1263. doi: 10.1093/jac/dku488. [DOI] [PubMed] [Google Scholar]