Abstract
Pyrazinamide (PZA) has important sterilizing activity in tuberculosis (TB) chemotherapy. We describe trends, risk factors, and molecular epidemiology associated with PZA-resistant (PZAr) Mycobacterium tuberculosis in New York City (NYC). From 2001 to 2008, all incident culture-positive TB cases reported by the NYC Department of Health and Mental Hygiene (DOHMH) were genotyped by IS6110-based restriction fragment length polymorphism and spoligotype. Multidrug-resistant (MDR) isolates underwent DNA sequencing of resistance-determining regions of pncA, rpoB, katG, and fabG1. Demographic and clinical information were extracted from the NYC DOHMH TB registry. During this period, PZAr doubled (1.6% to 3.6%) overall, accounting for 44% (70/159) of the MDR population and 1.4% (75/5511) of the non-MDR population. Molecular genotyping revealed strong microbial phylogenetic associations with PZAr. Clustered isolates and those from acid-fast bacillus (AFB) smear-positive cases had 2.7 (95% confidence interval [CI] = 1.71 to 4.36) and 2.0 (95% CI = 1.19 to 3.43) times higher odds of being PZAr, respectively, indicating a strong likelihood of recent transmission. Among the MDR population, PZAr was acquired somewhat more frequently via primary transmission than by independent pathways. Our molecular analysis also revealed that several historic M. tuberculosis strains responsible for MDR TB outbreaks in the early 1990s were continuing to circulate in NYC. We conclude that the increasing incidence of PZAr, with clear microbial risk factors, underscores the importance of routine PZA drug susceptibility testing and M. tuberculosis genotyping for the identification, control, and prevention of increasingly resistant organisms.
INTRODUCTION
Pyrazinamide (PZA) is a first-line antituberculosis (anti-TB) drug and the cornerstone of modern short-course chemotherapy. PZA acts synergistically with other TB drugs to accelerate culture conversion and reduce the risk of relapse among patients with drug-susceptible TB (1). In patients with multidrug-resistant (MDR) TB, defined as resistance to at least isoniazid (INH) and rifampin (RIF), inclusion of PZA is recommended to reduce the treatment duration (2, 3), while optimizing MDR TB treatment regimens based on PZA susceptibility may improve clinical outcomes (4). Due to its well-documented sterilizing capability, PZA has been included in several new TB drug regimens (5–7).
PZA is a prodrug that requires activation to pyrazinoic acid by the pyrazidamidase of M. tuberculosis under acidic conditions (8). While at least one other M. tuberculosis gene has been associated with PZA resistance (PZAr) (9), mutations in pncA, which encodes the pyrazidamidase, account for the majority of PZAr in vitro (10, 11). Most genes associated with drug resistance in M. tuberculosis, such as katG (INH) and rpoB (RIF), have clear mutational hot spot regions 7 to 66 nucleotides (nt) in length. In contrast, mutations observed in pncA span a region of ∼600 nt, comprising the entire gene and the putative promoter region (10, 12, 13). Growth-based assays of M. tuberculosis PZA susceptibility are the standard but are not always performed routinely, except in large referral laboratories, because they are technically challenging (14, 15). However, pncA mutations in clinical M. tuberculosis isolates have generally been found to correlate with phenotypic PZA drug susceptibility testing (DST) results, supporting the value of pncA sequence analysis as an alternative means to establish PZA resistance (13, 16).
Genetic markers have been used to confirm or refute TB outbreaks and to estimate the proportion of recent transmission in a population (17). For instance, due to the wide diversity of pncA mutations, these sequences can provide a genetic marker to confirm or resolve genotypic clusters, where the presence of identical pncA mutations in genotypically clustered strains is supportive of primary transmission while genotypic clusters with diverse pncA mutations suggest acquired (de novo) PZAr, indicating these cases may not be epidemiologically related (17).
Despite the unique role PZA plays in modern TB chemotherapy, few studies have investigated the epidemiology of PZAr in both general and MDR TB populations (18). Using TB case records and surveillance data provided by the New York City (NYC) Department of Health and Mental Hygiene (DOHMH) from 2001 to 2008, we conducted a population-based study of M. tuberculosis isolates from 6,260 culture-positive TB cases to examine PZAr in terms of clinical, microbial, and demographic risk factors. Due to the clinical and epidemiologic importance of MDR TB, we also performed a case-control study to identify PZAr risk factors and examine PZAr acquisition and clustering among the MDR population.
MATERIALS AND METHODS
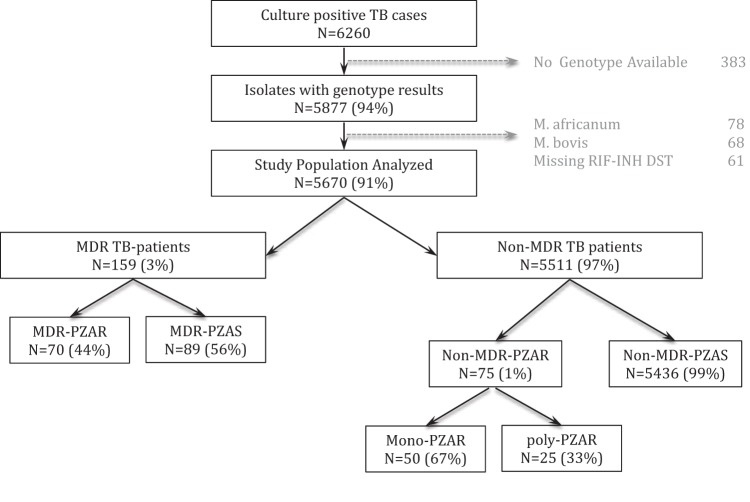
All incident culture-positive TB cases reported and verified by the NYC DOHMH between January 2001 and December 2008 (n = 6,260) were included in the study (Fig. 1). Routine genotyping was performed by the Public Health Research Institute (PHRI) Tuberculosis Center at Rutgers University (IS6110-restriction fragment length polymorphism [RFLP]) and New York State Wadsworth Center (spoligotype) (19). All strains with genotyping results available (n = 5,877) were assigned a molecular lineage using the taxonomic designation previously described by Gagneux and Small (20) and a strain code following a nomenclature system of the PHRI TB Center that has been described previously (21, 22). Strains identified as Mycobacterium bovis (n = 68) or Mycobacterium africanum (n = 78) (23) were omitted from our analysis. Clusters were defined as two or more strains sharing identical IS6110-RFLPs and spoligotypes. DST was performed at the NYC DOHMH Public Health Laboratories and the New York State Wadsworth Center (24) reference laboratories, which utilized the Bactec 460TB system (Becton, Dickinson and Company, Franklin Lakes, NJ) until 2003 and the BD Bactec MGIT 960 mycobacterial detection system thereafter. DST results were available for culture-positive TB cases for all first-line anti-TB agents: INH, RIF, ethambutol (EMB), and PZA. DST results for second-line drugs (SLD), including kanamycin, capreomycin, amikacin, fluoroquinolones, and ethionamide, were available for all cases with reduced susceptibility to any first-line agent. M. tuberculosis isolates were classified as MDR if they were resistant to at least INH and RIF (3). Isolates were classified as poly-PZAr if they were resistant to PZA and at least one other drug, excluding MDR isolates (Fig. 1).
FIG 1.
Study schema. MDR, resistant to at least isoniazid and rifampin; PZAR, PZA resistant; PZAS, PZA susceptible; mono-PZAR, resistant only to PZA and no other drug; MDR-PZAR, resistant to at least isoniazid, rifampin, and PZA; poly-PZAR, resistant to PZA and at least one other drug, excluding MDR-PZAR.
Data collection.
Demographic and clinical information was provided by the NYC DOHMH TB Registry, which contains information for each reported TB patient obtained by interview and medical-record abstraction performed by trained Bureau of Tuberculosis Control (BTBC) staff, using standard data collection forms. Demographic variables included age at TB diagnosis, sex, birthplace (United States or foreign born with country of birth), number of years since arrival in the United States for foreign-born patients, and race/ethnicity. Sociodemographic variables included reported homelessness; substance use (injection drug use), noninjection crack cocaine use, or noninjection drug use (consolidated into yes or no); alcohol abuse; and history of TB treatment at Rikers Island Prison Complex. Clinical variables included initial chest radiography results (normal/abnormal and absence/presence of cavities), the anatomical site of TB disease, respiratory acid-fast bacillus (AFB) smear status, final culture conversion (final conversion from positive to negative culture), HIV status (infected, uninfected, or unknown); and death from any cause (yes or no, excluding patients who refused treatment or were lost to follow-up).
All MDR isolates (n = 159) were subjected to PCR amplification (primers, 5′-ATGCGGCGTTGATCATCG-3′ and 5′-CAGGAGCTGCAAACCAACTCG-3′), followed by standard capillary sequencing of pncA promoter and coding DNA sequence (CDS), as previously described (12). Mutations were identified by alignment of nucleotide sequences to the M. tuberculosis H37Rv reference strain (NCBI accession number AL123456) (25) using ClustalW2 (26). All MDR isolates were also analyzed for the presence of known drug resistance-conferring mutations in the gene targets katG, fabG1 (promoter), and rpoB, using the IBIS platform (Ibis Biosciences Carlsbad, CA), which is based on PCR followed by electrospray ionization mass spectrometry, as described previously (27).
Statistical analysis.
All statistical analysis was performed using SAS (Cary, NC) 9.3. To examine aggregate patient risk factors associated with PZAr, unadjusted odds ratios (OR) were estimated using standard two-by-two contingency table univariate analysis with Fisher's exact test at a 0.05 significance level. We compared demographic and clinical characteristics of TB patients infected with PZAr M. tuberculosis to those of patients infected with PZA-sensitive (PZAs) M. tuberculosis, together with the microbial features (phylogenetic lineage) of the infecting isolates. Trend analysis was performed using the least-squares method, and R2 values are reported. The analysis of PZA susceptibility was stratified by MDR. In the case-control study, we compared the characteristics of MDR-PZAr cases to those of MDR-PZAs controls. A multivariate analysis by logistic regression was performed, using a priori variables reported in the literature to be associated with drug resistance, including age and HIV status. Variables with P values of ≤0.2 in the univariate analysis were included in the multivariate models. We further examined genotypic clustering of MDR-PZAr M. tuberculosis strains using pncA sequence data to support primary PZAr transmission events (defined by identical pncA mutations in genotypically clustered strains) and to distinguish independently acquired PZAr (IS6110-RFLP/spoligotype-based clusters showing diverse pncA mutations) within the MDR population (17). Finally, we evaluated the overall agreement between PZA susceptibility defined by phenotype (DST) and genotype (pncA sequence) among MDR isolates using a kappa statistic.
Ethical approval for this study was obtained from the Institutional Review Boards of the NYC DOHMH and Rutgers University (Newark, NJ).
RESULTS
PZA resistance trends and associated risk factors among NYC TB cases.
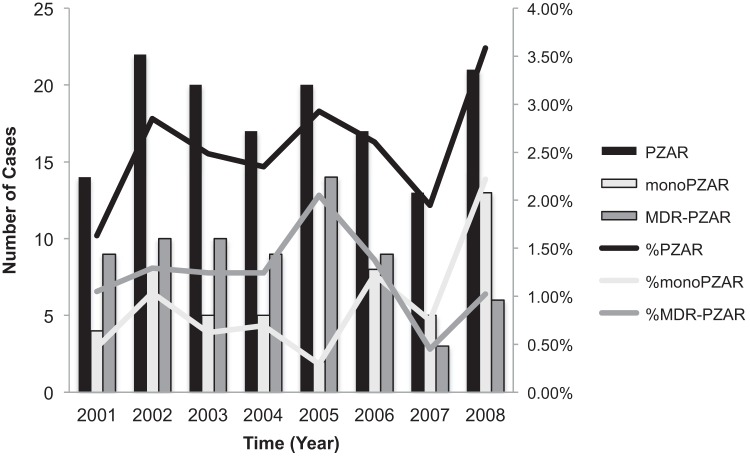
Culture-positive TB cases in New York City steadily declined from 950 cases in 2001 to 649 in 2008 (R2 = 0.9). During this time, a total of 6,260 culture-positive TB cases were reported, 5,670 (91%) of which were due to M. tuberculosis infection and had genotype and DST results available (Fig. 1). Of these, 145 (2.6%) cases involved infection with PZAr M. tuberculosis. The annual M. tuberculosis PZAr prevalence was between 1.6% and 3.6%, resulting in an average of 18 cases per year. The burden of PZAr fluctuated considerably, peaking in 2002, 2005, and 2008 (Fig. 2). From 2001 to 2005, MDR-PZAr (resistance to PZA, INH, and RIF only) accounted for an average of 60% of all PZAr in NYC, whereas mono-PZAr accounted for an average of 24%. Between 2006 and 2008, there was a shift in the relative proportions of mono-PZAr and MDR-PZAr. While the overall proportion of PZAr remained relatively constant during the study period (R2 = 0.2), by 2008, mono-PZAr accounted for 60% of PZAr in NYC, whereas MDR-PZAr accounted for less than 30% of PZAr.
FIG 2.
Distribution of PZAr, mono-PZAr, and MDR-PZAr TB in NYC from 2001 to 2008.
The univariate analysis of epidemiologic risk factors associated with PZAr is presented in Table 1. Predictors of PZAr included AFB smear positivity, history of TB, death, and strain clustering. Among drug-resistant strains (n = 915), MDR was strongly associated with PZAr (OR = 7.25; 95% confidence interval [CI] = 4.73 to 11.13) compared to any monoresistance. In the multivariate analysis (Table 2), AFB smear positivity (OR = 2.02; 95% CI = 1.19 to 3.43) and clustering (OR = 2.73; 95% CI = 1.71 to 4.36) maintained significant associations with PZAr TB caused by PZAr M. tuberculosis.
TABLE 1.
Epidemiologic characteristics associated with pyrazinamide resistance
| Characteristic | PZAr (n = 145) | PZAs (n = 5,525) | Unadjusted OR | 95% CI | P valuea |
|---|---|---|---|---|---|
| Median age, yr (IQR) | 40 (30–50) | 42 (30–57) | 0.99 | 0.98–1.00 | 0.0118 |
| Sexb | |||||
| M | 89 (61.38%) | 3,469 (62.79%) | 0.94 | 0.67–1.32 | 0.7292 |
| F | 56 (38.62%) | 2,056 (37.21%) | |||
| U.S. bornc | |||||
| Yes | 47 (32.64%) | 1,598 (29.03%) | 1.18 | 0.83–1.69 | 0.3472 |
| No | 97 (67.36%) | 3,906 (70.97%) | |||
| History of TB | |||||
| Yes | 8 (5.52%) | 139 (2.52%) | 2.26 | 1.09–4.71 | 0.0248 |
| No | 137 (94.48%) | 5,386 (97.48%) | |||
| History of LTBI | |||||
| Yes | 5 (3.45%) | 320 (5.79%) | 0.58 | 0.24–1.43 | 0.2308 |
| No | 140 (96.55%) | 5,205 (94.21%) | |||
| History of homelessness | |||||
| Yes | 18 (12.41%) | 518 (9.38%) | 1.37 | 0.83–2.26 | 0.2171 |
| No | 127 (87.59%) | 5,007 (90.62%) | |||
| History of substance abuse | |||||
| Yes | 19 (13.29%) | 494 (9.15%) | 1.52 | 0.93–2.49 | 0.0924 |
| No | 124 (86.71%) | 4,903 (90.85%) | |||
| History of alcohol abuse | |||||
| Yes | 24 (16.78%) | 875 (16.20%) | 1.04 | 0.67–1.63 | 0.8513 |
| No | 119 (83.22%) | 4,527 (83.80%) | |||
| History of Rikers treatment | |||||
| Yes | 3 (2.07%) | 106 (1.92%) | 1.08 | 0.34–3.44 | 0.8964 |
| No | 142 (97.93%) | 5,419 (98.08%) | |||
| HIV serostatus | |||||
| Positive | 31 (21.38%) | 825 (14.93%) | 1.39 | 0.92–2.11 | 0.1226 |
| Negative | 85 (58.62%) | 3,145 (56.92%) | Reference | ||
| Unknown | 29 (20.00%) | 1,555 (28.14%) | 0.69 | 0.45–1.06 | 0.0877 |
| TB infection site | |||||
| Pulmonary | 114 (78.62%) | 3,882 (70.26%) | Reference | ||
| Extrapulmonary | 14 (9.66%) | 986 (17.85%) | 0.48 | 0.28–0.85 | 0.0109 |
| Both | 17 (11.72%) | 657 (11.89%) | 0.88 | 0.53–1.48 | 0.6309 |
| Respiratory AFB smear status | |||||
| Positive | 104 (74.82%) | 2,967 (57.77%) | 2.17 | 1.48–3.2 | <0.0001 |
| Negative | 35 (25.18%) | 2,169 (42.23%) | |||
| Abnormal chest X-ray | |||||
| Yes | 59 (93.65%) | 2,091 (88.87%) | 1.85 | 0.67–5.13 | 0.2311 |
| No | 4 (6.35%) | 262 (11.13%) | |||
| Final culture conversion | |||||
| Yes | 106 (86.18%) | 3,563 (85.92%) | 1.02 | 0.61–1.72 | 0.9345 |
| No | 17 (13.82%) | 584 (14.08%) | |||
| Any cavitation | |||||
| Yes | 32 (25.00%) | 1,010 (21.62%) | 1.21 | 0.81–1.81 | 0.3606 |
| No | 96 (75.00%) | 3,661 (78.38%) | |||
| Any deathd | |||||
| Yes | 23 (17.83%) | 582 (11.18%) | 1.72 | 1.09–2.70 | 0.0187 |
| No | 106 (82.17%) | 4,623 (88.82%) | |||
| Drug resistancee | |||||
| Mono | 50 (34.48%) | 461 (59.87%) | Reference | ||
| Poly | 25 (17.24%) | 220 (28.57%) | 1.05 | 0.63–1.74 | 0.8568 |
| MDR | 70 (48.28%) | 89 (11.56%) | 7.25 | 4.73–11.13 | <0.0001 |
| Molecular epidemiology | |||||
| Clustered | |||||
| Yes | 83 (57.24%) | 2,414 (43.69%) | 1.73 | 1.24–2.41 | 0.0012 |
| No | 62 (42.76%) | 3,111 (56.31%) | |||
| Phylogenetic lineage | |||||
| 1 | 18 (12.50%) | 490 (8.90%) | 1.91 | 1.13–3.22 | 0.0159 |
| 2 | 44 (30.56%) | 878 (15.95%) | 2.60 | 1.78–3.81 | <0.0001 |
| 3 | 9 (6.25%) | 347 (6.31%) | 0.67 | 0.67–2.71 | 0.4063 |
| 4 | 73 (50.69%) | 3,788 (68.84%) | Reference | ||
Calculated using chi-square.
M, male; F, female.
Includes Puerto Rico.
Excludes patients who refused treatment or who were lost to follow-up.
Mono, resistance to any 1 drug only (isoniazid, rifampin, ethambutol, or second-line [fluoroquinolone, kanamycin, capreomycin, amikacin, or ethionamide)]; Poly, resistance to any combination of drugs excluding MDR; MDR, multidrug resistant only.
TABLE 2.
Adjusteda epidemiologic characteristics of pyrazinamide resistance
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| Age (yr) | 0.99 | 0.98–1.01 | 0.2007 |
| HIV positivity | 1.39 | 0.80–2.43 | 0.2467 |
| TB infection site | |||
| Extrapulmonary vs pulmonary | 0.84 | 0.33–2.14 | 0.7192 |
| Both vs pulmonary | 0.81 | 0.42–1.55 | 0.5272 |
| AFB smear positivity | 2.02 | 1.19–3.43 | 0.0094 |
| History of TB | 0.93 | 0.28–3.08 | 0.9032 |
| History of substance abuse | 1.29 | 0.70–2.40 | 0.4164 |
| Any death | 1.77 | 0.89–3.52 | 0.1033 |
| Clustered | 2.73 | 1.71–4.36 | <0.0001 |
| Lineage 1 vs 4 | 3.45 | 1.72–6.95 | 0.0005 |
| Lineage 2 vs 4 | 5.01 | 3.13–8.03 | <0.0001 |
| Lineage 3 vs 4 | 3.30 | 1.33–8.19 | 0.0101 |
Adjusted for known TB risk factors, including age, HIV, and any univariate variable with a P value of <0.2.
PZA resistance trends and risk factors among the NYC MDR population.
PZA resistance was high among patients with MDR TB, accounting for 44% (70/159) of all MDR cases, while only 1.4% (75/5511) of the non-MDR population was PZAr. Table 3 shows the odds ratios for PZAr according to clinical and demographic characteristics, stratified by patients with MDR and non-MDR TB. Factors independently associated with PZAr among patients with MDR TB in the stratified analysis were EMB-SLD resistance and AFB smear positivity. AFB smear positivity was the only significant characteristic among patients with non-MDR TB (Table 3). In the multivariate MDR TB analysis, only EMB-SLD resistance (OR = 3.48; 95% CI = 1.57 to 7.69) maintained significance (Table 4), while a history of latent M. tuberculosis infection (LTBI) trended toward significance (OR = 0.12; 95% CI = 0.01 to 1.07).
TABLE 3.
Epidemiologic characteristics associated with pyrazinamide resistance stratified by MDR
| Characteristic | Non-MDR TB Cases |
MDR TB cases |
Breslow-Day test for homogeneity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PZAr (n = 75) | PZAs (n = 5,436) | OR | 95% CI | P value | PZAr (n = 70) | PZAs (n = 89) | OR | 95% CI | P value | χ2 | P value | |
| Median age, yr (IQR) | 40 (31–51) | 42 (30–57) | 0.99 | 0.98–1.01 | 0.262 | 40 (28–49) | 42 (29–53) | 0.98 | 0.96–1.01 | 0.120 | NAa | |
| Sexb | ||||||||||||
| M | 49 (65.33%) | 3,425 (63.01%) | 1.11 | 0.69–1.79 | 0.678 | 40 (57.14%) | 44 (49.44%) | 1.36 | 0.73–2.56 | 0.334 | 0.2679 | 0.6047 |
| F | 26 (34.67%) | 2,011 (36.99%) | 30 (42.86%) | 45 (50.56%) | ||||||||
| U.S. born | ||||||||||||
| Yes | 17 (22.67%) | 1,563 (28.86%) | 0.72 | 0.42–1.24 | 0.239 | 30 (43.48%) | 35 (39.33%) | 1.19 | 0.63–2.25 | 0.599 | 1.3523 | 0.2449 |
| No | 58 (77.33%) | 3,852 (71.14%) | 39 (56.52%) | 54 (60.67%) | ||||||||
| History of TB | ||||||||||||
| Yes | 1 (1.33%) | 132 (2.43%) | 0.54 | 0.07–3.94 | 0.539 | 7 (10.00%) | 7 (7.87%) | 1.30 | 0.43–3.9 | 0.637 | 0.5967 | 0.4399 |
| No | 74 (98.67%) | 5,304 (97.57%) | 63 (90.00%) | 82 (92.13%) | ||||||||
| History of LTBI | ||||||||||||
| Yes | 3 (4.00%) | 313 (5.76%) | 0.68 | 0.21–2.18 | 0.516 | 2 (2.86%) | 7 (7.87%) | 0.34 | 0.07–1.71 | 0.175 | 0.4622 | 0.4966 |
| No | 72 (96.00%) | 5,123 (94.24%) | 68 (97.14%) | 82 (92.13%) | ||||||||
| History of homelessness | ||||||||||||
| Yes | 6 (8.00%) | 506 (9.31%) | 0.85 | 0.37–1.96 | 0.698 | 12 (17.14%) | 12 (13.48%) | 1.33 | 0.56–3.17 | 0.522 | 0.533 | 0.4654 |
| No | 69 (92.00%) | 4,930 (90.69%) | 58 (82.86%) | 77 (86.52%) | ||||||||
| History of substance abuse | ||||||||||||
| Yes | 5 (6.67%) | 481 (9.06%) | 0.72 | 0.29–1.79 | 0.473 | 14 (20.59%) | 13 (15.12%) | 1.46 | 0.63–3.35 | 0.375 | 1.2787 | 0.2581 |
| No | 70 (93.33%) | 4,830 (90.94%) | 54 (79.41%) | 73 (84.88%) | ||||||||
| History of alcohol abuse | ||||||||||||
| Yes | 11 (14.67%) | 853 (16.05%) | 0.90 | 0.47–1.71 | 0.747 | 13 (19.12%) | 22 (25.58%) | 0.69 | 0.32–1.49 | 0.273 | 0.6013 | 0.737 |
| No | 64 (85.33%) | 4,463 (83.95%) | 55 (80.88%) | 64 (74.42%) | ||||||||
| History of Rikers treatment | ||||||||||||
| Yes | 0 (0.00%) | 104 (1.91%) | 3 (4.29%) | 2 (2.25%) | 1.95 | 0.32–11.99 | 0.465 | 2.2788 | 0.1312 | |||
| No | 75 (100.00%) | 5,332 (98.09%) | 67 (95.71%) | 87 (97.75%) | ||||||||
| HIV serostatus | ||||||||||||
| Positive | 11 (14.67%) | 803 (14.77%) | 0.94 | 0.49–1.83 | 0.859 | 20 (28.57%) | 22 (24.72%) | 0.18 | 0.57–2.46 | 0.655 | 0.4642c | 0.4957c |
| Negative | 45 (60.00%) | 3,093 (56.90%) | Reference | 40 (57.14%) | 52 (58.43%) | Reference | ||||||
| Unknown | 19 (25.33%) | 1,540 (28.33%) | 0.85 | 0.49–1.46 | 0.549 | 10 (14.29%) | 15 (16.85%) | 0.87 | 0.35–2.13 | 0.755 | ||
| TB infection site | ||||||||||||
| Pulmonary | 55 (73.33%) | 3,816 (70.20%) | Reference | 59 (84.29%) | 66 (74.16%) | Reference | 1.4359c | 0.2308c | ||||
| Extrapulmonary | 8 (10.67%) | 976 (17.95%) | 0.57 | 0.27–1.20 | 0.138 | 5 (7.14%) | 10 (11.24%) | 0.67 | 0.23–1.96 | 0.466 | ||
| Both | 12 (16.00%) | 644 (11.85%) | 1.29 | 0.69–2.43 | 0.424 | 5 (7.04%) | 13 (14.61%) | 0.43 | 0.15–1.28 | 0.129 | ||
| Respiratory AFB smear status | ||||||||||||
| Positive | 50 (70.42%) | 2914 (57.74%) | 1.74 | 1.04–2.91 | 0.032 | 54 (79.41%) | 53 (59.55%) | 2.62 | 1.27–5.41 | 0.008 | 0.8126 | 0.3673 |
| Negative | 21 (29.58%) | 2,133 (42.26%) | 14 (20.59%) | 36 (40.45%) | ||||||||
| Abnormal chest X-ray | ||||||||||||
| Yes | 31 (91.18%) | 2,056 (88.81%) | 1.30 | 0.4–4.29 | 0.664 | 28 (96.55%) | 35 (92.11%) | 2.40 | 0.24–24.35 | 0.447 | 0.2147 | 0.6431 |
| No | 3 (8.82%) | 259 (11.19%) | 1 (3.45%) | 3 (7.89%) | ||||||||
| Final culture conversion | ||||||||||||
| Yes | 54 (88.52%) | 3,498 (85.90%) | 1.27 | 0.57–2.8 | 0.559 | 52 (83.87%) | 65 (86.67%) | 0.80 | 0.31–2.07 | 0.645 | 0.532 | 0.4658 |
| No | 7 (11.48%) | 574 (14.10%) | 10 (16.13%) | 10 (13.33%) | ||||||||
| Any cavitation | ||||||||||||
| Yes | 13 (19.70%) | 992 (21.60%) | 0.89 | 0.48–1.64 | 0.709 | 19 (30.65%) | 18 (23.08%) | 1.47 | 0.69–3.13 | 0.313 | 1.0368 | 0.3086 |
| No | 53 (80.30%) | 3,601 (78.40%) | 43 (69.35%) | 60 (76.92%) | ||||||||
| Any death | ||||||||||||
| Yes | 9 (12.50%) | 568 (11.07%) | 1.15 | 0.57–2.32 | 0.701 | 14 (24.56%) | 14 (19.18%) | 1.37 | 0.60–3.15 | 0.459 | 0.1021 | 0.7493 |
| No | 63 (87.50%) | 4,564 (88.93%) | 43 (75.44%) | 59 (80.82%) | ||||||||
| Drug resistanced | ||||||||||||
| INH | ||||||||||||
| Yes | 12 (40.00%) | 345 (57.02%) | 0.50 | 0.24–1.06 | 0.089 | |||||||
| No | 18 (60.00%) | 260 (42.98%) | ||||||||||
| INH-EMB | ||||||||||||
| Yes | 3 (10.00%) | 28 (4.63%) | 2.29 | 0.65–8.00 | 0.175 | |||||||
| No | 27 (90.00%) | 577 (95.37%) | ||||||||||
| INH-EMB-SLD | ||||||||||||
| Yes | 1 (3.33%) | 6 (0.99%) | 3.44 | 0.40–29.54 | 0.289 | |||||||
| No | 29 (96.67%) | 599 (99.01%) | ||||||||||
| EMB | ||||||||||||
| Yes | 22 (32.35%) | 22 (25.00%) | 1.43 | 0.71–2.89 | 0.371 | |||||||
| No | 46 (67.65%) | 66 (75.00%) | ||||||||||
| EMB-SLD | ||||||||||||
| Yes | 36 (52.94%) | 23 (26.14%) | 3.18 | 1.62–6.23 | <0.0001 | |||||||
| No | 32 (47.06%) | 65 (73.86%) | ||||||||||
| Molecular epidemiology | ||||||||||||
| Clustered | ||||||||||||
| Yes | 38 (50.67%) | 2,363 (43.47%) | 1.34 | 0.85–2.11 | 0.212 | 45 (64.29%) | 51 (57.30%) | 1.34 | 0.7–2.56 | 0.372 | 0.0001 | 0.9918 |
| No | 37 (49.33%) | 3,073 (56.53%) | 25 (35.71%) | 38 (42.70%) | ||||||||
| Phylogenetic lineage | ||||||||||||
| 1 | 17 (22.97%) | 488 (9.01%) | 3.10 | 1.75–5.49 | <0.0001 | 1 (1.43%) | 2 (2.25%) | 0.79 | 0.07–9.09 | 0.850 | 10.8398c | 0.0010c |
| 2 | 9 (12.16%) | 846 (15.63%) | 0.95 | 0.46–1.95 | 0.883 | 35 (50.00%) | 32 (35.96%) | 1.73 | 0.90–3.34 | 0.103 | ||
| 3 | 6 (8.11%) | 341 (6.30%) | 1.57 | 0.67–3.71 | 0.308 | 3 (4.29%) | 6 (6.74%) | 0.79 | 0.18–3.39 | 0.7515 | ||
| 4 | 42 (56.76%) | 3,739 (69.06%) | Reference | 31 (44.29%) | 49 (55.06%) | Reference | ||||||
NA, not applicable.
M, male; F, female.
Cochran-Mantel-Haenszel nonzero correlation statistic.
Drug resistance analysis excludes drug-susceptible M. tuberculosis strains and is independent of PZAr. The INH category excludes strains with additional RIFr, EMBr, or SLDr. INH-EMB excludes strains with additional RIFr or SLDr. INH-EMB-SLD excludes strains with additional RIFr. The EMB category excludes strains with additional SLDr.
TABLE 4.
Adjusteda epidemiologic characteristics of pyrazinamide resistance among MDR and non-MDR TB cases
| Characteristic | OR | 95% CI | P value |
|---|---|---|---|
| MDR | |||
| Age (yr%) | 0.98 | 0.95–1.01 | 0.1518 |
| HIV positivity | 1.38 | 0.57–3.37 | 0.4724 |
| TB infection site | |||
| Extrapulmonary vs pulmonary | 0.83 | 0.16–4.23 | 0.8211 |
| Both vs pulmonary | 0.44 | 0.12–1.60 | 0.2149 |
| AFB smear | 1.79 | 0.66–4.87 | 0.2556 |
| History of LTBI | 0.12 | 0.01–1.07 | 0.0572 |
| EMB-SLD | 3.48 | 1.57–7.69 | 0.0021 |
| Non-MDR | |||
| Age (yr) | 0.99 | 0.98–1.01 | 0.5188 |
| HIV positivity | 0.95 | 0.46–1.98 | 0.8891 |
| TB infection site | |||
| Extrapulmonary vs pulmonary | 0.89 | 0.28–2.81 | 0.8379 |
| Both vs pulmonary | 1.37 | 0.63–2.97 | 0.4256 |
| AFB smear | 1.82 | 0.93–3.58 | 0.0820 |
| Lineage 1 | 3.27 | 1.64–6.51 | 0.0008 |
Adjusted for known TB risk factors, including age, HIV, and any univariate variable with a P value of <0.2.
Molecular epidemiology of PZA resistance.
Overall, PZAr isolates accounted for 3.5% of lineage 1 (18/508), 4.8% of lineage 2 (44/922), 2.5% of lineage 3 (9/356), and 1.9% of lineage 4 (73/3861). Significant lineage-specific PZAr associations were identified in the univariate analysis, where lineage 1 and lineage 2 M. tuberculosis isolates were approximately twice as likely to be PZAr as lineage 4 isolates (Table 1). These associations maintained significance in the multivariate model and indicate a phylogenetic M. tuberculosis lineage association with PZAr: lineage 1 (OR = 3.45; 95% CI = 1.72 to 6.95), lineage 2 (OR = 5.01; 95% CI = 3.13 to 8.03), and lineage 3 (OR = 3.30; 95% CI = 1.33 to 8.19) compared to lineage 4 (Table 2). Among the non-MDR population, phylogenetic lineage 1 was the only PZAr predictor that maintained significance in the multivariate model (OR = 3.27; 95% CI = 1.64 to 6.51) (Table 4).
MDR TB case-control study.
To examine PZAr risk factors among the 159 MDR cases, we performed a case-control study. Sixty percent of MDR isolates (96/159) were considered genotypically clustered (identical IS6110-RFLPs and spoligotypes), with clusters ranging in size from 2 to 21 members. In contrast, only 43.6% (2,401/5,511) of the non-MDR strains were genotypically clustered (Table 3). Forty-four percent of MDR isolates (70/159) were PZAr based on DST, 64% (45/70) of which belonged to a genotypic cluster. The genetic markers pncA, katG, and rpoB were used to further resolve clustering within the MDR population. Sequence data for the fabG1 promoter did not provide additional cluster resolution and are not shown.
Complete sequence data (katG, rpoB, and pncA) were available for 88% (140/159) of MDR M. tuberculosis isolates (Fig. 1), while pncA sequence data were available for 143 isolates. Analysis of the pncA CDS-promoter region of MDR isolates identified 83 (58%) mutants. In total, there were 37 unique pncA mutations, which included insertions, deletions, and nonsynonymous substitutions. Some discordances between PZAr determined by DST and the presence of pncA mutations (PZAr-pncA) were observed (109/143; kappa = 0.54). However, the majority of these were accounted for by a single family of Beijing strains (termed W; n = 54) (29/54; kappa = 0.17), many of which carried a specific pncA mutation known to exhibit discordance with phenotypic PZAr (16, 28), as discussed below. Concordance between the pncA sequence and PZA DST results was significantly higher when W strains were excluded and the remaining 89 strains were evaluated (80/89; kappa = 0.79).
Table 5 shows the resolution of genotypic clusters, using DNA sequence data to distinguish primary transmission from independent acquisition of MDR TB. Identical sequences for katG, rpoB, and pncA confirmed the genotyping assignment of seven clusters (n = 38). Conversely, diverse pncA mutations were identified within four genotypic clusters possessing identical IS6110-RFLPs, spoligotypes, and mutations in katG and rpoB, while a fifth cluster with identical katG and pncA sequences (P strain; spoligotype S00086) was resolved by different rpoB mutations.
TABLE 5.
MDR cluster analysis using katG, rpoB, and pncA sequence data
| Infection | Genotypic cluster |
katG-rpoB |
pncA |
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| RFLP (spoligotype) | Lineage (spoligotype) | Count | Cluster | Count | Cluster | Count | ||
| Primary transmission | W (S00034) | 2 (Beijing, ST523, ST623) | 22 | S315T_H526Y | 21a | ACC(T)47GCC(A) | 21a | 28, 37 |
| W665 (S00034) | 2 (Beijing, ST523, ST623) | 3 | S315T_H526Y | 3 | ACC(T)47GCC(A) | 3 | ||
| P1 (S00086) | 4 (X, Harlem, LAM, Uganda) | 2 | S315T_H526Y | 2 | CTG(L)85CCG(P) | 2 | 40, 41 | |
| BW900 (S00005) | 4 (X, Harlem, LAM, Uganda) | 5 | WT_H526D | 5 | WTb | 5 | ||
| C (S00030) | 4 (X, Harlem, LAM, Uganda) | 2 | WT_S531L | 2 | WT | 2 | 38 | |
| DK22 (S00245) | 4 (X, Harlem, LAM, Uganda) | 2 | S315T_L511P | 2 | WT | 2 | ||
| BW230 (S00241) | 4 (X, Harlem, LAM, Uganda) | 2 | S315T_S531L | 2 | WT | 2 | 39 | |
| Independent acquisition | W283 (S00034) | 2 (Beijing, ST523, ST623) | 3 | S315T_S531L | 2 | 290 Δ G | 2 | |
| S315_WT | 1 | AGG(R)154GGG(G) | 1 | |||||
| W148 (S00034) | 2 (Beijing, ST523, ST623) | 3 | S315T_S531L | 3 | WT | 1 | 54 | |
| CAT(H)71CGT(R) | 1 | |||||||
| GGA(G)108CGA(R) | 1 | |||||||
| W1 (S00034) | 2 (Beijing, ST523, ST623) | 2 | S315T_H526Y | 2 | ACC(T)47GCC(A) | 1 | 28, 43 | |
| ACC(T)47AGC(S) | 1 | |||||||
| W33 (S00034) | 2 (Beijing, ST523, ST623) | 2 | S315T_H526Y | 2 | ACC(T)47GCC(A) | 1 | ||
| ACC(T)47AGC(S) | 1 | |||||||
| P (S00086) | 4 (X, Harlem, LAM, Uganda) | 3 | S315T_D516V | 1 | CTG(L)85CCG(P) | 3 | 40, 41 | |
| S315T_H526Y | 1 | |||||||
| S315T_S531L | 1 | |||||||
| Both | H (S00009) | 4 (X, Harlem, LAM, Uganda) | 11 | S315T_S531L | 9 | 70 Δ G | 10 | 40, 41 |
| WT_WT | 1 | |||||||
| WT_L511P | 1 | WT | 1 | |||||
| Unresolved | W738 (S00034) | 2 (Beijing, ST523, ST623) | 3 | S315T_WT | 2 | TCG(S)67CCG(P) | 1a | |
| S315T_S531L | 1 | WT | 1 | |||||
| AB (S00145) | 4 (X, Harlem, LAM, Uganda) | 2 | S315T_V146F | 2 | WT | 1a | 40 | |
| W269 (S00034) | 2 (Beijing, ST523, ST623) | 2 | WT_S531L | 1a | ACG(T)142ATG(M) | 1a | ||
| GD265 (S00474) | 3 (CAS) | 2 | S315T_S531L | 1a | CAG(Q)141CCG(P)c | 2 | ||
One bad sequence.
WT, wild type.
Also contains a lineage-specific synonymous mutation (TCC[S]65TCT[S]).
DISCUSSION
The prevalence of PZAr has been reported to range from 0.8 to 10% among patients with non-MDR TB and from 10 to 85% among patients with MDR TB worldwide (4, 29, 30). During the study period, TB caused by PZAr M. tuberculosis in NYC was 50% higher than the national average and 20% higher among patients with MDR TB (31). Moreover, the MDR TB burden in NYC was 2-fold higher than national estimates. Thus, the high prevalence of MDR TB could be an explanation for the high proportion of PZAr we observed. However, the MDR TB incidence in NYC declined over this period (32), while the incidence of PZAr TB cases increased, indicating that additional factors were contributing to PZAr in this population. To explain the national increase in PZAr, Kurbatova and colleagues proposed that a higher proportion of foreign-born TB patients may reflect international programmatic variation in TB treatment protocols, leading to increased levels of PZAr TB imported to the United States (31). While we observed high proportions of foreign-born TB patients annually (70 to 80%), we did not find being born outside the United States to be a statistically significant risk factor for PZAr in NYC. In contrast to our findings in NYC, a recent analysis of PZAr TB trends over 2 decades in San Francisco found rates similar to the national average and no significant association between PZAr and MDR TB (33).
Our study found AFB smear positivity, clustering, and death to be independently associated with PZAr. Together, these risk factors suggest that patients infected with PZAr strains were infectious, transmitting, and not responding well to treatment. A history of TB was also independently associated with PZAr, perhaps suggesting that a proportion of patients experienced relapses of drug-resistant TB, though relapse data were not available for this analysis (34, 35). Given the clinical importance of MDR TB and the high proportion of PZAr among these patients, we sought to determine PZAr risk factors in the MDR TB population. Concurrent resistance to EMB and SLDs was the only PZAr risk factor that maintained statistical significance in the adjusted case-control MDR model. While a strong association between MDR-PZAr and EMBr has been associated with the inappropriate use of standard short-course therapy in patients with MDR TB (36), we do not have sufficient evidence here to address this question. A larger proportion of MDR-PZAr among clustered versus nonclustered strains suggests that primary transmission was responsible for more PZAr than acquired resistance within the MDR TB population.
Based on our molecular examination of MDR strains, we were able to further refine our cluster analysis. In particular, we identified MDR TB clusters with IS6110-RFLP; spoligotype; and katG, rpoB, and pncA mutations identical to those previously described in a number of historic NYC drug-resistant outbreak strains (28, 37), e.g., strain C (S00030) (38), strain H (S00009), strain BW (S00241) (39), and strain P (S00086) (40, 41). The sequence-based confirmation of MDR genotypic clusters strongly suggests primary transmission and shows evidence that these historic strains were continuing to circulate and reactivate within NYC during the study period. For example, a 21-member cluster of the W (S00034; Beijing family) MDR-PZAr strain contained genetic markers identical to those of a highly clonal strain that was responsible for a large NYC MDR outbreak in the early 1990s (28, 37). Smaller clusters of historic W variant strains previously described (28, 37, 42, 43) were also identified. Our data suggest that PZAr also appeared independently in MDR TB (i.e., not as a result of primary transmission) (44). For example, the P strain was likely transmitted as a katG-pncA mutant prior to becoming MDR, which indicates PZAr was at times acquired prior to MDR development, as seen in other strains (45, 46).
In addition to clustering, further microbial phylogenetic lineage effects were observed. In the general NYC TB population, PZAr was associated with lineage 1 (East African/Indo-Oceanic), lineage 2 (East Asian/Beijing), and lineage 3 (East Africa/Central Asia) compared to lineage 4 (Euro-American). PZAr associations with lineages 1 and 2 were consistent with lineage effects reported in the national study conducted by the CDC (31). Our data indicate that PZAr among patients with MDR TB was associated with lineage 2, while PZAr was associated with lineage 1 among patients with non-MDR TB. Phylogenetic M. tuberculosis lineages are strongly associated with geographic locations. Therefore, the observed phylogenetic associations in this study are curious and may be a proxy for the importation of PZAr from specific non-U.S. locations. Additional studies would be needed to examine these microbial associations in light of social networks and neighborhood level effects.
Limitations of this study include moderate amounts of HIV data (70%), which is a well-established risk factor for drug-resistant TB. In addition, many demographic variables were self-reported, including a history of TB/LTBI, alcohol or substance abuse, and homelessness, which may have been subject to information bias and misclassification, though it is likely nondifferential. Furthermore, due to the technical complications of PZA-DST (8, 18), our findings may have been subject to some PZAr misclassification. However, pncA sequence data have been shown to be a useful tool to confirm PZAr-DST results (47). The most common pncA-PZAr-DST inconsistency we observed was that of W strains with a pncA mutation in codon 47 (ACC [Thr]→GCC [Ala]). This specific mutation has been shown to correlate poorly with PZA-DST in several previous studies (12, 16, 28, 48, 49), suggesting that the mutation confers borderline resistance at PZA concentrations used routinely in PZA-DST (16). Also, the molecular data suggest that IS6110-RFLP analysis and spoligotyping may have slightly overestimated the extent of clustering and primary transmission.
Despite a steady decline in the total number of TB cases in NYC from 2001 to 2008, the incidence of PZAr increased. Patients with PZAr TB were more infectious and actively transmitting and had poor clinical outcomes compared to patients with TB caused by PZAs M. tuberculosis. Among the MDR TB population, PZAr was acquired somewhat more frequently via primary transmission than independently. In addition, concurrent EMB-SLD resistance was the only risk factor for PZAr among patients with MDR TB. These observations have important clinical and public health implications for control of drug-resistant TB. Specifically, the strength of microbial risk factors for PZAr highlights the importance of routine PZA-DST, genotyping, and confirmatory sequence analysis for ensuring appropriate drug therapy and disrupting transmission. Finally, as clinical trials of new regimens to shorten treatment of drug-susceptible and MDR TB are expanding, these results support the need to consider PZAr in trial design (50–53).
ACKNOWLEDGMENTS
We thank Natalia Kurepina and Elena Shashkina for technical assistance; Zachary Schneider for editorial assistance; the New York City Public Health Laboratory and New York State Wadsworth Center for specimen tracking/testing; and the Bureau of TB Control field and clinic staff, who collected the data analyzed here.
This work was supported by the National Institutes of Health (AI106551 to B.K.).
REFERENCES
- 1.Chang KC, Leung CC, Yew WW, Leung EC, Leung WM, Tam CM, Zhang Y. 2012. Pyrazinamide may improve fluoroquinolone-based treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother 56:5465–5475. doi: 10.1128/AAC.01300-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 3.Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rusch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. 2011. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 38:516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Chang K, Leung C-C, Yew W, Gicquel B, Fallows D, Kaplan G, Chaisson R, Zhang W. 2012. ‘ZS-MDR-TB’ versus ‘ZR-MDR-TB’: improving treatment of MDR-TB by identifying pyrazinamide susceptibility. Emerg Microbes Infect 1:e5. doi: 10.1038/emi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lienhardt C, Davies G. 2010. Methodological issues in the design of clinical trials for the treatment of multidrug-resistant tuberculosis: challenges and opportunities. Int J Tuberc Lung Dis 14:528–537. [PubMed] [Google Scholar]
- 6.Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, Bantubani N, Narasimooloo R, De Marez T, van Heeswijk R, Lounis N, Meyvisch P, Andries K, McNeeley DF. 2012. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 56:3271–3276. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott W, Tompsett R. 1954. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc 70:748–754. [DOI] [PubMed] [Google Scholar]
- 9.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE III, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scorpio A, Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med 2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 11.Stoffels K, Mathys V, Fauville-Dufaux M, Wintjens R, Bifani P. 2012. Systematic analysis of pyrazinamide-resistant spontaneous mutants and clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:5186–5193. doi: 10.1128/AAC.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreevatsan S, Pan X, Zhang Y, Kreiswirth BN, Musser JM. 1997. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob Agents Chemother 41:636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano K, Takahashi M, Kazumi Y, Fukasawa Y, Abe C. 1997. Mutation in pncA is a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis. Tuber Lung Dis 78:117–122. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Permar S, Sun Z. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J Med Microbiol 51:42–49. [DOI] [PubMed] [Google Scholar]
- 15.Piersimoni C, Mustazzolu A, Giannoni F, Bornigia S, Gherardi G, Fattorini L. 2013. Prevention of false resistance results obtained in testing the susceptibility of Mycobacterium tuberculosis to pyrazinamide with the Bactec MGIT 960 system using a reduced inoculum. J Clin Microbiol 51:291–294. doi: 10.1128/JCM.01838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morlock GP, Crawford JT, Butler WR, Brim SE, Sikes D, Mazurek GH, Woodley CL, Cooksey RC. 2000. Phenotypic characterization of pncA mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 44:2291–2295. doi: 10.1128/AAC.44.9.2291-2295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathema B, Kurepina N, Fallows D, Kreiswirth BN. 2008. Lessons from molecular epidemiology and comparative genomics. Semin Respir Crit Care Med 29:467–480. doi: 10.1055/s-0028-1085699. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 19.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagneux S, Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7:328–337. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 21.Bifani PJ, Mathema B, Liu Z, Moghazeh SL, Shopsin B, Tempalski B, Driscol J, Frothingham R, Musser JM, Alcabes P, Kreiswirth BN. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 282:2321–2327. doi: 10.1001/jama.282.24.2321. [DOI] [PubMed] [Google Scholar]
- 22.Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. 2006. Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 19:658–685. doi: 10.1128/CMR.00061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streicher EM, Victor TC, van der Spuy G, Sola C, Rastogi N, van Helden PD, Warren RM. 2007. Spoligotype signatures in the Mycobacterium tuberculosis complex. J Clin Microbiol 45:237–240. doi: 10.1128/JCM.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.New York City Department of Health and Mental Hygiene. 2008. Bureau of Tuberculosis Control clinical policies and protocols. New York City Department of Health and Mental Hygiene, New York, NY. [Google Scholar]
- 25.Camus JC, Pryor MJ, Medigue C, Cole ST. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148:2967–2973. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. 6 April 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massire C, Ivy CA, Lovari R, Kurepina N, Li H, Blyn LB, Hofstadler SA, Khechinashvili G, Stratton CW, Sampath R, Tang YW, Ecker DJ, Kreiswirth BN. 2011. Simultaneous identification of mycobacterial isolates to the species level and determination of tuberculosis drug resistance by PCR followed by electrospray ionization mass spectrometry. J Clin Microbiol 49:908–917. doi: 10.1128/JCM.01578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bifani PJ, Plikaytis BB, Kapur V, Stockbauer K, Pan X, Lutfey ML, Moghazeh SL, Eisner W, Daniel TM, Kaplan MH, Crawford JT, Musser JM, Kreiswirth BN. 1996. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275:452–457. [PubMed] [Google Scholar]
- 29.Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsaker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol 46:3459–3464. doi: 10.1128/JCM.00973-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Kwak HK, Lee J, Yun YJ, Lee JS, Lee MS, Min SY, Park SK, Kang HS, Maeng YH, Kim SY, Kim SY, Kook YH, Kim YR, Lee KH. 2012. Patterns of pncA mutations in drug-resistant Mycobacterium tuberculosis isolated from patients in South Korea. Int J Tuberc Lung Dis 16:98–103. doi: 10.5588/ijtld.10.0739. [DOI] [PubMed] [Google Scholar]
- 31.Kurbatova EV, Cavanaugh JS, Dalton T, Click ES, Cegielski JP. 2013. Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999-2009. Clin Infect Dis 57:1081–1093. doi: 10.1093/cid/cit452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New York City Department of Health and Mental Hygiene. 2014. Bureau of Tuberculosis Control annual summary, 2013. New York City Department of Health and Mental Hygiene, New York, NY. [Google Scholar]
- 33.Budzik JM, Jarlsberg LG, Higashi J, Grinsdale J, Hopewell PC, Kato-Maeda M, Nahid P. 2014. Pyrazinamide resistance, Mycobacterium tuberculosis lineage and treatment outcomes in San Francisco, California. PLoS One 9:e95645. doi: 10.1371/journal.pone.0095645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. 1993. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med 328:521–526. (Erratum, 329:148, 1993.) doi: 10.1056/NEJM199307083290226. [DOI] [PubMed] [Google Scholar]
- 35.Costello HD, Caras GJ, Snider DE Jr. 1980. Drug resistance among previously treated tuberculosis patients, a brief report. Am Rev Respir Dis 121:313–316. [DOI] [PubMed] [Google Scholar]
- 36.Calver AD, Falmer AA, Murray M, Strauss OJ, Streicher EM, Hanekom M, Liversage T, Masibi M, van Helden PD, Warren RM, Victor TC. 2010. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg Infect Dis 16:264–271. doi: 10.3201/eid1602.090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munsiff SS, Nivin B, Sacajiu G, Mathema B, Bifani P, Kreiswirth BN. 2003. Persistence of a highly resistant strain of tuberculosis in New York City during 1990-1999. J Infect Dis 188:356–363. doi: 10.1086/376837. [DOI] [PubMed] [Google Scholar]
- 38.Macaraig M, Agerton T, Driver CR, Munsiff SS, Abdelwahab J, Park J, Kreiswirth B, Driscoll J, Zhao B. 2006. Strain-specific differences in two large Mycobacterium tuberculosis genotype clusters in isolates collected from homeless patients in New York City from 2001 to 2004. J Clin Microbiol 44:2890–2896. doi: 10.1128/JCM.00160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perri BR, Proops D, Moonan PK, Munsiff SS, Kreiswirth BN, Goranson C, Ahuja SD. 2011. Mycobacterium tuberculosis cluster with developing drug resistance, New York, New York, USA, 2003-2009. Emerg Infect Dis 17:372–378. doi: 10.3201/eid1703.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munsiff SS, Bassoff T, Nivin B, Li J, Sharma A, Bifani P, Mathema B, Driscoll J, Kreiswirth BN. 2002. Molecular epidemiology of multidrug-resistant tuberculosis, New York City, 1995-1997. Emerg Infect Dis 8:1230–1238. doi: 10.3201/eid0811.020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons LM, Salfinger M, Clobridge A, Dormandy J, Mirabello L, Polletta VL, Sanic A, Sinyavskiy O, Larsen SC, Driscoll J, Zickas G, Taber HW. 2005. Phenotypic and molecular characterization of Mycobacterium tuberculosis isolates resistant to both isoniazid and ethambutol. Antimicrob Agents Chemother 49:2218–2225. doi: 10.1128/AAC.49.6.2218-2225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bifani PJ, Mathema B, Kurepina NE, Kreiswirth BN. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol 10:45–52. doi: 10.1016/S0966-842X(01)02277-6. [DOI] [PubMed] [Google Scholar]
- 43.Moss AR, Alland D, Telzak E, Hewlett D Jr, Sharp V, Chiliade P, LaBombardi V, Kabus D, Hanna B, Palumbo L, Brudney K, Weltman A, Stoeckle K, Chirgwin K, Simberkoff M, Moghazeh S, Eisner W, Lutfey M, Kreiswirth B. 1997. A city-wide outbreak of a multiple-drug-resistant strain of Mycobacterium tuberculosis in New York. Int J Tuberc Lung Dis 1:115–121. [PubMed] [Google Scholar]
- 44.Xu C, Kreiswirth BN, Sreevatsan S, Musser JM, Drlica K. 1996. Fluoroquinolone resistance associated with specific gyrase mutations in clinical isolates of multidrug-resistant Mycobacterium tuberculosis. J Infect Dis 174:1127–1130. (Erratum, 175:1027, 1997.) [DOI] [PubMed] [Google Scholar]
- 45.Mokrousov I. 2013. Insights into the origin, emergence, and current spread of a successful Russian clone of Mycobacterium tuberculosis. Clin Microbiol Rev 26:342–360. doi: 10.1128/CMR.00087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drobniewski F, Balabanova Y, Nikolayevsky V, Ruddy M, Kuznetzov S, Zakharova S, Melentyev A, Fedorin I. 2005. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 293:2726–2731. doi: 10.1001/jama.293.22.2726. [DOI] [PubMed] [Google Scholar]
- 47.Alexander DC, Ma JH, Guthrie JL, Blair J, Chedore P, Jamieson FB. 2012. Gene sequencing for routine verification of pyrazinamide resistance in Mycobacterium tuberculosis: a role for pncA but not rpsA. J Clin Microbiol 50:3726–3728. doi: 10.1128/JCM.00620-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewlett D Jr, Horn DL, Alfalla C. 1995. Drug-resistant tuberculosis: inconsistent results of pyrazinamide susceptibility testing. JAMA 273:916–917. [PubMed] [Google Scholar]
- 49.Dormandy J, Somoskovi A, Kreiswirth BN, Driscoll JR, Ashkin D, Salfinger M. 2007. Discrepant results between pyrazinamide susceptibility testing by the reference BACTEC 460TB method and pncA DNA sequencing in patients infected with multidrug-resistant W-Beijing Mycobacterium tuberculosis strains. Chest 131:497–501. doi: 10.1378/chest.06-1899. [DOI] [PubMed] [Google Scholar]
- 50.Miotto P, Cabibbe AM, Feuerriegel S, Casali N, Drobniewski F, Rodionova Y, Bakonyte D, Stakenas P, Pimkina E, Augustynowicz-Kopec E, Degano M, Ambrosi A, Hoffner S, Mansjo M, Werngren J, Rusch-Gerdes S, Niemann S, Cirillo DM. 2014. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. mBio 5:e01819–01814. doi: 10.1128/mBio.01819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL. 2014. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 52.Conde MB, Lapa ESJR. 2011. New regimens for reducing the duration of the treatment of drug-susceptible pulmonary tuberculosis. Drug Dev Res 72:501–508. doi: 10.1002/ddr.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon YS, Jeong BH, Koh WJ. 2014. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med 20:280–286. doi: 10.1097/MCP.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 54.Narvskaya O, Otten T, Limeschenko E, Sapozhnikova N, Graschenkova O, Steklova L, Nikonova A, Filipenko ML, Mokrousov I, Vyshnevskiy B. 2002. Nosocomial outbreak of multidrug-resistant tuberculosis caused by a strain of Mycobacterium tuberculosis W-Beijing family in St. Petersburg, Russia. Eur J Clin Microbiol Infect Dis 21:596–602. doi: 10.1007/s10096-002-0775-4. [DOI] [PubMed] [Google Scholar]