Abstract
Current treatments for cutaneous and visceral leishmaniasis are toxic, expensive, difficult to administer, and limited in efficacy and availability. Disulfiram has primarily been used to treat alcoholism. More recently, it has shown some efficacy as therapy against protozoan pathogens and certain cancers, suggesting a wide range of biological activities. We used an ex vivo system to screen several thiuram disulfide compounds for antileishmanial activity. We found five compounds (compound identifier [CID] 7188, 5455, 95876, 12892, and 3117 [disulfiram]) with anti-Leishmania activity at nanomolar concentrations. We further evaluated these compounds with the addition of divalent metal salts based on studies that indicated these salts could potentiate the action of disulfiram. In addition, clinical studies suggested that zinc has some efficacy in treating cutaneous leishmaniasis. Several divalent metal salts were evaluated at 1 μM, which is lower than the normal levels of copper and zinc in plasma of healthy individuals. The leishmanicidal activity of disulfiram and CID 7188 were enhanced by several divalent metal salts at 1 μM. The in vitro therapeutic index (IVTI) of disulfiram and CID 7188 increased 12- and 2.3-fold, respectively, against L. major when combined with ZnCl2. The combination of disulfiram with ZnSO4 resulted in a 1.8-fold increase in IVTI against L. donovani. This novel combination of thiuram disulfides and divalent metal ions salts could have application as topical and/or oral therapies for treatment of cutaneous and visceral leishmaniasis.
INTRODUCTION
The leishmaniases are vector-borne parasitic diseases with a significant global impact. These diseases occur in more than 88 countries of the world, where approximately 350 million people are at risk. In the Old World, visceral and cutaneous leishmaniasis are caused by the intracellular protozoa Leishmania donovani and Leishmania major, respectively. Visceral leishmaniasis is characterized by impaired parasite-specific cell-mediated immunity and progressive hepatosplenomegaly, anemia, and weight loss and is frequently fatal if left untreated (1). In cutaneous leishmaniasis (CL), the disease severity varies considerably from single self-resolving skin nodules or ulcers to one or more nonhealing lesions, which can be disfiguring (2). The healing response is generally associated with expansion of parasite-specific gamma interferon (IFN-γ)-producing T cells (3).
All currently available drug therapies for leishmaniasis have potentially harmful side effects and documented limitations in efficacy. The pentavalent antimony compounds, which are still widely used in many countries, are suboptimal because of the difficulty of administration, well-known host toxicity, and increasing parasite drug resistance. Cure rates have been noted recently to be unacceptably low (4). Other therapies, including amphotericin B desoxycholate and its liposomal formulation, and miltefosine, have been increasingly used in the treatment of visceral or cutaneous leishmaniasis. Their use is also limited by the difficulty of administration, toxicity, high cost, and/or potential to develop drug resistance (5–7). Topical treatment of CL with paromomycin plus methylbenzethonium chloride or gentamicin ointment, or application of heat have been effective in selected areas of the Old and New World (8, 9). The lack of industry interest in developing new anti-Leishmania drugs is one of the reasons why the World Health Organization listed the leishmaniases among the most neglected diseases. Therefore, it is imperative to develop new oral drugs to treat all clinical forms of leishmaniasis. In addition, topical treatments need to be further developed for the treatment of CL.
Disulfiram has been used extensively in patients to treat alcoholism (10). However, recent studies also identified therapeutic activity against Mycobacterium tuberculosis, Plasmodium falciparum, and Giardia lamblia (11–13). It also has cytotoxic activity against some cancer cells (14–16). We and others have reported that disulfiram is active at nanomolar concentrations against intracellular amastigotes of L. donovani (17) and against L. major amastigotes (18) or promastigotes (19, 20). Studies in BALB/c mice infected with L. major showed that administration of disulfiram at 160 mg/kg of resulted in significant reduction (50%) of the footpad lesion in comparison to sham-treated mice (19). The in silico evaluation of L. major metabolic networks predicted that the antileishmanial activity of disulfiram may reside in its ability to disturb the transmembrane proton transport system encoded by the LmjF25.1170 and LmjF25.1180 genes (20). The proteins coded by these genes are part of the F-type ATPase β chain complex, which participates in the synthesis of parasite ATP, specifically transporting protons (H+) across the membrane to generate an electrochemical gradient that powers ATP synthesis (21). In L. donovani, the inhibition of F-ATP synthase depolarizes the mitochondrial membrane potential so that ATP synthesis is impaired. This increases the generation of cellular reactive oxygen species (ROS), which induces parasite DNA fragmentation (22).
The anti-infective properties of zinc sulfate have been exploited by clinicians to treat different skin infections, including cutaneous leishmaniasis (23). There is evidence that zinc has direct and indirect (via host immunity) antiparasitic effects. In vitro experiments indicated that zinc inhibits several Leishmania enzymes, including those involved in the Embden-Meyerhof pathway (24, 25). On the other hand, zinc is an essential trace element for humans and its deficiency leads to decreased recruitment of naive T cells and reduced expression of Th1 cytokines (IFN-γ, interleukin-2, and tumor necrosis factor alpha) (26). This immune impairment could explain in part observations made in patients with mucocutaneous and visceral leishmaniasis in whom significantly lower plasma levels of zinc were found compared to normal endemic controls (27). Therefore, supplementing zinc in a deficient host could favor the development of a protective Th1-mediated response against Leishmania.
A strong anticancer effect in vitro and in vivo of disulfiram was observed when combined with divalent metal ions such as zinc and copper (28–30). These complexes had improved influx into cancer cells lines compared to disulfiram alone and triggered the generation of ROS (28, 31). In contrast to cancer cells, normal cells can tolerate higher levels of ROS (32). ROS damage induces DNA, protein, and lipid membrane damage, leading to apoptosis in cancer cell lines via cJun N-terminal kinase and p38 mitogen-activated protein kinase pathways (33). ROS are also highly toxic for Leishmania amastigotes (34). Therefore, we hypothesized that disulfiram in combination with divalent metal ions can increase the production of intracellular ROS and synergize its anti-Leishmania activity.
Immune mechanisms play an important role in both the pathogenesis and healing response to Leishmania infection in patients and experimental models (35–37). Therefore, to screen antileishmanial compounds within the infected host microenvironment, we developed ex vivo culture systems derived from skin-draining lymph nodes of BALB/c mice infected with L. major or spleen cells from hamsters infected with L. donovani (17, 18). The aim of the present study was to evaluate the antileishmanial activity of thiuram disulfide analogs alone and in combination with divalent metal ion salts for the treatment of cutaneous and visceral leishmaniasis.
MATERIALS AND METHODS
Animals and parasites.
All of the procedures involving animals were approved by the IACUC of the University of Texas Health Science Center and the University of Texas Medical Branch. Male BALB/c mice and Golden Syrian hamsters (6 to 8 weeks old; Harlan Laboratories, Indianapolis, IN) were used in all of the experiments. Leishmania donovani (MHOM/SD/001S-2D) and L. major (MHOM/IL/81/Friedlin) promastigotes were transfected with an episomal vector containing the luciferase (luc) reporter gene (38). The parasites were cultured at 26 to 28°C in complete M199 (Gibco, Grand Island, NY; 0.12 mM adenine, 0.0005% hemin, 20% fetal bovine serum [FBS]) with the addition of the selective antibiotic Geneticin (10 μg/ml, G418; Gibco). Parasite virulence was maintained by subinoculating hamsters (L. donovani) or mice (L. major) every 2 to 3 months, from which the strains were recovered for subsequent in vitro determinations.
Thiuram disulfide analogs.
A total of nine thiuram disulfide analogs identified by CID in Pubchem (Fig. 1) were obtained from commercial sources as follows: 7188 and 30054 from Acros Organics (Thermo Fisher Scientific, Pittsburgh, PA); 3110809 and 3097317 from Asinex (Moscow, Russia); and 3117 (disulfiram), 5455, 95876, 12892, and 15412 from Sigma (St. Louis, MO). The compounds were dissolved in dimethyl sulfoxide (DMSO [Sigma]; cell culture tested) at a stock concentration of 20 mM and stored at −20°C until thawed shortly before each experiment. All divalent metal salts were obtained from Sigma and dissolved in culture medium (see below). We used miltefosine and amphotericin B (Sigma) dissolved in DMSO (Sigma) as positive controls for antileishmanial activity.
FIG 1.
Chemical structures of disulfiram/thiuram disulfide analogs by CID (Pubchem).
Animal infection and determination of drug activity using the ex vivo explant culture and in vitro-infected macrophages.
An ex vivo explant culture to evaluate disulfiram and analogs activity against L. donovani was obtained by inoculating hamsters through the intracardial route with 106 purified metacyclic promastigotes (17). For cutaneous leishmaniasis, mice were intradermally inoculated on the rump with 107 highly virulent metacyclic L. major promastigotes (18). At 3 weeks postinfection, the spleen (L. donovani) or subiliac lymph nodes (L. major) of infected animals were aseptically removed and placed in 2 ml of collagenase solution (collagenase D [2 mg/ml; Roche, Indianapolis, IN] in 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES [pH 7.4]). The spleens or lymph nodes were infiltrated with collagenase solution and incubated for 30 min at 37°C. The cell suspension and remaining tissue fragments were gently passed through a 100-μm-pore-size cell strainer (BD, Bedford MA) to obtain a single cell suspension. The cells were washed in Dulbecco modified Eagle medium (DMEM), centrifuged at 500 × g for 7 min at 4°C and resuspended in 2× supplemented culture medium composed of DMEM (Cellgro), 10% heat-inactivated FBS (Atlanta Biologicals, Lawrenceville, GA), 2 mM sodium pyruvate (Sigma, St. Louis, MO), 2× MEM amino acid solution (Sigma), and 20 mM HEPES buffer (Cellgro). The ex vivo cultures (100 μl) were exposed to 2-fold serial dilutions of test compounds in 100 μl of DMEM (to make the final medium 1×).
The mouse macrophage cell line RAW 264.7 (ATCC TIB-71) was cultured in DMEM, infected at 1:5 ratio (cells/parasites) with stationary-phase LUC-transfected L. major or LUC-transfected-L. donovani promastigotes, and incubated overnight at 34 or 37°C, respectively, with 5% CO2. The extracellular parasites were then removed by washing with prewarmed Dulbecco phosphate-buffered saline (PBS; Gibco). Parasitized RAW 264.7 cell monolayers were detached using 1× trypsin-EDTA (Gibco), washed, and adjusted to 105 cells/well in 100 μl in 2× supplemented DMEM culture medium using flat-bottom 96-well plates. Infected RAW 264.7 macrophages were exposed to 2-fold serial dilutions of test compounds as described above for the ex vivo culture. The infected RAW 264.7 macrophages were incubated for 48 h at 34°C (L. major) or 37°C (L. donovani) in 5% CO2.
To calculate the effective concentration of compound that killed 50% of the parasites (EC50), we determined the parasite burden by luminometry of serial concentrations of disulfiram and its analogs as described before for L. donovani (spleen cell explant or RAW 264.7) (17) or L. major (lymph node cell explant or RAW 264.7) (18). Briefly, after 48 h of culture, the cells were lysed, and the luciferase signal was read in a plate luminometer (FLUOstar Omega; BMG Labtech) after adding the luciferin substrate (Promega). The percentage of parasite inhibition with regard to controls was calculated as = 100 − [(parasite counts in treated cells/parasite counts in untreated cells) × 100]. The EC50 was determined by regression analysis using GraphPad (Prism 5.0), and the means and standard errors from three different experiments were utilized to estimate the final EC50.
Cell toxicity (CC50) and calculation of the IVTI.
We used the HepG2 cell line (human hepatocellular carcinoma, ATCC HB-8065) as a cell-based assay and an alternative to animal testing to determine toxicity of disulfiram and its analogs (39, 40). The cells were maintained in MEM (Gibco) supplemented with 5% heat-inactivated FBS, 1 mM sodium pyruvate (Gibco), and 1× MEM amino acids solution (Sigma). Cell monolayers were detached using 1× trypsin-EDTA (Gibco), washed, and adjusted to 20,000 cells/well in 100 μl of supplemented MEM. RAW 264.7 macrophages were also detached using 1× trypsin-EDTA, washed twice by centrifugation, and adjusted to 20,000 in 100 μl of supplemented DMEM. Then, both types of cells followed the same protocol: cells were added to white-bottom 96-well plates containing 100 μl of serial 2-fold dilutions of the test compounds (0.24 to 250 μM) or 2.5% DMSO as control. After 24 h of culture at 37°C, the number of viable cells was determined by quantification of ATP present in the cells using a CellTiter-Glo luminescent cell viability assay (Promega). The luminescence values were transformed to the percentage of cytotoxicity compared to the controls, which allowed constructing a regression model to calculate the cytotoxic concentration that killed 50% of the cells (CC50) using GraphPad (Prism 5.0). At least three different assays were carried out to determine the in vitro therapeutic index (IVTI) of each molecule per experiment, which was calculated as the ratio between the CC50 obtained in the HepG2 or RAW 264.7 cell line and the corresponding EC50 determined in the ex vivo explant culture or parasitized RAW 264.7 cells.
In vitro metabolic stability.
To estimate the metabolic stability of disulfiram and its analogs, we incorporated the hamster or mouse S9 fraction of liver enzymes (Moltox, Boone, NC) to both the HepG2 cytotoxicity assay and the ex vivo explant culture for antileishmanial efficacy assays as described by Peniche et al. (18). The liver S9 fraction, which contains drug-metabolizing enzymes, including the cytochrome P450, flavin monooxygenases, and UDP glucuronyl transferases, was prepared and kept on ice right before use. The final concentration of this preparation was as follows: 0.4 mg of S9 protein/ml (Moltox), 3.1 mM KCl, 6.3 mM glucose 6-phosphate, and 1 mM NADPH (Sigma) in supplemented DMEM (ex vivo explant culture) or MEM (HepG2 cells). Determination of the IVTI (CC50/EC50) upon incorporation of the S9 fraction to both HepG2 cells and ex vivo explant culture allowed us to estimate the in vitro metabolic stability of compounds.
RESULTS
Antileishmanial activity and metabolic stability of thiuram disulfide analogs.
Six of the nine thiuram disulfide compounds evaluated in the L. major ex vivo system were found to be active at nanomolar concentrations. Five of these compounds (CID 3117 [disulfiram], 7188, 5455, 95876, and 12892) were identified as having an excellent therapeutic window (IVTI > 100) (Table 1). Four of the compounds were active against L. donovani. Three of the six compounds active against L. major were substantially less active against L. donovani. Compound stability is an essential characteristic of therapeutic molecules and has a significant role in guiding which active molecules will be selected to move forward to preclinical trials. To test the metabolic stability of compounds in the ex vivo systems, we added the S9 liver enzyme fraction which contains the drug-metabolizing cytochrome P450 enzymes. The S9 fraction does not affect parasite or cell viability in the absence of drug (data not shown). In the L. major ex vivo system, compounds CID 7188 and 3117 (disulfiram) maintained high activity after exposure to liver enzymes (Table 1), but these compounds showed significantly reduced activity against L. donovani after exposure to the hamster liver enzymes. Disulfiram was the least affected by exposure to the drug-metabolizing enzymes.
TABLE 1.
Antileishmanial efficacy of thiuram disulfide analogsa
| CID with or without S9b | Mean CC50 (μM) ± SE in HepG2 cells |
L. major |
L. donovani |
||
|---|---|---|---|---|---|
| Mean EC50 (μM) ± SE | IVTI | Mean EC50 (μM) ± SE | IVTI | ||
| Without S9 | |||||
| 7188 | 31.14 ± 3.27 | 0.044 ± 0.03 | 701 | 0.023 ± 0.01 | 1,372 |
| 3117* | 38.08 ± 1.35 | 0.058 ± 0.03 | 661 | 0.062 ± 0.01 | 615 |
| 5455 | 8.02 ± 0.08 | 0.035 ± 0.00 | 230 | 2.931 ± 0.04 | 3 |
| 95876 | 139.61 ± 32.69 | 0.636 ± 0.63 | 220 | 17.350 ± 3.24 | 8 |
| 12892 | 3.27 ± 0.42 | 0.030 ± 0.03 | 109 | 0.618 ± 0.00 | 5 |
| 15412 | 56.58 ± 9.23 | 2.879 ± 0.85 | 20 | 18.440 ± 1.29 | 3 |
| 30054 | 3.64 ± 0.81 | 0.278 ± 0.30 | 13 | 0.390 ± 0.10 | 9 |
| 3110809 | 46.43 ± 3.31 | 1.699 ± 0.03 | 27 | 16.85 ± 2.87 | 3 |
| 3097317 | 1.88 ± 0.77 | 1.490 ± 0.09 | 1 | 9.06 ± 1.48 | 0 |
| Miltefosine | 72.08 ± 5.79 | 1.59 ± 0.36 | 45 | 0.995 ± 0.04 | 72 |
| Amphotericin B | 9.94 ± 0.99 | 0.242 ± 0.01 | 41 | 0.076 ± 0.01 | 131 |
| With S9 | |||||
| 7188 | 51.27 ± 3.09 | 0.154 ± 0.04 | 333 | 0.659 ± 0.34 | 78 |
| 3117* | 44.93 ± 5.80 | 0.056 ± 0.00 | 801 | 1.179 ± 0.83 | 38 |
| 5455 | 2.45 ± 0.72 | 0.059 ± 0.00 | 42 | 0.278 ± 0.02 | 9 |
| 95876 | 119.44 ± 4.15 | 2.053 ± 0.26 | 58 | 16.665 ± 4.74 | 7 |
| 12892 | 3.18 ± 0.29 | 0.237 ± 0.02 | 13 | 0.970 ± 0.17 | 3 |
| 15412 | 27.75 ± 6.58 | 4.796 ± 0.57 | 6 | 19.734 ± 0.38 | 1 |
| 30054 | 1.99 ± 1.51 | 0.556 ± 0.43 | 4 | 1.946 ± 0.50 | 1 |
| 3110809 | 102.42 ± 4.45 | 1.704 ± 0.31 | 60 | 16.836 ± 0.96 | 6 |
| 3097317 | 2.87 ± 0.67 | 1.413 ± 0.11 | 2 | 9.588 ± 0.85 | 1 |
| Miltefosine | 74.94 ± 5.01 | 1.638 ± 0.26 | 46 | 1.243 ± 0.43 | 60 |
| Amphotericin B | 16.27 ± 1.49 | 0.501 ± 0.01 | 33 | 0.098 ± 0.01 | 166 |
The data represent means from two or three different experiments performed with or without exposure to S9 (hepatic metabolic enzymes) and using luminescence to calculate the 50% effective concentration (EC50) in L. major and L. donovani ex vivo systems. The 50% cytotoxicity concentration (CC50) was determined using the HepG2 cell line, and the IVTI of each compound was calculated as the ratio between the CC50 obtained in the HepG2 cell line and the corresponding parasite EC50. The EC50 was determined by regression analysis using GraphPad (Prism 5.0) software.
EC50 and CC50 values without the addition of S9 were previously published for thiuram disulfide analogs by the author in supplemental file S1 in a previous publication (18). *, Disulfiram.
Dose-dependent antileishmanial activity and cellular cytotoxicity of disulfiram.
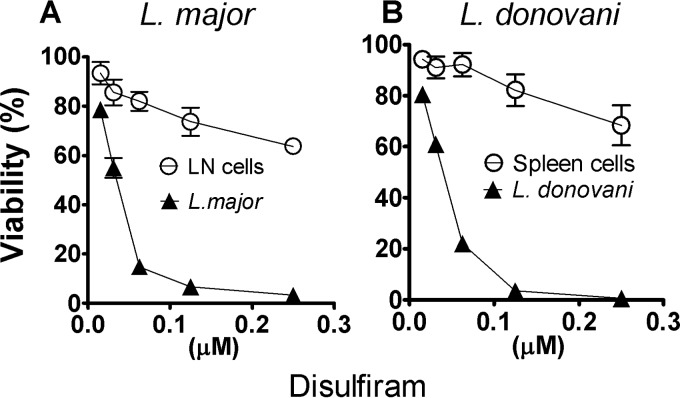
Disulfiram was further studied to determine whether the decrease in parasite burden conferred by thiuram disulfide analogs was indirectly due to host cell toxicity in the ex vivo explant assay. We determined the parasite and host cell dose/survival curves using luminometry to assess parasite load or the trypan blue exclusion test to estimate host cell toxicity. This assay indicated that disulfiram killed intracellular amastigotes of L. major and L. donovani with little toxic effect on host cells, even at doses much higher than the respective antileishmanial EC50 (Fig. 2).
FIG 2.
Viability of host cells and Leishmania exposed to disulfiram in the spleen and lymph node explant culture system. The percent viability of L. major or L. donovani and lymph node and spleen cells was determined in the ex vivo system upon exposure to disulfiram. At 21 days postinfection, lymph node explants from mice infected with L. major or spleen explants from hamsters infected with L. donovani were harvested and then cultured for 48 h. Parasite viability was determined by luminometry, and lymph node or spleen cell viability was assessed by microscopy using trypan blue exclusion. The percentage of parasite survival compared to controls was calculated as follows: (parasite counts in treated cells/parasite counts in untreated cells) × 100. The percentage of viable cells was calculated as follows: (total number of viable cells per ml of aliquot/total number of cells per ml of aliquot) × 100. The results are shown as the mean percentages ± the standard deviations of the mean from a representative experiment.
Intrinsic host cytotoxicity and leishmanicidal activity of divalent metal ions salts.
The toxicity of each divalent metal ion salt toward HepG2 cells was determined by luminescence quantifying ATP. Culture medium alone (untreated control) was used as reference of 100% viability. Using the average luminescence after exposure to each salt, we calculated the percentage of cell survival for the salt relative to the untreated control. Magnesium, lithium, and nickel salts yielded the maximum reductions of HepG2 viability (23 to 31%), followed by zinc, copper, and silver salts at 14 to 19% reduction (Table 2). A smaller decrease in viability was obtained with manganese and silver salts (3 to 7%; Table 2). Although the antileishmanial activity of disulfiram salts paralleled their cellular toxicity (Table 3), the increased IVTI of the salt-disulfiram complex suggests that the ex vivo system is measuring the antileishmanial activity over the cellular toxicity.
TABLE 2.
Cytotoxicity of divalent metal ion saltsa
| Culture medium or salt (1 μM) | Relative viability (%) |
|
|---|---|---|
| Mean ± SE | P | |
| Culture medium | 100 | |
| CuSO4 | 85.7 ± 2.7 | * |
| ZnCl2 | 80.8 ± 2.9 | ** |
| ZnSO4 | 81.0 ± 4.6 | ** |
| AgNO3 | 93.9 ± 2.3 | NS |
| MnCl2 | 97.8 ± 4.7 | NS |
| MgCl2 | 74.1 ± 5.0 | *** |
| MgSO4 | 85.2 ± 3.6 | * |
| NiSO4 | 77.7 ± 1.5 | *** |
| LiCl | 77.1 ± 3.6 | *** |
The HepG2 survival for each divalent metal ion salt was determined using culture medium as a reference of 100% viability; the average luminescence of each salt was used to calculate the percent viability compared to the control. The data represent the means from three different experiments. Statistical differences were calculated using the Mann-Whiney test (GraphPad, Prism 5.0 software). *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
TABLE 3.
Toxicity of divalent metal ion salts against intracellular amastigotes of L. major and L. donovania
| Culture medium or salt (1 μM) | Relative viability (%) |
|||
|---|---|---|---|---|
|
L. major |
L. donovani |
|||
| Mean ± SE | P | Mean ± SE | P | |
| Culture medium | 100 | 100 | ||
| CuSO4 | 79.7 ± 10.4 | NS | 80.0 ± 0.9 | *** |
| ZnCl2 | 83.8 ± 8.7 | NS | 91.0 ± 0.5 | NS |
| ZnSO4 | 84.4 ± 4.1 | NS | 91.8 ± 2.9 | NS |
| AgNO3 | 94.9 ± 4.1 | NS | 91.5 ± 1.7 | NS |
| MnCl2 | 97.4 ± 8.9 | NS | 88.9 ± 2.7 | * |
| MgCl2 | 96.0 ± 2.6 | NS | 88.6 ± 5.4 | * |
| MgSO4 | 94.2 ± 2.1 | NS | 94.0 ± 2.9 | NS |
| NiSO4 | 80.1 ± 5.5 | NS | 81.2 ± 1.8 | *** |
| LiCl | 67.5 ± 3.1 | *** | 70.7 ± 3.5 | *** |
Parasite survival was determined in mouse lymph node explant or ex vivo for L. major or in hamster spleen explant or ex vivo for L. donovani ex vivo systems upon addition of 1 μM divalent metal ion salts to the culture medium. The average parasite luminescent signal of wells exposed to the different salts was compared to the signal of untreated wells, considered as a reference of 100% viability. The data represent the means from three different experiments. Statistical differences were calculated using the Mann-Whitney test (GraphPad, Prism 5.0 software). *, P < 0.05; ***, P < 0.001; NS, not significant.
Divalent metal ion salts enhance the antileishmanial efficacy of disulfiram and thiuram disulfide analog CID 7188.
Disulfiram is known to bind divalent metal ions, and this chelation may enhance the transport of metals across biological membranes (41). We therefore explored the effects of combining thiuram analogs with the divalent metal salts. We selected for these studies the two highest ranked compounds, disulfiram and CID 7188, based on our measured in vitro therapeutic index for both species of Leishmania (Table 1). To determine the cytotoxic concentration of the compounds combined with divalent metal ion salts, we quantified the ATP present in metabolically active HepG2 cells. The addition of divalent metal ion salts increased the cytotoxicity of disulfiram and CID 7188 (CC50). This was greatest with AgNO3 and CuSO4 (CC50 ≤ 10 μM; Tables 4 and 5).
TABLE 4.
Additive leishmanicidal effect of disulfiram combined with divalent metal ion saltsa
| Control or salt (1 μM) | HepG2 or RAW 264.7 cellsb |
L. major |
L. donovani |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 |
IVTI |
EC50 |
IVTI |
|||||||
| Mean CC50 (μM) ± SE | P | Mean concn (μM) ± SE | P | Mean ± SE | P | Mean concn (μM) ± SE | P | Mean ± SE | P | |
| Ex vivo systems | ||||||||||
| Control | 36.0 ± 2.6 | 0.058 ± 0.013 | 665 ± 70 | 0.062 ± 0.007 | 591 ± 35 | |||||
| CuSO4 | 7.9 ± 0.6 | *** | 0.018 ± 0.005 | NS | 483 ± 67 | NS | 0.052 ± 0.006 | NS | 155 ± 9 | NS |
| ZnCl2 | 21.0 ± 1.5 | *** | 0.003 ± 0.0008 | *** | 8,337 ± 1,169 | *** | 0.033 ± 0.004 | NS | 644 ± 38 | NS |
| ZnSO4 | 20.1 ± 1.5 | *** | 0.060 ± 0.016 | NS | 379 ± 53 | NS | 0.019 ± 0.002 | *** | 1,091 ± 65 | *** |
| MgSO4 | 28.6 ± 2.1 | * | 0.010 ± 0.003 | NS | 3,140 ± 440 | *** | 0.045 ± 0.005 | NS | 647 ± 39 | NS |
| MgCl2 | 37.0 ± 2.7 | NS | 0.045 ± 0.012 | NS | 937 ± 131 | NS | 0.058 ± 0.007 | NS | 653 ± 39 | NS |
| AgNO3 | 0.74 ± 0.05 | *** | 0.152 ± 0.042 | NS | 5 ± 1 | NS | 0.076 ± 0.009 | NS | 10 ± 1 | NS |
| MnCl2 | 19.0 ± 1.4 | *** | 0.167 ± 0.046 | NS | 128 ± 18 | NS | 0.166 ± 0.019 | NS | 116 ± 7 | NS |
| NiSO4 | 20.6 ± 1.5 | *** | 0.060 ± 0.016 | NS | 620 ± 87 | NS | 0.060 ± 0.007 | NS | 350 ± 21 | NS |
| LiCl | 29.9 ± 2.2 | NS | 0.054 ± 0.015 | NS | 388 ± 55 | NS | 0.073 ± 0.008 | NS | 417 ± 25 | NS |
| In vitro system | ||||||||||
| Control | 14.1 ± 0.65 | 0.068 ± 0.006 | 209 ± 9 | 0.066 ± 0.008 | 217 ± 13 | |||||
| ZnCl2 | 22.1 ± 1.02 | ** | 0.015 ± 0.002 | *** | 1,540 ± 97 | *** | 0.023 ± 0.016 | * | 1,917 ± 536 | *** |
| ZnSO4 | 22.1 ± 1.02 | ** | 0.052 ± 0.020 | NS | 522 ± 88 | *** | 0.029 ± 0.010 | * | 920 ± 157 | *** |
The data represent mean CC50 and EC50 values from three different experiments, using the HepG2 or RAW 264.7 cells and ex vivo or in vitro mouse macrophage systems for each Leishmania species (see details in Materials and Methods). The CC50 and EC50 were determined by regression analysis using GraphPad software (Prism 5.0). P values reflect comparisons of disulfiram with divalent salts versus disulfiram alone (control). *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
HepG2 cells were used for ex vivo systems, and RAW 264.7 cells were used for the in vitro mouse macrophage system.
TABLE 5.
Additive leishmanicidal effect of CID 7188 combined with divalent metal ion salts in ex vivo systemsa
| Control or salt (1 μM) | HepG2 cells |
L. major |
L. donovani |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 |
IVTI |
EC50 |
IVTI |
|||||||
| Mean CC50 (μM) ± SE | P | Mean concn (μM) ± SE | P | Mean ± SE | P | Mean concn (μM) ± SE | P | Mean ± SE | P | |
| Control | 32.2 ± 2.1 | 0.044 ± 0.017 | 934 ± 184 | 0.023 ± 0.007 | 1,620 ± 231 | |||||
| CuSO4 | 4.5 ± 0.3 | *** | 0.003 ± 0.001 | ** | 2,050 ± 404 | * | 0.012 ± 0.003 | NS | 436 ± 62 | NS |
| ZnCl2 | 23.0 ± 1.5 | *** | 0.014 ± 0.005 | NS | 2,100 ± 413 | * | 0.027 ± 0.008 | NS | 974 ± 139 | NS |
| ZnSO4 | 18.8 ± 1.2 | *** | 0.025 ± 0.010 | NS | 970 ± 191 | NS | 0.024 ± 0.007 | NS | 897 ± 128 | NS |
| MgSO4 | 24.4 ± 1.6 | *** | 0.010 ± 0.004 | NS | 3,242 ± 638 | *** | 0.026 ± 0.008 | NS | 1,086 ± 155 | NS |
| MgCl2 | 24.9 ± 1.6 | ** | 0.021 ± 0.008 | NS | 1,545 ± 304 | NS | 0.021 ± 0.006 | NS | 1,324 ± 189 | NS |
| AgNO3 | 0.58 ± 0.04 | *** | 0.190 ± 0.074 | NS | 4 ± 1 | NS | 0.036 ± 0.011 | NS | 18 ± 3 | NS |
| MnCl2 | 16.2 ± 1.1 | *** | 0.023 ± 0.009 | NS | 897 ± 177 | NS | 0.074 ± 0.022 | NS | 251 ± 36 | NS |
| NiSO4 | 16.0 ± 1.0 | *** | 0.030 ± 0.012 | NS | 675 ± 133 | NS | 0.025 ± 0.007 | NS | 724 ± 103 | NS |
| LiCl | 26.0 ± 1.7 | ** | 0.026 ± 0.010 | NS | 1,291 ± 254 | NS | 0.022 ± 0.007 | NS | 1,343 ± 192 | NS |
Data represent the mean CC50 and EC50 values from three different experiments, using the HepG2 and the ex vivo systems for each Leishmania species, respectively (see details in Materials and Methods). The CC50 and EC50 were determined by regression analysis using GraphPad software (Prism 5.0). Values reflect comparisons of disulfiram with divalent salts versus disulfiram alone (control). *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant.
The addition of ZnCl2 significantly enhanced disulfiram activity (EC50) against L. major and ZnSO4 enhanced activity against L. donovani (Table 4). The addition of ZnCl2 led to a 12-fold improvement of the IVTI against L. major. The large difference between the EC50s of disulfiram-ZnCl2 against L. donovani and L. major was surprising. It may be due to differences in host cell cytotoxicity between the hamster (L. donovani) and mouse (L. major) ex vivo explant screening systems. To address this, we infected mouse macrophages (RAW 264.7 cell line) with L. major or L. donovani and determined the EC50. Again, we found very good enhancement activity of the compounds with ZnCl2 (>3-fold reduction in the EC50 for both Leishmania species). ZnSO4 also modestly improved the EC50. Both ZnCl2 and ZnSO4 significantly improved the CC50 in the mouse macrophage system, which positively impacted the IVTI (7- and 9-fold improvements, respectively) (Table 4). However, the striking difference between the activity of ZnCl2 against L. donovani and L. major in the ex vivo model systems was not evident in the in vitro-infected macrophages (Table 4). Collectively, these data indicated that the combination of disulfiram with zinc salts significantly enhanced the antileishmanial activity in multiple screening models but that the type and origin of the host cell influenced the level of activity.
The addition of magnesium (MgSO4) sulfate to disulfiram showed a modest improvement of the IVTI against L. major (4.7-fold), but not L. donovani (Table 4). On the other hand, the combination of CuSO4, MgCl2, AgNO3, MnCl2, NiSO4, and LiCl either did not change the therapeutic index of disulfiram or reduced it due to the significant increase in cytotoxicity toward HepG2 cells (Table 4). With the exception of AgNO3, the combination of all divalent salts with compound CID 7188 improved the EC50 against L. major (Table 5). Similar to disulfiram, the therapeutic index of compound CID 7188 was improved 1.5- to 3.5-fold against L. major when combined with CuSO4, ZnCl2, and MgSO4. No improvement in activity against L. donovani was observed with any combination of CID 7188 and divalent metal salts (Table 5).
DISCUSSION
Using an ex vivo system derived from skin-draining lymph nodes from BALB/c mice infected with L. major or spleen cells from hamsters infected with L. donovani, we identified four new thiuram disulfide compounds with antileishmanial activity at nanomolar concentrations. Disulfiram and CID 7188 exhibited the best metabolic stability, as judged by retention of antileishmanial efficacy upon exposure to liver enzymes. Thus, these compounds may be suitable for systemic administration. Because of previous reports of enhanced in vitro anti-infective and anticancer activity of disulfiram when coupled with some divalent metal salts (11–16), we tested disulfiram and one of its analogs in combination with additional salts including copper, lithium, manganese, magnesium, nickel, silver, and zinc ex vivo and in vitro at a concentration of 1 μM (28, 41, 42). In our experiments, disulfiram-ZnCl2 was the most effective combination against both Leishmania species. This combination improved the activity (EC50) by 12-fold in the mouse-L. major ex vivo system and 4.6-fold in the mouse macrophage RAW 264.7 cell line. On the other hand, in L. donovani, the improvement was observed only in RAW 264.7 cells (2.9-fold). The quantitative differences found in the mouse and hamster ex vivo tissue explant systems and mouse macrophages demonstrated the influence of the host cell origin on the activity of antileishmanial compounds. This may be due to differences in host cell toxicity or immune effector mechanisms. Our previous work found that hamster macrophages showed decreased expression of inducible nitric oxide synthase and production of the leishmanicidal molecule nitric oxide compared to mouse macrophages (43). Similarly, parasitized hamster macrophages showed some features of a permissive “alternatively activated” phenotype, with a high expression of host arginase 1 (17, 44).
Multiple in vitro studies suggested that disulfiram has a broad antipathogen potential. Upon screening of diverse chemical libraries, we and others reported the activity of disulfiram against intracellular amastigotes of L. donovani (17) and both parasite stages of L. major (18–20). Disulfiram also showed activity against Mycobacterium tuberculosis, Plasmodium falciparum, and Giardia lamblia (11–13). In combination with divalent metals, such as copper, disulfiram had enhanced antimalarial activity, probably by acting as a toxic molecule for the parasite membrane (11).
Divalent salts have been used by both topical and by local injection for treatment of skin conditions (45). Their use in the treatment of cutaneous leishmaniasis has yielded mixed results, supporting the notion that they may be more effective in combination with other therapies. In vitro studies showed that ZnSO4 at 600 μM was 5-fold more active (EC50) against promastigotes and axenic and intracellular amastigotes of L. major and L. tropica than meglumine antimoniate and sodium stibogluconate (24, 25, 46). Limited clinical trials using intralesional 2% ZnSO4 in patients infected with L. major or L. tropica showed encouraging results, with cure rates ranging between 83.3 and 94.7%. However, these trials also showed an unusually low efficacy of pentavalent antimonials (47, 48). More recent studies found that only 10.5 to 33.3% of the patients healed when intralesional 2% ZnSO4 was injected weekly for 6 weeks compared to 61.3 to 80% cure rate with intralesional Glucantime (49, 50). Similar variable results were found upon oral administration of ZnSO4 as monotherapy at concentrations of 10 mg/kg in patients infected with L. major or L. tropica (51, 52). These discrepant treatment outcomes, which ranged from 96.9% down to 30.2%, may be due to differences in parasite species, the sensitivities of the strain populations, and therapeutic regimens. Host factors such as zinc deficiency or dietary factors affecting zinc absorption may also play a role (52).
At present, no clinical trials have been carried out to assess the efficacy of disulfiram as monotherapy or in combination to treat cutaneous or visceral leishmaniasis. Its combination with zinc gluconate could be assessed since the latter salt has the capacity to reach the dermis and exert anti-inflammatory and antibacterial effects against Propionibacterium acnes when given by the oral route (53). The implementation of a clinical trial is feasible since the combination of oral zinc and copper gluconate and disulfiram is U.S. Food and Drug Administration approved to treat alcoholism. Combination therapy is also being explored for patients with glioblastoma, refractory solid tumors in liver, metastatic melanoma, and prostate cancer (clinicaltrials.gov identifiers NCT00742911, NCT01777919, NCT00256230, and NCT01118741, respectively).
Overall, our results demonstrated the possibility that disulfiram or other new thiuram disulfide compounds in combination with divalent metal ion salts could expand the therapeutic alternatives to treat visceral or cutaneous leishmaniasis. Further study of the in vivo pharmacodynamics and antileishmanial efficacy are warranted.
ACKNOWLEDGMENTS
This study was funded by the U.S. Department of Defense, Air Force contracts AF-SGR-8-31-09 (B.L.T.) and FA7014-07-C-0034 (P.C.M.). The funding source had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.McCall LI, Zhang WW, Matlashewski G. 2013. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog 9:e1003053. doi: 10.1371/journal.ppat.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weina PJ, Neafie RC, Wortmann G, Polhemus M, Aronson NE. 2004. Old world leishmaniasis: an emerging infection among deployed US military and civilian workers. Clin Infect Dis 39:1674–1680. doi: 10.1086/425747. [DOI] [PubMed] [Google Scholar]
- 3.von Stebut E. 2007. Cutaneous Leishmania infection: progress in pathogenesis research and experimental therapy. Exp Dermatol 16:340–346. doi: 10.1111/j.1600-0625.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Monge-Maillo B, López-Vélez R. 2013. Therapeutic options for visceral leishmaniasis. Drugs 73:1863–1888. doi: 10.1007/s40265-013-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Singh RT, Sundar S. 2003. Novel mechanism of drug resistance in kala azar field isolates. J Infect Dis 188:600–607. doi: 10.1086/377133. [DOI] [PubMed] [Google Scholar]
- 6.Minodier P, Parola P. 2007. Cutaneous leishmaniasis treatment. Travel Med Infect Dis 5:150–158. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Murray HW. 2010. Treatment of visceral leishmaniasis in 2010: direction from Bihar State, India. Future Microbiol 5:1301–1303. doi: 10.2217/fmb.10.92. [DOI] [PubMed] [Google Scholar]
- 8.González U, Pinart M, Reveiz L, Alvar J. 2008. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev 2008:CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, Gharbi A, Belhadj Hamida N, Boukthir A, Chlif S, Abdelhamid K, El Ahmadi Z, Louzir H, Mokni M, Morizot G, Buffet P, Smith PL, Kopydlowski KM, Kreishman-Deitrick M, Smith KS, Nielsen CJ, Ullman DR, Norwood JA, Thorne GD, McCarthy WF, Adams RC, Rice RM, Tang D, Berman J, Ransom J, Magill AJ, Grogl M. 2013. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med 368:524–532. doi: 10.1056/NEJMoa1202657. [DOI] [PubMed] [Google Scholar]
- 10.Skinner MD, Lahmek P, Pham H, Aubin HJ. 2014. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One 9:e87366. doi: 10.1371/journal.pone.0087366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meshnick SR, Scott MD, Lubin B, Ranz A, Eaton JW. 1990. Antimalarial activity of diethyldithiocarbamate. Potentiation by copper. Biochem Pharmacol 40:213–216. [DOI] [PubMed] [Google Scholar]
- 12.Horita Y, Takii T, Yagi T, Ogawa K, Fujiwara N, Inagaki E, Kremer L, Sato Y, Kuroishi R, Lee Y, Makino T, Mizukami H, Hasegawa T, Yamamoto R, Onozaki K. 2012. Antitubercular activity of disulfiram, an anti-alcoholism drug, against multidrug- and extensively drug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 56:4140–4145. doi: 10.1128/AAC.06445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash T, Rice WG. 1998. Efficacies of zinc-finger-active drugs against Giardia lamblia. Antimicrob Agents Chemother 42:1488–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, McLeod HL, Cassidy J. 2003. Disulfiram-mediated inhibition of NF-κB activity enhances cytotoxicity of 5-fluorouracil in human colorectal cancer cell lines. Int J Cancer 104:504–511. doi: 10.1002/ijc.10972. [DOI] [PubMed] [Google Scholar]
- 15.Wickström M, Danielsson K, Rickardson L, Gullbo J, Nygren P, Isaksson A, Larsson R, Lövborg H. 2007. Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients. Biochem Pharmacol 73:25–33. doi: 10.1016/j.bcp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen CH, Pedersen B, Tønnesen H. 2011. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res 35:1749–1758. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 17.Osorio Y, Travi BL, Renslo AR, Peniche AG, Melby PC. 2011. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. PLoS Negl Trop Dis 5:e962. doi: 10.1371/journal.pntd.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peniche AG, Osorio Y, Renslo AR, Frantz DE, Melby PC, Travi BL. 2014. Development of an ex vivo lymph node explant model for identification of novel molecules active against Leishmania major. Antimicrob Agents Chemother 58:78–87. doi: 10.1128/AAC.00887-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharlow ER, Close D, Shun T, Leimgruber S, Reed R, Mustata G, Wipf P, Johnson J, O'Neil M, Grögl M, Magill AJ, Lazo JS. 2009. Identification of potent chemotypes targeting Leishmania major using a high-throughput, low-stringency, computationally enhanced, small molecule screen. PLoS Negl Trop Dis 3:e540. doi: 10.1371/journal.pntd.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavali AK, Blazier AS, Tlaxca JL, Jensen PA, Pearson RD, Papin JA. 2012. Metabolic network analysis predicts efficacy of FDA-approved drugs targeting the causative agent of a neglected tropical disease. BMC Syst Biol 6:27. doi: 10.1186/1752-0509-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyva JA, Bianchet MA, Amzel LM. 2003. Understanding ATP synthesis: structure and mechanism of the F1-ATPase. Mol Membr Biol 20:27–33. doi: 10.1080/0968768031000066532. [DOI] [PubMed] [Google Scholar]
- 22.Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, Jaisankar P, Majumder HK. 2008. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol 74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- 23.Haase H, Overbeck S, Rink L. 2008. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol 43:394–408. doi: 10.1016/j.exger.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mulla Hummadi YM, Najim RA, Al-Bashir NM. 2005. The mechanism behind the antileishmanial effect of zinc sulfate. I. An in-vitro study. Ann Trop Med Parasitol 99:27–36. doi: 10.1179/136485905X19900. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mulla Hummadi YM, Al-Bashir NM, Najim RA. 2005. The mechanism behind the antileishmanial effect of zinc sulfate. II. Effects on the enzymes of the parasites. Ann Trop Med Parasitol 99:131–139. [DOI] [PubMed] [Google Scholar]
- 26.Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. 1997. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol 272:E1002–E1007. [DOI] [PubMed] [Google Scholar]
- 27.Van Weyenbergh J, Santana G, D'Oliveira A, Santos AF, Costa CH, Carvalho EM, Barral A, Barral-Netto M. 2004. Zinc/copper imbalance reflects immune dysfunction in human leishmaniasis: an ex vivo and in vitro study. BMC Infect Dis 4:50. doi: 10.1186/1471-2334-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. 2011. Disulfiram modulated ROS-MAPK and NF-κB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer 104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu B, Shi P, Fombon IS, Zhang Y, Huang F, Wang W, Zhou S. 2011. Disulfiram/copper complex activated JNK/c-Jun pathway and sensitized cytotoxicity of doxorubicin in doxorubicin resistant leukemia HL60 cells. Blood Cells Mol Dis 47:264–269. doi: 10.1016/j.bcmd.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Brar SS, Grigg C, Wilson KS, Holder WD, Dreau D, Austin C, Foster M, Ghio AJ, Whorton AR, Stowell GW, Whittall LB, Whittle RR, White DP, Kennedy TP. 2004. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther 3:1049–1060. [PubMed] [Google Scholar]
- 31.Wadhwa S, Mumper RJ. 2013. d-Penicillamine and other low molecular weight thiols: review of anticancer effects and related mechanisms. Cancer Lett 337:8–21. doi: 10.1016/j.canlet.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Fruehauf JP, Meyskens FL. 2007. Reactive oxygen species: a breath of life or death? Clin Cancer Res 13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 33.López-Lázaro M. 2007. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett 252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. 2011. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med 51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Murray HW, Oca MJ, Granger AM, Schreiber RD. 1989. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Invest 83:1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray HW, Delph-Etienne S. 2000. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun 68:288–293. doi: 10.1128/IAI.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabors GS, Farrell JP. 1996. Successful chemotherapy in experimental leishmaniasis is influenced by the polarity of the T cell response before treatment. J Infect Dis 173:979–986. doi: 10.1093/infdis/173.4.979. [DOI] [PubMed] [Google Scholar]
- 38.Roy G, Dumas C, Sereno D, Wu Y, Singh AK, Tremblay MJ, Ouellette M, Olivier M, Papadopoulou B. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol 110:195–206. doi: 10.1016/S0166-6851(00)00270-X. [DOI] [PubMed] [Google Scholar]
- 39.Schoonen WG, Westerink WM, de Roos JA, Débiton E. 2005. Cytotoxic effects of 100 reference compounds on HepG2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol In Vitro 19:505–516. [DOI] [PubMed] [Google Scholar]
- 40.Gerets HH, Hanon E, Cornet M, Dhalluin S, Depelchin O, Canning M, Atienzar FA. 2009. Selection of cytotoxicity markers for the screening of new chemical entities in a pharmaceutical context: a preliminary study using a multiplexing approach. Toxicol In Vitro 23:319–332. doi: 10.1016/j.tiv.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Cen D, Brayton D, Shahandeh B, Meyskens FL, Farmer PJ. 2004. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem 47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 42.Morrison BW, Doudican NA, Patel KR, Orlow SJ. 2010. Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res 20:11–20. doi: 10.1097/CMR.0b013e328334131d. [DOI] [PubMed] [Google Scholar]
- 43.Saldarriaga OA, Travi BL, Choudhury GG, Melby PC. 2012. Identification of hamster inducible nitric oxide synthase (iNOS) promoter sequences that influence basal and inducible iNOS expression. J Leukoc Biol 92:205–218. doi: 10.1189/jlb.1010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osorio EY, Zhao W, Espitia C, Saldarriaga O, Hawel L, Byus CV, Travi BL, Melby PC. 2012. Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase 1 expression. PLoS Pathog 8:e1002417. doi: 10.1371/journal.ppat.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spann CT, Taylor SC, Weinberg JM. 2004. Topical antimicrobial agents in dermatology. Dis Mon 50:407–421. doi: 10.1016/j.disamonth.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Najim RA, Sharquie KE, Farjou IB. 1998. Zinc sulphate in the treatment of cutaneous leishmaniasis: an in vitro and animal study. Mem Inst Oswaldo Cruz 93:831–837. doi: 10.1590/S0074-02761998000600025. [DOI] [PubMed] [Google Scholar]
- 47.Sharquie KE, Najim RA, Farjou IB. 1997. A comparative controlled trial of intralesionally administered zinc sulphate, hypertonic sodium chloride and pentavalent antimony compound against acute cutaneous leishmaniasis. Clin Exp Dermatol 22:169–173. doi: 10.1046/j.1365-2230.1997.2510668.x. [DOI] [PubMed] [Google Scholar]
- 48.Iraji F, Vali A, Asilian A, Shahtalebi MA, Momeni AZ. 2004. Comparison of intralesionally injected zinc sulfate with meglumine antimoniate in the treatment of acute cutaneous leishmaniasis. Dermatology 209:46–49. doi: 10.1159/000078586. [DOI] [PubMed] [Google Scholar]
- 49.Firooz A, Khatami A, Khamesipour A, Nassiri-Kashani M, Behnia F, Nilforoushzadeh M, Pazoki-Toroudi H, Dowlati Y. 2005. Intralesional injection of 2% zinc sulfate solution in the treatment of acute old world cutaneous leishmaniasis: a randomized, double-blind, controlled clinical trial. J Drugs Dermatol 4:73–79. [PubMed] [Google Scholar]
- 50.Maleki M, Karimi G, Tafaghodi M, Raftari S, Nahidi Y. 2012. Comparison of intralesional two percent zinc sulfate and glucantime injection in treatment of acute cutaneous leishmaniasis. Indian J Dermatol 57:118–122. doi: 10.4103/0019-5154.94279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharquie KE, Najim RA, Farjou IB, Al-Timimi DJ. 2001. Oral zinc sulphate in the treatment of acute cutaneous leishmaniasis. Clin Exp Dermatol 26:21–26. doi: 10.1046/j.1365-2230.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 52.Yazdanpanah MJ, Ghayour-Mobarhan M, Taji A, Javidi Z, Pezeshkpoor F, Tavallaie S, Momenzadeh A, Esmaili H, Shojaie-Noori S, Khoddami M, Sahebkar A. 2011. Serum zinc and copper status in Iranian patients with pemphigus vulgaris. Int J Dermatol 50:1343–1346. doi: 10.1111/j.1365-4632.2011.04968.x. [DOI] [PubMed] [Google Scholar]
- 53.Dréno B, Nocera T, Verrière F, Vienne MP, Ségard C, Vitse S, Carré C. 2005. Topical retinaldehyde with glycolic acid: study of tolerance and acceptability in association with anti-acne treatments in 1,709 patients. Dermatology 210(Suppl 1):S22–S29. [DOI] [PubMed] [Google Scholar]