Abstract
We report on the in vitro effects of the bumped kinase inhibitor 1294 (BKI-1294) in cultures of virulent Neospora caninum isolates Nc-Liverpool (Nc-Liv) and Nc-Spain7 and in two strains of Toxoplasma gondii (RH and ME49), all grown in human foreskin fibroblasts. In these parasites, BKI-1294 acted with 50% inhibitory concentrations (IC50s) ranging from 20 nM (T. gondii RH) to 360 nM (N. caninum Nc-Liv), and exposure of intracellular stages to 1294 led to the nondisjunction of newly formed tachyzoites, resulting in the formation of multinucleated complexes similar to complexes previously observed in BKI-1294-treated N. caninum beta-galactosidase-expressing parasites. However, such complexes were not seen in a transgenic T. gondii strain that expressed CDPK1 harboring a mutation (G to M) in the gatekeeper residue. In T. gondii ME49 and N. caninum Nc-Liv, exposure of cultures to BKI-1294 resulted in the elevated expression of mRNA coding for the bradyzoite marker BAG1. Unlike in bradyzoites, SAG1 expression was not repressed. Immunofluorescence also showed that these multinucleated complexes expressed SAG1 and BAG1 and the monoclonal antibody CC2, which binds to a yet unidentified bradyzoite antigen, also exhibited increased labeling. In a pregnant mouse model, BKI-1294 efficiently inhibited vertical transmission in BALB/c mice experimentally infected with one of the two virulent isolates Nc-Liv or Nc-Spain7, demonstrating proof of concept that this compound protected offspring from vertical transmission and disease. The observed deregulated antigen expression effect may enhance the immune response during BKI-1294 therapy and will be the subject of future studies.
INTRODUCTION
Neospora caninum is a cyst-forming apicomplexan parasite that is closely related to Toxoplasma gondii but exhibits distinct differences in transmission patterns, virulence, host specificity, immunogenetic aspects, and the pathology it induces. T. gondii causes toxoplasmosis in humans and many domestic and wildlife animals, with great economic impact especially in sheep but also in many other animal species (1). Human toxoplasmosis causes serious pathology in immune-suppressed individuals. In addition, if a seronegative mother acquires primary infection during pregnancy, human toxoplasmosis can lead to abortion, microcephalus and hydrocephalus, and other fetal abnormalities causing intellectual disability (2). N. caninum is a veterinary health problem and represents one of the most important infectious causes of bovine abortion, stillbirth, and the birth of weak calves, with an economic impact of over $1.3 billion (3–5). In addition, N. caninum causes neuromuscular disease in dogs, and neosporosis has also been detected in a wide range of other species of livestock and wild animals worldwide.
Despite their differences, an important common feature of these parasites is their ability to invade and replicate within a wide range of cell types and tissues, where they reside in an intracellular parasitophorous vacuole, surrounded by a parasitophorous vacuole membrane. Repeated cycles of invasion, proliferation, and egress of the disease-causing tachyzoites are responsible for inducing the pathological effects that occur during the acute stage of infection. In turn, chronic infection is characterized by the formation of intracellular tissue cysts in brain and muscular tissues that harbor slowly proliferating bradyzoites. Calcium-dependent protein kinases (CDPKs), encoded by apicoplast-associated genes and, thus, only found in apicomplexan parasites and plants, represent excellent drug targets in several apicomplexans such as Plasmodium falciparum (6), Cryptosporidium parvum (7, 8) where novel drug targets are of crucial interest (9), N. caninum (10), Eimeria tenella, and Babesia bovis (11). Excellent correlations between cell activity and CDPK1 inhibition were achieved by compounds from a focused bumped kinase inhibitor (BKI) library. In many apicomplexan CDPK1 enzymes, including T. gondii and N. caninum, the ATP binding pocket is characterized by a glycine as the smallest possible gatekeeper residue. The T. gondii strain CDPK1_G128M, overexpressing a CDPK1 version with a mutation (G to M) in the ATP binding pocket, was found to be much less sensitive to BKIs (12).
We have previously demonstrated the outstanding efficacy of BKI-1294 against transgenic beta-galactosidase-expressing N. caninum tachyzoites (Nc-betaGal) in vitro and in a nonpregnant mouse model for cerebral infection (10). In addition, we have shown that for the BKI-1294-inhibited egress of Nc-betaGal tachyzoites in vitro, once located intracellularly, parasites underwent nuclear division but cytokinesis was not achieved, which resulted in the formation of large multinucleated complexes that remained trapped within the host cell and eventually died (10). However, it is currently unclear whether this incomplete cytokinesis was caused specifically by CDPK1 inhibition or whether another target may be potentially involved (13).
In this study, we compared in vitro effects of BKI-1294 in tachyzoite cultures of the virulent N. caninum isolates Nc-Liverpool (Nc-Liv) and Nc-Spain7 and in two strains of T. gondii (RH and ME49). In order to investigate the direct role of CDPKI, we have included the T. gondii strain overexpressing the gatekeeper mutation G128M and a control strain overexpressing the wild-type CDPKI in our study. We show that BKI-1294 does not only interfere in tachyzoite invasion but also causes incomplete cytokinesis resulting in the formation of multinucleated complexes in the two species but not in the transgenic T. gondii strain expressing CDPK1 harboring a mutation (G to M) in the gatekeeper residue. Moreover, we present data on antigen expression in these multinucleate complexes. We also show that BKI-1294 inhibits vertical transmission of Nc-Liv and Nc-Spain7 in a pregnant mouse model for N. caninum infection. Our data provide a proof of concept for the treatment of N. caninum infection and the protection of offspring by BKI-1294 and related compounds.
MATERIALS AND METHODS
Tissue culture media, biochemicals, and drugs.
If not stated otherwise, all tissue culture media were purchased from Gibco-BRL (Zürich, Switzerland), and biochemical reagents were from Sigma (St. Louis, MO). Kits for molecular biology were purchased from Qiagen (Hilden, Germany). BKI-1294 was obtained from the Center for Emerging and Reemerging Infectious Diseases (CERID), Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington (Seattle, WA, USA). For in vitro studies, the compound was stored as a 20 mM stock solution in dimethyl sulfoxide (DMSO) at −20°C; for application in mice, BKI-1294 powder was suspended either in 0.5% carboxymethyl cellulose (CMC) in water or in corn oil, and the suspensions were applied by gavage.
Host cell cultivation and parasite cultures.
Human foreskin fibroblasts (HFF) were maintained in Dulbecco's modified Eagle medium (DMEM), and Vero cells were cultured in RPMI 1640 medium, each with phenol red supplemented with 10% heat-inactivated and sterile filtered fetal calf serum (FCS), 50 U of penicillin/ml, and 50 μg streptomycin/ml (culture medium). Cultures were maintained at 37°C and 5% CO2 in tissue culture flasks (Sarstedt, Sevelen, Switzerland) and were passaged at least once a week.
Tachyzoites of N. caninum isolates Nc-Liverpool (Nc-Liv) and Nc-Spain7, T. gondii strains ME49 and RH, the T. gondii strain CDPK1_G128M overexpressing a CDPK1 with the gatekeeper mutation G128M, and an isogenic strain, T. gondii CDPK_wt, expressing the wild-type CDPK1 (12) were all maintained by serial passages in either Vero cells or HFF in their respective media (14, 15). Tachyzoites were harvested by removing infected cell layers with a rubber cell scraper followed by repeated passages through a 25-gauge needle at 4°C. For in vitro studies, tachyzoites were separated from cell debris on a Sephadex G-25 column (16). For in vivo experiments, purification over Sephadex G-25 columns was omitted.
Determination of 50% inhibitory concentrations.
HFF were grown in 6-well plates for 24 h until a confluent monolayer was formed. Just prior to infection, the BKI-1294 was added at a final concentration of 2.5 μM for initial experiments and ranged between 0.5 nM and 2.5 μM for 50% effective concentration (EC50) determinations (the effective concentration to reduce proliferation by 50%). Controls received the corresponding amounts of DMSO. HFF were then infected with 5 × 104 freshly purified tachyzoites of N. caninum Nc-Liv, Nc-Spain7, T. gondii ME49, T. gondii CDPK1_G128M, or T. gondii CDPK1_wt in 5 ml medium. After 4 days, cells were collected with a cell scraper, centrifuged, washed once more in phosphate-buffered saline (PBS), and the pellet was stored at −20°C prior to quantification of parasite proliferation. DNA purification was performed employing the DNeasy blood and tissue kit (Qiagen, Basel, Switzerland) according to the standard protocol suitable for animal cells. N. caninum parasite load was determined by real-time PCR as previously described (15). Proliferation of T. gondii ME49, T. gondii CDPK1_G128M, and T. gondii CDPK1_wt tachyzoites was quantified as described previously (17). The parasite counts were calculated by interpolation from a standard curve with DNA equivalents from 1,000, 100, and 10 culture-derived tachyzoites included in each run.
Transmission electron microscopy.
HFF were grown to confluence in T25 flasks and were infected with T. gondii ME49, T. gondii RH, T. gondii CDPK1_G128M, T. gondii CDPK1_wt, or N. caninum Nc-Liv tachyzoites. After 24 h, cultures were supplemented with 2.5 μM BKI-1294; infected cultures treated with DMSO served as controls. After 2, 4, and 6 days of treatment, the monolayers were washed with 100 mM sodium cacodylate buffer (pH 7.3) and fixed with cacodylate buffer containing 2.5% glutaraldehyde for 10 min (18). Cells were collected using a rubber cell scraper and centrifuged for 10 min at 1,200 rpm and room temperature. The supernatant was removed, and infected cells were fixed further in glutaraldehyde-cacodylate at 4°C overnight. Postfixation in 2% OsO4, dehydration, embedding in Epon 820 epoxy resin, and cutting of ultrathin sections was done as previously described (18, 19). Specimens were viewed on a Phillips 400 transmission electron microscope (TEM) operating at 80 kV.
Measurement of BAG1 and SAG1 RNA expression during drug treatment by reverse transcriptase real-time PCR.
Confluent HFF monolayers grown in 6-well plates were infected with 2.5 × 104 N. caninum or T. gondii tachyzoites. Treatments with 5 μM BKI-1294 or DMSO alone were initiated 2 days postinfection (p.i.). After different time points, medium supernatant was removed, the monolayers were washed once in PBS, and the adherent cells were carefully removed with a rubber cell scraper. The suspension was transferred to a tube, and after centrifugation at 600 × g for 10 min at 4°C, the supernatant was removed and the pellet resuspended in 350 μl RLT buffer of the RNeasy minikit (Qiagen, Hilden, Germany) and stored at −20°C until further use. RNA extraction was carried out according to the instructions provided by the manufacturers. Synthesis of cDNA for quantitative reverse transcriptase PCR (RT-PCR) was performed with 2 μg of RNA using the Qiagen Omniscript kit with random primers according to the manufacturer's instructions. Quantitative PCR was performed with 10 μl of cDNA (diluted 1:50 in water) using the QuantiTect SYBR green PCR kit (Qiagen) in 20 μl standard reaction mixtures containing 0.5 μM forward and reverse primers (MWG Biotech, Ebersberg, Germany) as listed in Table 1. Furthermore, control PCRs with RNA equivalents from samples that had not been reverse transcribed into cDNA (data not shown) confirmed that no DNA was amplified from any residual genomic DNA that might have resisted DNase I digestion (see above). PCR was started by initiating the hot-start Taq DNA-polymerase reaction at 95°C (10 min). Subsequent DNA amplification was performed in 40 cycles, including denaturation (94°C for 15 s), annealing (58°C for 15 s), and extension (72°C for 30 s); temperature transition rates in all cycle steps were 20°C/s. Fluorescence was measured at 79°C on a Corbett cycler (Corbett Research, Mortlake, Australia). From the quantitative RT-PCR, mean values (MV) (±standard error [SE]) from four replicates were assessed, and expression levels were given as values in arbitrary units relative to the amount of Gra rRNA.
TABLE 1.
Overview of primers used in this study
| Primer | Gene product (accession no.a) | Sequence |
|---|---|---|
| NcSAG1-F | N. caninum SAG1 (FR823385; region 1666638–1673366) | GGGTCGTAGGCTTGATGG |
| NcSAG1-R | GCAGCAGCGGCAAGGGG | |
| TgSAG1-F | T. gondii SAG1 (TGME49_264660) | CTTCAGGACGTCGTTCCC |
| TgSAG1-R | CACCTTTGCAGGCTTGCC | |
| NcBAG1_F | N. caninum BAG1 (NCLIV_chrVIIb; region 2868187–2869925) | CTCGACTTCATGGATGAGG |
| NcBAG1_R | CTTCTATGGTAACGTCATCC | |
| TgBAG1-F | T. gondii BAG1 (TGME49_259020) | CTAGACTATTTGGAT |
| TgBAG1-R | CTGTCGAACTCCACG | |
| NcGra2_F | N. caninum GRA2 (AF196293.1) | GCCATGCGTGTATCGCCC |
| NcGra2_R | TTCGCAGTAAACTGCTTGAG | |
| TgGra2_F | T. gondii GRA2 (TGME49_227620) | CCAGAAGAACCGGTTTCCC |
| TgGra2_R | AACGGTCACCATGCCCCT |
The accession numbers for T. gondii SAG1, BAG1, and GRA2 are from ToxoDB (http://www.toxodb.org), and the accession number for N. caninum BAG1 is from GeneDB (http://www.genedb.org).
Immunofluorescence staining.
HFF monolayers grown in 24-well plates on poly-l-lysine coated coverslips were infected with 2 × 104 N. caninum Nc-Liv or T. gondii ME49 tachyzoites. After initial growth for 24 h, specimens were exposed to 2.5 μM BKI-1294 at periods of 2, 4, or 6 days. They were then washed once in PBS and fixed and processed for immunofluorescence as previously described (18). The following antibodies (Abs) were used: (i) a whole N. caninum extract rabbit antiserum (16), diluted 1:2,500; (ii) mAbCC2, a rat monoclonal Ab reacting with a yet uncharacterized cyst wall antigen (20), diluted 1:300; (iii) α-BAG1, a polyclonal rabbit Ab directed against BAG1, a classical bradyzoite-specific antigen (21–23), diluted 1:300; (iv) α-SAG1, a mouse monoclonal Ab directed against the major immunodominant tachyzoite surface antigen (24), diluted 1:300; and (v) a whole T. gondii extract rabbit antiserum, diluted 1:2,000. Primary antibodies were applied for 45 min at room temperature in PBS with 0.3% bovine serum albumin (BSA). After three washes in PBS for 5 min each, the corresponding secondary antibody conjugates (anti-mouse fluorescein isothiocyanate [FITC] or anti-rabbit Texas Red [Sigma-Aldrich]) were applied at a dilution of 1:300 in PBS with 0.3% BSA. After three washes in PBS for 5 min each, specimens were incubated in 4′,6-diamidino-2-phenylindole (diluted 1:300 in PBS) and then embedded in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). All specimens were viewed on a Nikon Eclipse E800 digital confocal fluorescence microscope. Processing of images was performed using the Openlab 5.5.2 software (Improvision, PerkinElmer, Waltham MA, USA).
Animal experimentation.
All protocols involving animals were approved by the Animal Welfare Committee of the Canton of Bern under the license BE115/14. All animals used in this study were handled in strict accordance with practices made to minimize suffering.
Assessment of potential effects of the BKI-1294 on pregnancy outcome in noninfected BALB/c mice.
Animal procedures were approved by the Animal Welfare Committee of the Canton of Bern (approval no. BE115/14). Forty female and 20 male BALB/c mice, 8 weeks of age, were purchased from a commercial breeder (Charles River, Sulzberg, Germany) and were maintained in a common room under controlled temperature and a 14-h dark and 10-h light cycle according to the guidelines set up by the animal welfare legislation of the Swiss Veterinary Office (approval no. BE 105/14). Female mice were oestrus-synchronized by the Whitten effect and were mated for three nights by housing 1 male with 2 females (25). At day 9 of pregnancy, the pregnant females were randomly distributed into 2 experimental groups of 10 females each. One group was treated with the BKI-1294 (50 mg/kg of body weight per day emulsified in 100 μl 0.5% CMC in water) for a period of 8 days by gavage once a day, and the other group was treated identically with CMC but without drug. Mice were separated into individual cages at day 18 of pregnancy, and they gave birth on days 20 to 22. Dams and offspring were maintained for at least 1 month after birth and were closely monitored to rule out any adverse effects due to drug treatment.
Assessment of the efficacy of treatment with the BKI-1294 in mice infected with the N. caninum Nc-Liv isolate.
Thirty female and 15 male BALB/c mice, 8 weeks of age, were maintained as described above. Pregnancy was achieved after synchronization of oestrus followed by mating (1 male housed with 2 females) for 3 nights. They were all infected by intraperitoneal (i.p.) inoculation of 2 × 106 N. caninum Nc-Liv tachyzoites on day 7 postmating. Following infection and for the duration of the entire experiment, mice were inspected twice daily for clinical signs (ruffled coat, apathy, and hind limb paralysis) and were categorized according to a score sheet approved by the local authorities. Two days after infection, 12 pregnant and 12 nonpregnant mice were randomly allocated into experimental groups of 6 (2 pregnant and 2 nonpregnant groups). One pregnant and one nonpregnant group were given the BKI-1294 (50 mg/kg/day emulsified in 100 μl 0.5% CMC in water) for a period of 8 days by oral gavage once a day, while the other two control groups received CMC in water. Pregnant mice were placed into individual cages on day 18 of pregnancy, and they all gave birth on days 20 to 22. Dams were euthanized in a CO2 chamber and offspring decapitated immediately after birth. The nonpregnant mice were euthanized in a CO2 chamber at 15 days p.i. Brains and sera were sampled and stored at −20°C for subsequent analyses.
Assessment of the efficacy of treatment with the BKI-1294 in nonpregnant and pregnant BALB/c mice experimentally infected with the N. caninum Nc-Spain7 isolate.
Sixty female and 30 male BALB/c mice were housed, and pregnancy was achieved after synchronization of oestrus as described above. Subsequently, female mice were randomly distributed in 3 groups of 16 mice each. Mice from groups 1 and 2 were subcutaneously challenged with 105 tachyzoites of the Nc-Spain7 isolate at midgestation (days 7 after mating), while mice from group 3 were left unchallenged and received a culture medium inoculation (26). Two days after infection, all mice from group 1 were given the BKI-1294 (50 mg/kg/day emulsified in 100 μl corn oil) for a period of 6 days by gavage once a day, while the group 2 mice received corn oil alone. Prior to gavage, the corn oil-drug mixture was heated to 37°C to enhance solubility of the drug. Group 3 remained untouched. Pregnancy was confirmed between days 15 and 18 of gestation, and pregnant mice were then allocated into single cages to give birth on days 20 to 22 and to rear their pups for an additional 30 days. During this time, nonpregnant mice were maintained in cages of 3 to 5. Nonpregnant mice, dams, and their offspring were evaluated for clinical signs of disease twice a day from the day of birth to day 30 postpartum (p.p.) according to the scoring scheme as above. Data on the number of female mice that became pregnant, litter size (number of delivered pups per dam), early pup mortality (number of full-term dead pups from birth until day 2 p.p.), postnatal mortality (number of dead pups from day 3 to 30 p.p.), and clinical signs in dams and nonpregnant mice were recorded. Dams were weighed at days 15 and 30 p.p., and neonates were weighed every second day from day 15 p.p. onwards until the end of the experiment (day 30 p.p.). Nonpregnant mice were weighed once a week. Surviving dams, nonpregnant mice, and pups were euthanized in a CO2 chamber latest at 30 days p.p. (approximately day 44 p.i.). Blood from dams and nonpregnant mice was recovered by cardiac puncture, and serum samples were obtained to assess humoral immune responses.
Analysis of biological samples from in vivo experiments.
To quantify the parasite load in brains, DNA purification was performed employing the DNeasy blood and tissue kit (Qiagen, Basel, Switzerland) according to the standard protocol suitable for animal tissues. The DNA concentrations in all samples were determined using the QuantiFluor dsDNA system (Promega, Madison, WI, USA) according to the manufacturer's instructions and adjusted to 5 ng/μl with sterile DNase-free water. Quantification of parasite loads in the brains of nonpregnant mice and dams was done as described previously (10, 27). Seropositivity for N. caninum was assessed as described previously (28, 29).
Statistics.
Fifty percent inhibitory concentrations (IC50s) were calculated after the logit log-transformation of the relative growth (RG; control = 1) according to the formula ln(RG/[1 − RG]) = a × ln(drug concentration) + b and subsequent regression analysis by the corresponding software tool contained in the Excel software package (Microsoft, Seattle, WA, USA). Statistical analysis of the parasite burdens in brains was done using the Kruskal-Wallis test. Comparison of positive and negative animals was performed using the chi-square test. All analyses were performed using the software package R (30).
RESULTS
N. caninum and T. gondii infections display similar sensitivities to treatment with BKI-1294 in vitro.
Different concentrations of BKI-1294 were added to in vitro cultures of N. caninum Nc-Liv and Nc-Spain7, two wild-type T. gondii strains (RH and ME49), and the two transgenic T. gondii strains (CDPK1_wt and CDPK1_G128M). The drug was added to HFF monolayers just prior to inoculation of tachyzoites. This resulted in IC50s ranging from 20 nM (T. gondii RH) up to 360 nM (N. caninum Nc-Liv) (Table 2). The T. gondii strain CDPK1_G128M overexpressing a plasmid-encoded CDPK1 enzyme with a mutated (G to M) gatekeeper residue at position 128 was much less susceptible compared to the T. gondii RH and ME49 wild-type strains. The corresponding transgenic wild-type cell line, expressing an ectopic copy of nonmutated CDPK1, also exhibited a slightly higher IC50 than the wild-type strains in accordance to previous findings (12).
TABLE 2.
IC50 of BKI-1294 in N. caninum and T. gondii strainsa
| Strain | IC50 (nM) |
|---|---|
| N. caninum Nc-Liv | 360 ± 50 |
| N. caninum Nc-Spain7 | 270 ± 20 |
| T. gondii RH | 20 ± 10 |
| T. gondii ME49 | 220 ± 60 |
| T. gondii RH CDPK1-wt | 480 ± 100 |
| T. gondii RH CDPK1-G128 M | 2,260 ± 400 |
HFF monolayers were treated with different concentrations of BKI-1294 or DMSO as a solvent control, infected with 5 × 104 tachyzoites, and incubated for 3 days. Cells were harvested, parasite load was determined, and IC50s were calculated as described.
Treatments of N. caninum and T. gondii infections with BKI-1294 interferes with the separation of daughter zoites and leads to the formation of multinucleated complexes.
The effects of continued BKI-1294 treatments in already infected HFF monolayers were visualized under a light microscope and by TEM. T. gondii ME49 and RH tachyzoites were allowed to invade HFF monolayers for 2 h prior to addition of 2.5 μM BKI-1294 or the corresponding amount of DMSO. Inspection by light microscopy showed that in treated and untreated cultures, large parasitophorous vacuoles were formed, indicating that parasite replication took place. However, the interior of the vacuoles in BKI-1294-treated cultures exhibited clear alterations compared to parasitophorous vacuoles from cultures maintained in the absence of the drug (see Fig. 1A through D). In untreated HFF, large vacuoles containing numerous tachyzoites were present after 2 to 3 days (Fig. 1A and C) followed by egress of tachyzoites from day 3 onwards. In BKI-1294-treated cultures, parasitophorous vacuoles had also increased in size, but egress of tachyzoites was blocked, and the interior of the vacuoles was not filled with individual parasites but larger structures of unknown nature (Fig. 1B and D). Similar findings were obtained in cultures infected with N. caninum Nc-Liv and Nc-Spain7 (data not shown). These observations were in line with earlier findings on transgenic Nc-betaGal treated with BKI-1294. Thus, TEM was used for further visualization of these interior complexes.
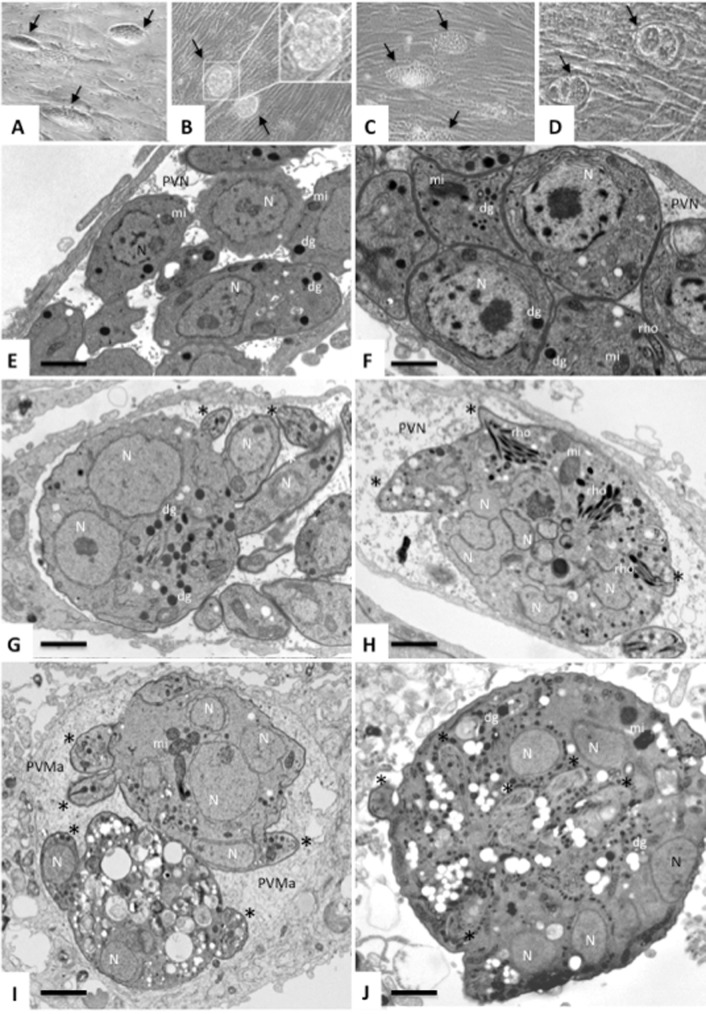
FIG 1.
Effects of BKI-1294 treatment on intracellular T. gondii and N. caninum tachyzoites. (A to D) Phase contrast images of T. gondii ME49 (A, B) and RH (C, D) cultured in HFF in the absence (A, C) or presence (B, D) of BKI-1294. Arrows point toward parasitophorous vacuoles. (E, F) Electron micrographs of T. gondii ME49 and RH tachyzoites, respectively, cultured in HFF in the absence of the drug for 3 days. Tachyzoites exhibit mostly single nuclei (N), and rhoptries (rho), dense granules (dg), and mitochondria (mi) are visible. The interior of the parasitophorous vacuole is filled with a parasitophorous vacuole tubular network (PVN). (G, H, and I) Transition of T. gondii ME49 tachyzoites into multinucleated complexes upon exposure to BKI-1294 for 2 (G), 4 (H), and 6 (I) days. Emerging apical complexes are marked with an asterisk (*), and multiple nuclei are visible (N), as well as rhoptries (rho) and dense granules (dg). Note that at 6 days of BKI-1294 treatment, the matrix of the parasitophorous vacuole becomes more granular (PVMa). (J) Intracellular N. caninum Nc-Liv complex formed after 6 days of BKI-1294 treatment. (E) Bar = 1 μm; (F) bar = 0.8 μm; (G) bar = 1.2 μm; (H) bar = 1.8 μm; (I) bar = 2.5 μm; (J) bar = 2.1 μm.
Figures 1E and F show nontreated T. gondii ME49 and RH tachyzoites cultured in HFF in the absence of drug at day 3 p.i. Tachyzoites proliferate intracellularly within a parasitophorous vacuole, surrounded by a parasitophorous vacuole membrane, and mostly individual tachyzoites are seen. Upon treatment with BKI-1294, the first signs of an inhibition of separation of newly formed tachyzoites in the two T. gondii strains were already observed on day 2 of treatment, as seen for T. gondii ME49 in Fig. 1G, and complexes increased in size and number of nuclei on day 4 (Fig. 1H). Figure 1I shows a vacuole containing two complexes of T. gondii ME49 treated with BKI-1294 for 6 days, with the two complexes embedded in a granular vacuolar matrix. One of these complexes already exhibited clear signs of structural impairment such as extensive intracellular vacuolization, while the other one still had more intact features, with a higher number of detectable nuclei and morphologically intact mitochondria and apical conoids being formed and protruding from its body. Figure 1J shows a multinucleated complex formed by N. caninum Nc-Liv tachyzoites treated with BKI-1294 for 6 days. Similar features were observed when T. gondii RH tachyzoites were treated with BKI-1294 for 6 days (Fig. 2A and B). In contrast, BKI-1294 treatment of the T. gondii cell line CDPK1_G128M overexpressing a mutated gatekeeper residue with a transition from G to M at position 128 did not lead to the multinucleated complex phenotype as observed for the other cell lines (Fig. 2D). These tachyzoites underwent proliferation within a parasitophorous vacuole and separated in a normal manner, in contrast to their respective wild type, T. gondii CDPK_wt overexpressing the wild-type CDPK1 (Fig. 2C). However, T. gondii CDPK1_G128M exhibited a slightly altered phenotype with increased intracytoplasmic vacuole formation (Fig. 2D).
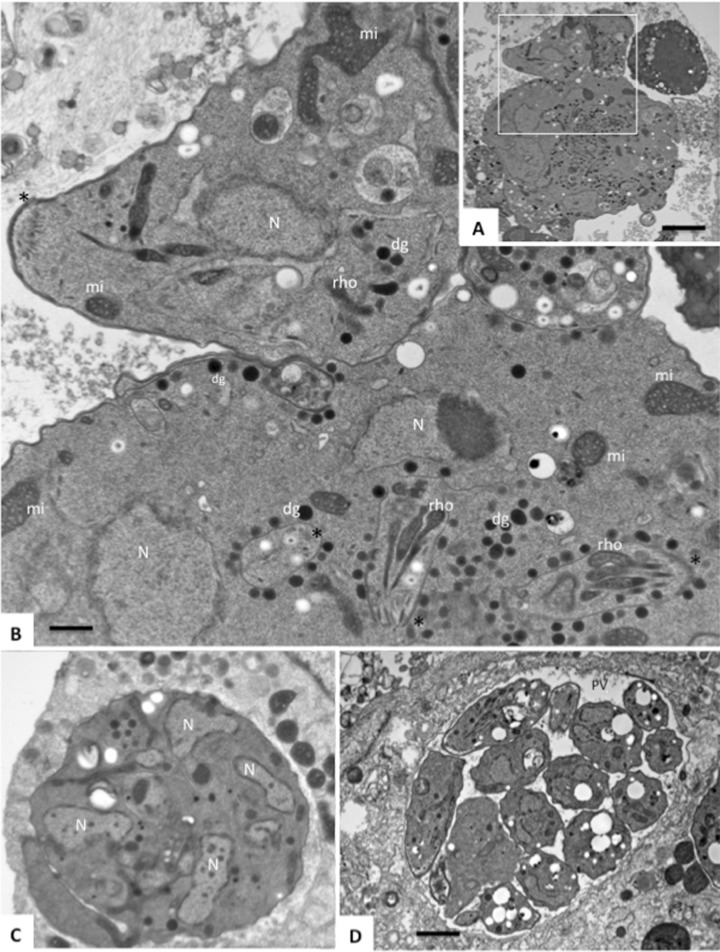
FIG 2.
Complex formation of wild-type T. gondii RH and CDPK1_wt but not T. gondii CDPK1_G128M upon exposure to BKI-1294. (A) Low-magnification view of a large multinucleated complex formed by T. gondii RH; (B) the boxed area is shown enlarged. (C) T. gondii CDPK1_wt treated with BKI-1294. (D) No complexes are formed upon BKI-1294 treatment of T. gondii CDPK1_G128M. N, nucleus; mi, mitochondria; rho, rhoptry; dg, dense granules. (A) Bar = 2.5 μm; (B) bar = 0.6 μm; (C) bar = 2 μm; (D) bar = 1 μm.
BKI-1294 treatment results in deregulated antigen expression in N. caninum and T. gondii.
In order to investigate whether bradyzoite-specific gene expression was induced by BKI-1294, HFF monolayers were infected with Nc-Liv or T. gondii ME49, cultured for 2 days and treated with BKI-1294 or with DMSO as a solvent control. During the next 4 days, the infected cell layers were harvested, and mRNA levels of the bradyzoite-specific antigen BAG1 and the tachyzoite-specific antigen SAG1 were determined in relation to Gra2 mRNA, which is expressed in bradyzoites and tachyzoites. After 2 and 4 days, BAG1 levels were strongly increased in cells infected with N. caninum Nc-Liv or T. gondii ME49 treated with BKI-1294. SAG1 levels remained constant in Nc-Liv and were even increased in T. gondii ME49 upon BKI-1294 treatment (Table 3). Conversely, BAG1 expression was reduced in T. gondii CDPK1_G128M compared to its isogenic strain overexpressing wild-type CDPK1 (Fig. 3).
TABLE 3.
BAG1 and SAG1 gene expression during treatment with BKI-1294a
| Transcript | Relative mRNA level |
|
|---|---|---|
| N. caninum Liverpool | T. gondii ME49 | |
| BAG1 | ||
| Day 2 | 13 ± 4 | 729 ± 220 |
| Day 4 | 7 ± 1 | 413 ± 77 |
| SAG1 | ||
| Day 2 | 1.4 ± 0.4 | 17 ± 1 |
| Day 4 | 1.3 ± 0.2 | 31 ± 2 |
Confluent HFF monolayers grown in 6-well plates were infected with 2.5 × 104 Nc-Liv or TgME49 tachyzoites. Treatment with 5 μM BKI-1294 or DMSO (control) was initiated 2 days postinfection. After different time points, the adherent cells were harvested for RNA extraction. mRNA levels of BAG1, SAG1, and Gra2 were quantified by real-time RT-PCR. Mean values (±SE) from four replicates were assessed, and expression levels of BAG1 (bradyzoite specific) or SAG1 (tachyzoite specific) relative to the amount of GRA2 (overall) mRNA were given as multiples of the corresponding DMSO control values (MV ± SE for quadruplicates).
FIG 3.

Quantification of BAG1 and SAG1 gene expression during treatment with BKI-1294. Confluent HFF monolayers grown in 6-well plates were infected with 2.5 × 104 tachyzoites of T. gondii RH overexpressing CDPK1_G128M or wild-type CDPK1. Treatments with 5 μM BKI-1294 or DMSO (control) were initiated 2 days postinfection. After different time points, the adherent cells were harvested for RNA extraction. mRNA levels of BAG1, SAG1, and GRA2 were quantified by real-time RT-PCR. Mean values (±SE) from four replicates were assessed, and expression levels of BAG1 (bradyzoite specific) or SAG1 (tachyzoite specific) relative to the amount of GRA2 (overall) mRNA were given as multiples of the corresponding DMSO control values (MV ± SE for quadruplicates).
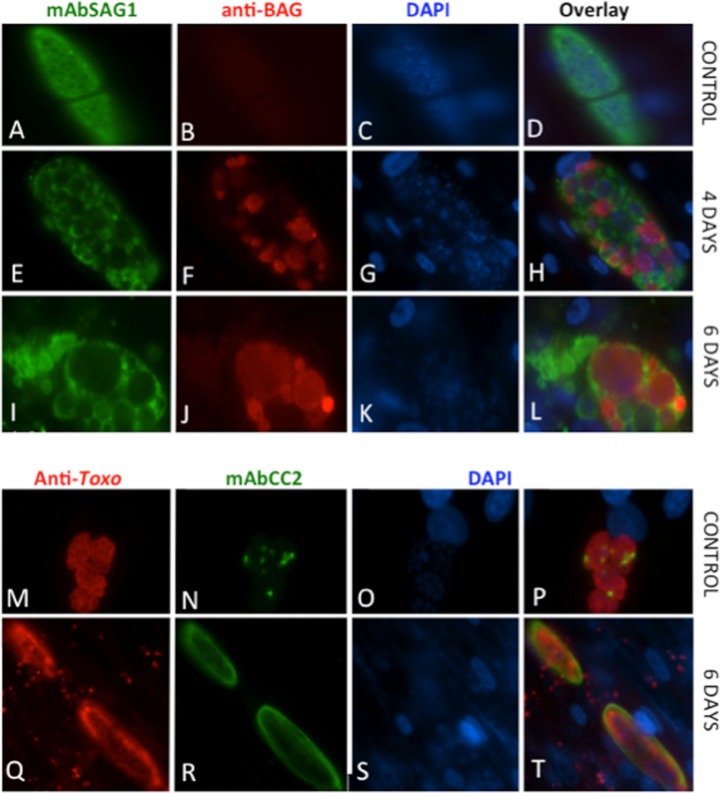
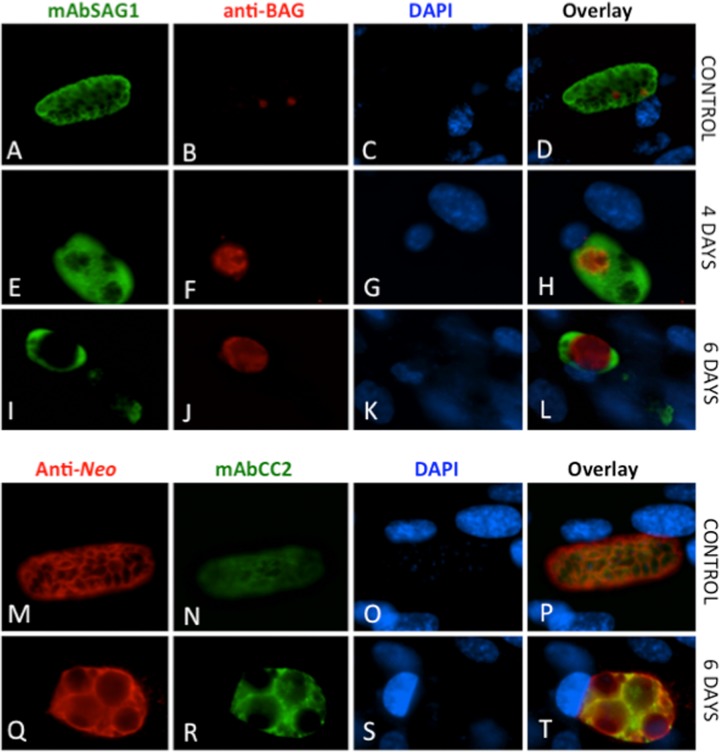
Moreover, the expression of tachyzoite-specific antigen SAG1 and the bradyzoite marker BAG1 in BKI-1294-treated and untreated T. gondii ME49 and N. caninum Nc-Liv was investigated by immunofluorescence (Fig. 4 and 5). In addition, a second bradyzoite marker recognized by the mAbCC2 (18, 31) was also studied. Toxoplasma (Fig. 4A through D and M through P) and Neospora (Fig. 5A through D and M through P) control cultures maintained in the absence of the drug are shown at 3 days postinfection, as they were normally lysed completely after 4 to 5 days. In these controls, SAG1 expression was always evident on tachyzoites localized in large parasitophorous vacuoles, while staining with antibodies against BAG1 and the mAbCC2 were either sparse or nonexistent, indicating that in nontreated cultures the protein expression levels of these antigens were low. In contrast, BKI-1294 treatment led to increased BAG1 protein expression and mAbCC2 labeling in T. gondii (Fig. 4E through L and Q through T) and N. caninum (Fig. 5E through L and Q through T). Most notably, BAG1 (Fig. 4A through L and Fig. 5A through L) was detected already after 4 days of BKI-1294 treatment, and staining intensity increased consistently with time. Within these multinucleated complexes, SAG1 and BAG1 were distributed unevenly, and in many instances, staining of BAG1 antigen occurred in regions with lower SAG1 expression. MAbCC2 labeling was comparatively assessed to staining of complexes with a polyclonal antiserum raised against crude T. gondii (Fig. 4M through T) and N. caninum antigens (Fig. 5M through T), and staining intensity also increased with time.
FIG 4.
BKI-1294 treatment of T. gondii ME49 cultured in HFF induces BAG1 protein expression and increased staining with mAbCC2. Controls (A to D and M to P) were fixed and processed after 3 days, and BAG1 labeling is shown after 4 and 6 days of BKI-1294 treatment. Staining with mAbCC2 is shown after 6 days of treatment (E to L and Q to T). Note the increased staining of the two bradyzoite markers in BKI-treated cells.
FIG 5.
BKI-1294 treatment of N. caninum Nc-Liv cultured in HFF induces BAG1 protein expression and increased staining with mAbCC2. Controls (A to D and M to P) were fixed and processed after 3 days, and BAG1 labeling is shown after 4 and 6 days of BKI-1294 treatment. Staining with mAbCC2 is shown after 6 days of treatment. Note the increased staining of the two bradyzoite markers.
Treatment of pregnant mice with BKI-1294 has no side effects and protects pups from vertical transmission of N. caninum.
In order to investigate whether BKI-1294 affected pregnancy and the number of offspring, pregnant mice were treated with BKI-1294 (50 mg/kg/day) during 8 days or with the vehicle (CMC, 0.5%) alone. The numbers of offspring in the different treatment groups were virtually identical. Fifty-four offspring were born in the BKI-1294 treatment group, and 46 pups were born in the control group (with 2 stillborn pups in each group). No negative impact on the further development of offspring mice was detected during at least 1 month after birth (data not shown). Thus, BKI-1294 treatment did not affect pregnancy outcome.
These findings prompted us to proceed to experimental treatments of infected mice with BKI-1294. In a first experiment, nonpregnant mice and pregnant mice were infected at the same time (7 days postmating) by intraperitoneal injection of 2 × 106 N. caninum Nc-Liv tachyzoites. Two days later, BKI-1294 treatment was initiated by oral gavage (50 mg/kg/day for 8 days) formulated in 0.5% CMC, while the control groups received CMC only. None of the mice died during the 2-week period following infection, but 4 out of 6 dams in the control group exhibited mild symptoms such as ruffled coat and moderate weight loss, while in all other groups, no clinical signs were detected. After the dams gave birth, all mice including offspring were euthanized, and the effects of the treatment were evaluated. Thirty-nine pups were born in the control group, and 27 pups were born in the BKI-1294-treated group (the difference was not statistically significant). All infected mice were seropositive for N. caninum. All control mice (nonpregnant and dams) were tested PCR-positive in the brain, whereas only one nonpregnant female and one dam were positive in the BKI-1294-treated groups. Of the 27 pups born in the BKI-1294-treated group, all brains were real-time PCR-negative, while in the 39 pups born in the control group, 21 tested positive. These differences were highly significant (Table 4).
TABLE 4.
Effects of BKI-1294 treatments on cerebral N. caninum Nc-Liv infection in nonpregnant mice, dams, and pupsa
| Parameter | BKI-1294 | Placebo control |
|---|---|---|
| Nonpregnant females (no./total no.) | ||
| Total no. | 6 | 6 |
| Seropositive | 6/6 | 6/6 |
| N. caninum positive | 1/6b | 6/6 |
| Dams and pups (no./total no.) | ||
| Total no. of dams | 6 | 6 |
| Seropositive dams | 6/6 | 6/6 |
| N. caninum-positive dams | 1/6b | 6/6 |
| Total no. of pups | 27 | 39 |
| N. caninum-positive pups | 0/27c | 21/39 |
| Stillborn pups | 6/27d | 8/39 |
Mice were treated with BKI-1294 in CMC or with CMC alone as a placebo control, infected with Nc-Liv, and sacrificed as described in Materials and Methods. Adults and pups were tested for the presence of N. caninum in their brains with a specific real-time PCR. Respective numbers of animals in control and 1294-treated groups were compared by chi-square tests.
P < 0.005.
P < 0.001.
P > 0.1.
In order to assess the reproducibility of these findings using another virulent N. caninum isolate, another mode of infection, and another formulation of the compound, a second experiment was performed according to the recently standardized infection model established by Arranz-Solís et al. (26). Mice were mated as described above and infected 7 days postmating by subcutaneous injection of 105 N. caninum Nc-Spain7 tachyzoites. Two days later, BKI-1294 treatment was initiated by oral gavage (50 mg/kg/day formulated in corn oil for a period of 6 days). Pregnant and nonpregnant mice were separated and analyzed separately as described. All infected mice were seropositive for N. caninum. None of the nonpregnant mice exhibited clinical signs of neosporosis during the 3 weeks following infection. After the dams gave birth, offspring and dams were maintained for 30 days in order to investigate how the treatments had an impact in the long term during the postnatal phase in dams and offspring. Whereas 5 of 8 dams of the placebo control group showed clinical signs and one died before the end of the experiment, only 2 of 10 mice from the treated group showed mild clinical signs and none of them died before the end of the experiment (Table 5). Cerebral parasite loads in nonpregnant mice and dams as quantified by PCR were significantly lower in the treated group, demonstrating that BKI-1294 treatment has essentially prevented cerebral infection with N. caninum Nc-Spain7 (Fig. 6A).
TABLE 5.
Effects of BKI-1294 treatments on clinical signs, mortality, fertility, and cerebral N. caninum Nc-Spain7 infection in nonpregnant mice, dams, and pupsa
| Parameter | BKI-1294 | Placebo control |
|---|---|---|
| Nonpregnant females (no./total no.) | ||
| Total no. | 6 | 8 |
| Clinical signs | 0/6 | 0/8 |
| Mortality | 0/6 | 0/8 |
| Seropositive | 6/6 | 8/8 |
| N. caninum positive | 1/6b | 8/8 |
| Dams and pups (no./total no.) | ||
| Total no. of dams | 10 | 8 |
| Clinical signs | 2/10c | 5/8 |
| Mortality | 0/10d | 1/8 |
| Seropositive dams | 10/10 | 8/8 |
| N. caninum-positive dams | 3/10b | 8/8 |
| Pregnancy rate | 10/16 | 8/16 |
| Total no. of pups | 58 | 43 |
| Litter size avg (no. of pups/no. of dams) | 58/10d | 43/8 |
| Neonatal mortalitye | 2/58d | 2/43 |
| Postnatal mortalityf | 4/56g | 40/41 |
| N. caninum-positive pups | 9/58g | 43/43 |
Mice were treated with BKI-1294 in corn oil or with corn oil alone as a placebo control, infected with Nc-Spain7, and sacrified as described in Materials and Methods. Adults and surviving pups were tested for the presence of N. caninum in their brains with a specific real-time PCR. Pups that had died before the end of the experiment were considered N. caninum positive. Respective numbers of animals in control and 1294-treated groups were compared by chi-square tests.
P < 0.005.
P < 0.1.
P > 0.1.
Proportion of pups born dead or that died within the 2 first days postpartum.
Proportion of pups dead from days 3 to 30 postpartum.
P < 0.001.
FIG 6.
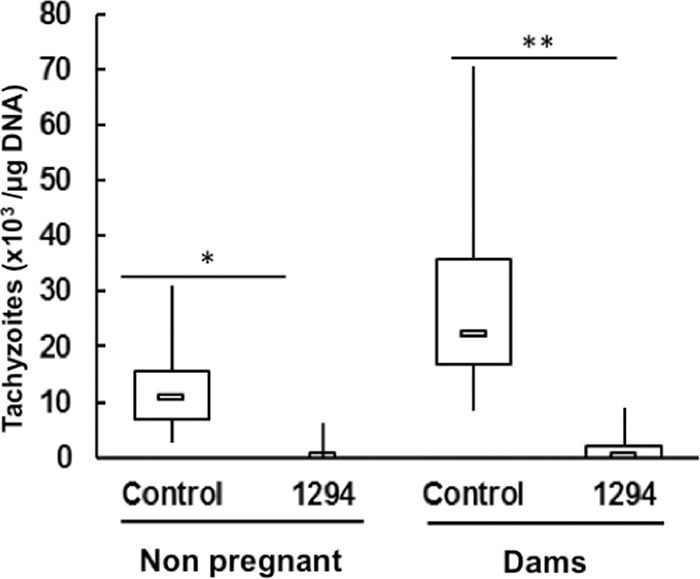
Mice treated with BKI-1294 have a lower cerebral parasite load. BALB/c mice were infected with Nc-Spain7 tachyzoites and subsequently treated with BKI-1294 in corn oil (1294) or corn oil only (control) as detailed in Materials and Methods. Nonpregnant mice were euthanized 3 weeks p.i., and dams were euthanized 30 days postpartum. After euthanasia, brains were collected and the amount of tachyzoites was determined by quantitative PCR (*, P < 0.005; **, P < 0.001; Kruskal-Wallis test).
After 30 days p.p., all surviving mice including offspring were euthanized, and the effects of BKI-1294 exposure were evaluated with regard to cerebral infection in surviving dams and nonpregnant mice and pups. The number of pups per dam was not significantly affected by the treatment. In the two groups, two pups died immediately after birth. Later, 40 of the remaining 41 pups died in the control group but only 4 of 56 in the treated group (see Fig. 6B). Based on previous results (26), the dead pups were considered PCR-positive for N. caninum. After 30 days, all survivors were tested by PCR for the presence of N. caninum in their brains. The last survivor from the control group was brain positive, as well as 5 of 52 surviving pups from the treated dams. Thus, mortality and transmission of N. caninum to pups was strongly reduced by BKI-1294 treatment of the dams (Fig. 7). The differences were highly significant (Table 5).
FIG 7.
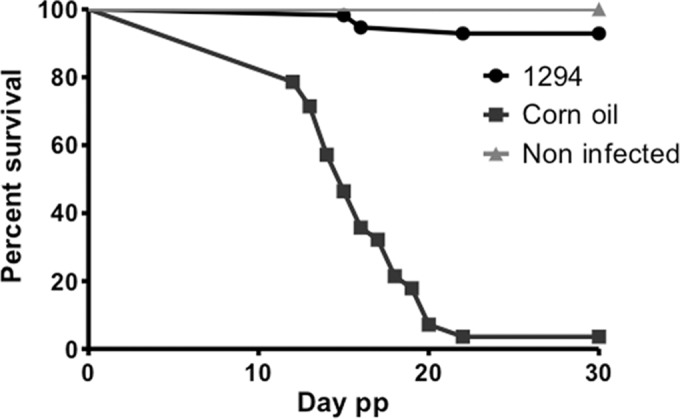
Offspring from BKI-1294-treated mice exhibit a similar survival rate as mice from uninfected and nontreated mice. Survival curves of pups during 30 days postpartum (pp) of BKI-1294-treated (1294) and placebo-treated (corn oil only) groups, each of which was infected with Nc-Spain7 isolates of N. caninum. A group that was not infected and remained untreated with any compound (noninfected) exhibited 100% survival.
DISCUSSION
In apicomplexan parasites, calcium-mediated signaling plays a major role in a variety of crucial activities, including events that ensure progression in the life cycle such as secretion, gliding motility, and entry into host cells (32), as well as intracellular proliferation, cytokinesis, and stage differentiation (33). Calcium also activates CDPKs, which are found in plants, ciliates, and in apicomplexan parasites but not in mammalian cells (34). They are thus promising targets for chemotherapeutical intervention. In T. gondii, it was shown that CDPK1, a member of the serine/threonine kinase family, regulates the calcium-dependent pathway of microneme secretion and governs cell attachment (35), migration, and microneme secretion (36). The ATP-binding site of T. gondii CDPK1, but also of other related apicomplexans, such as N. caninum and Cryptosporidium parvum, is characterized by a small glycine gatekeeper residue (10, 37), while most mammalian kinases have larger residues such as methionine or phenylalanine at that position. Selective and potent inhibitors have been generated that take advantage of the enlarged space in the CDPK1 ATP-binding site (37–39). Benzoyl benzimidazole-based selective inhibitors and BKIs based on a pyrazolopyrimidine scaffold can block T. gondii CDPK1 and affect host cell invasion (40–42).
Here, we focus on the pyrazolopyrimidine compound BKI-1294. BKI-1294 was reported earlier to inhibit host cell invasion of transgenic T. gondii RH and N. caninum tachyzoites expressing beta-galactosidase, with IC50s of 137 and 32 nM, respectively, if the compound was added to the cultures at the time point the parasites were allowed to invade their host cell (10, 39). We show here that BKI-1294 also exhibited in vitro activity against the virulent N. caninum isolates Nc-Liv and Nc-Spain7 (IC50s = 360 and 270 nM, respectively) and similarly against the cyst-forming T. gondii ME49 strain, while T. gondii RH tachyzoites were much more susceptible (IC50 = 20 nM). The value we obtained for T. gondii RH inhibition (20 nM) differs substantially from those reported previously (39). However, it is important to note that in this study parasite loads were quantified by real-time PCR while in earlier reports transgenic parasites expressing beta-galactosidase were used, and some degree of variation may be attributed to different culture conditions, host cells, and slight variations in protocols for parasite isolation prior to infection of HFF. In addition, strains and isolates that have been maintained in culture for extended periods of time may exhibit various drug susceptibilities. However, in accordance with an earlier report (12), the sensitivity to BKI-1294 was entirely lost in T. gondii CDPK1_G128M, which overexpresses a mutated TgCDPK1. Crystallographic confirmation of the interaction between BKI-1294 and NcCDPK1 showed that binding was entirely consistent with that previously determined for the TgCDPK1 homologue, which is 96% identical and differs in the active site by only one residue (10). This clearly indicates that CDPK1 represents the main target for BKI-1294 in T. gondii and N. caninum.
However, BKIs might also affect other targets. We have recently shown that exposure of intracellular Nc-betaGal tachyzoites to BKI-1294 did not only inhibit egress but led to the formation of large multinucleated complexes within 5 to 9 days of culture, which can be visualized by TEM (10). Here, we demonstrate that similar multinucleated complexes occurred upon exposure of T. gondii ME49 and T. gondii RH tachyzoites if treatment with BKI-1294 was initiated after host cell invasion has already taken place. These complexes contained several nuclei and protrusions carrying features of the apical complex such as conoid, dense granules, rhoptries, and micronemes. Thus, DNA replication and daughter nucleus formation took place; however, cytokinesis of the daughter cells was not completed. Many complexes were still clearly viable after 6 days of in vitro treatment, while others showed signs of deterioration. However, BKI-1294 treatment of the resistant T. gondii cell line CDPK1_G128M did not lead to the multinucleated complex phenotype seen in the wild-type cell lines, suggesting that the observed effect may have some association with CDPK1 inhibition. The various metabolic pathways for which TgCDPK1 and NcCDPK1 are associated have not been fully defined. We have however shown that TgCDPK1 was highly localized in the cytoplasm but also found in the nucleus (12). Nuclear localization suggests that the TgCDPK1 may be responsible for phosphorylating nuclear proteins, potentially relaying a shift in transcription associated with invasion and/or other yet to be defined functions associated with cytokinesis of the daughter cells. We also show that intracellular Neospora and Toxoplasma exposed to BKI-1294 treatment exhibited markedly elevated BAG1 transcript levels and increased labeling by antibodies directed against BAG1 and by the mAbCC2, another marker for bradyzoite stage differentiation (18).
Abnormally enlarged parasites and upregulation of bradyzoite-specific gene expression upon protein kinase inhibitor treatments have been reported by others as well (43, 44). Moreover, BKI analogs 1NM-PP1, 3MB-PP1, and 3BrB-PP1 increased BAG1 gene promoter activity (45). Protocols for in vitro induction of tachyzoite-to-bradyzoite stage conversion such as high pH and CO2 depletion also cause defects in cytokinesis and asynchronous cell division (46). However, rather than postulating that BKI-1294 treatment causes bradyzoite stage conversion, we hypothesize that exposure to BKI-1294 leads to deregulated gene expression, which does not necessarily interfere in DNA replication but severely hampers cytokinesis and thus the separation of newly formed daughter zoites.
A secondary BKI target that is actually involved in cytokinesis has been defined (47), showing that mutations in the T. gondii mitogen-activated protein kinase-like 1 (TgMAPKL-1; TGME49_312570) resulted in a 3.5-fold increased resistance of these parasites against the BKI 1NM-PP1. Treatment of T. gondii tachyzoites with the BKI 1NM-PP1 also led to enlarged, multinucleated parasites that were severely impaired in coordinated cell cycle progression. Furthermore, a single mutation in the gatekeeper residue of this kinase restored the inhibitory effect of the BKI 1NM-PP1 (13). Comparison of the genomes of N. caninum and T. gondii reveals the presence of a TgMAPKL-1 orthologue in N. caninum (NcMAPK-3; NCLIV_056080), with a highly conserved kinase domain of 80% identity. However, whether the BKI-1294 also inhibits TgMAPKL-1 and NcMAPK-3 activities is not known to date. In the N. caninum genome, other enzymes with small gatekeeper residues were identified that BKI-1294 may potentially inhibit, including NcCamK (NCLIV_046430) with an alanine gatekeeper, an NcAGC-like kinase (NCLIV_016060) with a serine gatekeeper, and an NcROP-like kinase (NCLIV_030990) with a threonine gatekeeper (10). Engagement of multiple parasite targets may be a potential advantage, especially since BKI-1294 was shown to be parasite specific and exhibits very low toxicity in mammalian cells and no off-target inhibition of small gatekeeper (threonine) human protein kinases (39, 48).
To provide a proof of concept that the BKI-1294 can prevent Neospora-related abortion in cattle, we investigated the effects of BKI-1294 treatments in a pregnant mouse model. The first in vivo study showed that oral application of BKI-1294 at a dosage of 50 mg/kg/day for a period of 8 days in pregnant, but noninfected, mice had no negative impact, neither on litter size, neonatal mortality, nor subsequent development of the offspring during 1 month postpartum. This is in line with earlier studies demonstrating that mice receiving 100 mg/kg BKI-1294 twice daily for 5 days did not show any signs of toxicity or weight loss, and no alterations in tissue histology, metabolic enzymes, and complete blood counts (48). Subsequently, the two in vivo studies in N. caninum Nc-Liv- and Nc-Spain7-infected mice showed that BKI-1294 prevented vertical transmission of N. caninum from dams to pups without impairing vitality and fertility of the dams. Due to the close phylogenetic relationship between N. caninum and T. gondii and the similarities of the effects in the two parasites exerted by BKI-1294 treatments, the current results in the pregnant model for Neospora infection should motivate testing the drug in a pregnant T. gondii mouse model to extend these results to that parasite.
Although BKI-1294 was surprisingly efficacious, the outcome of these experiments is not a surprise as such. The in vivo efficacy of this compound against other apicomplexan parasites in mice has been previously reported, such as activity against cryptosporidiosis (8) and experimental toxoplasmosis inflicted by the virulent T. gondii RH strain (49). The BKI 1NM-PP1 also inhibited T. gondii RH strain infection in mice, although only when given in high doses by i.p. injection prior to infection via the intraperitoneal route (50). A similar BKI series was assessed in vivo against the cyst-forming type II strain T. gondii Pru, and these compounds prolonged survival and decreased the numbers of brain cysts at 30 days p.i. (38). However, in this study, compound administration was started 1 day prior to infection and lasted for a period of 10 days. In contrast, in our experiments the BKI-1294 was administered 48 h after infection, hence, when a robust infection and dissemination of the parasite in the mice has already taken place. This clearly indicates that BKI-1294 probably exerts effects in vivo that go far beyond its expected impact on host cell invasion as the primary target, and this may be well in line with our in vitro observations. Thus, those effects seen in vitro may also affect parasite viability in vivo since BKI-1294 achieved therapeutic concentrations in mice that mimic those of our in vitro treatment experiments; the mean serum concentration after administration of 40 mg/kg of body weight for 6 consecutive days in mice resulted in a serum concentration of 6.3 ± 1.8 μM, and the therapeutic concentrations in the brain are about 30% of the serum concentration (49). Pharmacokinetic studies showed that hepatic metabolism of BKI-1294 becomes saturated with repeated administration and increased dosage (48). The fact that these therapeutic concentrations in vivo are sufficiently high to potentially induce the formation of rather long-lived multinucleated complexes in an infected animal may have important implications for the formation of a sustainable immunological response. For instance, the Nc-Spain7-infected dams were maintained for a period of 38 days following BKI-1294 treatment, and still only minimal cerebral parasite loads were noted. Such an immune response, directed against antigens expressed by parasites blocked in the process of cytokinesis but still viable, may be effective for extended periods of time after the end of dosing, and may well contribute to the excellent efficacy of BKI-1294. This hypothesis of an enhanced antigen expression causing a strong immune response during BKI-1294 therapy and leading to better outcomes in immunocompetent animals will be the subject of future studies.
In conclusion, the broad-spectrum anti-apicomplexan compound BKI-1294 exhibits excellent potency and specificity and does not cause toxicity in pregnant mice. Its impact on CDPK1 and host cell invasion is undisputed, but potentially other targets related to the completion of cytokinesis may also be affected. Intracellular parasites exposed to BKI-1294 exhibit a deregulated gene expression and build up multinucleated and rather long-lived complexes. Further investigations will focus on the expression profile of these complexes and their role in the immune response in BKI-1294-treated animals.
ACKNOWLEDGMENTS
This study was financially supported by a combined NIH/USDA grant (Dual use therapeutics for cryptosporidiosis, toxoplasmosis and neosporosis; no. 1 R01 HD080670_01) and the Swiss National Science Foundation (no. 310030_146162).
We thank Diana Williams (University of Liverpool) for the gift of the Nc-Liv isolate, Milton Mcallister (University of Adelaide) for providing a polyclonal antiserum against recombinant TgBAG1, Camilla Björkman (University of Uppsala) for monoclonal anti-NcSAG1 antibodies, and Wolfgang Bohne (University of Göttingen) for mAbCC2. We also thank Norbert Müller for expert advice during many experiments.
REFERENCES
- 1.Lim SS, Othman RY. 2014. Recent advances in Toxoplasma gondii immunotherapeutics. Korean J Parasitol 52:581–593. doi: 10.3347/kjp.2014.52.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos FA, Andrade GM, Lanna Ade P, Lage BF, Assumpcao MV, Pinto JA. 2014. Incidence of congenital toxoplasmosis among infants born to HIV-coinfected mothers: case series and literature review. Braz J Infect Dis 18:609–617. doi: 10.1016/j.bjid.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monney T, Hemphill A. 2014. Vaccines against neosporosis: what can we learn from the past studies? Exp Parasitol 140:52–70. doi: 10.1016/j.exppara.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Reichel MP, Alejandra Ayanegui-Alcerreca M, Gondim LF, Ellis JT. 2013. What is the global economic impact of Neospora caninum in cattle—the billion dollar question. Int J Parasitol 43:133–142. doi: 10.1016/j.ijpara.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP, Schares G, Ortega-Mora LM. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM, Reid MC, Johnson SM, Murphy RC, Kennedy M, Mann H, Leibly DJ, Hewitt SN, Verlinde CL, Kappe S, Merritt EA, Maly DJ, Billker O, Van Voorhis WC. 2012. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest 122:2301–2305. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lendner M, Bottcher D, Delling C, Ojo KK, Van Voorhis WC, Daugschies A. 2015. A novel CDPK1 inhibitor—a potential treatment for cryptosporidiosis in calves? Parasitol Res 114:335–336. doi: 10.1007/s00436-014-4228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellanos-Gonzalez A, White AC Jr, Ojo KK, Vidadala RS, Zhang Z, Reid MC, Fox AM, Keyloun KR, Rivas K, Irani A, Dann SM, Fan E, Maly DJ, Van Voorhis WC. 2013. A novel calcium-dependent protein kinase inhibitor as a lead compound for treating cryptosporidiosis. J Infect Dis 208:1342–1348. doi: 10.1093/infdis/jit327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojo KK, Reid MC, Kallur Siddaramaiah L, Müller J, Winzer P, Zhang Z, Keyloun KR, Vidadala RS, Merritt EA, Hol WG, Maly DJ, Fan E, Van Voorhis WC, Hemphill A. 2014. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS One 9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyloun KR, Reid MC, Choi R, Song Y, Fox AM, Hillesland HK, Zhang Z, Vidadala R, Merritt EA, Lau AO, Maly DJ, Fan E, Barrett LK, Van Voorhis WC, Ojo KK. 2014. The gatekeeper residue and beyond: homologous calcium-dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitology 141:1499–1509. doi: 10.1017/S0031182014000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, Napuli AJ, Maly DJ, Verlinde CL, Buckner FS, Parsons M, Hol WG, Merritt EA, Van Voorhis WC. 2010. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugi T, Kawazu S, Horimoto T, Kato K. 2015. A single mutation in the gatekeeper residue in TgMAPKL-1 restores the inhibitory effect of a bumped kinase inhibitor on the cell cycle. Int J Parasitol Drugs Drug Resist 5:1–8. doi: 10.1016/j.ijpddr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barna F, Debache K, Vock CA, Küster T, Hemphill A. 2013. In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob Agents Chemother 57:5747–5754. doi: 10.1128/AAC.02446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorer M, Debache K, Barna F, Monney T, Müller J, Boykin DW, Stephens CE, Hemphill A. 2012. Di-cationic arylimidamides act against Neospora caninum tachyzoites by interference in membrane structure and nucleolar integrity and are active against challenge infection in mice. Int J Parasitol Drugs Drug Resist 2:109–120. doi: 10.1016/j.ijpddr.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemphill A, Gottstein B, Kaufmann H. 1996. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitology 112:183–197. [DOI] [PubMed] [Google Scholar]
- 17.Kropf C, Debache K, Rampa C, Barna F, Schorer M, Stephens CE, Ismail MA, Boykin DW, Hemphill A. 2012. The adaptive potential of a survival artist: characterization of the in vitro interactions of Toxoplasma gondii tachyzoites with di-cationic compounds in human fibroblast cell cultures. Parasitology 139:208–220. doi: 10.1017/S0031182011001776. [DOI] [PubMed] [Google Scholar]
- 18.Alaeddine F, Hemphill A, Debache K, Guionaud C. 2013. Molecular cloning and characterization of NcROP2Fam-1, a member of the ROP2 family of rhoptry proteins in Neospora caninum that is targeted by antibodies neutralizing host cell invasion in vitro. Parasitology 140:1033–1050. doi: 10.1017/S0031182013000383. [DOI] [PubMed] [Google Scholar]
- 19.Guionaud C, Hemphill A, Mevissen M, Alaeddine F. 2010. Molecular characterization of Neospora caninum MAG1, a dense granule protein secreted into the parasitophorous vacuole, and associated with the cyst wall and the cyst matrix. Parasitology 137:1605–1619. doi: 10.1017/S0031182010000442. [DOI] [PubMed] [Google Scholar]
- 20.Gross U, Bormuth H, Gaissmaier C, Dittrich C, Krenn V, Bohne W, Ferguson DJ. 1995. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin Diagn Lab Immunol 2:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonlaufen N, Guetg N, Naguleswaran A, Müller N, Bjorkman C, Schares G, von Blumroeder D, Ellis J, Hemphill A. 2004. In vitro induction of Neospora caninum bradyzoites in Vero cells reveals differential antigen expression, localization, and host-cell recognition of tachyzoites and bradyzoites. Infect Immun 72:576–583. doi: 10.1128/IAI.72.1.576-583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonlaufen N, Müller N, Keller N, Naguleswaran A, Bohne W, McAllister MM, Bjorkman C, Muller E, Caldelari R, Hemphill A. 2002. Exogenous nitric oxide triggers Neospora caninum tachyzoite-to-bradyzoite stage conversion in murine epidermal keratinocyte cell cultures. Int J Parasitol 32:1253–1265. doi: 10.1016/S0020-7519(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 23.McAllister MM, Parmley SF, Weiss LM, Welch VJ, McGuire AM. 1996. An immunohistochemical method for detecting bradyzoite antigen (BAG5) in Toxoplasma gondii-infected tissues cross-reacts with a Neospora caninum bradyzoite antigen. J Parasitol 82:354–355. doi: 10.2307/3284181. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkman C, Hemphill A. 1998. Characterization of Neospora caninum iscom antigens using monoclonal antibodies. Parasite Immunol 20:73–80. doi: 10.1046/j.1365-3024.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- 25.Monney T, Grandgirard D, Leib SL, Hemphill A. 2013. Use of a Th1 stimulator adjuvant for vaccination against Neospora caninum infection in the pregnant mouse model. Pathogens 2:193–208. doi: 10.3390/pathogens2020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arranz-Solís D, Aguado-Martínez A, Müller J, Regidor-Cerrillo J, Ortega-Mora LM, Hemphill A. 2015. Dose-dependent effects of experimental infection with the virulent Neospora caninum Nc-Spain7 isolate in a pregnant mouse model. Vet Parasitol 211:133–140. doi: 10.1016/j.vetpar.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Müller J, Aguado-Martinez A, Manser V, Balmer V, Winzer P, Ritler D, Hostettler I, Arranz-Solís D, Ortega-Mora L, Hemphill A. 2015. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int J Parasitol Drugs Drug Resist 5:16–25. doi: 10.1016/j.ijpddr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debache K, Alaeddine F, Guionaud C, Monney T, Müller J, Strohbusch M, Leib SL, Grandgirard D, Hemphill A. 2009. Vaccination with recombinant NcROP2 combined with recombinant NcMIC1 and NcMIC3 reduces cerebral infection and vertical transmission in mice experimentally infected with Neospora caninum tachyzoites. Int J Parasitol 39:1373–1384. doi: 10.1016/j.ijpara.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Debache K, Guionaud C, Alaeddine F, Mevissen M, Hemphill A. 2008. Vaccination of mice with recombinant NcROP2 antigen reduces mortality and cerebral infection in mice infected with Neospora caninum tachyzoites. Int J Parasitol 38:1455–1463. doi: 10.1016/j.ijpara.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Core Team R. 2012. R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 31.Bohne W, Gross U, Ferguson DJ, Heesemann J. 1995. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol 16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacot D, Soldati-Favre D. 2012. Does protein phosphorylation govern host cell entry and egress by the Apicomplexa? Int J Med Microbiol 302:195–202. doi: 10.1016/j.ijmm.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Wei F, Wang W, Liu Q. 2013. Protein kinases of Toxoplasma gondii: functions and drug targets. Parasitol Res 112:2121–2129. doi: 10.1007/s00436-013-3451-y. [DOI] [PubMed] [Google Scholar]
- 34.Harper JF, Harmon A. 2005. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol 6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 35.Kieschnick H, Wakefield T, Narducci CA, Beckers C. 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem 276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 36.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. 2010. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson ET, Ojo KK, Murphy RC, Johnson SM, Zhang Z, Kim JE, Leibly DJ, Fox AM, Reid MC, Dale EJ, Perera BG, Kim J, Hewitt SN, Hol WG, Verlinde CL, Fan E, Van Voorhis WC, Maly DJ, Merritt EA. 2012. Multiple determinants for selective inhibition of apicomplexan calcium-dependent protein kinase CDPK1. J Med Chem 55:2803–2810. doi: 10.1021/jm201725v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lourido S, Zhang C, Lopez MS, Tang K, Barks J, Wang Q, Wildman SA, Shokat KM, Sibley LD. 2013. Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J Med Chem 56:3068–3077. doi: 10.1021/jm4001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, Fox AM, Mueller NR, Merritt EA, Fan E, Parsons M, Van Voorhis WC, Maly DJ. 2012. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem 55:2416–2426. doi: 10.1021/jm201713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Ojo KK, Vidadala R, Huang W, Geiger JA, Scheele S, Choi R, Reid MC, Keyloun KR, Rivas K, Siddaramaiah LK, Comess KM, Robinson KP, Merta PJ, Kifle L, Hol WG, Parsons M, Merritt EA, Maly DJ, Verlinde CL, Van Voorhis WC, Fan E. 2014. Potent and selective inhibitors of CDPK1 from and based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 5:40–44. doi: 10.1021/ml400315s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Ojo KK, Johnson SM, Larson ET, He P, Geiger JA, Castellanos-Gonzalez A, White AC Jr, Parsons M, Merritt EA, Maly DJ, Verlinde CL, Van Voorhis WC, Fan E. 2012. Benzoylbenzimidazole-based selective inhibitors targeting Cryptosporidium parvum and Toxoplasma gondii calcium-dependent protein kinase-1. Bioorg Med Chem Lett 22:5264–5267. doi: 10.1016/j.bmcl.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BG, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CL, White AC Jr, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown KM, Suvorova E, Farrell A, McLain A, Dittmar A, Wiley GB, Marth G, Gaffney PM, Gubbels MJ, White M, Blader IJ. 2014. Forward genetic screening identifies a small molecule that blocks Toxoplasma gondii growth by inhibiting both host- and parasite-encoded kinases. PLoS Pathog 10:e1004180. doi: 10.1371/journal.ppat.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei S, Marches F, Daniel B, Sonda S, Heidenreich K, Curiel T. 2002. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. Int J Parasitol 32:969–977. doi: 10.1016/S0020-7519(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 45.Sugi T, Masatani T, Murakoshi F, Kawazu S, Kato K. 2014. Microplate assay for screening Toxoplasma gondii bradyzoite differentiation with DUAL luciferase assay. Anal Biochem 464:9–11. doi: 10.1016/j.ab.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Dzierszinski F, Nishi M, Ouko L, Roos DS. 2004. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell 3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugi T, Kobayashi K, Takemae H, Gong H, Ishiwa A, Murakoshi F, Recuenco FC, Iwanaga T, Horimoto T, Akashi H, Kato K. 2013. Identification of mutations in TgMAPK1 of Toxoplasma gondii conferring resistance to 1NM-PP1. Int J Parasitol Drugs Drug Resist 3:93–101. doi: 10.1016/j.ijpddr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ojo KK, Eastman RT, Vidadala R, Zhang Z, Rivas KL, Choi R, Lutz JD, Reid MC, Fox AM, Hulverson MA, Kennedy M, Isoherranen N, Kim LM, Comess KM, Kempf DJ, Verlinde CL, Su XZ, Kappe SH, Maly DJ, Fan E, Van Voorhis WC. 2014. A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J Infect Dis 209:275–284. doi: 10.1093/infdis/jit522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC. 2014. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother 58:3547–3549. doi: 10.1128/AAC.01823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugi T, Kato K, Kobayashi K, Kurokawa H, Takemae H, Gong H, Recuenco FC, Iwanaga T, Horimoto T, Akashi H. 2011. 1NM-PP1 treatment of mice infected with Toxoplasma gondii. J Vet Med Sci 73:1377–1379. doi: 10.1292/jvms.11-0085. [DOI] [PubMed] [Google Scholar]