Abstract
Colistin has found increasing use in treating drug-resistant bacterial lung infections, but potential interactions with pulmonary biomolecules have not been investigated. We postulated that colistin, like aminoglycoside antibiotics, may bind to secretory mucin in sputum or epithelial mucin that lines airways, reducing free drug levels. To test this hypothesis, we measured binding of colistin and other antibiotics to porcine mucin, a family of densely glycosylated proteins used as a surrogate for human sputum and airway mucin. Antibiotics were incubated in dialysis tubing with or without mucin, and concentrations of unbound antibiotics able to penetrate the dialysis tubing were measured over time using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The percentage of antibiotic measured in the dialysate after 4 h in the presence of mucin, relative to the amount without mucin, was 15% for colistin, 16% for polymyxin B, 19% for tobramycin, 52% for ciprofloxacin, and 78% for daptomycin. Antibiotics with the strongest mucin binding had an overall polybasic positive charge, whereas those with comparatively little binding were less basic. When comparing MICs measured with or without added mucin, colistin and polymyxin B showed >100-fold increases in MICs for multiple Gram-negative bacteria. Preclinical evaluation of mucin binding should become a standard procedure when considering the potential pulmonary use of new or existing antibiotics, particularly those with a polybasic overall charge. In the airways, mucin binding may reduce the antibacterial efficacy of inhaled or intravenously administered colistin, and the presence of sub-MIC effective antibiotic concentrations could result in the development of antibiotic resistance.
INTRODUCTION
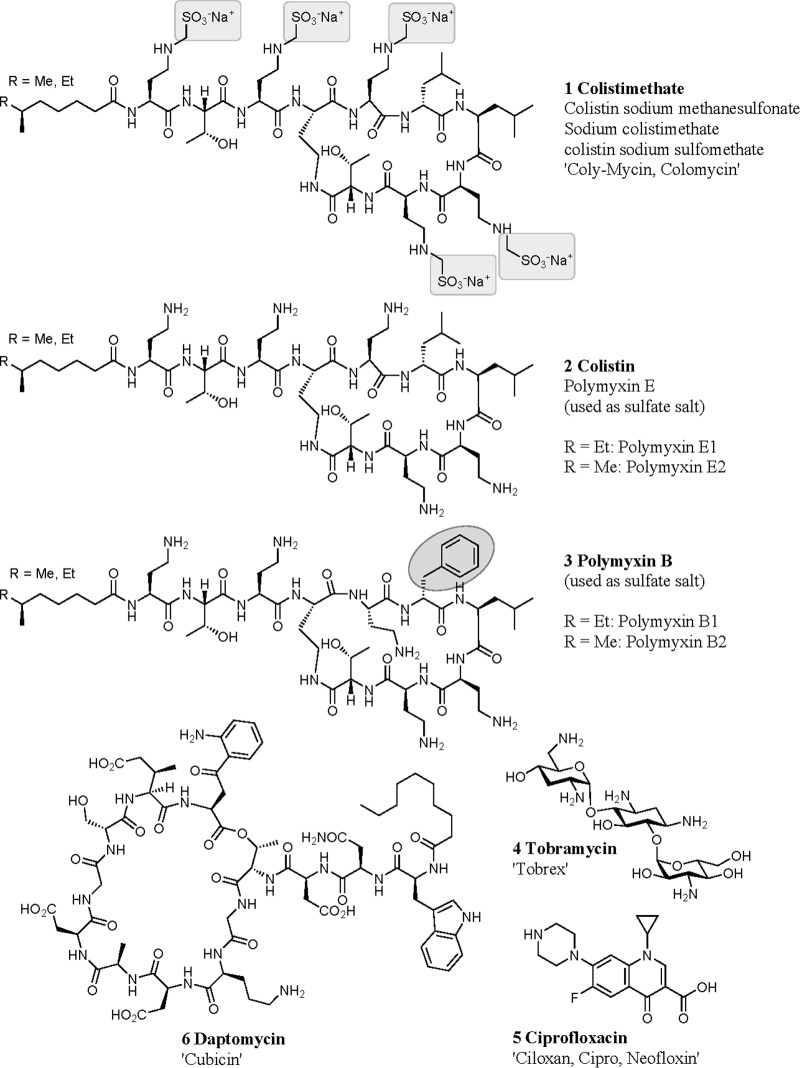
Colistimethate (also known as colistin methanesulfate) (Fig. 1, structure 1), often referred to by the name of the active hydrolysis product, colistin (Fig. 1, structure 2) (1-4), is widely used as an aerosol therapy in patients with cystic fibrosis (CF) and chronic airway infection with Pseudomonas aeruginosa (5–8). Use of nebulized colistimethate has also been reported in patients with bronchiectasis or with ventilator-associated pneumonia due to Gram-negative bacteria (9, 10). The initial use of colistin in patients with CF was reported in 1987 in a small study from Denmark (5). The subsequent widespread adoption of colistin treatment and more than 25 years of therapy without major adverse effects reported, with the exception of bronchospasm, have likely contributed to the improvement of care in patients with CF and chronic endobronchial pseudomonal infection (6).
FIG 1.
Antibiotic structures.
Colistimethate is an inactive prodrug of the antibiotic colistin in which the five basic amine groups are nominally masked by methanesulfonylation, although multiple partially derivatized species are likely present (11). It can be administered intravenously, intramuscularly, or by nebulization (note that the nebulized form has regulatory approval in many European countries but not in the United States, though a phase I clinical trial sponsored by the U.S. National Institute of Allergy and Infectious Diseases [12] is currently recruiting 39 participants to compare aerosolized and intravenous colistimethate sodium). While nebulization of an intravenous formulation of colistimethate is generally employed for inhalation therapy, dry powder formulations of colistimethate have also been administered (13, 14). After administration, the prodrug is activated by slow nonspecific hydrolysis, releasing formaldehyde-bisulfite adducts while progressing through a variety of partially methanesulfonylated intermediates to eventually generate the active form of the drug, colistin sulfate (1, 2, 8, 15). The active drug is a collection of closely related cyclic cationic peptides, also known as polymyxin E, with the 2 major components, known as polymyxin E1 and E2, differing only in the fatty acid moiety (Fig. 1) (1, 2, 4, 15). The cationic peptides disrupt the cell membranes of Gram-negative bacteria via an initial specific interaction with the membrane component lipid A (1, 15). These peptides are also cytotoxic to mammalian cells (16) and can cause airway and alveolar damage at high concentrations (17). Closely related polymyxin B (Fig. 1, structure 3) is used directly as the active compound (polymyxin B sulfate) for intravenous, intramuscular, intrathecal, or topical dosing; it is also a mixture of peptides, with the major peptides known as polymyxin B1 and B2 (1, 2). In contrast to polymyxin B sulfate, colistin sulfate is normally not administered to humans without being masked as the polymethanesulfonylated prodrug.
Examination of preclinical toxicology studies raises questions about why colistimethate is so safe for pulmonary clinical use. Development of one of the major active components of colistin (polymyxin E1) as a potential drug for inhalation therapy was previously abandoned, because a tolerated dose was not found in rat preclinical studies that evaluated inhaled doses ranging from 3 to 32 mg/kg body weight/day; significant local respiratory tract irritation was found at the lowest tested dose without any evidence of systemic toxicity (17). A 2002 study of inhalation of a dry powder formulation of colistin sulfate in six healthy patients and five with CF, comparing dosing of 25 mg of powdered colistin sulfate (approximately 0.4 mg/kg, lower than the lowest dose in the rat study) to 160 mg of colistimethate in nebulized solution, found reductions in pulmonary function in most patients and mild to severe cough in all patients dosed with the powder but no adverse effects in the nebulized group (18). A 2004 study compared nebulized colistin sulfate (100-mg dose, approximately 1.6 mg/kg) with nebulized colistimethate (160-mg dose, with equivalent colistin content to the colistin sulfate dose) in nine CF patients chronically infected with P. aeruginosa; seven of the patients could not complete the colistin sulfate dose due to throat irritation and severe cough and showed significant reductions in lung function compared to those who received the colistimethate dose (19). Furthermore, in an unfortunate accident in a patient with CF, colistimethate (75-mg dose) was reconstituted and allowed to convert to active colistin before administration, which led to fatal acute respiratory distress syndrome (20). This established that high doses of inhaled colistin can be toxic to humans. The actual dose of active colistin delivered by nebulization of colistimethate solution is unknown; however, assuming (i) a conservative 15% of the nominal dose in the nebulizer (nebulized dose is generally 1 to 2 million IU of colistin, equivalent to 80 to 160 mg of colistimethate or 33 to 67 mg of colistin [colistin base activity, the active moiety] [21]) is delivered to the lung (a deposition efficiency of approximately 10% has been found for aerosolized tobramycin [22, 23]) and (ii) subsequent 50% conversion to active colistin (for intravenous dosing, only 20% to 25% is converted, due largely to significant renal clearance of colistimethate before conversion [1]; the amount converted in the lung before being absorbed or cleared has not been reported), then this generates an estimate of approximately 8% of the nominal dose in the nebulizer, or 5 to 10 mg of colistin, as the exposure. However, even this exposure would not be predicted to be safely tolerated based on clinical studies of direct inhalation of colistin sulfate. The discrepancy in the results may result from treatment with the entire dose of colistin at once, instead of administering the precursor that slowly converts to colistin over time (24). In addition, colistin, like aminoglycosides (25-28), may be bound to mucin found in sputum, and, because of the resulting reduction in unbound antibiotic, the active and clinically effective dose of colistin in humans may be far smaller than the previously reported concentrations in sputum (21, 29). Slow release of colistin from colistimethate may allow for increased mucin binding compared to a rapid high dose, further reducing the exposure to free colistin. Pharmacokinetic studies of inhaled colistimethate in humans show that high concentrations of colistin are maintained in the sputum (over 10 mg/liter of polymyxin E1 at 8 h after a single dose of 66 mg), while serum concentrations peak at 1.5 h at 0.15 mg/liter and are below 0.05 mg/liter by 6 h (21).
The mucin glycoproteins form the major macromolecular constituent of mucus, though sputum also contains other components, such as DNA, proteins, lipids, and cellular debris (30, 31). To test the hypothesis that colistin is bound to mucin, we measured the binding of colistin and other antibiotics to porcine stomach mucin, a family of densely glycosylated proteins used as a surrogate for the mucin component of human sputum. Porcine mucin has previously been employed as a surrogate in studies measuring the binding of tobramycin (25), where it showed similar levels of antibiotic inhibition as sputum from a CF patient (40% to 50% versus 60% recovery after 250 min), and has been utilized as a component of artificial sputum (32).
The effect of sputum on antibiotic availability has previously been studied by a number of methods, including dialysis (26), ultrafiltration (28), and addition of sputum to bactericidal measurements (27, 33).
MATERIALS AND METHODS
Study design.
Studies were conducted on colistin sulfate, polymyxin B sulfate, tobramycin, ciprofloxacin, and daptomycin (Fig. 1, structures 2 through 6) to measure binding to porcine stomach mucin. A solution of 400 μg of each antibiotic was incubated in dialysis tubing with or without 125 mg of porcine stomach mucin, and the concentration of the unbound antibiotic able to penetrate the dialysis tubing was subsequently measured in the dialysates over time, with a total solution volume of 11 ml. Initial testing was conducted at 4°C, with the colistin sulfate assay repeated at 37°C.
Antibiotic and mucin preparation.
Colistin sulfate (Sigma-Aldrich, catalog no. C4461), polymyxin B sulfate (Sigma-Aldrich, catalog no. P0972), tobramycin (Sigma-Aldrich, catalog no. T4014), ciprofloxacin (Sigma-Aldrich, catalog no. 17850), and daptomycin (Enzo Life Sciences, catalog no. BML-A201-0100) were prepared as 4-mg/ml solutions in water. A 12.5% (wt/vol) solution of mucin was prepared by dissolving 125 mg of porcine stomach mucin (Sigma-Aldrich, catalog no. M1778, type III, bound sialic acid 0.5% to 1.5%, partially purified powder) in 1.0 ml of Dulbecco's phosphate buffered saline (PBS) (Life Technologies Australia Pty Ltd., Invitrogen division, catalog no. 14190250).
Kinetics of efflux.
Assessment of the kinetics of the efflux of each antibiotic from a dialysis bag was performed as previously described (25), with slight modifications. In brief, 400 μg of each antibiotic (100 μl of a 4-mg/ml solution) was added to 1 ml of calcium- and magnesium-free PBS with or without 12.5% (wt/vol) porcine stomach mucin (Sigma-Aldrich, catalog no. M1778). The resulting mixtures were placed in Spectra/Por dialysis bags (6- to 8-kDa molecular mass cutoff, 32-mm flat width, 20.4-mm diameter; 3.3-ml/cm volume; Spectrum Laboratories, catalog no. 132655, Rancho Dominguez, CA), with the tube ends closed using standard dialysis bag closures (Spectra/Por, catalog no. 5160190). Dialysis was conducted in 10 ml of PBS at 4°C in a 10-cm polystyrene petri dish (Nunc, catalog no. P7741) covered with a lid and gently agitated on an orbital shaker at 20 rpm. A second colistin sulfate sample was tested at 37°C. Dialysate samples (200 μl) were collected at 10 min and after 1, 2, and 4 h, and the antibiotic concentration within each dialysate was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. The percentage of each antibiotic detected in the dialysate was calculated relative to the theoretical total concentration based on 400 μg of antibiotic in an 11-ml total volume.
LC-MS/MS analyses.
Analyses of colistin sulfate, polymyxin B sulfate, tobramycin, ciprofloxacin, and daptomycin were undertaken using the 4000 Qtrap LC-MS/MS system mass spectrometer (AB SCIEX, Framingham, MA, USA). The LC-MS/MS parameters were as follows: mobile phase A, 0.1% (vol/vol) formic acid in water; mobile phase B, 0.1% (vol/vol) formic acid in acetonitrile; column, Atlantis T3 (2.1 mm by 50 mm; 5-μm particle size; Waters Corp., Milford, MA, USA); column temperature, 40°C; flow rate, 0.35 ml/min with a gradient of 2% B for 1 min then 2% to 100% B for 4 min; and injection volume, 5 μl with an autosampler cooled to 12°C. Direct infusion initially provided the molecular ion, followed by selective reaction monitoring (SRM) (monitoring multiple parent fragment ions in a single MS run) in positive ionization mode, with declustering potential (DP) and collision energy (CE) optimized to generate a good response signal. In these analyses, DP = 60 V, but CE varied depending upon the compound: colistin sulfate at m/z 385.9→101.3 (CE, 30 V), polymyxin B sulfate at m/z 402.0→101.3 (CE, 30 V), tobramycin at m/z 468.2→324.4 (CE, 28 V) and m/z 468.2→163.3 (CE, 28 V), ciprofloxacin at m/z 332.1→288.1 (CE, 28 V), and daptomycin at m/z 811.1→313.3 (CE, 40 V).
For each antibiotic, standards of 40, 20, 4, 2, 0.4, 0.2, 0.04, and 0.02 μg/ml were prepared in PBS solvent. Both the standards and samples were diluted 1/10 with water before LC-MS/MS analysis.
MIC measurement.
Bacteria were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and included Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 13883), Acinetobacter baumannii (ATCC 19606), P. aeruginosa (ATCC 27853), and Staphylococcus aureus (ATCC 25923). Bacteria were cultured in Mueller-Hinton broth (MHB; Bacto Laboratories, catalog no. 211443) at 37°C overnight. A sample of each culture was then diluted 50-fold in fresh MHB and incubated at 37°C for 2 to 3 h. Antibiotic stock solutions were prepared at 1.28 mg/ml in water, then serially diluted 2-fold across the wells of 96-well nonbinding surface plates (NBS; Corning, catalog no. 3641). After the 2- to 3-h incubation, the mid-log-phase bacterial cultures were diluted to a final concentration of 5 × 105 CFU/ml, and 50 μl was added to each well, giving a final antibiotic concentration range of 64 μg/ml to 0.03 μg/ml. MICs were determined visually after 24 h of incubation at 37°C, with the MIC defined as the lowest antibiotic concentration at which no bacterial growth was visible. To measure MICs in the presence of mucin, 0.4% and 4% mucin (Sigma-Aldrich, catalog no. M1778) was added to the mid-log-phase bacterial cultures and mixed gently before adding the mixture to the 96-well plates to achieve final concentrations of 0.2% and 2% mucin. After 24 h of incubation at 37°C, the plates with mucin were particularly cloudy, making it difficult to determine growth/lack of bacterial growth. Therefore, 30 μl of resazurin, an oxidation-reduction indicator dye for cell viability determination (34, 35), was added to each well, and wells were incubated for a further 3 h at 37°C. Following incubation with resazurin, MICs were determined visually, with blue coloration indicating lack of cell viability and pink indicating live cells/bacterial growth.
RESULTS
Binding to mucin.
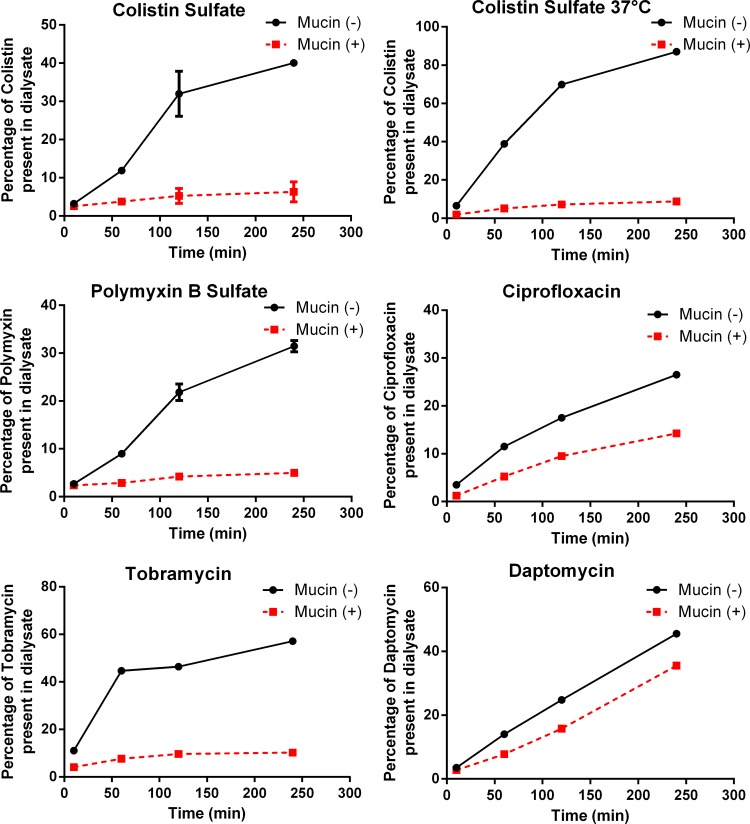
Previous studies measuring the binding of antibiotics to sputum/mucin using the dialysis method have indirectly assessed the amount of effluxed antibiotic by radioenzymatic assay (26), by MIC determination of the solution (26), or by immunofluorescence (25); we have used a much more versatile direct measurement of concentration, LC-MS/MS. The amounts of binding to mucin varied between the tested antibiotics (Fig. 2). Colistin sulfate, polymyxin B sulfate, and tobramycin showed strong interactions with mucin at 4°C; after 4 h of incubation in the presence of mucin, 6% (colistin sulfate), 5% (polymyxin B sulfate), and 11% (tobramycin) of the compounds diffused out of the dialysis bag and were detected in the dialysate, compared with 40% (colistin sulfate), 31% (polymyxin B sulfate), and 57% (tobramycin) detected in the dialysate in the absence of mucin. This was equivalent to 15%, 16%, and 19% efflux in the presence of mucin relative to efflux in the absence of mucin. Colistin sulfate efflux in the absence of mucin was significantly increased at 37°C, with 9% of the compound moving into the dialysate when incubated with mucin compared with 87% moving into the dialysate when mucin was absent. However, the relative recovery of colistin in the presence of mucin remained relatively constant at both temperatures (15% at 4°C, 10% at 37°C).
FIG 2.
Antibiotic concentrations present in the dialysates. Antibiotic concentrations were measured in dialysates from samples of 400 μg of each antibiotic (in 1 ml PBS) that were placed into dialysis tubing with (+) or without (−) 125 mg porcine gastric mucin at 4°C, except for the top right panel, for which data were obtained at 37°C. The percentage of the antibiotic present in each dialysate was calculated relative to the theoretical concentration based on the total volume of the solution. Mean and standard deviation (SD) values are plotted for assays performed multiple times. Colistin sulfate dialysis at 4°C was performed in triplicate; dialysis of ciprofloxacin and daptomycin was performed once, and the others were performed twice. Error bars for colistin sulfate at 37°C and tobramycin were too small to show.
The interactions with mucin at 4°C were much weaker for ciprofloxacin and daptomycin than for the other compounds, with 14% (ciprofloxacin) and 36% (daptomycin) detected in the dialysates in the presence of mucin, compared with 27% and 46% detected, respectively, in the absence of mucin. This was equivalent to 52% and 79% efflux in the presence of mucin relative to efflux in the absence of mucin.
Effect of mucin on MICs.
Binding to mucin, which reduces the free concentration of antibiotic, would be expected to reduce the measured MIC potency of the antibiotics, just as high protein binding is known to reduce antibiotic efficacy. Indeed, MICs measured in the presence of 0.2% or 2% porcine mucin showed decreased antibiotic potency (Table 1), which was generally consistent with the extent of mucin binding observed in the dialysis experiments (Fig. 2). Colistin sulfate and polymyxin B sulfate, which had the highest mucin binding in the dialysis results, showed >100-fold increases in MICs in the presence of mucin relative to MICs in the absence of mucin. These increases in MIC values were observed across multiple Gram-negative bacterial organisms. The presence of mucin caused tobramycin MIC values to consistently increase 8- to 64-fold against both Gram-negative and Gram-positive organisms. In contrast, ciprofloxacin was much less affected by the presence of mucin, with MIC values increasing 2- to 4-fold for all tested organisms except E. coli. Daptomycin was also much less affected by the presence of mucin, with a 2-fold increase in MIC when tested with one Gram-positive organism (S. aureus).
TABLE 1.
MIC of antibiotics in MHB with and without added mucin
| Isolate and antibiotic | MIC (μg/ml) in: |
||
|---|---|---|---|
| MHB alone | +0.2% mucin | +2% mucin | |
| E. coli ATCC 25922 | |||
| Colistin sulfate | ≤0.03 | 0.5 | >64 |
| Polymyxin B sulfate | ≤0.03 | 1 | >64 |
| Tobramycin | 0.5/1 | 1 | 16 |
| Ciprofloxacin | ≤0.03 | 0.06 | 0.25 |
| K. pneumoniae ATCC 13883 | |||
| Colistin sulfate | 0.5 | 16 | >64 |
| Polymyxin B sulfate | 0.5 | 8 | >64 |
| Tobramycin | 0.25 | 0.5 | 8 |
| Ciprofloxacin | 0.06 | ≤0.03 | 0.25 |
| A. baumannii ATCC 19606 | |||
| Colistin sulfate | 0.125 | 0.5 | >64 |
| Polymyxin B sulfate | 0.03 | 0.5 | >64 |
| Tobramycin | 4 | 4 | 32 |
| Ciprofloxacin | 0.5 | 0.5 | 2 |
| P. aeruginosa ATCC 27853 | |||
| Colistin sulfate | 0.06 | 4 | >64 |
| Polymyxin B sulfate | 0.06 | 1 | >64 |
| Tobramycin | 0.125 | 0.5 | 8 |
| Ciprofloxacin | 0.25 | 0.25 | 1 |
| S. aureus ATCC 25923 | |||
| Tobramycin | 0.125 | 0.5 | 8 |
| Ciprofloxacin | 0.5 | 0.5 | 1 |
| Daptomycin | 1 | 1 | 2 |
DISCUSSION
The efflux of colistin sulfate and a closely related antibiotic, polymyxin B sulfate, from a dialysis bag in the presence of mucin was less than 20% of the efflux observed in the absence of mucin. This strong binding to mucin may partly explain the discrepancy between the human experience of aerosolized (inhaled) colistin (as the prodrug colistimethate) as a safe drug and the toxicity of colistin (as the active component, colistin or polymyxin E1) observed in preclinical models (17) and clinical studies (18, 19). It is consistent with the high sputum concentration and low systemic levels of polymyxin E measured after a single inhaled dose of colistimethate (21), as the strong binding would prevent systemic absorption. Secretory mucins are found in high abundance in sputum as a result of mucociliary clearance from the lung. Indeed, mucin overproduction and hypersecretion in sputum are common features of chronic pulmonary infection (30). Sputum also contains other macromolecular components, such as DNA (31), which have been shown to also bind to antibiotics such as tobramycin (25, 27, 28); therefore, the colistin binding measured in this study may be underestimated. Normal rats have approximately 100-fold less density of submucosal glands than humans (36, 37); therefore, their exposure to an aerosol of polymyxin E1 in the preclinical study (17) could have been 5- to 10-fold higher than the predicted exposure in humans if mucin binding had occurred. In Europe, the currently used nebulizer dose of the colistimethate prodrug is 2 million units, or 160 mg, although it is important to note that the labeling of colistimethate dosing varies with the product, as does the exact composition of different brands of colistimethate (1, 2, 11). However, the actual exposure to the active drug polymyxin E is far less because of loss due to inefficiency of the nebulizer, an unknown but potentially low rate of conversion of prodrug to active polymyxin E, and the additional >5-fold reduction in the concentration of active polymyxin E caused by binding to mucin.
The results showing that tobramycin binds to mucin are confirmatory of previous studies that showed reduced activity of tobramycin in the presence of sputum from patients with CF (26). Further, the moderate binding of ciprofloxacin to mucin that was observed (52% recovery), though less strong than the binding of colistin (15% recovery) and tobramycin (19% recovery), may help explain the rapid emergence of ciprofloxacin-resistant bacteria in CF patients receiving oral ciprofloxacin therapy (38), since mucin binding of ciprofloxacin may lead to airway concentrations that are below the MICs required to inhibit bacterial growth (39).
One limitation of these studies is that, in the absence of mucin, efflux did not reach 100% in the dialysates for any of the antibiotics, with the highest levels of efflux (87%) achieved with colistin sulfate at 37°C. This could be due to insufficient incubation time or to the binding of the antibiotics to the polystyrene plates, dialysis bags (regenerated cellulose), or bag closures; such nonspecific binding might have been reduced by the addition of a surfactant (polysorbate 80) (40). Another limitation is that mucin only represents one of the components of sputum; binding to DNA components, as observed for tobramycin, could potentially reduce the amount of free colistin even more. Further, antibiotic activity can also be inhibited by other substances found in the airways; e.g., daptomycin is inhibited by pulmonary surfactant (41).
The levels of binding to mucin generally showed good correspondence with decreases in MIC values measured in the presence of mucin. Colistin sulfate and polymyxin B sulfate, with 15% to 16% recovery in mucin binding experiments, exhibited >100-fold increases in measured MIC values in the presence of mucin. Tobramycin (19% recovery in the presence of mucin) exhibited 8- to 64-fold increases in MIC. In contrast, ciprofloxacin, with less binding to mucin (52% recovery), generally had 2- to 4-fold increases in MICs in the presence of mucin, and daptomycin (78% recovery) had a 2-fold increase for the one organism against which it was tested.
Examination of the drug structures tested in these analyses (Fig. 1) shows that those with the strongest mucin binding (colistin sulfate, polymyxin B sulfate, and tobramycin) have multiple primary amine groups, with an overall polybasic positive charge. In contrast, the small molecule ciprofloxacin and the large peptide antibiotic daptomycin, with comparatively little mucin binding, are less basic in character.
The finding that colistin binds to mucin, a key component in sputum, means that modeling the pharmacodynamics of aerosolized colistimethate has now become even more complicated. It will be necessary to account for (i) the time from reconstitution to administration (because active drug is being created after reconstitution; while analytical studies suggest that this rate is exceptionally low [24], the amount of conversion to active drug may depend on how the reconstituted drug is stored [20, 24]); (ii) the amount of aerosol deposited from the nominal dose in the nebulizer; (iii) the rate of conversion to the active drug in the airway (which is complicated by the presence of partially deprotected, partially active intermediates [11]); (iv) the airway clearance of both the active drug and the prodrug; and now (v) the effect of mucin binding. Furthermore, the use of colistimethate aerosols in diseases other than CF, such as ventilator-associated pneumonia, may require a much lower dose to be both safe and effective, since patients with other diseases are likely have sputum volumes and mucin concentrations that differ from those of CF patients or to have sputum volumes that are variable across the course of the disease. Pharmacokinetic evaluation of colistin is also complicated by the fact that samples can continue to convert from the prodrug to the active form after recovery from the patient, thereby making elucidation of active drug levels in vivo inaccurate (42). However, the strong binding of colistin to mucin suggests that preclinical evaluation of such binding should become a standard procedure when considering the potential use of any antibiotic as an aerosol, particularly those with a polybasic overall positive charge.
With the development of multidrug resistant (MDR) Gram-negative bacteria, colistin has been highlighted as an effective choice of antibiotics (43, 44). However, mucin binding in the airways may reduce the antibacterial efficacy of both inhaled and intravenously administered colistin, leading to insufficient antibiotic levels in sputum to kill bacteria once mucin binding is taken into account, fostering the generation of resistant populations due to constant exposure to sublethal doses. This may help explain why resistance arises within patients undergoing colistin therapy, as shown in a recent study demonstrating the emergence of colistin-resistant A. baumannii following treatment of carbapenem-resistant, colistin-susceptible infections with intravenous and/or inhaled colistimethate (45). Further examination of the kinetics of colistin exposure, the rate of colistimethate unmasking in sputum after inhalation delivery, and the rate of colistin and colistimethate clearance is required in order to determine whether a slow but sustained release of colistin from colistimethate is more effective than a rapidly achieved high concentration of colistin produced via inhalation of colistin and to determine whether a smaller, tolerable, yet effective dose of inhaled colistin sulfate can be found.
ACKNOWLEDGMENTS
This research was supported financially by NHMRC grants APP1005350, APP1045326, and AF511105 and by NIH grant 1R21AI098731-01. J.X.H., M.A.T.B., R.P., and M.S.B. were supported by Wellcome Trust seeding drug discovery award 094977/Z/10/Z, and M.A.C. was supported by NHMRC professorial research fellowship APP1059354.
Medical writing assistance was provided by Kate Loughney under the sponsorship of Cardeas Pharma.
M.A.C. and A.B.M. are members of the SAB of Adenium Biotech, and A.B.M. is the CEO of Cardeas Pharma.
REFERENCES
- 1.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassamali Z, Rotschafer JC, Jones RN, Prince RA, Danziger LH. 2013. Polymyxins: wisdom does not always come with age. Clin Infect Dis 57:877–883. doi: 10.1093/cid/cit367. [DOI] [PubMed] [Google Scholar]
- 3.Nation RL, Li J, Turnidge JD. 2013. The urgent need for clear and accurate information on the polymyxins. Clin Infect Dis 57:1656–1657. doi: 10.1093/cid/cit522. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the reemerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 5.Jensen T, Pedersen SS, Garne S, Heilmann C, Høiby N, Koch C. 1987. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother 19:831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 6.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A, Touw DJ. 2000. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 16:749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurjar M. 2015. Colistin for lung infection: an update. J Intensive Care 3:3. doi: 10.1186/s40560-015-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velkov T, Abdul Rahim N, Zhou QT, Chan HK, Li J. 2015. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev 85:65–68. doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. 2014. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Resp Crit Care Med 189:975–982. doi: 10.1164/rccm.201312-2208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, Falagas ME. 2008. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study. Respir Med 102:407–412. doi: 10.1016/j.rmed.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 11.He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J. 2013. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68:2311–2317. doi: 10.1093/jac/dkt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health. April 2015. ClinicalTrials.gov identifier NCT01863719: aerosolized and intravenous colistin in healthy adults. https://clinicaltrials.gov/ct2/show/NCT01863719. [Google Scholar]
- 13.Westerman EM, de Boer AH, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. 2007. Dry powder inhalation of colistin sulphomethate in healthy volunteers: a pilot study. Int J Pharm 335:41–45. doi: 10.1016/j.ijpharm.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Westerman EM, De Boer AH, Le Brun PP, Touw DJ, Roldaan AC, Frijlink HW, Heijerman HG. 2007. Dry powder inhalation of colistin in cystic fibrosis patients: a single dose pilot study. J Cyst Fibros 6:284–292. doi: 10.1016/j.jcf.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multiresistant Gram-negative bacteria. Int J Antimicrob Agents 25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Das D, Buyck J, Van Bambeke F, Lorent J, Mingeot-Leclercq M, Gobin P, Couet W, Tulkens PM. 2011. Comparative assessment of antibacterial and cytotoxic effects of colistin (CST) colistin methanesulfonate (CMS) and polymyxin B (PMB) using Pseudomonas aeruginosa, macrophages and renal cells, and liposomes, abstr F2-170 51st Intersci Conf Antimicrob Agents Chemother, 17 to 20 September 2011, Chicago, IL http://www.abstractsonline.com/plan/ViewAbstract.aspx?mID=2789&sKey=5b319c87-0c75-4ca9-9ee9-762aa8a4a65c&cKey=e1f391f6-ef61-4e88-8613-537cbdfd7a9e&mKey=0c918954-d607-46a7-8073-44f4b537a439. [Google Scholar]
- 17.VanDevanter DR, Rose LM, Sprugel KH. 2001. 28-day inhalation toxicology of polymyxin E1, the major active component of colistin, in rats and dogs, poster P236 24th European Cystic Fibrosis Congress, Vienna, Austria. [Google Scholar]
- 18.Le Brun PP, de Boer AH, Mannes GP, de Fraîture DM, Brimicombe RW, Touw DJ, Vinks AA, Frijlink HW, Heijerman HG. 2002. Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2. Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur J Pharm Biopharm 54:25–32. doi: 10.1016/S0939-6411(02)00044-9. [DOI] [PubMed] [Google Scholar]
- 19.Westerman EM, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. 2004. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: a pilot study. J Cyst Fibros 3:23–28. doi: 10.1016/j.jcf.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 20.McCoy KS. 2007. Compounded colistimethate as possible cause of fatal acute respiratory distress syndrome. N Engl J Med 357:2310–2311. doi: 10.1056/NEJMc071717. [DOI] [PubMed] [Google Scholar]
- 21.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 22.Touw DJ, Jacobs FA, Brimicombe RW, Heijerman HG, Bakker W, Briemer DD. 1997. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis. Antimicrob Agents Chemother 41:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Brun PP, Vinks AA, Touw DJ, Hekelaar N, Mannes GP, Brimicombe RW, Frijlink EH, Heijerman HG. 1999. Can tobramycin inhalation be improved with a jet nebulizer? Ther Drug Monit 21:618–624. doi: 10.1097/00007691-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. 2008. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother 52:3047–3051. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt BE, Weber A, Berger A, Ramsey B, Smith AL. 1995. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother 39:34–39. doi: 10.1128/AAC.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL. 1985. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am Rev Respir Dis 132:761–765. [DOI] [PubMed] [Google Scholar]
- 27.King P, Lomovskaya O, Griffith DC, Burns JL, Dudley MN. 2010. In vitro pharmacodynamics of levofloxacin and other aerosolized antibiotics under multiple conditions relevant to chronic pulmonary infection in cystic fibrosis. Antimicrob Agents Chemother 54:143–148. doi: 10.1128/AAC.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramphal R, Lhermitte M, Filliat M, Roussel P. 1988. The binding of anti-pseudomonal antibiotics to macromolecules from cystic fibrosis sputum. J Antimicrob Chemother 22:483–490. doi: 10.1093/jac/22.4.483. [DOI] [PubMed] [Google Scholar]
- 29.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voynow JA, Rubin BK. 2009. Mucins, mucus, and sputum. Chest 135:505–512. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 31.Roussel P, Degand P, Lamblin G, Laine A, Lafitte JJ. 1978. Biochemical definition of human tracheobronchial mucus. Lung 154:241–260. [DOI] [PubMed] [Google Scholar]
- 32.Sriramulu DD, Lünsdorf H, Lam JS, Römling U. 2005. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol 54(Part 7):667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 33.Bucki R, Namiot DB, Namiot Z, Savage PB, Janmey PA. 2008. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J Antimicrob Chemother 62:329–335. doi: 10.1093/jac/dkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarker SD, Nahar L, Kumarasamy Y. 2007. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann CM, Markham JL. 1998. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84:538–544. doi: 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 36.Mizgerd JP, Skerrett SJ. 2008. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol 294:L387–L398. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Driskell RR, Engelhardt JF. 2004. Airway glandular development and stem cells. Curr Top Dev Biol 64:33–56. doi: 10.1016/S0070-2153(04)64003-8. [DOI] [PubMed] [Google Scholar]
- 38.Ball P. 1990. Emergent resistance to ciprofloxacin amongst Pseudomonas aeruginosa and Staphylococcus aureus: clinical significance and therapeutic approaches. J Antimicrob Chemother 26(Suppl F):165–179. [DOI] [PubMed] [Google Scholar]
- 39.Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 40.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 42.Coulthard K. 2008. Maximizing the efficacy and safety of colistimethate therapy. Pediatr Pulmonol 43(Suppl):193–195. doi: 10.1002/ppul.20938. [DOI] [Google Scholar]
- 43.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 44.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, Pasculle AW, Ernst RK, Doi Y. 2015. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]