Abstract
Background
Obesity is associated with higher risk of atrial fibrillation (AF), but the impact of behavioral weight loss interventions on atrial fibrillation (AF) risk in persons with diabetes is unknown. We addressed this question in the Look AHEAD randomized trial.
Methods and Results
5067 overweight or obese individuals 45-76 years old with type 2 diabetes without prevalent AF were randomized to either an intensive lifestyle intervention (ILI) designed to achieve and maintain weight loss through caloric reduction and increased physical activity or to a diabetes support and education (DSE) usual care group. AF was ascertained from electrocardiograms at study exams and hospitalization discharge summaries. Multivariable Cox models were used to estimate the intention to treat effect of the intervention adjusting for baseline covariates. During a mean follow-up of 9.0 years, 294 incident AF cases were identified. Rates of AF were comparable in the ILI and DSE groups (6.1 and 6.7 cases per 1,000 person-years, respectively, p=0.42). The intervention did not affect AF incidence (multivariable hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.77, 1.28). Similarly, neither weight loss nor improvement in physical fitness during the first year of the intervention were significantly associated with AF incidence: multivariable HR (95%CI) comparing top versus bottom quartile were 0.70 (0.41, 1.18) for weight loss and 0.88 (0.55, 1.43) for physical fitness improvement.
Conclusion
In a large randomized trial of overweight and obese individuals with type 2 diabetes, an ILI that induced modest weight loss did not reduce the risk of developing AF.
Keywords: atrial fibrillation, prevention, weight loss, type 2 diabetes, randomized trial
INTRODUCTION
Atrial fibrillation (AF) is a common arrhythmia in the general population, with an estimated lifetime risk of 25% and affecting >33 million individuals worldwide.1, 2 AF increases the risk of stroke, heart failure, coronary heart disease, and total mortality, and is associated with added healthcare costs.3, 4
The etiology of AF is likely to be multifactorial, with hypertension and intrinsic cardiac causes, such as valvular disease and heart failure identified as independent risk factors. Obesity is also a well-established risk factor for AF.5 In the Atherosclerosis Risk in Communities (ARIC) study, a large community-based cohort, presence of borderline or elevated cardiovascular risk factors explained >55% of the AF cases in the cohort, with overweight and obesity accounting for 18% of AF incidence.6 Several other studies have found overweight and obesity to be strong determinants of AF risk.7-10 Diabetes has also long been recognized as a risk factor for AF, increasing the risk of new-onset AF by 40%.11 Physical activity and cardiorespiratory fitness, in contrast, show a more complex association with the risk of AF. Epidemiologic studies have shown that extreme levels of physical activity in endurance athletes increase AF incidence,12 but recent reports suggest that higher levels of cardiorespiratory fitness in the range observed in the general population may be associated with lower AF risk.13, 14
As type 2 diabetes and AF share many common antecedents, including hypertension, atherosclerosis, and obesity, it is plausible that weight reduction may reduce the risk of developing new-onset AF. Indeed, a structured weight management program has been shown to significantly reduce symptom burden and severity in highly symptomatic individuals with AF, as compared to an intervention that attempted to optimize risk factors alone.15 Similarly, long-term sustained weight loss in AF patients has been associated with reduction of arrhythmia burden and maintenance of sinus rhythm.16 To our knowledge, no previous randomized studies, including trials of bariatric surgery or lifestyle-based weight-loss intervention, have determined whether a sustained weight loss intervention reduces the risk of new-onset AF in persons with type 2 diabetes.
Look AHEAD (Action for Health in Diabetes) was a multicenter, randomized clinical trial of an intensive lifestyle intervention (ILI) designed to achieve weight loss through caloric restriction and physical activity compared with a diabetes support and education (DSE) group in overweight and obese adults with type 2 diabetes. In the Look AHEAD trial, the ILI did not reduce the incidence of the primary endpoint of cardiovascular morbidity and mortality.17 AF, however, was not part of the primary outcome. In the present secondary analysis of the Look AHEAD trial, we examined the impact of the ILI on the risk of newly-diagnosed AF. We hypothesized that individuals randomized to ILI, versus DSE, would have a lower risk of AF. In addition, we hypothesized that greater weight loss and improvement in physical fitness would be associated with lower AF incidence.
METHODS
Participants, Study Design, and Interventions
Look AHEAD randomly assigned 5,145 overweight or obese individuals with type 2 diabetes to ILI or DSE at 16 clinical sites across the United States. The study methods and baseline characteristics are described elsewhere,18, 19 and the protocol is available at https://www.lookaheadtrial.org/public/LookAHEADProtocol.pdf. Participating centers received local IRB approval. Written informed consent was obtained from all participants.
Individuals were eligible if they were 45 to 76 years old, overweight or obese (body mass index, [BMI] ≥25 kg/m2, or BMI ≥27 kg/m2 in those taking insulin), able to complete a maximal exercise test to safely exercise, with treated or untreated systolic/diastolic blood pressure <160/100 mmHg, glycated hemoglobin (HbA1c) ≤11%, and had a primary health care provider. Exclusion criteria included inability to walk two blocks, non-traumatic amputation of a lower limb, urine dipstick protein of 4+ (equivalent to approximately >1 g protein/day), serum creatinine exceeding 1.4 mg/dl in women or 1.5 mg/dl in men, or current treatment with dialysis. There were no exclusions based on other complications of diabetes. Glucose-lowering medicines of any type were allowed and the use of insulin was limited to <30% of the cohort. Participants with and without a history of cardiovascular disease (CVD) were included. Additional inclusion and exclusion criteria have been published elsewhere.19 For the present analysis, we also excluded participants with prevalent AF or atrial flutter at baseline (self-reported or present in the baseline study ECG).
At baseline, participants were randomized 1:1 to ILI or DSE. Initial treatment allocation was concealed to clinic staff; the assignment was revealed only after the participant was enrolled in the clinical trial. Outcomes assessors and laboratory staff were masked to treatment, but participants and interventionists were not because the intervention was behavioral. Those performing the statistical analyses for this report were not masked.
A detailed description of the interventions has been previously published.20 In brief, the ILI aimed to achieve and maintain weight loss of at least 7% through reduced caloric intake and increased physical activity. The program included both group and individual counseling sessions, occurring weekly during the first 6 months, with decreasing frequency over the course of the trial. Strategies included a calorie intake goal of 1200 to 1800 kcal per day (with <30% of calories from fat and >15% from protein), meal-replacement products, and a physical activity goal of at least 175 minutes of moderate-intensity activity per week. Participants assigned to the DSE group received 3 group sessions per year that focused on diabetes education, diet, exercise, and social support for years 1 through 4 of the trial. In subsequent years, the frequency was reduced to 1 session annually. Participant’s diabetes and cardiovascular risk factors were managed by their health care providers outside of Look AHEAD, with the exception of temporary changes in glucose-lowering medications that were made by study staff according to a specific algorithm to reduce the risk of hypoglycemia in the ILI group during periods of weight loss. Results of risk factor assessment at annual Look AHEAD data collection visits were provided to primary care physicians along with reminders of American Diabetes Association recommended targets for risk factor management. At the end of the study, mean weight loss from baseline was 6.0% in the ILI group and 3.5% in the DSE group; similarly, at year 4, physical fitness improved 3.7% in the ILI group and decreased 2.0% in the DSE group.17
On September 14, 2012, the intervention was terminated on the basis of a futility analysis and the subsequent recommendation from the Data and Safety Monitoring Board.17 For the present analysis, we included AF cases identified through that date.
Covariate Assessment
At baseline and at subsequent annual visits, height and weight were measured in duplicate using a digital scale and stadiometer. Cardiorespiratory fitness was assessed at baseline, and years 1 and 4 using a graded exercise test. Details of the exercise tests and assessment of cardiorespiratory fitness in the Look AHEAD trial have been published elsewhere.21 Briefly, a maximal graded exercise test was administered at baseline and a submaximal test at years 1 and 4. In both the maximal and submaximal exercise tests, cardiorespiratory fitness was defined similarly as the estimated metabolic equivalent (MET) level based on the treadmill workload (speed and grade) in which 80% of the maximal heart rate was attained among participants not taking β-blockers or a rating of 16 was reached on the rating of perceived exertion scale. Baseline-year 1 changes in fitness were computed as the percent difference between MET levels estimated using the approach described above.
Age, sex, race, smoking status, education, family income, and prior history of coronary heart disease and heart failure were self-reported at baseline. HbA1c was assessed from a fasting blood sample using standard methods at a central laboratory. Blood pressure was measured in duplicate using an automated device following a standardized protocol, and the average of the two measurements was used. Participants were asked to bring all their prescription medications to their assessments.
Study Outcome
Diagnosis of AF was obtained from 2 different sources: study electrocardiograms (ECGs) and hospitalization discharge diagnosis. Information on outpatient diagnosis of AF was not available. Participants underwent standard 12-lead ECG performed by a trained technician, masked to the intervention, at baseline and subsequently every 2 years. ECGs were electronically sent to the Look AHEAD ECG reading center at EPICARE (Wake Forest School of Medicine, Winston-Salem, NC) and were processed with the GE Marquette 12-SL program (GE Marquette, Inc., Milwaukee, WI). ECGs automatically coded as AF or atrial flutter were visually inspected by a trained cardiologist to confirm the diagnosis. Only visually confirmed new cases of AF or atrial flutter were considered to be events.
Information on AF occurrence was additionally collected from hospitalization discharge summaries. During annual study visits and telephone calls every 6 months, staff interviewers unaware of study-group assignment asked participants about medical events and hospitalizations. All relevant medical records were retrieved for adjudication of the trial primary and secondary endpoints. AF was considered present if the ICD-9-CM codes 427.31 or 427.32 were listed in any hospitalization not in the context of open cardiac surgery.
Incident time to AF was the study exam date in which the arrhythmia was diagnosed or the first hospitalization with a relevant ICD-9-CM discharge code, whichever occurred earlier.
Statistical Analyses
We estimated the effect of the ILI (vs the DSE intervention) on AF incidence using multivariable Cox proportional hazards regression, with time to AF as the main outcome variable. Time of follow-up was defined as the time in days from baseline to the earliest of AF incidence, death, lost to follow-up, last outcomes ascertainment or September 14, 2012, whichever was earlier. An initial model only adjusted for clinical site. A second model adjusted for clinical site, age, sex, and race. A third model adjusted additionally for education, family income, smoking, BMI, height, systolic and diastolic blood pressure, use of antihypertensive medications, HbA1c, prevalent coronary heart disease, and prevalent heart failure (all variables assessed at baseline). We tested the proportional hazards assumption including interaction terms between intervention status and time. No violations were observed. To assess the impact of method of case ascertainment on the estimates of association, we conducted additional analyses using AF events identified through each source (study ECG, hospital discharge codes) in separate models. We explored whether baseline BMI (dichotomized at 31.5 kg/m2, the median value) or prevalent cardiovascular disease modified the effect of the ILI intervention including multiplicative terms between the effect modifier and the intervention group in the model and also performing a stratified analysis.
To assess the association of weight loss and improved fitness during the first year with the incidence of AF, we fit similar Cox models starting follow-up at the time of the year 1 visit, excluding individuals with AF occurring before that visit. Weight loss and improved fitness were categorized in quintiles, with the lowest quintile used as reference in multivariable models. P-values for trend were calculated modeling the quintile as a continuous variable. Additional models included weight loss and improved fitness as continuous variables. Finally, since previous observational studies have reported an interaction between physical activity and adiposity,10, 22 we assessed the joint association of change in weight and physical fitness by categorizing participants into nine categories based on separate tertiles of weight change and physical fitness change, with those in the bottom tertile of both variables as the reference group.
Funding
Funded by the National Institutes of Health and other Department of Health and Human Services agencies through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
RESULTS
Of the 5145 participants randomized, 61 were excluded due to presence of prevalent AF at baseline and an additional 17 were excluded because AF was reported solely on the basis of a severe adverse event during the follow-up. Table 1 shows baseline characteristics among 5067 eligible participants by intervention assignment. During a mean (median) follow-up of 9.0 (9.6) years, 294 cases of new-onset AF were identified. Of these 294 cases, 272 were identified from discharge hospitalizations and 97 from study ECGs (75 cases were identified through both sources) (Supplementary Table S1). The associations of selected baseline characteristics with risk of AF in this sample of overweight individuals with type 2 diabetes were similar to those observed in other populations (Table 2). Older age, higher BMI and height, use of antihypertensive medications, higher levels of HbA1c, and prevalent coronary heart disease were associated with significantly higher rates of AF.
Table 1.
Baseline characteristics of study participants by intervention group, Look AHEAD, 2001-2004
| % or Mean (SD) | ||
|---|---|---|
| DSE | ILI | |
| N | 2539 | 2528 |
| Age, years | 59 (7) | 59 (7) |
| Women | 60% | 60% |
| Race | ||
| White | 63% | 63% |
| Black | 16% | 16% |
| Hispanic | 13% | 13% |
| Other | 8% | 8% |
| Completed high school | 93% | 93% |
| Family Income | ||
| < $20K | 12% | 13% |
| $20K-$40K | 21% | 21% |
| $40K-$60K | 20% | 21% |
| $60K-$80K | 16% | 16% |
| > $80K | 30% | 29% |
| Current smoking | 4% | 5% |
| Baseline maximum METS | 7.2 (2.0) | 7.2 (2.0) |
| Body mass index, kg/m2 | 36.0 (5.8) | 35.9 (6.0) |
| Height, m | 1.67 (0.10) | 1.67 (0.10) |
| Systolic Blood pressure, mmHg | 129 (17) | 128 (17) |
| Diastolic Blood pressure, mmHg | 70 (10) | 70 (10) |
| Use of antihypertensive medication | 72% | 73% |
| Use of statins | 45% | 45% |
| HbA1c, % | 7.3 (1.2) | 7.2 (1.1) |
| Prevalent CHD | 11% | 11% |
| Prevalent heart failure | 1% | 1% |
Table 2.
Association of selected baseline characteristics with risk of AF, Look AHEAD, 2001-2012
| Variable | HR (95% CI) |
|---|---|
| Age, per 5 years | 1.45 (1.29, 1.64) |
| Sex (male vs female) | 1.19 (0.77, 1.84) |
| Race/ethnicity | |
| White | 1 (ref.) |
| Black | 0.60 (0.39, 0.93) |
| Hispanic | 0.65 (0.30, 1.39) |
| Other | 1.10 (0.54, 2.25) |
| Body mass index, per 5 kg/m2 | 1.23 (1.08, 1.39) |
| Baseline physical fitness, per 1 MET | 0.90 (0.82, 0.99) |
| Height, per 10 cm | 1.59 (1.28, 1.96) |
| Systolic blood pressure, per 15 mmHg | 1.05 (0.92, 1.20) |
| Diastolic blood pressure, per 10 mmHg | 0.96 (0.80, 1.15) |
| Use of antihypertensive medication | 1.49 (1.04, 2.15) |
| Hb1Ac, per 1% increase | 1.14 (1.02, 1.27) |
| Prevalent CHD | 1.75 (1.27, 2.39) |
| Prevalent heart failure | 2.05 (0.82, 5.10) |
Multivariable Cox model simultaneously adjusted for variables in the table, education, income, intervention group, smoking status, and study site. CHD: Coronary heart disease; CI: Confidence interval; HbA1c: Glycated hemoglobin; HR: Hazard ratio
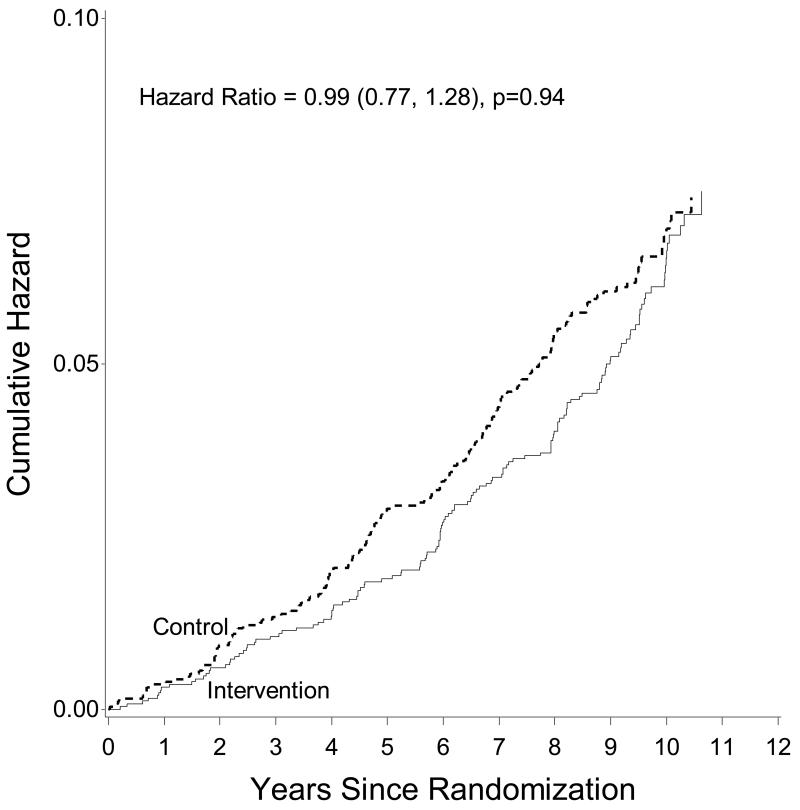
Incidence of AF was comparable in both intervention arms (6.1 per 1,000 person-years in ILI and 6.7 per 1,000 person-years in DSE, p=0.42 for the comparison of the two rates). In fully-adjusted multivariable models, ILI did not affect the risk of developing newly-diagnosed AF compared to DSE (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.77-1.28) (Table 3 and Figure 1). A similar lack of effect was observed in additional analyses considering AF cases ascertained through each independent source, that is, study ECGs and hospital discharges (Supplementary Table S2). The effect of the ILI intervention did not differ by levels of baseline BMI (Supplementary Table S3), baseline physical fitness (Supplementary Table S4), or by prevalent CVD at baseline (Supplementary Table S5), p>0.05 for interaction in all analyses.
Table 3.
Effect of intervention on risk of AF, Look AHEAD, 2001-2012
| DSE | ILI | ||
|---|---|---|---|
| AF events, n | 153 | 141 | |
| Person-years | 22,691 | 22,957 | |
| Incidence rate, per 1000 person-year | 6.7 | 6.1 | |
| HR (95% Confidence interval) | P-value | ||
| Model 1 | 1 (ref.) | 0.91 (0.72, 1.14) | 0.42 |
| Model 2 | 1 (ref.) | 0.92 (0.73, 1.16) | 0.47 |
| Model 3 | 1 (ref.) | 0.99 (0.77, 1.28) | 0.94 |
DSE: Diabetes and Support Education group; HR: Hazard ratio; ILI: Intensive lifestyle intervention Model 1: Adjusted for clinic. Model 2: Adjusted for clinic, age, sex, and race. Model 3: Adjusted for clinic, age, sex, race, education, family income, smoking, body mass index, height, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, glycated hemoglobin, prevalent coronary heart disease, and prevalent heart failure.
Figure 1.
Cumulative hazard of atrial fibrillation by intervention group, Look AHEAD, 2001-2012
We examined whether weight loss and improved fitness during the first year after randomization was associated with the risk of AF. Compared to participants in the bottom quintile of weight loss, those in the top quintile did not have a significantly lower risk of AF (HR 0.70, 95%CI 0.41-1.18), after adjustment for multiple potential confounders (Table 4). Results were essentially unchanged when we used absolute weight loss rather than percent weight loss (data not shown). Similar lack of significant associations was observed for improved fitness (HR 0.88, 95%CI 0.55-1.43, comparing top versus bottom quintile; Table 5). We also explored the joint association of change in weight and physical fitness with the risk of AF categorizing individuals by tertiles of both variables. Risk of AF was not significantly different in any of these categories compared to individuals in the lowest tertiles of change in weight and physical fitness (Supplementary Table S6). Change in weight and change in physical fitness, modeled as continuous variables, did not interact in their association with AF (p for interaction = 0.80).
Table 4.
Association of weight change during first year of follow-up with subsequent risk of AF, Look AHEAD, 2001-2012
| Quintiles of weight change in first year of follow-up | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-value* | ||
| Range (% weight loss) | 29.2,0.9 | 0.9,−2.0 | −2.0,−5.2 | −5.2,−10.2 | −10.2,−43.4 | ||
| AF events, n | 54 | 53 | 60 | 46 | 51 | ||
| Person-years | 8750 | 8920 | 8796 | 8815 | 8874 | ||
| Incidence, per 1000 py | 6.2 | 5.9 | 6.8 | 5.2 | 5.8 | ||
| Hazard ratio (95% confidence interval) | 1-SD** | ||||||
| Model 1 | 1 (ref.) | 0.95 (0.65, 1.38) | 1.11 (0.77, 1.61) | 0.84 (0.57, 1.24) | 0.91 (0.62, 1.33) | 0.48 | 0.98 (0.87, 1.11) |
| Model 2 | 1 (ref.) | 0.88 (0.60, 1.29) | 1.07 (0.74, 1.55) | 0.80 (0.54, 1.19) | 0.78 (0.53, 1.14) | 0.18 | 0.95 (0.83, 1.06) |
| Model 3 | 1 (ref.) | 0.90 (0.59, 1.38) | 1.09 (0.71, 1.67) | 0.79 (0.48, 1.30) | 0.70 (0.41, 1.18) | 0.20 | 0.93 (0.78, 1.10) |
Model 1: Adjusted for clinic. Model 2: Adjusted for clinic, age, sex, and race. Model 3: Adjusted for clinic, age, sex, race, intervention group, education, family income, smoking, body mass index, height, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, glycated hemoglobin, prevalent CHD, and prevalent HF.
P-value obtained from including quintile of weight loss as a continuous variable in the Cox model.
1-SD: Hazard ratio and 95% CI of AF per 1-standard deviation (7% weight loss) change in weight.
Table 5.
Association of fitness change during first year of follow-up with incidence of atrial fibrillation, hazard ratios (95% confidence intervals), Look AHEAD, 2001-2012
| Improved fitness in first year of follow-up | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-value* | ||
| Range (% improved fitness) | −52.9, −6.9 | −6.8, 0 | 0.1, 13.5 | 13.6, 31.7 | 31.8, 185.7 | ||
| AF events, n | 53 | 54 | 41 | 47 | 41 | ||
| Person-years | 7948 | 8613 | 7300 | 8118 | 7982 | ||
| Incidence, per 1000 py | 6.3 | 6.3 | 5.6 | 5.8 | 5.1 | ||
| Hazard ratio (95% confidence interval) | 1-SD** | ||||||
| Crude | 1 (ref.) | 0.91 (0.62, 1.34) | 0.83 (0.55, 1.26) | 0.87 (0.58, 1.29) | 0.76 (0.50, 1.15) | 0.20 | 0.89 (0.78, 1.03) |
| Age, sex, race adjusted | 1 (ref.) | 0.88 (0.60, 1.30) | 0.89 (0.59, 1.34) | 0.89 (0.60, 1.33) | 0.80 (0.53, 1.22) | 0.37 | 0.92 (0.80, 1.05) |
| Multivariable adjusted | 1 (ref.) | 0.82 (0.53, 1.26) | 0.86 (0.55, 1.36) | 0.89 (0.56, 1.39) | 0.88 (0.55, 1.43) | 0.76 | 0.98 (0.84, 1.16) |
Model 1: Adjusted for clinic. Model 2: Adjusted for clinic, age, sex, and race. Model 3: Adjusted for clinic, age, sex, race, intervention group, education, family income, smoking, body mass index, height, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, glycated hemoglobin, prevalent coronary heart disease, and prevalent heart failure.
P-value obtained from including quintile of fitness change as a continuous variable in the Cox model.
1-SD: Hazard ratio and 95% CI of AF per 1-standard deviation (27% improved fitness) change in improved fitness.
DISCUSSION
In this secondary analysis of the Look AHEAD randomized trial, an ILI designed to induce weight loss in people with type 2 diabetes did not reduce the risk of newly-diagnosed AF compared to a control intervention. These findings are consistent with the results for the Look AHEAD primary endpoint, in which the ILI did not reduce the risk of a composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and hospitalized angina (HR 0.95, 95%CI 0.82, 1.09).17 The lack of effect was consistent across subgroups of baseline BMI or prevalent CVD. Similarly, we did not find a significant association between weight loss or improved fitness during the first year of the intervention and the incidence of AF thereafter.
Numerous prospective cohorts have identified an increased risk of AF in overweight and obese individuals, compared to those with normal weight.5-10, 23 Moreover, weight gain has also been found to be linked with increased AF risk. In the ARIC study, men who gained >10% of baseline weight over a 9 year period experience a 61% higher rate of AF compared to those gaining <5% (no association was found for weight gain and AF in women).10 Similarly, in the Women’s Health Study, risk of AF in women who became obese during a 5 year period was 41% higher during subsequent follow-up than among those who maintained BMI <30 kg/m2.9 Additional evidence has shown that higher levels of physical activity could ameliorate the adverse association between increased body mass and AF risk, with obesity being a weaker risk for AF among those physically more active.10, 22, 24 Given the association between overweight/obese status and an increased risk of AF, we expected to observe a lower risk of AF with weight reduction. However, we failed to see a difference in the risk of AF between the ILI and DSE interventions, despite the fact that the ILI resulted in modest reduction in body weight. Additionally, we did not observe a statistically significant lower risk of AF in the subgroup of participants who lost the most weight or experienced the greatest improvement in physical fitness. To our knowledge, this analysis is the first to report the effect of a weight loss-focused lifestyle intervention in the risk of newly-diagnosed AF.
The lack of effect of weight loss and lifestyle modification in the Look AHEAD trial seems to be at odds with results from randomized and observational studies exploring the association of weight reduction lifestyle interventions in individuals with existing AF.15, 16 In a randomized trial including 150 AF patients, Abed and colleagues showed that participants assigned to a weight loss intervention, compared to those in the control group (general lifestyle advice), experienced significant reductions in symptom burden scores, symptom severity scores, number of AF episodes, cumulative duration of AF, as well as reductions in interventricular septal thickness and left atrial area.15 Similarly, the observational LEGACY Study reported that a sustained weight loss >10% was associated with lower arrhythmia burden and maintenance of sinus rhythm.16 However, the participants in these studies were different from those in the Look AHEAD trial (patients with AF versus AF-free individuals with diabetes) and the degree of weight loss with the intervention was considerably higher in the LEGACY and Abed studies (>10%) compared to the Look AHEAD trial (6% at the end of the trial).
In light of the previous evidence, why was the ILI intervention in the Look AHEAD trial ineffective in reducing the risk of AF? Several reasons can be offered. First, the modest weight loss achieved in the intervention group and the minimal difference between groups may have not been enough to reduce the risk of AF. Second, as shown in the manuscript reporting the primary Look AHEAD results, use of cardioprotective drugs was lower in the ILI group compared to the DSE group, which could have contributed to the null results.17, 25 Third, epidemiologic studies have found that very high levels of physical activity, such as that found in endurance athletes, are associated with increased risk of AF.12 Higher risk of AF associated with increased physical activity in Look AHEAD participants may have masked a potential protective effect of weight loss. This mechanism, however, is unlikely given that the achieved levels of physical activity, generally brisk walking, are not comparable to those from studies in athletes, and also considering the lack of association of changes in physical fitness with AF risk in the Look AHEAD trial. Fourth, the lifestyle modification in the Look AHEAD trial included recommendations to consume 30% or fewer calories from fat.18 Though there is no direct evidence to suggest that following this particular dietary advice could increase AF risk, other dietary patterns, such as the Mediterranean diet, have demonstrated efficacy in the primary prevention of AF compared to low-fat diets.26 Fifth, all participants in Look AHEAD had type 2 diabetes. Therefore, our results do not rule out that a similar weight loss intervention could be effective in persons without diabetes. Sixth, we could not separate weight loss due to the intervention from that due to underlying comorbidities that may increase AF risk. Seventh, a potential effect of weight loss on the risk of AF may occur in a longer timeframe than that provided by the Look AHEAD trial, requiring extended follow-up. Finally, due to the limited number of events, our results cannot exclude a small effect of the intervention on AF risk.
The existing epidemiologic evidence suggests a complex interplay between physical activity, cardiorespiratory fitness, and the risk of AF. Very high levels of physical activity may be associated with increased risk of AF, particularly among younger individuals,12, 27 while other studies have shown that moderate levels of physical activity could be protective.10, 28 Our results, even though not showing a protective effect of increased cardiorespiratory fitness, suggest that moderate increases in physical activity levels are not associated with increased risk of AF. These findings should reassure clinicians and patients that initiating an exercise program, even in a high-risk population as people with type 2 diabetes, will not likely increase their risk of developing AF.
Incidence rates of AF in Look AHEAD (6.1-6.7 per 1000 person-years) were lower than AF rates observed in persons with diagnosed diabetes in the ARIC cohort (14.8 per 1000 person-years).29 Differences in AF ascertainment, in the age and race composition, in the proportion of patients with diabetes using insulin (<30% by design in Look AHEAD), and in the distribution of cardiovascular risk factors between the two studies make this comparison problematic. However, in agreement with results from observational studies in community-based cohorts,30 we identified age, white race, higher BMI and height, use of antihypertensive medication, and prevalent coronary heart disease as risk factors for the incidence of AF in the Look AHEAD trial. Higher levels of HbA1c at baseline were also associated with increased AF incidence, similar to findings in other populations.29, 31
Strengths and limitations
The current analysis has major strengths, such as being performed in the context of a large, well-conducted randomized trial with long follow-up. The study intervention led to clinically significant and sustained changes in weight and fitness and the ascertainment of AF cases was based on two independent sources.
While Look AHEAD had many strengths, there are limitations. AF was not a primary trial endpoint, and the specific analyses of AF were not pre-specified at the beginning of the trial. In addition, our method of AF ascertainment is certain to miss some individuals with paroxysmal or asymptomatic AF. Nonetheless, previous studies suggest that AF ascertainment using hospital discharge codes, one of the sources for case identification in the Look AHEAD trial, has adequate sensitivity and positive predictive value,32, 33 and results were similar across different definitions of incident AF. Also, the number of events was probably insufficient to detect small effects: the estimated 95% confidence intervals are consistent with effects ranging from a 23% AF risk reduction to a 28% AF risk increase. The current results are from a secondary analysis of a completed trial, and future studies specifically designed to test the effect of lifestyle interventions on AF risk should be performed. Lastly, the results from the Look AHEAD trial may not generalize to individuals who do not have type 2 diabetes.
Conclusion
To conclude, among overweight and obese individuals with type 2 diabetes, an ILI that induced a modest weight loss did not reduce the risk of AF compared to diabetes support and education. Future studies should explore the inconsistencies between our results and those from previous interventional and observational studies.
Supplementary Material
Acknowledgments
The full membership of the Look AHEAD Research Group is shown in the Appendix. We thank Andrea Anderson, MS, for performing some of the statistical analysis.
Role of the Funding Source
The primary sponsor, the NIDDK, was represented on the Steering Committee and played a part in design and management of the Look AHEAD trial. The statistician (S.G.) had access to the raw data. Alvaro Alonso had full access to all results. All authors shared the final responsibility to submit for publication.
Funding and Support
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: clinicaltrials.gov identifier: NCT00017953
DISCLOSURES
None
REFERENCES
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation. The Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayana K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y-H, McAnulty JH, Jr., Zheng Z-J, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJL. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis Je, Ellinor PT, Benjamin EJ. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013;138:401–405. doi: 10.1161/CIRCULATIONAHA.113.001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr., Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 8.Dublin S, French B, Glazer NL, Wiggins K, Lumley T, Psaty BM, Smith NL, Heckbert SR. Risk of new-onset atrial fibrillation in relation to body mass index. Arch Intern Med. 2006;166:2322–2328. doi: 10.1001/archinte.166.21.2322. [DOI] [PubMed] [Google Scholar]
- 9.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation: the Women’s Health Study. J Am Coll Cardiol. 2010;55:2319–2327. doi: 10.1016/j.jacc.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities Study. Circ Arrhythm Electrophysiol. 2014;7:620–625. doi: 10.1161/CIRCEP.113.001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace. 2009;11:11–17. doi: 10.1093/europace/eun289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al-Mallah MH. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation. 2015;131:1827–1834. doi: 10.1161/CIRCULATIONAHA.114.014833. [DOI] [PubMed] [Google Scholar]
- 14.Khan H, Kella D, Rauramaa R, Savonen K, Lloyd MS, Laukkanen JA. Cardiorespiratory fitness and atrial fibrillation: a population-based follow-up study. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.03.024. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: A randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 16.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term Effect of Goal directed weight management in an Atrial fibrillation Cohort: a long-term follow-up studY (LEGACY Study) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Look AHEAD Research Group Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diabetes and Vascular Disease Research. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Look AHEAD Research Group. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG, Ribisl PM, Look AHEAD Study Group Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. International Journal of Obesity. 2009;33:305–316. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, Manson JE, Albert CM, LaMonte MJ, Larson JC, Li W, Martin LW, Nassir R, Garcia L, Assimes TL, Tharp KM, Hlatky MA, Perez MV. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3:e001127. doi: 10.1161/JAHA.114.001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Grundvold I, Skretteberg PT, Liestøl K, Gjesdal K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Importance of physical fitness on predictive effect of body mass index and weight gain on incident atrial fibrillation in healthy middle-aged men. Am J Cardiol. 2012;110:425–432. doi: 10.1016/j.amjcard.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC. Do lifestyle changes reduce serious outcomes in diabetes? N Engl J Med. 2013;369:189–190. doi: 10.1056/NEJMe1306987. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Gonzalez MA, Toledo E, Arós F, Fiol M, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fernández-Crehuet J, Lapetra J, Muñoz MA, Fitó M, Serra-Majem L, Pintó X, Lamuela-Raventos RM, Sorlí JV, Babio N, Buil-Cosiales P, Ruiz-Gutiérrez V, Estruch R, Alonso A. Extra-virgin olive oil consumption reduces risk of atrial fibrillation: the PREDIMED trial. Circulation. 2014;130:18–26. [Google Scholar]
- 27.Aizer A, Gaziano JM, Cook NR, Manson JE, Buring JE, Albert CM. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol. 2009;103:1572–1577. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2008;118:800–807. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98:133–138. doi: 10.1136/heartjnl-2011-300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JoD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF Consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–858. doi: 10.1007/s11606-010-1340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.