Abstract
Background:
Melissa officinalis, an herbal drug, is well known and frequently applied in traditional and modern medicine. Yet, there is inadequate information regarding its effects on electrical properties of the heart. The present study attempted to elucidate the effects of Melissa officinalis aqueous extract on electrocardiogram (ECG) in rat.
Objectives:
ECG is an easy, fast and valuable tool to evaluate the safety of used materials and drugs on heart electrical and conductivity properties. Many drugs with no cardiovascular indication or any overt cardiovascular effects of therapeutic dosing become cardiotoxic when overdosed (16). On the other hand, there are numerous substances and drugs that can cause ECG changes, even in patients without a history of cardiac disease. Therefore, this study was conducted to elucidate safety and outcome of one-week administration of M. officinalis aqueous extract on blood pressure and ECG parameters of rats.
Materials and Methods:
Four animal groups received tap water (control group), aqueous extracts of Melissa officinalis 50 (M50), 100 (M100) and 200 (M200) mg/kg/day, respectively and orally for a week. ECG and blood pressure were recorded on the eighth day of experiment.
Results:
Consumption of Melissa officinalis extract associated with prolonged QRS interval (P < 0.05 for M50 and M100 groups and P < 0.01 for M200 group versus the control group, respectively), prolonged QTc and JT intervals (P < 0.01 for different M groups versus the control group) and prolonged TpTe interval (P < 0.001 when M groups compared with the control group) of ECG. However, different doses of the extract had no significant effect on RR interval, PR interval, amplitudes of ECG waves, heart rate and blood pressure.
Conclusions:
For the first time, this study revealed that consumption of Melissa officinalis extract is associated with significant ECG alterations in rat. Future studies are necessary to determine potential clinical outcomes.
Keywords: Melissa officinalis, Electrocardiography, Blood Pressure
1. Background
Melissa officinalis (M. officinalis) belongs to the genus Melissa (family Lamiaceae) (1) known as “Badrangboya” in Persian, “Mufarrehal qhalb” and “Utrajul Raihath” in Arabic, “Billi lotan” in Hindi, “Mountain balm” and “Lemon balm” in English (2). It is a salient medicinal and commercial herb popular worldwide (3). M. officinalis is used in traditional medicine for various illnesses such as menstrual problems, hypertension, migraines, vertigo and fever (4, 5), depression and melancholy (4), bronchitis and asthma (6), eczema and gout (4), epilepsy, paralysis, bell's palsy and arthritis (7). Advanced researches showed its neuroprotective (8), anxiolytic (9, 10), antispasmodic (11), antihyperlipidemic and hepatoprotective effects (12). The beneficial effect of lemon balm on Alzheimer’s disease (13) and heart palpitation was also reported (4). Gazola et al. (14) indicated negative chronotropic but no inotropic effect of M. officinalis extract on isolated rat heart. In an experimental study, we showed that acute injection of M. officinalis aqueous extract had a mild protective effect against reperfusion-induced lethal ventricular arrhythmias in rats (15). Nowadays, M. officinalis is commonly used for its therapeutic properties; however, there is a lack of information about its long intake on electrical properties of the heart.
ECG is an easy, fast and valuable tool to evaluate the safety of used materials and drugs on heart electrical and conductivity properties. Many drugs with no cardiovascular indication or any overt cardiovascular effects of therapeutic dosing, become cardiotoxic when overdosed (16). On the other hand, there are numerous substances and drugs that can cause ECG changes, even in patients without a history of cardiac disease.
2. Objectives
Therefore, this study was conducted to elucidate the safety and outcome of one-week administration of M. officinalis aqueous extract on blood pressure and ECG parameters of rats.
3. Materials and Methods
The present study was conducted in accordance with the guidelines of animal studies provided by the Ethics committee permission No K/90/486, Kerman University of Medical Sciences, Kerman, Iran.
3.1. Extract Preparation
Melissa officinalis was collected in spring from Sirjan area (Kerman, Iran). After its identification, a voucher specimen (KF1429-1) was delivered to the Herbarium Center, Faculty of Pharmacy, and Kerman University of Medical Sciences. The dried aerial parts of the plants were ground and powdered to a particle size of about 0.5 mm. Later, 10 mL boiling distilled water was added to the powder and left for 10 minutes to brew the M. officinalis. The mixture was then filtered and its liquid evaporated under vacuum at 45 - 50°C. The resulting material was then dried completely at 70°C (14, 15). Finally, the prepared extract was stored in glass vials and kept at 20°C before use. On each day of the experiment, the required amounts of extract were weighed, dissolved in distilled water and then orally given to the animals.
3.2. Animals Groups and Experimental Protocol
Thirty-two male Wistar rats (250 - 300 grams) were purchased and kept in appropriate condition of temperature (21 ± 2°C), light-dark cycle (12/12 hour) and free access to rat regular diet and water. After adaptation, they were divided into four groups (8 in each) and were treated for seven days as: Control (CTL) group, which was gavaged with 1mL of distilled water/daily (equal to the volume of the extract), Melissa officinalis 50 (M 50), Melissa officinalis100 (M 100) and Melissa officinalis 200 (M 200) groups, which were gavaged with the aqueous extract of Melissa officinalis 50, 100 and200 mg/kg/daily, respectively. These doses of extract were chosen based on previous in vivo studies.
On the eighth day, animals were anesthetized with 50 mg/kg sodium thiopental (i.p.) (17). Selection of the study period, surgical preparation and recording method of parameters were performed based on previous studies (18). Briefly, the trachea of the animal was cannulated while spontaneous breathing was allowed throughout the experiment. The left common carotid artery was cannulated with a filled catheter (saline with 15 IU/mL heparin). The catheter was connected to a pressure transducer of a PowerLab system (AD Instruments, Australia) to allow arterial pressure recording. After connection of electrodes, standard limb lead II of ECG and arterial blood pressure (BP) were recorded following recovery time from surgery (15 minutes). ECG parameters (RR, PR, QRS, QTc, JT and TpTe intervals and waves amplitude) were assessed using ECG analysis module for PowerLab and LabChart from five minutes continuous ECG recorded strip. PR interval embraces the atrial depolarization (P wave) plus the duration of the electric current conduction through AV node and the specialized His-Purkinje system to the ventricles. It is the interval time from the P-wave onset to QRS complex (Q- or R wave) onset (19). The QT interval includes both depolarization and repolarization of the heart and it is the interval time from the earliest Q or R-wave onset to the end of T wave. By using the Bazett’s formula normalized as QTcn - B = QT/(RR/ f) 1/2, a modified and applicable formula for QT correction in rat, corrected QT (QTc) interval was calculated to prevent the dependence of QT interval on heart rate. RR is R-R interval and f = 150 ms (20). JT interval an interval from junction point J to T wave end and T peak to T end interval (TpTe), the period from the peak of the T wave until the end of the T wave, were also calculated.
3.3. Statistical Analysis
Data was shown as Mean ± SD. Normal distribution of data was confirmed by Kolmogorov-Smirnov test. One-way ANOVA and the post hoc Tukey test were used to compare data using IBM SPSS Statistics 20 for Windows (IBM Inc., Armonk, NY, USA). The significance level was set at 0.05.
4. Results
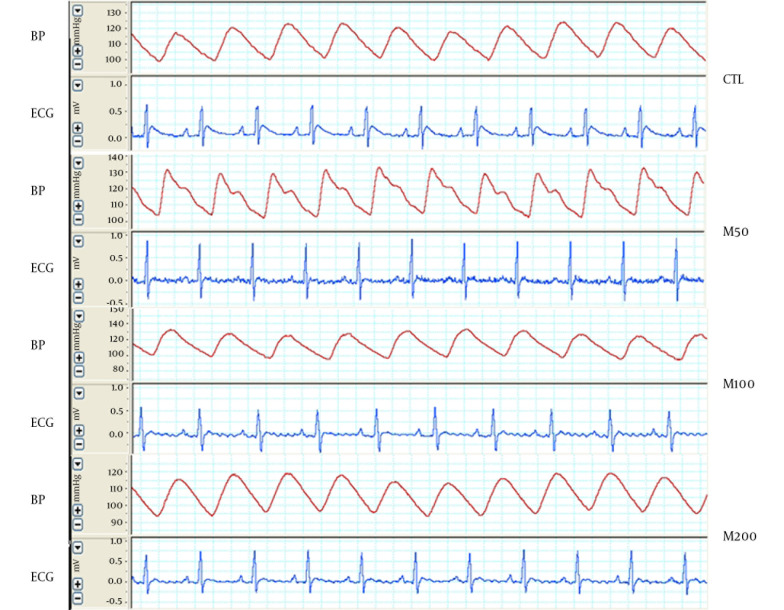
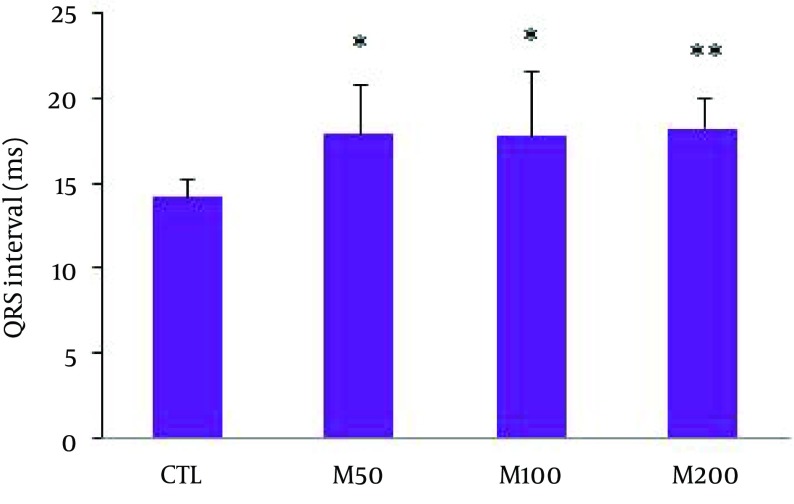
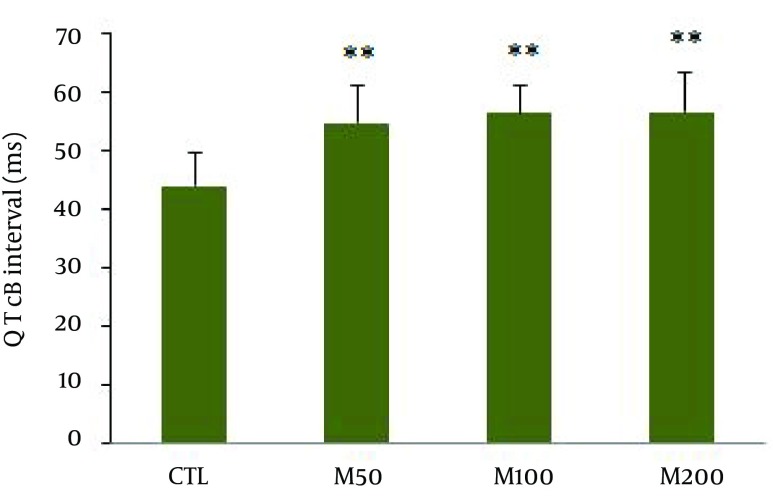
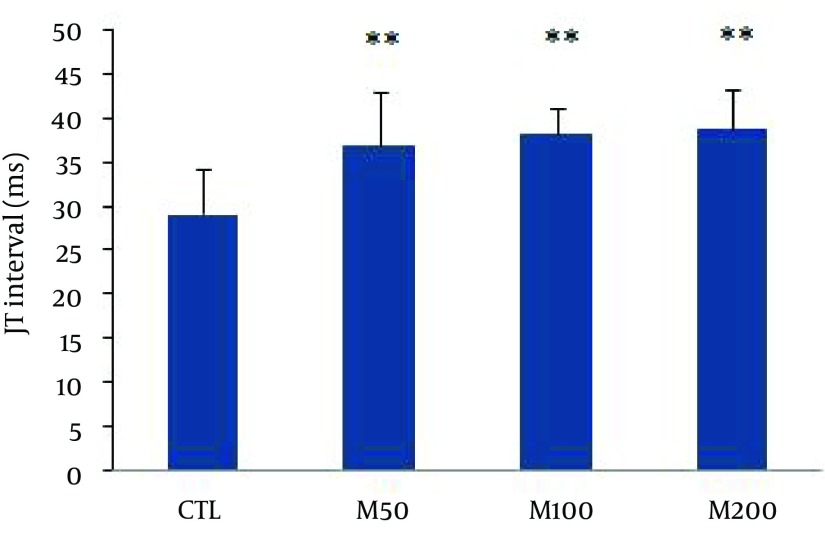
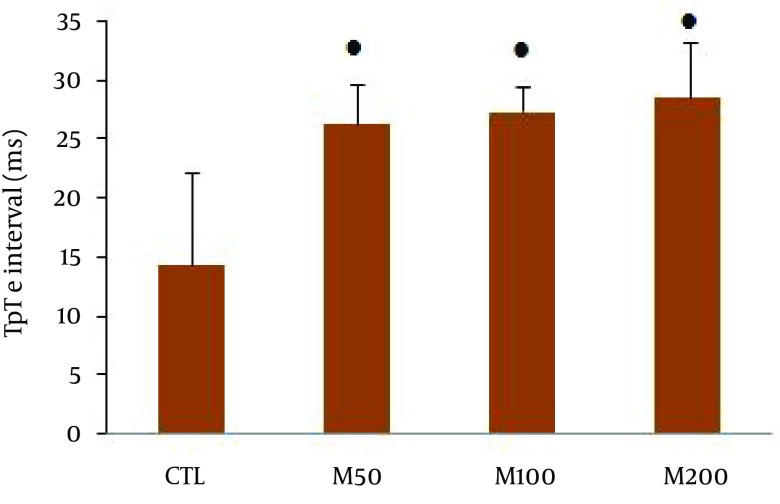
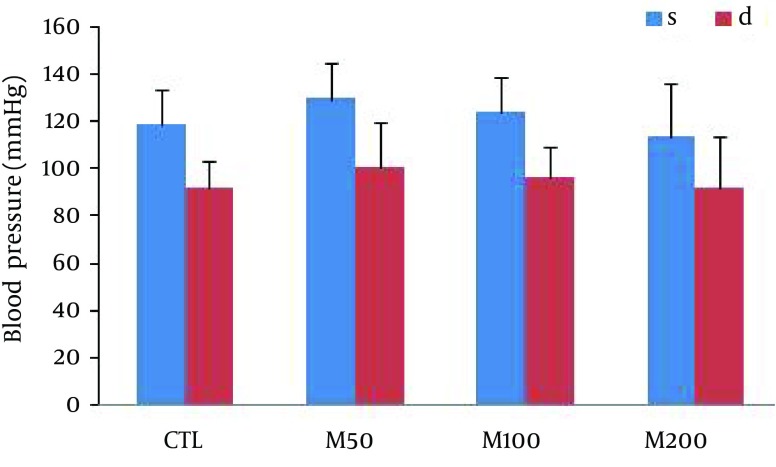
Figure 1 demonstrates samples of the traces of the arterial blood pressure and lead II of ECG recorded simultaneously in a rat selected from each of the animal groups. Administration of different doses of M. officinalis extract did not induce significant alteration in the heart rate and also RR interval, P duration, P amplitude, Q amplitude, R amplitude, S amplitude, T amplitude and ST height of ECG in rats. M. officinalis extract was associated with an incremental trend in PR interval; however, this alteration was not statistically significant (Table 1). In addition, M. officinalis extract significantly prolonged the QRS interval (P < 0.05 for M50 and M100 group and P < 0.01 for M200 group versus CTL group, respectively, Figure 2), the QTc and JT intervals (P < 0.01 for different M groups than the CTL group, Figures 3 and 4), also the TpTe interval (P < 0.001 when M groups were compared with the CTL group, Figure 5). In the present study, different doses of M. officinalis extract had no significant effect on blood pressure (Figure 6).
Figure 1. The Strips of Arterial Blood Pressure and Lead II of ECG Simultaneously Recorded From an Animal in Each Group.
CTL: the control group, M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract.
Table 1. Heart Rate and Some ECG Parameters of Animal Groups a.
| Groups | Number | Heart rate, Beat/Min | RR Interval, ms | PR Interval, ms | P Duration, ms | P Amplitude, mV | Q Amplitude, mV | R Amplitude, mV | S Amplitude, mV | ST Height, mV | T Amplitude, mV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTL b | 8 | 393 ± 25 | 151 ± 13 | 42 ± 2 | 15 ± 4 | 0.09 ± 0.04 | -0.02 ± 0.01 | 0.66 ± 0.24 | -0.41 ± 0.15 | 0.03 ± 0.06 | 0.07 ± 0.08 |
| M50 b | 8 | 400 ± 45 | 152 ± 17 | 44 ± 4 | 18 ± 2 | 0.11 ± 0.03 | -0.02 ± 0.02 | 0.78 ± 0.22 | -0.32 ± 0.11 | 0.03 ± 0.04 | 0.08 ± 0.02 |
| M100 b | 8 | 395 ± 16 | 152 ± 6 | 46 ± 3 | 17 ± 3 | 0.13 ± 0.02 | -0.02 ± 0.01 | 0.85 ± 0.19 | -0.38 ± 0.09 | 0.04 ± 0.04 | 0.09 ± 0.02 |
| M200 b | 8 | 390 ± 31 | 155 ± 13 | 45 ± 4 | 16 ± 3 | 0.13 ± 0.02 | -0.02 ± 0.01 | 0.84 ± 0.08 | -0.37 ± 0.13 | 0.04 ± 0.05 | 0.09 ± 0.03 |
aValues are presented as Mean ± SD.
bCTL: control, M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract, n = 8. Differences were not significant among the groups.
Figure 2. QRS Interval in All Groups.
Values are expressed in Mean ± SD, CTL: the control group, M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract. n = 8, * P < 0.05 and ** P < 0.01 vs. CTL group.
Figure 3. Corrected QT Interval Measured Using the Bazett’s Formula Normalized in Animals Groups.
Values are expressed in Mean ± SD, CTL: the control group, M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract. n = 8, ** P < 0.01 vs. CTL group.
Figure 4. JT Interval in Animals Groups.
Values are expressed in Mean ± SD, CTL: the control group, M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract. n = 8, ** P < 0.01 vs CTL group.
Figure 5. TpTe Interval in All Groups.
Values are expressed in Mean ± SD, CTL, the control group; M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M.officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract. n = 8. ● P < 0.001 vs. CTL group.
Figure 6. Arterial Blood Pressure in Different Experimental Groups.
Values are Mean ± SD. CTL, the control group; M50: animal group which received 50 mg/kg/day of M. officinalis extract, M100: animal group which received 100 mg/kg/day of M. officinalis extract, M200: animal group which received 200 mg/kg/day of M. officinalis extract. n = 8.
5. Discussion
This study was conducted to elucidate the effect of M. officinalis extract on BP and ECG parameters of rat heart. The results showed that compared to the control group, doses of 50, 100 and 200 (mg/kg/day) M. officinalis significantly increased QRS, QTc, TpTe and JT intervals in rats.
QRS complex indicates depolarization of ventricles and its prolongation may stem from a wide variety of processes involved in the conduction system, the myocardium or both. Hypertrophic or infiltrative diseases, drugs or focal damage from infarction may be associated with prolonged QRS (19). Sodium channel blockers including some class IA or 1C antiarrhythmics by blocking inward sodium current, can slow depolarization, reduce conduction velocity, prolong the QRS interval and consequently it may increase the risk of arrhythmia (19, 21). Inward rectifier potassium channel blockers such as chloroquine cause a more positive resting membrane potential, partial inactivation of sodium channels and hence can prolong the QRS interval (22).
Based on QRS interval prolongation induced by M. officinalis extract, it is possible that consumption of this extract can slow ventricular conductivity by blocking sodium or potassium (IK1) or both currents. The blockade of the sodium channels, in a manner similar to the action of Class 1 antiarrhythmic drugs, could be potentially an antiarrhythmic effect. However, in high dosage of these drugs, this beneficial effect may convert to an arrhythmogenic adverse effect.
QTc interval is equal to the sum of depolarization and repolarization periods of the heart. JT interval is an interval in electrocardiogram made from the junction point J to the T wave end and describes the duration of ventricular repolarization (23). TpTe is a mark of transmural dissemination of repolarization in the left ventricle (24, 25). Some drugs or agents such as Class 3 antiarrhythmic drugs may induce QTc and JT prolongation by blocking the potassium channels (26). However, very long QT interval may trigger early after-depolarizations (EADs), a phenomenon that may be associated with ventricular extrasystoles and in turn increase the risk of reentry and torsade de pointes (TdP) tachyarrhythmia (27).
There are three cell types identified in the ventricular myocardium: the endocardial, epicardial and subendocardial M cells (Masonic mid-myocardial Moe cells) (28, 29). Likely, due to larger late sodium and sodium/calcium exchange currents and a weaker slowly activating delayed rectifier current (IKs) (30), the action potential of the M cells is more vulnerable to prolongation compared to the other two cell types (25). During the period of TpTe interval in which the epicardium has been repolarized, M cells are still in the process of repolarization and vulnerable to the occurrence of early after depolarizations (EADs) (31) and in turn it can lead to reentry and consequently ventricular tachycardia or ventricular fibrillation. Therefore, prolongation of QRS, JT, QTc and TpTe intervals following Melissa officinalis pretreatment, can be a double-edged sword, indicating antiarrhythmic or proarrhythmic effect. If the proarrhythmic effect be dominant, it could increase the potential vulnerability to re-entry ventricular arrhythmias (32-34) and also elevate the risk of sudden cardiac death (35, 36). In this regard, we previously showed mild anti-arrhythmic effect of single doses of Melissa officinalis (15). Recent studies have also shown the electrophysiological effects of some herbal medicine, which led to expansion of our approach in the use of herbal medicines in the field of cardiac electrophysiology. Some examples include: (Ι) suppressing and preventing effect of Wenxin Keli, a herbal extract, on atrial and ventricular arrhythmias by depression of Ito and INa-dependent electrophysiological parameters (37, 38), (ΙΙ) anti-arrhythmic effect of saffron extract (39) and its effects on the Wenckebach block cycle length and nodal functional refractory period in in vitro model (40), (ΙΙΙ) anti-arrhythmic and electrophysiological effects of European Lamiaceae Leonurus cardiaca extract as calcium channels I (Ca.L) blockade, reduction of repolarizing current I (K.r) and prolongation of action potential duration (41) and (IV) bidirectional tachycardia induced by aconites present in a Chinese herbal decoction (42).
The present study demonstrated that a week consumption of Melissa officinalis, a famous well-known herbal medicine, can cause ECG alteration in rat. Changes included prolongation of QRS, QTc and JT and TpTe intervals. These effects may result from modulation in expression or conductance properties of involving ion channels in the heart. Further studies are necessary to elucidate the exact mechanisms and determine potential clinical outcomes.
Acknowledgments
We wish to thank Mrs. Mahdavi, The head of Herbarium Center, Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran for helping in extract preparation of plant. We would like to thank Nadia Ghazanfari-Moghaddam for proofreading of the manuscript.
Footnotes
Authors’ Contributions:Haleh Asadipour contributed in preparation, treatment and training of animals. She also helped in the experimental procedure and sampling. Siyavash Joukar developed the original idea and the protocol, supervised the study, helped in experimental procedure and data analysis and also wrote the manuscript.
References
- 1.Moradkhani H, Sargsyan E, Bibak H, Naseri B, Sadat-Hosseini M, Fayazi-Barjin A, et al. Melissa officinalis L., a valuable medicine plant: A review. Med Plant Res J. 2010;4:2753–9. [Google Scholar]
- 2.Basar SN, Zaman R. An Overview of Badranjboya (Melissa officinalis). Int. Res. J Biol Sci. 2013;2:107–9. [Google Scholar]
- 3.Aharizad S, Rahimi MH, Moghadam M, Mohebalipour N. Study of genetic diversity in lemon balm (Melissa officinalis l.) populations based on morphological traits and essential oils content. Annals Biological Res. 2012;3(12):5748–53. [Google Scholar]
- 4.Lemon Balm: An Herb Society of America Guide. Kirtland, Ohio: The Herb Society of America; 2007. Available from: http://www.herbsociety.org/factsheets/Lemon%20Balm%20Guide.pdf. [Google Scholar]
- 5.Duke J. Handbook of Medicinal Herbs. Florida: CRC Press; 2002. [DOI] [Google Scholar]
- 6.Mamedov N, Craker LE. Medicinal Plants Used for the Treatment of Bronchial Asthma in Russia and Central Asia. Journal of Herbs, Spices & Medicinal Plants. 2001;8(2-3):91–117. [Google Scholar]
- 7.Kabeeruddin HM. Maghzanul Mufarradat Almaroof Khawasul Advia. 2nd ed. Delhi: Aijaz Publishing house; 2000. [Google Scholar]
- 8.Lopez V, Martin S, Gomez-Serranillos MP, Carretero ME, Jager AK, Calvo MI. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. 2009;34(11):1955–61. doi: 10.1007/s11064-009-9981-0. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy DO, Little W, Scholey AB. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm). Psychosom Med. 2004;66(4):607–13. doi: 10.1097/01.psy.0000132877.72833.71. [DOI] [PubMed] [Google Scholar]
- 10.Cases J, Ibarra A, Feuillere N, Roller M, Sukkar SG. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med J Nutrition Metab. 2011;4(3):211–8. doi: 10.1007/s12349-010-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadraei H, Ghannadi A, Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74(5):445–52. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 12.Bolkent S, Yanardag R, Karabulut-Bulan O, Yesilyaprak B. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: a morphological and biochemical study. J Ethnopharmacol. 2005;99(3):391–8. doi: 10.1016/j.jep.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 13.Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomised, placebo controlled trial. J Neurol Neurosurg Psychiatry. 2003;74(7):863–6. doi: 10.1136/jnnp.74.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazola R, Machado D, Ruggiero C, Singi G, Macedo Alexandre M. Lippia alba, Melissa officinalis and Cymbopogon citratus: effects of the aqueous extracts on the isolated hearts of rats. Pharmacol Res. 2004;50(5):477–80. doi: 10.1016/j.phrs.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Joukar S, Zarisfi Z, Sepehri G, Bashiri A. Efficacy of Melissa officinalis in suppressing ventricular arrhythmias following ischemia-reperfusion of the heart: a comparison with amiodarone. Med Princ Pract. 2014;23(4):340–5. doi: 10.1159/000363452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionte C, Bologa C, Sorodoc L. Advances in Electrocardiograms - Clinical Applications. Intech. 2014:271–97. [Google Scholar]
- 17.Joukar S, Najafipour H, Dabiri S, Sheibani V, Esmaeili-Mahani S, Ghotbi P, et al. The effect of chronic co-administration of morphine and verapamil on isoproterenol-induced heart injury. Cardiovasc Hematol Agents Med Chem. 2011;9(4):218–24. doi: 10.2174/187152511798120930. [DOI] [PubMed] [Google Scholar]
- 18.Joukar S. Electrocardiogram alterations following one-week consumption of Crocus sativus L.(saffron). EXCLI J. 2012;11:480–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Nada A, Gintant GA, Kleiman R, Gutstein DE, Gottfridsson C, Michelson EL, et al. The evaluation and management of drug effects on cardiac conduction (PR and QRS intervals) in clinical development. Am Heart J. 2013;165(4):489–500. doi: 10.1016/j.ahj.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641(2-3):187–92. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AE, Hallstrom AP, Rogers WJ, Liebson PR, Seals AA, Anderson JL, et al. Mortality following ventricular arrhythmia suppression by encainide, flecainide, and moricizine after myocardial infarction. The original design concept of the Cardiac Arrhythmia Suppression Trial (CAST). JAMA. 1993;270(20):2451–5. [PubMed] [Google Scholar]
- 22.Rodriguez-Menchaca AA, Navarro-Polanco RA, Ferrer-Villada T, Rupp J, Sachse FB, Tristani-Firouzi M, et al. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc Natl Acad Sci U S A. 2008;105(4):1364–8. doi: 10.1073/pnas.0708153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zumbakyte-Sermuksniene R, Kajeniene A, Berskiene K, Daunoraviciene A, Sedereviciute-Kandrataviciene R. Assessment of the effect of anthropometric data on the alterations of cardiovascular parameters in Lithuanian elite male basketball players during physical load. Medicina (Kaunas). 2012;48(11):566–71. [PubMed] [Google Scholar]
- 24.Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, et al. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001;50(3):454–62. doi: 10.1016/s0008-6363(01)00223-1. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, et al. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991;69(6):1427–49. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 26.Brunton L, Chabner B, Knollman B. Goodman & Gilmans The pharmacological basis of therapeutics. 12th ed. New York: McGraw–Hill; 2011. [Google Scholar]
- 27.Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153(6):891–9. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Antzelevitch C, Shimizu W, Yan G. Electrical heterogeneity and the development of arrhythmias. Dispersion of ventricular repolarization: state of the art. Armonk (NY): Futura Publishing Company, Inc. 2000:3–21. [Google Scholar]
- 29.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68(6):1729–41. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 30.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293(4):H2024–38. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41(6):567–74. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37(3):191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98(18):1928–36. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 34.Antzelevitch C, Shimizu W, Yan G, Sicouri S. Cellular basis for QT dispersion. Journal of Electrocardiology. 1998;30:168–75. doi: 10.1016/s0022-0736(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 35.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119(5):663–70. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 37.Minoura Y, Panama BK, Nesterenko VV, Betzenhauser M, Barajas-Martinez H, Hu D, et al. Effect of Wenxin Keli and quinidine to suppress arrhythmogenesis in an experimental model of Brugada syndrome. Heart Rhythm. 2013;10(7):1054–62. doi: 10.1016/j.hrthm.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burashnikov A, Petroski A, Hu D, Barajas-Martinez H, Antzelevitch C. Atrial-selective inhibition of sodium-channel current by Wenxin Keli is effective in suppressing atrial fibrillation. Heart Rhythm. 2012;9(1):125–31. doi: 10.1016/j.hrthm.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joukar S, Ghasemipour-Afshar E, Sheibani M, Naghsh N, Bashiri A. Protective effects of saffron (Crocus sativus) against lethal ventricular arrhythmias induced by heart reperfusion in rat: a potential anti-arrhythmic agent. Pharm Biol. 2013;51(7):836–43. doi: 10.3109/13880209.2013.767362. [DOI] [PubMed] [Google Scholar]
- 40.Khori V, Alizadeh AM, Yazdi H, Rakhshan E, Mirabbasi A, Changizi S, et al. Frequency-dependent electrophysiological remodeling of the AV node by hydroalcohol extract of Crocus sativus L. (saffron) during experimental atrial fibrillation: the role of endogenous nitric oxide. Phytother Res. 2012;26(6):826–32. doi: 10.1002/ptr.3643. [DOI] [PubMed] [Google Scholar]
- 41.Ritter M, Melichar K, Strahler S, Kuchta K, Schulte J, Sartiani L, et al. Cardiac and electrophysiological effects of primary and refined extracts from Leonurus cardiaca L. (Ph.Eur.). Planta Med. 2010;76(6):572–82. doi: 10.1055/s-0029-1240602. [DOI] [PubMed] [Google Scholar]
- 42.Tai YT, Lau CP, But PP, Fong PC, Li JP. Bidirectional tachycardia induced by herbal aconite poisoning. Pacing Clin Electrophysiol. 1992;15(5):831–9. doi: 10.1111/j.1540-8159.1992.tb06849.x. [DOI] [PubMed] [Google Scholar]