Dear Editor,
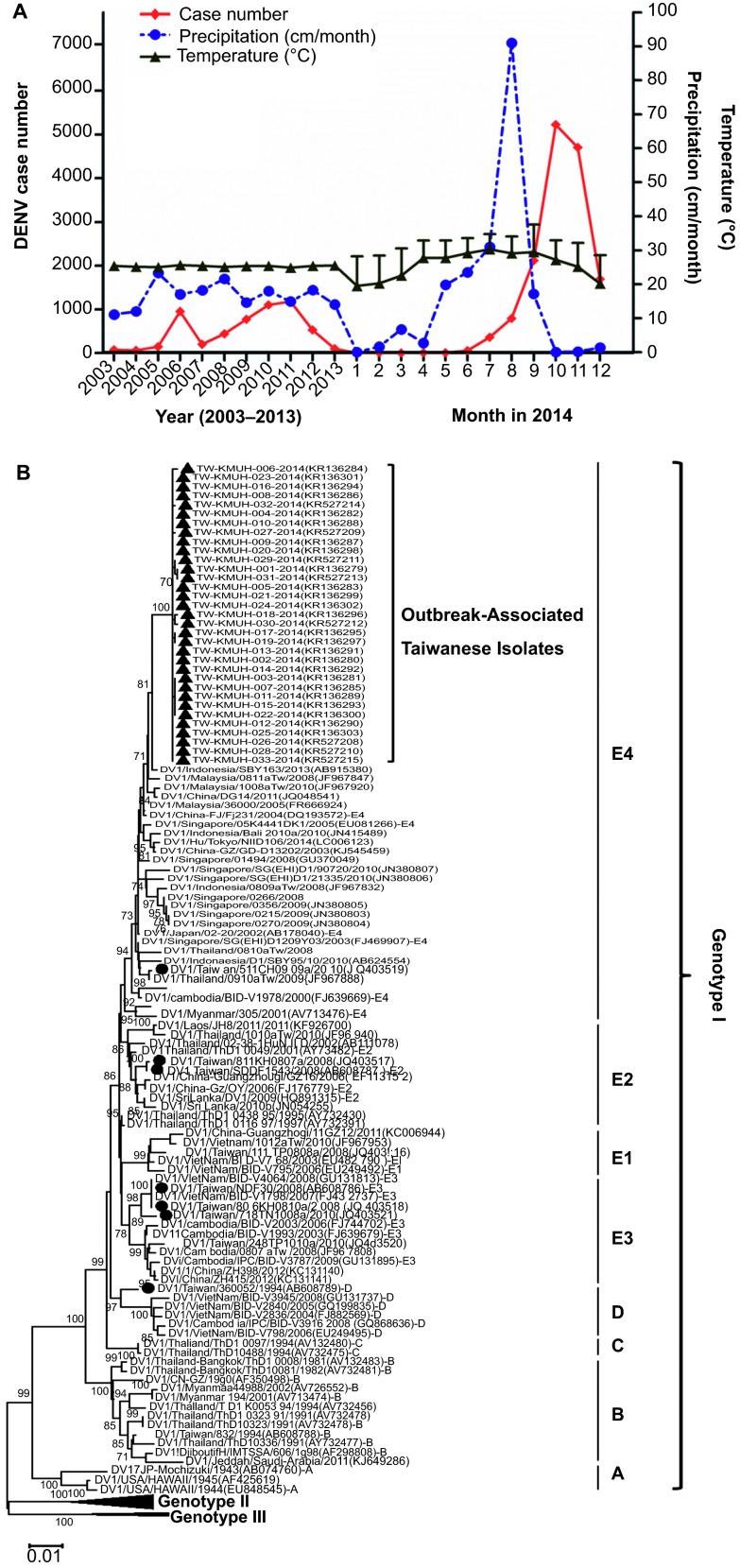
In 2014, Taiwan experienced its largest dengue virus (DENV) outbreak since formal records were kept starting in 1981.1 According to Taiwan's Centers for Disease Control (Taiwan CDC),2 there were 15 732 reported DENV infections in 2014, including 15 492 indigenous and 240 imported cases. Among them, 15 043 cases (95.6%) occurred in Kaohsiung city, which is located in southern Taiwan (Supplementary Figure S1). There were around 60–1183 cases yearly in Kaohsiung during 2003–2013. In 2014, the number of cases dramatically increased from July and peaked in October (5533 cases) (Figure 1). The exact cause of this large DENV outbreak in Taiwan is still unclear.
Figure 1.
Dengue virus outbreaks in Taiwan in 2014. (A) The accumulative dengue infection cases in the past decade reported by Taiwan CDC (left) and reported cases monthly in 2014 (right) in Kaohsiung city. The association of dengue case numbers with temperature and precipitation is also illustrated. The asterisk “*” indicates the time of gas explosion in Kaohsiung city. (B) Phylogenetic tree analyses of Taiwanese 2014 dengue outbreak-associated isolates. The nucleotide sequences of complete E-genes of DENV strains were aligned, edited, and analyzed using ClustalW software. The phylogenetic analysis was performed using MEGA version 5 (http://www.megasoftware.net/). Consensus neighbor-joining trees were obtained from 1000 bootstrap replicates. The black filled triangles indicate 2014 Taiwanese outbreak-associated isolates and the black-filled circles indicate previous Taiwanese DENV-1 endemic or epidemic strains.
DENV is the most important arthropod-borne viral infectious disease in tropical and subtropical areas of the world. Its incidence has increased dramatically in the past two decades.3,4 There are an estimated over 100 million cases of dengue infection per year in tropical and subtropical regions. Taiwan is located in the western Pacific Ocean and has a tropical and subtropical climate. DENV has been circulating in southern Taiwan, and epidemics occur intermittently.4 Compared to 2013, the total number of DENV infections in 2014 increased more than 147-fold in Kaohsiung (Figure 1). DENV belongs to the genus Flavivirus of the family Flaviviridae and contains four distinct serotypes. Recently, the detection of the serotype fifth has further increased its complexity.5 In order to identify which DENV serotypes caused the outbreak in Kaohsiung in 2014, 4478 serum samples from laboratory confirmed dengue cases in Kaohsiung Municipal Siaogang Hospital, Municipal Ta-Tung Hospital and Kaohsiung Medical University Hospital were collected. Patients who had any of the following condition will be considered to have DENV infection: (i) positive virus isolation; (ii) positive result of real-time polymerase chain reaction; (iii) positive result of higher titers of dengue-specific IgM and IgG antibody in which cross-reaction to Japanese encephalitis had been excluded; or (iv) positive seroconversion or ≥4-fold rise in dengue-specific IgM or IgG antibody in the convalescent phase and positive result in the NS1 antigen test.4,6 Testing by Taiwan CDC showed that all 4478 specimens were serotype 1. Of these, 224 serum samples were randomly selected for viral isolation using C6/36 cells. DENV serotypes were further confirmed by real-time reverse transcription polymerase chain reaction of nonstructural protein gene with specific primers.7
Previously, subgenotypes or clusters in different genotypes of DENV had been identified, and genotype I of DENV-1 was further classified into subgenotypes A, B, C, D, and E.8 To elucidate the origin and dissemination pathway of DENV in Kaohsiung, the E gene was amplified from 33 DENV isolates using real-time reverse transcription polymerase chain reaction for phylogenetic analysis.4,9 During phylogenetic analysis, sequences of the isolates were aligned on the basis of translated nucleotide sequences (accession NOs KR136279-R136303 and KR527208-KR527215), and a neighbor-joining tree with p-distance was inferred with 1000 bootstrap replicates in MEGA version 5 for a 1485-nt fragment spanning the full gene of the envelope glycoprotein.10 Our results showed that all the outbreak-associated DENV isolates belonged to subgenotype E4 of genotype I (bootstrap value of 100) (Figure 1B). According to Taiwan's CDC, there were four DENV serotypes circulating in Kaohsiung city during 2012–2013.2 Unfortunately, due to the low incidence of cases in 2011–2013, no nucleotide sequences of DENV from that period were available for our analysis. The 2008 and 2010 Taiwanese DENV-1 strains were subgenotypes E1-E3 and E3-E4, respectively (Figure 1B).
It is interesting to note that all the Taiwanese isolates clustered with an Indonesian strain (accession NO AB915380) isolated in 2013 with a bootstrap value of 81 (Figure 1B). Furthermore, the variations of nucleotide and amino acid sequences in E region between our 2014 Taiwanese isolates and the 2013 Indonesian strain were 0.7%–0.9% and 0.2%–0.4%, respectively. Taiwan CDC reported that there were 240 imported DENV cases in Taiwan in 2014, of which 44 were detected in Kaohsiung city.2 It is important to know whether these imported cases were linked to this outbreak. Previous reports indicated that epidemics or local outbreaks of DENV in Taiwan are usually caused by imported DENV strains.4,11 There were 1328 imported cases during 2002–2010, of which 362 (27.3%) were from Indonesia, 347 (26.1%) from Vietnam, 161 (12.1%) from Thailand, 157 (11.8%) from the Philippines, and 77 (5.8%) from Cambodia. These imported strains led to subsequent indigenous infections via Aedes mosquito's transmissions.11
Environmental factors may have also contributed to this large outbreak of DENV in Kaohsiung. Kaohsiung is a center of the petrochemical industry in Taiwan and there are many factories in the city. Their pipelines run underneath Kaohsiung's streets. An underground gas explosion occurred in Qianzhen and Lingya districts of Kaohsiung at midnight of July 31, 2014. The cause of the blast was suspected to be a chemical leak – most likely of propylene. This underground pipeline explosions caused at least 26 deaths and injured 269 persons (http://www.bbc.com/news/world-asia-28594693). Subsequently, continuous rain fell for several days in Kaohsiung (Figure 1). Combined with the hot weather, the activities and breeding of mosquitoes may have increased dramatically. We found that the Breteau index increased from 10%–19% to 35%–49% in Kaohsiung from July to September 2014. The major DENV cases in Kaohsiung were reported in the underground pipeline regions affected by the explosion (Supplementary Table S1). The higher number and earlier annual peak incidence of dengue cases were reported to be related to the climate. The average temperatures in Kaohsiung from June to September were 0.5 °C–1.3 °C higher in 2014 than in previous years (Figure 1). Detailed temporal and geographic analyses of DENV in different districts of Kaohsiung need to be conducted to confirm the association between this accident and this DENV outbreak.
Some risk factors are thought to contribute to the clinical manifestation of dengue infection. Studies have revealed that dengue hemorrhagic fever or dengue shock syndrome occur in individuals with secondary heterotypic DENV infections. The hypothesis has been made that during heterotypic secondary infections, pre-existing cross-reactive antibodies may enhance virus entry by forming virus-antibody complexes and binding to Fcγ receptor expressing cells. In this outbreak, 19 deaths and 132 dengue hemorrhagic fever cases were reported. Despite of the severity of the DENV-1 outbreak, the incidence of dengue hemorrhagic fever was only 0.88%, which was lower than the incidences in 2012 and 2013 (5.91% and 5.71%, respectively) (Supplementary Table S2). This suggests that although four types of DENVs were detected in Kaohsiung during 2011–2013, the dominant strains may not be type 1 and the genotype may also be different. In addition, the outbreak-associated strains may disseminate easily via Aedes mosquitoes.
In 2014, Kaohsiung experienced its largest dengue outbreak and both viral and environmental factors may have contributed to the epidemic. The Ministry of Health and Welfare of Taiwan has implemented a series of mosquito control strategies in dengue affected-areas. Meanwhile, active surveillance systems, vector monitoring and case reporting should also be reinforced for effective control of DENV infection and dissemination as well as early detection of future dengue epidemics. Epidemics or outbreaks of dengue fever have also been reported in our neighbors mainland China6,12,13 and Japan14 in 2013–2014. Therefore, this emerging problem demands further attention.
Acknowledgments
The authors wish to thank the staffs at Taiwan CDC for their assistance in DENV serotyping and Dr. Day-Yu Chao at the Institute of Microbiology and Public Health, School of Veterinary Medicine, National Chung-Hsing University, Taichung, Taiwan for her helpful discussions critiquing the manuscript. This work was supported by grants from the Center for Infectious Disease and Cancer Research, Kaohsiung Medical University (KMUTP103E00, KMUTP103E03 and KMUTP103E20) and also partially supported by a grant from the Kaohsiung Medical University Research Foundation (KMU-Q104001).
Footnotes
Supplementary information of this article can be found on the Emerging Microbes & Infections' website (http://www.nature.com/emi).
Supplementary Information
References
- 1Chao DY, Lin TH, Hwang KP, Huang JH, Liu CC, King CC. 1998 dengue hemorrhagic fever epidemic in Taiwan. Emerg Infect Dis 2004; 10: 552–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Center for Disease Control (Taiwan). Taiwan National Infectious Disease Statistics System for Dengue Virus Surveillance. Taiwan: CDC, 2014. Available at http://nidss.cdc.gov.tw/en/SingleDisease.aspx?dc=1&dt=2&disease=061 (accessed 4 June 2015). [Google Scholar]
- 3Lin CH, Schioler KL, Jepsen MR, Ho CK, Li SH, Konradsen F. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003-2009. Emerg Infect Dis 2012; 18: 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Huang JH, Su CL, Yang CF, Liao TL, Hsu TC et al. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2008-2010. Am J Trop Med Hyg 2012; 87: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India 2015; 71: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Jin X, Lee M, Shu J. Dengue fever in China: an emerging problem demands attention. Emerg Microbes Infect 2015; 4: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Kong YY, Thay CH, Tin TC, Devi S. Rapid detection, serotyping and quantitation of dengue viruses by TaqMan real-time one-step RT-PCR. J Virol Methods 2006; 138: 123–130. [DOI] [PubMed] [Google Scholar]
- 8Chu PY, Ke GM, Chen PC, Liu LT, Tsai YC, Tsai JJ. Spatiotemporal dynamics and epistatic interaction sites in dengue virus type 1: a comprehensive sequence-based analysis. PLoS One 2013; 8: e74165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Hwang KP, Chu PY, Tung YC, Wang HL, Yueh YY et al. Molecular epidemiological study of dengue virus type 1 in Taiwan. J Med Virol 2003; 70: 404–409. [DOI] [PubMed] [Google Scholar]
- 10Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Shu PY, Su CL, Liao TL, Yang CF, Chang SF et al. Molecular characterization of dengue viruses imported into Taiwan during 2003-2007: geographic distribution and genotype shift. Am J Trop Med Hyg 2009; 80: 1039–1046. [PubMed] [Google Scholar]
- 12Wang B, Li Y, Feng Y, Zhou H, Liang Y et al. Phylogenetic analysis of dengue virus reveals the high relatedness between imported and local strains during the 2013 dengue outbreak in Yunnan, China: a retrospective analysis. BMC Infect Dis 2015; 15: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Ooi EE. The re-emergence of dengue in China. BMC Med 2015; 13: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Tsuda Y, Maekawa Y, Ogawa K, Itokawa K, Komagata O et al. Biting density and distribution of Aedes albopictus during the September 2014 outbreak of dengue fever in Yoyogi Park and the vicinity in Tokyo Metropolis, Japan. Jpn J Infect Dis 2015. March 13. doi: 10.7883/yoken.JJID.2014.576 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.