Abstract
P21-activated kinases (PAKs) are multifunctional effectors of Rho GTPases with both kinase and scaffolding activity. Here, we investigated the effects of inflammation on PAK1 signaling and its role in colitis-driven carcinogenesis. PAK1 and p-PAK1 (Thr423) were assessed by immunohistochemistry, immunofluorescence, and Western blot. C57BL6/J wildtype mice were treated with a single intraperitoneal TNFα injection. Small intestinal organoids from these mice and from PAK1-KO mice were cultured with TNFα. NF-κB and PPARγ were analyzed upon PAK1 overexpression and silencing for transcriptional/translational regulation. PAK1 expression and activation was increased on the luminal intestinal epithelial surface in inflammatory bowel disease and colitis-associated cancer. PAK1 was phosphorylated upon treatment with IFNγ, IL-1β, and TNFα. In vivo, mice administered with TNFα showed increased p-PAK1 in intestinal villi, which was associated with nuclear p65 and NF-κB activation. p65 nuclear translocation downstream of TNFα was strongly inhibited in PAK1-KO small intestinal organoids. PAK1 overexpression induced a PAK1–p65 interaction as visualized by co-immunoprecipitation, nuclear translocation, and increased NF-κB transactivation, all of which were impeded by kinase-dead PAK1. Moreover, PAK1 overexpression downregulated PPARγ and mesalamine recovered PPARγ through PAK1 inhibition. On the other hand PAK1 silencing inhibited NF-κB, which was recovered using BADGE, a PPARγ antagonist. Altogether these data demonstrate that PAK1 overexpression and activation in inflammation and colitis-associated cancer promote NF-κB activity via suppression of PPARγ in intestinal epithelial cells.
Abbreviations: PAK1, p-21 activated kinase 1; PPARγ, peroxisome proliferator associated receptor gamma; IBD, inflammatory bowel disease; NF-κB, nuclear factor-kappa B; CRC, colorectal cancer; UC, ulcerative colitis; CD, Crohn's disease; CAC, colitis-associated cancer; EV, empty vector; WT, wild type; KD, kinase dead; KO, knock out; SIO, small intestinal organoids; SB, small bowel; LB, large bowel; IEC, intestinal epithelial cells; Rosi, Rosiglitazone
Keywords: PAK1, NF-κB, PPARγ, Inflammation, Ulcerative colitis, Colitis-associated cancer
Highlights
-
•
PAK1 is overexpressed and activated in inflammatory bowel diseases and in colitis-associated cancer.
-
•
PAK1 is activated by pro-inflammatory cytokines.
-
•
PAK1 regulates NF-κB signaling through modulation of PPAR-γ.
1. Introduction
Inflammatory bowel diseases (IBD) such as Crohn's disease (CD) and ulcerative colitis (UC) are associated with an increased risk of developing colorectal cancer (CRC). Mesalamine, 5-aminosalicyclic acid (5-ASA), is an anti-inflammatory drug used to treat UC, and epidemiological evidence suggests that it has chemopreventive effects [1]. We previously identified p-21 activated kinase-1 (PAK1) as a 5-ASA target [2]. PAK1 is a serine/threonine kinase effector of the small Rho GTPases Rac1/Cdc42 [3], which regulates cytoskeletal dynamics and epithelial cell (IEC) migration and homeostasis. Recently, we demonstrated that PAK1 is overexpressed in IBD and CAC and promotes cell survival pathways [4–6]. However, the exact cause and consequence of PAK1 overexpression in intestinal inflammation have yet to be defined.
Several studies support the notion that canonical NF-κB activation promotes intestinal tumorigenesis through the upregulation of pro-inflammatory cytokines, proliferation, and cell survival [7–10]. NF-κB activation is regulated by the transcription factor RelA (p65). At basal levels, p65 is sequestered in the cytoplasm by its inhibitors IKKα/β and IκB. Upon pathway activation, IκB is degraded, and p65 translocates into the nucleus [11]. NF-κB signaling in immune cells drives the expression of pro-inflammatory cytokines such as TNFα or IL-1β, which subsequently activate NF-κB in IECs thereby promoting cell survival [12]. In support of this, TNFα-administered NF-κB1−/− mice show increased IEC apoptosis [12]. PAK1 was previously reported to stimulate NF-κB [13], albeit its exact mechanism within the canonical pathway is unknown.
Here, we have investigated the effect of PAK1 activation in IECs upon inflammation and its relevance for NF-κB signaling. We observed that several cytokines including TNFα can activate PAK1 and induce its nuclear localization. In IECs, such PAK1 activation induces a p65 interaction and assists its nuclear translocation through inhibition of PPARγ.
2. Materials and methods
2.1. Animal experiments
9 week old, C57BL/6 mice were provided by Charles River (Margate, UK) and housed at the University of Liverpool. All procedures were performed according to UK Home Office license guidelines. Mice were euthanized 1.5 h after receiving an intraperitoneal injection of TNFα at 0.33 mg/kg body weight. The large (LB) and small bowel (SB) were dissected and paraffin embedded as previously described.
2.2. Cell culture, isolation of small intestinal epithelial organoids, and reagents
Primary human colon epithelial cells (HCEC-1CT), a generous gift from Dr. Jerry W. Shay, were cultured as described previously [14]. HCT116 cells were purchased from ATCC. Small intestinal organoids (SIO) were isolated, from C57BL/6 wild type (WT) and PAK1−/− (PAK1 KO) mice (purchased from MMRRC UNC, Chapel Hills, NC), and maintained as previously described [15]. All cells were serum starved in incomplete medium for 12 h prior to treatment with recombinant protein. Reagents (Supplementary Table S2) were applied 2–12 h prior to inflammatory cytokines. Cells were harvested at 60–70% confluency.
2.3. Co-culture
Polymorphonuclear cells (PMN) were isolated, activated, and cultured using a previously established co-culture system [16]. Briefly, isolated PMNs were activated using 50 ng/mL phorbol 12-myristate 13-acetate (PMA) at 37 °C for 30 min. PMNs were washed twice with Ca/Mg free-Hank's buffer salt solution (HBSS), and applied to the upper chamber of a 0.45 μm Transwell insert in a 1:50 epithelial/PMN ratio. Following treatment, PMNs were discarded, and colonic epithelial cells were harvested for western blot analysis.
2.4. Immunohistochemical analysis
Immunohistochemistry (IHC) was performed on 3–5 μm tissue sections from human samples, and mouse SB or LB [6]. The human samples were obtained from the Clinical Institute of Pathology at the Medical University of Vienna. The study was approved by the local ethics committee. Samples were selected from endoscopic biopsies or surgical specimens of active IBD and CAC patients. Control specimens were taken from normal colon tissue. CD patients displayed active colonic disease, and CAC included both adenoma and invasive tumors.
2.5. Immunoreactivity score
An immunoreactivity score (IRS) was generated by two blinded investigators. Separate IRSs were generated for the small bowel (SB) and the large bowel (LB). The SB included villus and crypt epithelial staining, while the LB included surface and crypt epithelial staining. The intensity of staining included a score from 0–3, 0 (no staining), 1 (low), 2 (medium), 3 (high). The percentage of positively stained epithelial cells was evaluated on a scale from 0–4, 0 (< 1%), 1 (1–10%), 2 (10–50%), 3 (51–80%), and 4 (> 80%) in 4 separate fields of view. The mean intensity and percentage of positively stained epithelial cells was multiplied to generate the IRS, and the highest score attainable was 12. Data shown represent the mean of the 2 scorers' findings.
2.6. Quantitative real-time PCR
RNA was isolated using TRIzol reagent (Life Technologies). cDNA synthesis was carried out using Thermoscript RT-PCR System (Invitrogen) according to manufacturer's protocol. Quantitative RT-PCR was carried out in triplicate using Fast SYBR Green Master Mix with primers (Supplementary Table S3). All data were normalized to the endogenous control 36b4. Relative quantification of transcripts were calculated using the comparative CT method.
2.7. Western blotting (WB) and co-immunoprecipitation (co-IP)
Cells were washed in PBS and harvested in RIPA buffer for whole cell lysates. Nuclear and cytoplasmic extracts were collected as previously described [17]. SIO were released from matrigel in ice cold PBS, and centrifuged at 1000 RPM for 5 min. The matrigel was discarded and the organoids were sonicated for 2–3 s in RIPA buffer. All lysates were harvested for 30 min on ice and centrifuged at 4 °C for 30 min. Protein concentration was determined by Bradford assay and measured on an (Anthos 2010) photometer. For co-immunoprecipitation (co-IP), 750 μg of protein were incubated with 2 μg rabbit or mouse IgG or primary antibody shaking at 4 °C overnight followed by incubation with A/G agarose beads for 2 h at room temp. Samples were washed 5 × with PBS and added to SDS sample buffer. For WB, 25–50 μg protein were added to SDS sample buffer for 10 min at 95 °C. Protein samples were separated using SDS-PAGE and transferred on a PVDF membrane. Membranes were blocked in Western blocker solution (Sigma) for 1 h, and incubated with primary antibodies (Supplementary Table S1) overnight at 4 °C. Protein bands were visualized using IRDye coupled secondary antibodies and imaged on an Odyssey infrared imaging system (LI-COR). Images were obtained and densitometry was calculated using Odyssey application software version 3.0.21.
2.8. Electroporation, PAK1 overexpression, and RNA interference
Transient transfection in HCEC-1CT was achieved via electroporation using Amaxa basic nucleofector 2B kit and device for primary mammalian epithelial cells (Lonza) [17]. PAK1 was overexpressed with 5 μg of pCMV6M-PAK1 (WT-PAK1), a generous gift from Dr. Jonathan Chernoff, pCMV6M-PAK1 K299R (KD-PAK1) (Addgene) or a PCMV6M empty vector (EV) (Addgene) for 72 h. PAK1 knockdown using RNA interference was achieved using 100 nM siPAK1 (Dharmacon) or 100 nM scrambled siRNA (Santa Cruz) for 48 h.
2.9. Immunofluorescence
HCEC-1CT and SIO were fixed in 4% paraformaldehyde at room temperature for 30 min and blocked in 0.1% Triton-X-100/2% horse serum for 1 h. Samples were incubated with primary antibody in 1% BSA/PBS overnight at 4 °C, then washed and incubated with fluorescent secondary antibody for 1 h at room temperature. Samples were washed, dried, and mounted in medium containing DAPI (Vector Laboratories), and imaged on an Olympus IX81 or BX51 microscope.
2.10. Luciferase assay
HCEC-1CT cells were transiently transfected with a TkpGL3 NF-κB or PPAR luciferase response element, or co-transfected with scrRNA, siPAK1, EV, WT-PAK1, or KD-PAK1 and seeded into 24 well plates. Cells were lysed with a lysis buffer containing 4% Triton X-100, 100 mM Glycyl-glycine, 100 mM MgSO4, and 250 mM EGTA for 20 min on a shaker at room temperature. 20 μL of lysate were added into a 96 well plate and analyzed on a Plate Chameleon V photometer (Hidex) in which 100 μL of luciferase activity buffer containing 100 mM Glycyl-glycine, 100 mM MgSO4, 20 mM ATP (Sigma), and 2.5 mM d-luciferin (Sigma) were injected per well. All measurements were performed using technical triplicates.
2.11. Statistics
Statistical analysis was performed using SPSS (version 21.0). Metric outcome variables were compared using univariate analysis of variance (ANOVA) and Tukey HSD post-hoc tests. RT-PCR data and immunoreactivity scores were analyzed using a 2-tailed t-test. p-Values less than 0.05 were considered significant (*p < 0.05, **p < 0.01, ***p < 0.001). All data are expressed as mean ± standard deviation.
3. Results
3.1. Pro-inflammatory cytokines promote PAK1 activation in IEC
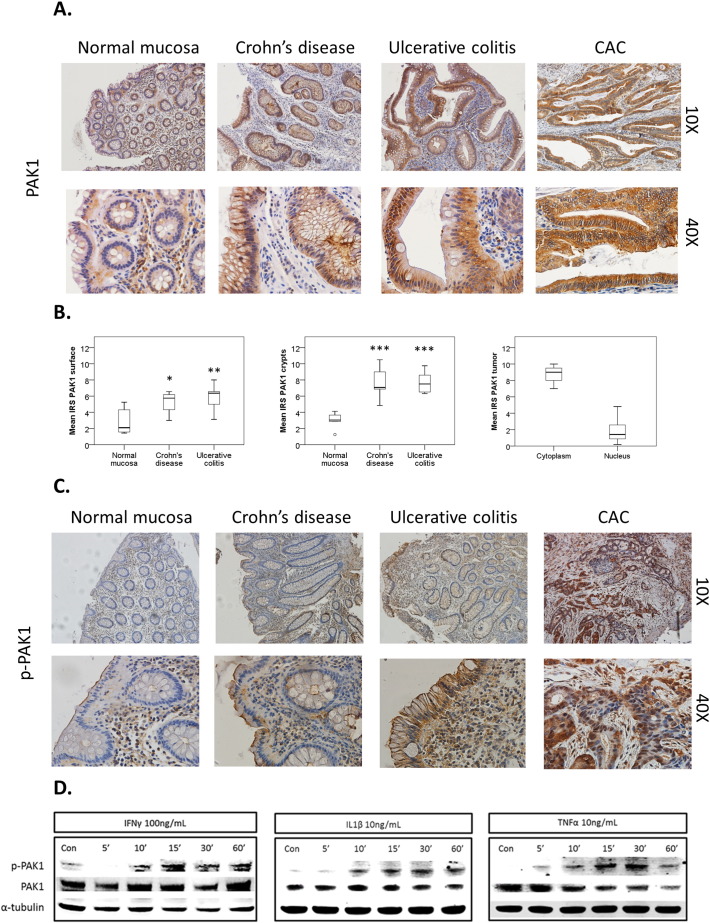
PAK1 is associated with gut tumorigenesis [5,18,19] and we recently demonstrated its overexpression in IBD and colitis-associated cancer (CAC) [6]. Here we investigated PAK1 activation and signaling in IBD and CAC. Normal intestinal mucosa, CD, UC, and CAC samples were stained with both a PAK1 and p-PAK1 antibody that detects phosphorylation at Thr-423, a residue that is essential for PAK1 activation [20]. In comparison to normal mucosa, total and nuclear PAK1 was overexpressed at the luminal epithelial surface and crypts in CD and UC, as well as in tumors of CAC (Fig. 1A–B). PAK1 activation was absent within the normal mucosa, and cytoplasmic p-PAK1 levels increased at the luminal intestinal epithelial surface in both UC and CD, but not within the crypts. CAC tumor samples displayed increased PAK1 phosphorylation in both the cytoplasm and nucleus (Fig. 1C, Supplementary Fig. S1A).
Fig. 1.
(A–D). PAK1 is overexpressed and activated in IBD. (A) Human colonic tissue stained for PAK1. PAK1 expression increases in comparison to normal mucosa at the luminal epithelial surface and crypts in both Crohn's disease (CD), ulcerative colitis (UC), and in tumors of colitis associated cancer (CAC). (B) Box plots compare mean PAK1 immunoreactivity scores (IRS) in normal mucosa (n = 6), CD (n = 7), and UC (n = 6) at the luminal surface and crypts. PAK1 is overexpressed in CAC (n = 8). The circle is the outlier from the boxplot. (C) Human colonic tissue stained for p-PAK1. In CD and UC, PAK1 activation in epithelial cells is found at the membrane, specifically at the luminal surface, but not in the crypts. In CAC, PAK1 phosphorylation is found within the nuclei and cytoplasm of tumor cells. (D) Inflammatory cytokines activate PAK1 in HCEC-1CT. WB of HCEC-1CT RIPA whole cell lysates using 50 μg protein. Cells were either untreated (Con) or treated with IFNγ, IL-1β, or TNFα for 5–60 min and analyzed for PAK1 activation with a p-PAK1 Thr 423 antibody. TNFα induced the most profound effect in PAK1 activation at 30 min. α-Tubulin was utilized as a loading control.
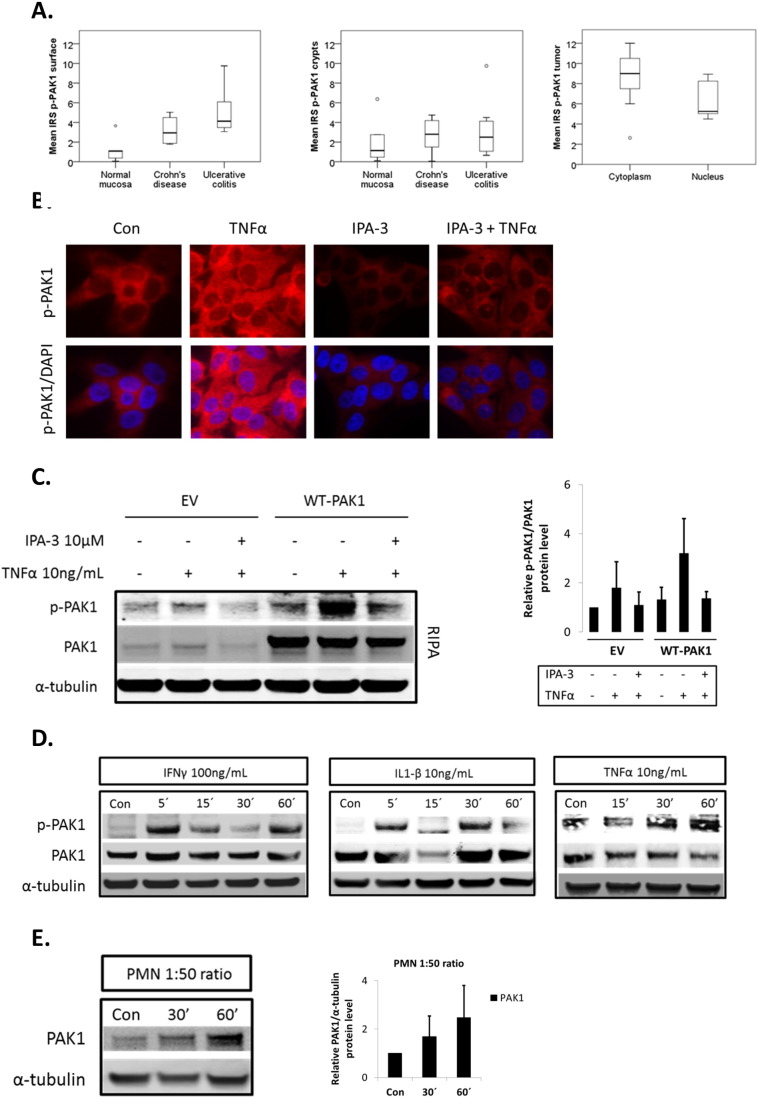
Supplementary Fig. S1.
(A–E). Inflammation promotes PAK1 activation. (A) Box plots compare PAK1 activation at the luminal intestinal surface and crypts in normal mucosa (n = 5), CD (n = 6), UC (n = 8), and also in CAC samples (n = 7). Tukey HSD; *p < 0.05, **p < 0.01, ***p < 0.001. (B) Immunofluorescence of p-PAK1 in TNFα-treated cells with DMSO (Con) or IPA-3 pretreatment. Upper panel shows p-PAK1; lower panel is p-PAK1 merged with DAPI staining. (C) WB of whole cell lysates (RIPA) in EV or WT-PAK1 overexpressing cells. TNFα activation of PAK1 was blocked upon IPA-3 pretreatment. Densitometry of relative p-PAK1 expression upon IPA-3 pretreatment, data are mean and SD from 2 independent experiments. (D) Inflammatory cytokines activate PAK1 in HCT116 cells. WB of RIPA lysates. Cells were left untreated (Con) or treated with IFNγ, IL-1β, or TNFα for 5–60 min and analyzed for PAK1 activation using p-PAK1 Thr 423 antibody. PAK1 activation increased from 5–60 min. Total PAK1 expression was also evaluated and α-tubulin was utilized as a loading control. (E) HCEC-1CT cells were co-cultured with PMNs in a 1:50 epithelial to neutrophil ratio and analyzed by WB for PAK1 expression. Total PAK1 expression increased from 30–60 min. Relative densitometry of PAK1 expression upon co-culture treatment. Data are mean and SD of 2 independent experiments.
IFNγ, IL-1β, and TNFα are pro-inflammatory cytokines which contribute to gut inflammation in IBD [21]. We investigated their effect on PAK1 activation in HCEC-1CT cells. IFNγ, IL-1β, and TNFα induced phosphorylation of PAK1 at Thr-423 within 10 to 30 min (Fig. 1D). Immunofluorescence of p-PAK1 revealed cytoplasmic levels of p-PAK1 increased upon TNFα stimulation, and this effect was abolished upon PAK1 kinase inhibition using IPA-3 (Supplementary Fig. S1B). We further verified the specificity of our p-PAK1 antibody using IPA-3 upon WT-PAK1 overexpression in the presence of TNFα (Supplementary Fig. S1C). PAK1 overexpression also resulted in PAK1 auto-phosphorylation. IFNγ, IL-1β, and TNFα also induced p-PAK1 in the colorectal cancer cell line HCT116 as early as 5 min peaking within the 30–60 min range (Supplementary Fig. S1D). Accumulation of polymorphonuclear leukocytes (PMNs) within colonic crypts is a key feature of UC [16]. HCEC-1CT cells were co-cultured with freshly isolated PMNs using Transwell inserts. Total PAK1 expression increased more than two-fold at 60 min (Supplementary Fig. S1E). Taken together, these data demonstrate that pro-inflammatory cytokines and activated PMNs regulate PAK1 activation and expression in human colonic epithelial cells.
3.2. PAK1 regulates NF-κB activation downstream of TNFα
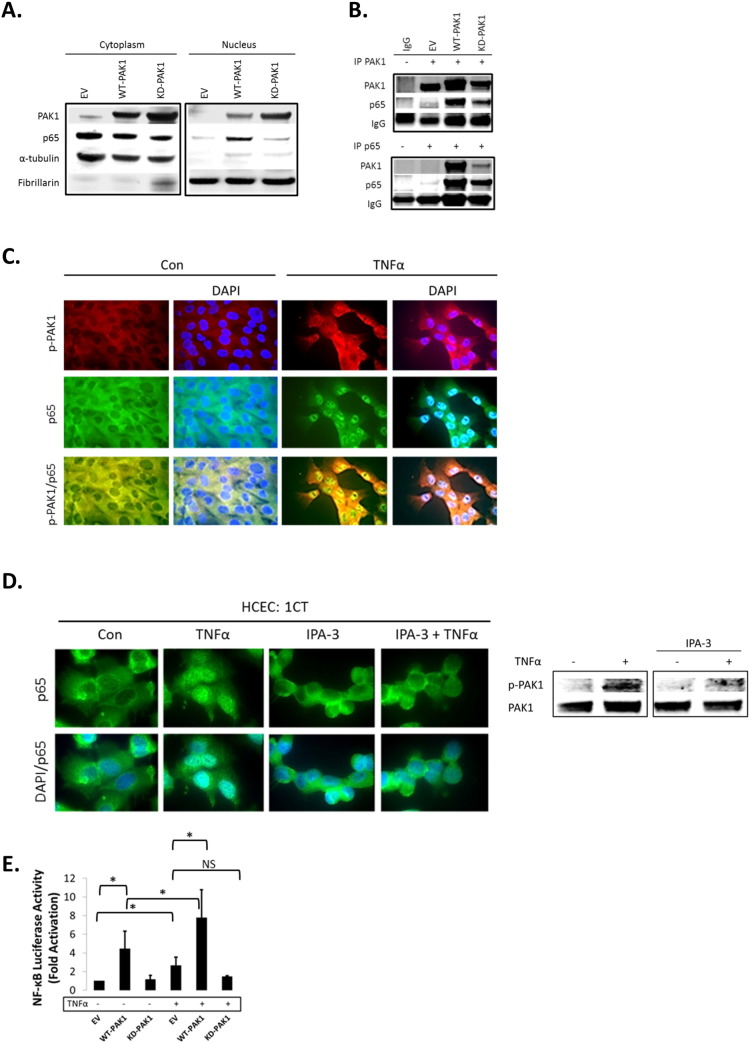
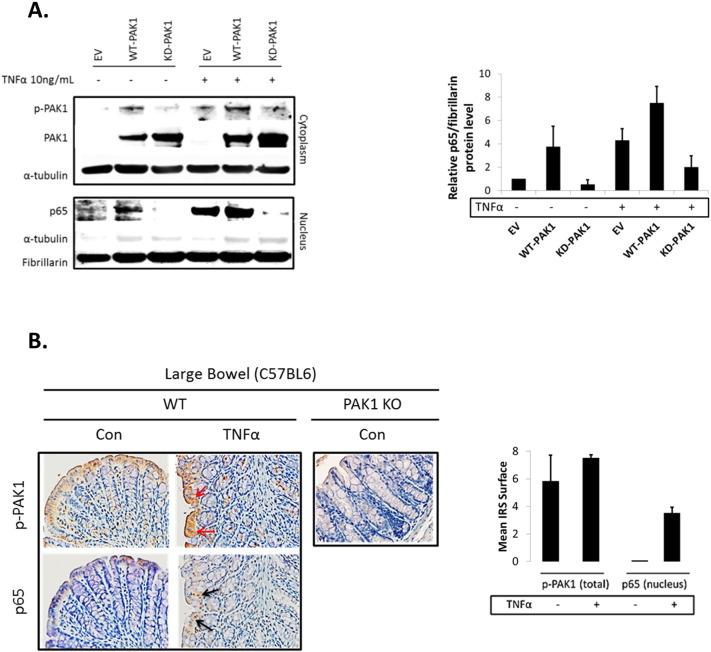
Our data showed that TNFα, which plays a key role in the pathogenesis of IBD [21], and activates canonical NF-κB signaling, induced PAK1 activation. Next, we investigated the role of PAK1 in NF-κB signaling. Control (EV), WT-PAK1, and KD-PAK1 vectors were overexpressed in HCEC-1CT and Western blot analysis was performed on cytoplasmic and nuclear fractions. WT-PAK1 overexpression induced p65 nuclear translocation in the absence of TNFα, whereas KD-PAK1 did not (Fig. 2A). Co-immunoprecipitation showed a direct interaction of p65 and PAK1 upon WT-PAK1 overexpression which was reduced upon KD-PAK1 indicating the importance of auto-phosphorylation of PAK1 for interaction with p65 (Fig. 2B). In the absence of TNFα, immunofluorescence staining of p-PAK1 and p65 revealed localization of both proteins in the cytoplasm. TNFα induced translocation and co-localization of p-PAK1 and p65 in the nucleus (Fig. 2C). Immunofluorescence in HCEC-1CT revealed that TNFα activated nuclear translocation of p65 was blocked upon PAK1 kinase inhibition using IPA-3 (Fig. 2D). P-PAK1 inhibition by IPA-3 was confirmed by Western blot (Fig. 2D). Western blot analysis supported these observations and demonstrated that KD-PAK1 abrogated the effect of TNFα on p65 nuclear translocation (Supplementary Fig. S2A). Densitometry of nuclear p65 revealed that WT-PAK1 overexpressing cells had increased nuclear p65 and were more sensitive to TNFα in comparison to EV or KD-PAK1 (Supplementary Fig. S2A). Further, the effect of PAK1 overexpression and the dependence of PAK1 kinase activity on NF-κB transactivation were confirmed using an NF-κB luciferase reporter assay (Fig. 2E). These data demonstrate that PAK1 overexpression or its activation by TNFα results in a p-PAK1–p65 interaction, nuclear translocation, and transcriptional activation of NF-κB.
Fig. 2.
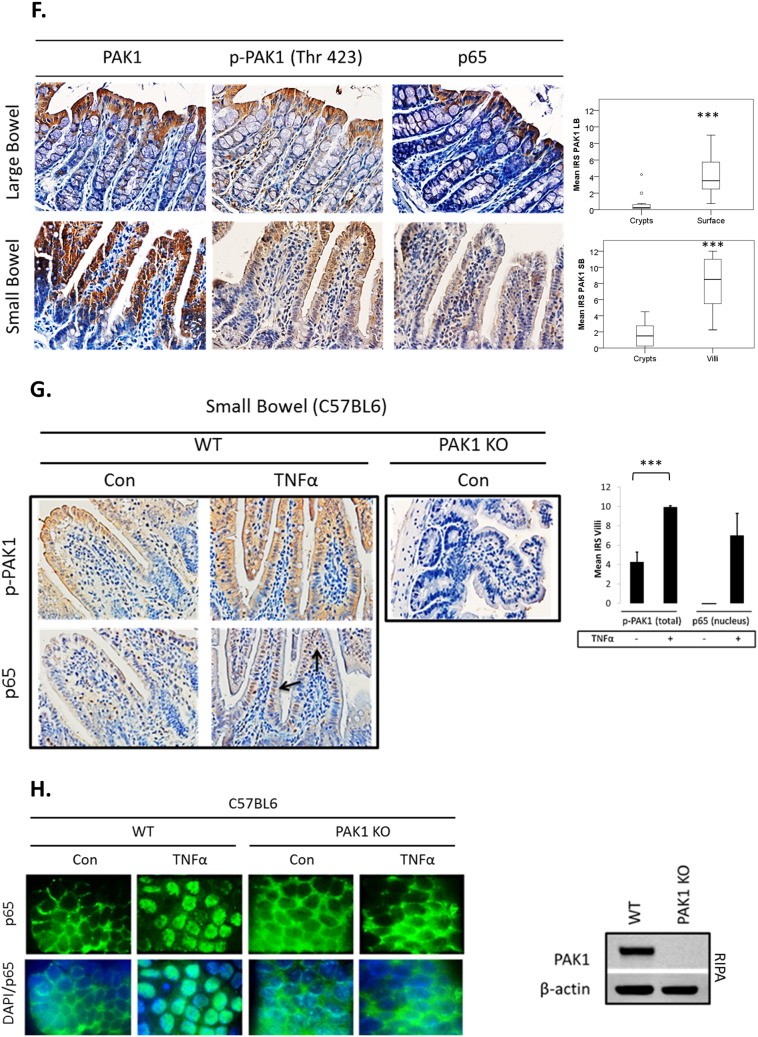
(A–H). PAK1 overexpression regulates NF-kB activation downstream of TNFα in HCEC-1CT. (A) WB of HCEC-1CT cytoplasmic and nuclear lysates. Cells were transfected with EV, WT-PAK1 or KD-PAK1 and probed for PAK1 and p65. WT-PAK1 but not KD-PAK1 overexpression increased nuclear translocation of p65. Loading controls included α-tubulin (cytoplasm) and Fibrillarin (nucleus). (B) Co-IP of PAK1 (top) and p65 (bottom) probed for PAK1 and p65 demonstrating a PAK1–p65 interaction. (C) Immunofluorescence (IF) of p-PAK1 and p65 co-staining in untreated (Con) and TNFα treated cells. Upper panel p-PAK1, middle panel p65, and lower panel merged p-PAK1/p65 images with or without merged DAPI channel. PAK1 and p65 colocalized upon TNFα. (D) IF of p65 or merged DAPI/p65 in HCEC-1CT. Cells were pre-treated with 10 μM IPA-3 (2 h) followed by 10 ng/mL TNFα for 30 min. Nuclear accumulation of p65 by TNFα is impeded by p-PAK1 inhibition. The WB verified p-PAK1 activation by TNFα and its inhibition by IPA-3 in HCEC-1CT. (E) NF-κB luciferase assay of EV, WT-PAK1, and KD-PAK1 overexpression in untreated and TNFα treated cells. Data are representative of 3 independent experiments, ANOVA, Tukey HSD; *p < 0.05. (F) Immunohistochemistry of large bowel (LB) or small bowel (SB) mouse tissue sections stained for PAK1, p-PAK1, or p65. Box plots are mean PAK1 immunoreactivity scores (IRS) comparing SB crypt and villi expression, and LB crypt and surface expression in control mice (n = 3) mice (t-test, 2 tailed; ***p < 0.001). (G) IHC of p-PAK1 and p65 within small intestinal tissue in TNFα injected mice. TNFα increases p-PAK1 expression and p65 (black arrow) nuclear translocation within villi, but not in crypts. PAK1 KO mice were stained for p-PAK1 as a negative control. Graphs show mean villi p-PAK1 and p65 IRSs (± SD) (n = 3) mice per group (t-test, 2 tailed; ***p < 0.001) (H) IF of p65 or merged DAPI/p65 in wild type (WT) and PAK1 KO mouse small intestinal organoids (SIO) with or without TNFα. WB of PAK1 protein expression in WT or PAK1 KO SIO.
Supplementary Fig. S2.
(A–B). PAK1 overexpression regulates NF-kB activation downstream of TNFα in HCEC-1CT. (A) WB of HCEC-1CT cytoplasmic and nuclear lysates. Cells were transfected with EV, WT-PAK1 or KD-PAK1 then treated with TNFα and probed for p-PAK1, PAK1, and p65. WT-PAK1 but not KD-PAK1 overexpression increased nuclear translocation of p65. In the presence of TNFα, KD-PAK1 overexpression impedes nuclear accumulation of p65. Loading controls included α-tubulin (cytoplasm) and Fibrillarin (nucleus). Densitometry of nuclear p65 protein levels upon PAK1 overexpression in untreated or TNFα-treated cells. Data are mean and SD of 2 independent experiments. (B) IHC of p-PAK1 and p65 within large bowel tissue in TNFα injected mice. A modest increase in p-PAK1 expression (red arrows) and p65 (black arrows) nuclear translocation was observed at the luminal surface following TNFα treatment. PAK1 KO mice were stained for p-PAK1 as a negative control. Graphs show mean villi p-PAK1 and p65 IRSs (± SD) (n = 3) mice per group.
As the findings above indicate a concerted action of PAK1 and p65, we further assessed the distribution of such a PAK1–p65 interaction in the mouse intestine. PAK1 and p-PAK1 were expressed in the cytoplasm within cells of small bowel (SB) villi and of the large bowel (LB) luminal surface (Fig. 2F). P65 was also cytoplasmic and its localization resembled PAK1 and p-PAK1 in the LB (Fig. 2F). Less than 1% of IECs had nuclear p65. These data suggest that without inflammation, PAK1 and p65 are co-expressed in differentiated IEC of SB villi and LB luminal surface and canonical NF-κB signaling is not active.
Considering that PAK1 and p65 expression was associated throughout the mouse intestine, we investigated the effect of TNFα on PAK1 expression and activation in vivo. Mice were i.p. injected with TNFα 1.5 h before sacrificing and the SB and LB were collected for immunohistochemistry. Total PAK1 expression was not altered upon TNFα treatment (data not shown). We observed a modest increase in p-PAK1 at the LB epithelial surface (Supplementary Fig. S2B). Nuclear p65, which was not present in control mice, also increased upon TNFα treatment at the luminal surface (Supplementary Fig. S2B).
PAK1 activation upon TNFα treatment within the SB was even more profound (Fig. 2G). This effect was marked towards the villus tip with an observable gradient along the villus axis. In parallel with PAK1 activation, a robust accumulation of nuclear p65 was observed, which was absent in untreated mice. Ex vivo immunofluorescence in small intestinal organoids (SIO) from WT and PAK1 KO mice confirmed that PAK1 is required for nuclear p65 translocation following TNFα treatment (Fig. 2H). Complete deletion of PAK1 in PAK1 KO SIO was verified by Western blot (Fig. 2H). Inhibition of PAK1 kinase activity using IPA-3 also impeded nuclear accumulation of p65 on TNFα in SIO (data not shown). These data support a role for PAK1 in NF-κB signaling downstream of TNFα in IECs.
3.3. PAK1 is required for complete activation of NF-κB downstream of IκB in IECs
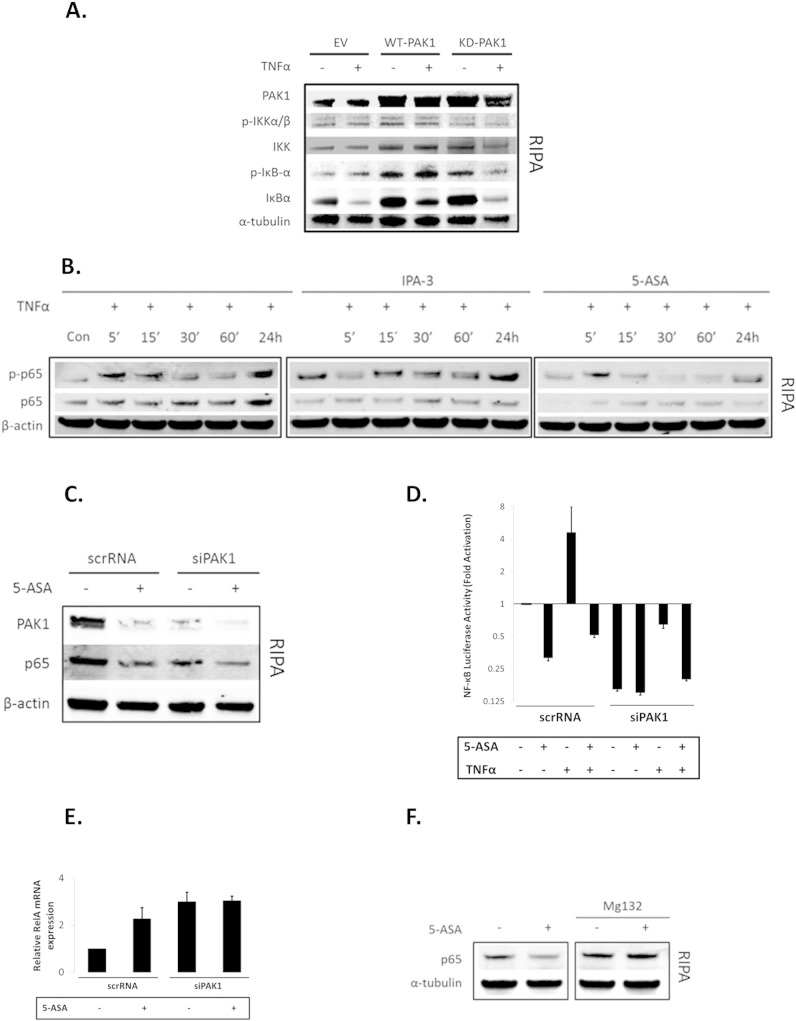
In order to delineate PAK1's role within canonical NF-κB signaling, we further investigated the IKKα/β-IκB cascade in HCEC-1CT cells. If PAK1 was upstream of IKKα/β or IκB, PAK1 overexpression would modulate IkB degradation. Western blot analysis revealed that neither WT-PAK1 nor KD-PAK1 mediated TNFα's effect on IκB degradation (Fig. 3A). PAK1 knockdown (siPAK1) reduced p65 nuclear translocation upon TNFα treatment, but did not affect IκB phosphorylation or degradation further suggesting that PAK1 acts downstream of IκB (Supplementary Fig. S3A).
Fig. 3.
(A–F). PAK1 is required for complete activation of NF-κB downstream of IκB in HCEC-1CT. (A) EV, WT-PAK1, or KD-PAK1 overexpressing cells were treated with 10 ng/mL TNFα (30 min). RIPA lysates were analyzed by WB for PAK1, p-IKKα/β, IKKα, p-IκB-α, and IκBα expression. WT or KD-PAK1 overexpression did not interfere with the degradation of IκB following TNFα treatment. (B) WB of RIPA lysates analyzed for p-p65 and p65. Cells were treated with TNFα or pretreated (12 h) with 10 μM IPA-3 or 20 mM 5-ASA. Pretreatment with IPA-3 or 5-ASA blocked the effect of TNFα on p65. (C) WB of whole cell (RIPA) lysates probed for PAK1, p65, and β-actin. PAK1 knock down by both 5-ASA and siPAK1 blocked total p65 protein levels. (D) Cells were co-transfected with a NF-κB luciferase reporter and siPAK1 or scrRNA. Pretreatment with 5-ASA and PAK1 knockdown impeded the effect of TNFα on NF-κB transcriptional activation. Data are representative of 3 independent experiments. (E) Relative mRNA expression of RelA upon siPAK1 or scrRNA with or without 5-ASA treatment. PAK1 inhibition increased transcription of RelA. (F) HCEC-1CT cells were treated with 20 mM 5-ASA (24 h) with or without the proteasomal inhibitor MG132 (6 h). RIPA lysates were analyzed for p65 and α-tubulin by WB. 5-ASA inhibits p65 but not upon pretreatment with MG132. All data are representative of 3 independent experiments.
Supplementary Fig. S3.
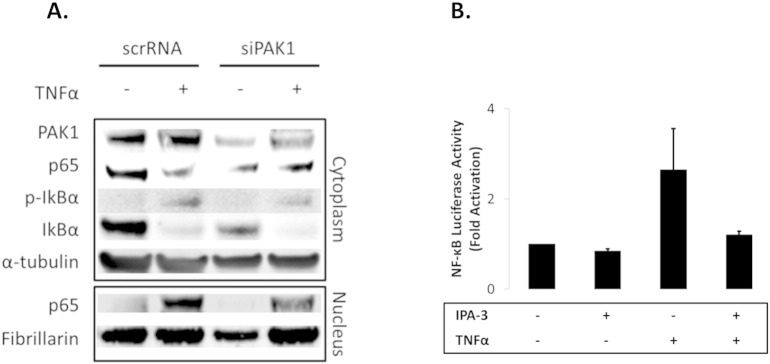
(A–B). PAK1 is required for complete activation of NF-κB downstream of IκB in HCEC-1CT. (A) The effect of PAK1 on p65 was evaluated using siPAK1 or scrRNA. Cells were treated with TNFα and analyzed for PAK1, p65, p-IκB-α, and IκBα expression in nuclear and cytoplasmic cell fractions by WB. α-Tubulin and Fibrillarin were utilized as loading controls. siPAK1 impeded nuclear accumulation of p65 on TNFα treatment independent of IκB degradation. (B) NF-kB luciferase assay of TNFα treated cells with or without IPA-3 pretreatment. IPA-3 blocked TNFα induction of NF-kB.
Considering that PAK1 associates with p65 upon TNFα stimulation, we investigated its effect on p65 stability. PAK1 inhibitors IPA-3 as well as 5-ASA reduced total p65 protein levels with and without TNFα (Fig. 3B), although 5-ASA but not IPA-3 inhibited p65 phosphorylation. Compared to TNF-α treatment, 5-ASA treatment reduced p65 levels over time upon stimulation with TNF-α and this effect was more prominent at 24 h. IPA-3 however, inhibited total p65 levels but did not reduce p65 phosphorylation. This suggested that the effect of PAK1 inhibition on p65 is a result of p65 stability, but not phosphorylation. Mechanistically, 5-ASA reduced PAK1 expression to a similar extent as siPAK1 and both resulted in reduction of p65 total protein levels (Fig. 3C) and p65 transcriptional activity (Fig. 3D). 5-ASA did not display an additive effect to siPAK1 alone suggesting that 5-ASA utilizes PAK1 to reduce p65. TNFα transactivation of NF-κB was blocked upon 5-ASA, siPAK1, and their combination. NF-κB transactivation by TNFα was also blocked by IPA-3 (Supplementary Fig. S3B).
We further investigated whether siPAK1 or 5-ASA reduced p65 at the transcriptional level. Surprisingly, both siPAK1 and 5-ASA increased RelA mRNA (Fig. 3E). Inhibition of the proteasome with MG132, however, blocked 5-ASA's effect on p65 suggestive that PAK1's role within the canonical NF-κB pathway is rather posttranslational (Fig. 3F). These data demonstrate that (1) PAK1 stabilizes p65 protein downstream of IkB, (2) PAK1 is required for complete activation of NF-κB by TNFα, and (3) 5-ASA inhibits NF-κB transcriptional activity via inhibition of total PAK1 in IECs.
3.4. PAK1 modulates a PPARγ/p65 cascade within IECs
Our data indicated that 5-ASA blocks NF-κB in IECs through inhibition of PAK1. 5-ASA is also a known agonist of PPAR gamma (PPARγ) [22] and PPARγ polyubiquitinates and degrades p65 [23]. PPARγ is highly expressed in the gut, and regulates IEC differentiation and migration within SB villi [24]. Within the LB, PPARγ activation has anti-inflammatory and chemopreventive effects [22,25].
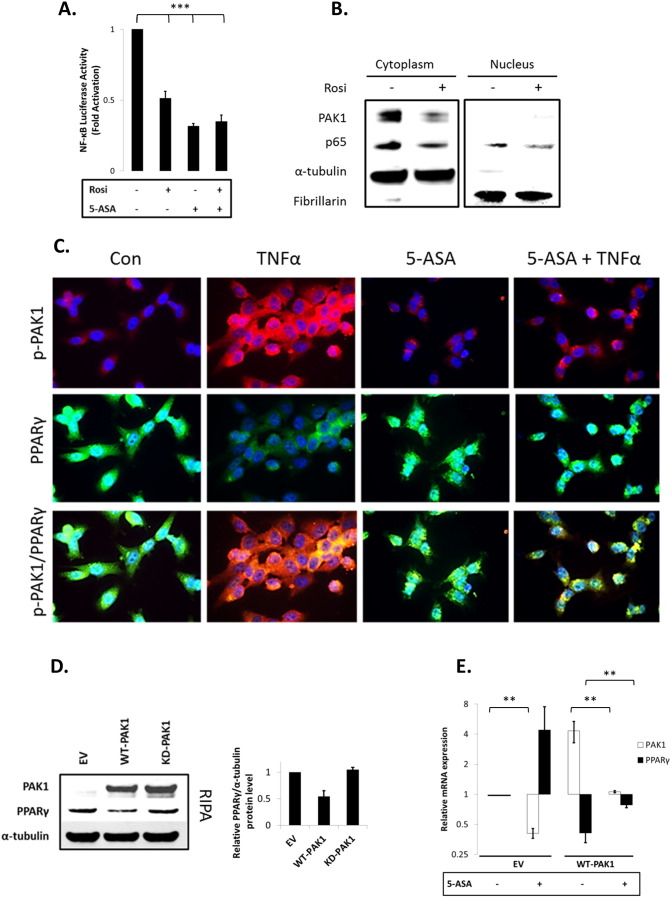
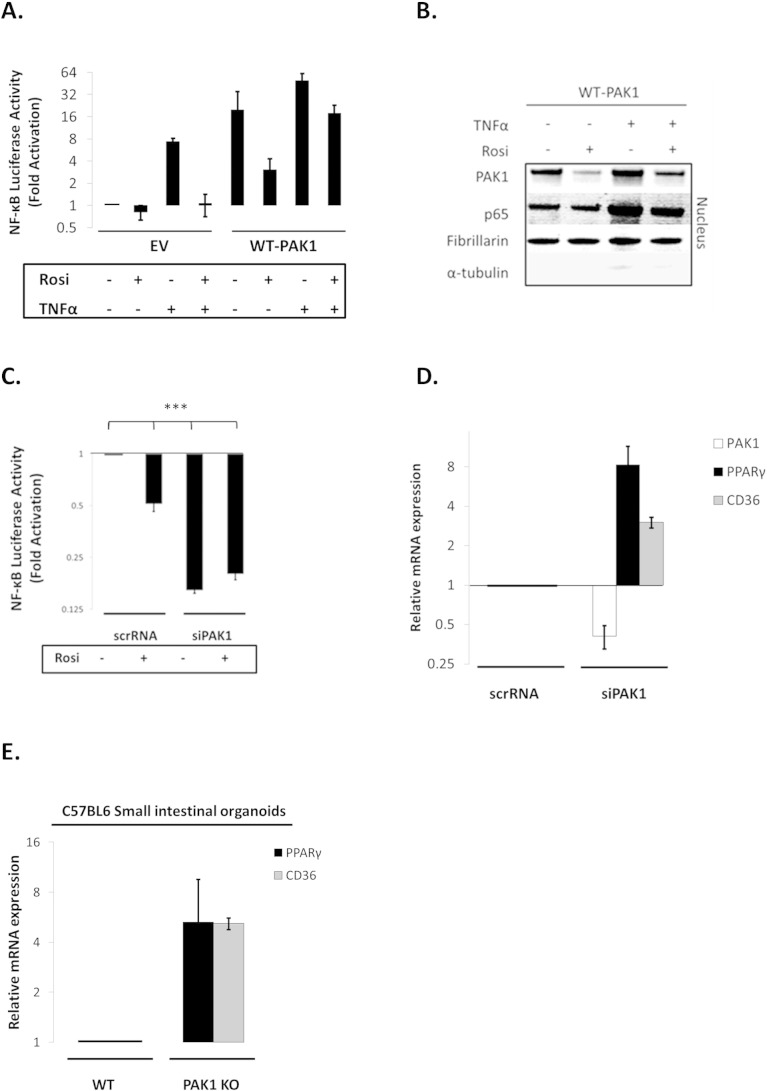
First, we confirmed that both Rosiglitazone (Rosi; a PPARγ agonist) and 5-ASA – to a lesser degree – increased PPAR transactivation (data not shown). Both also blocked NF-κB signaling in our system (Fig. 4A). Interestingly, Rosi did not add to 5-ASA's effect on NF-κB inhibition, suggesting that both agonists utilize PPARγ activation to inhibit NF-κB (Fig. 4A). These data were confirmed at the protein level in which PPARγ activation by Rosi reduced p65 levels in both cytoplasm and nucleus (Fig. 4B). Rosi also reduced PAK1 protein abundance.
Fig. 4.
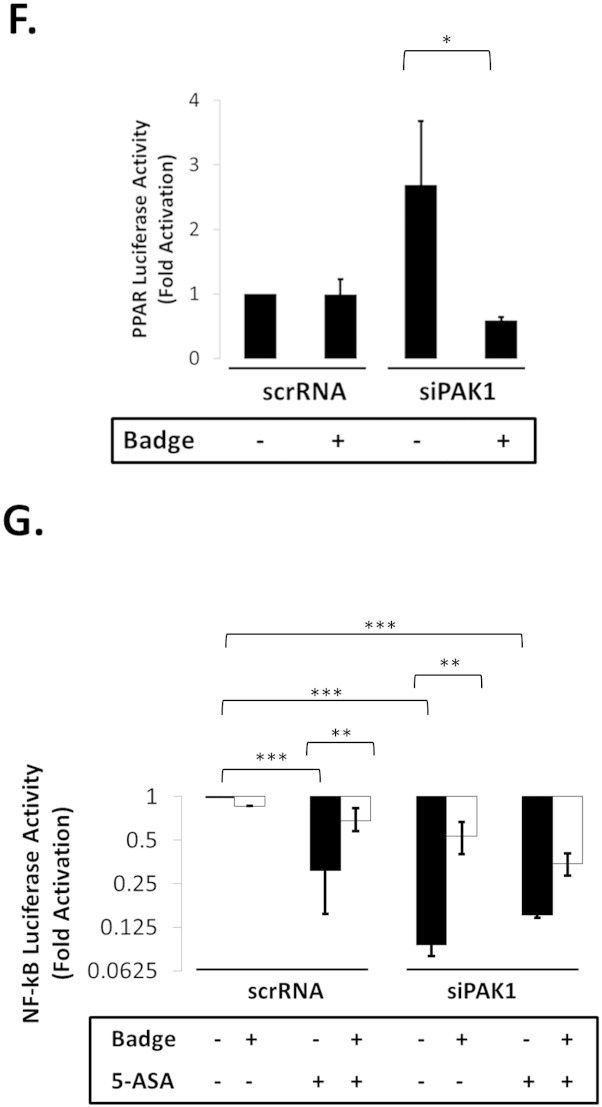
(A–G). PAK1 modulates a PPARγ/p65 cascade. (A) HCEC-1CT cells were transfected with a NF-kB luciferase reporter and treated with 1 μM Rosi ± 20 mM 5-ASA (24 h). Rosi and 5-ASA blocked NF-kB transcriptional activity ANOVA, Tukey HSD; ***p < 0.001. (B) Rosi treated cells were fractionated and analyzed by WB for PAK1 and p65. Rosi blocked PAK1 and p65 expression. (C) IF of p-PAK1 and PPARγ upon TNFα (30 min) with or without 20 mM 5-ASA pretreatment (24 h). TNFα resulted in p-PAK1 activation and PPARγ nuclear export and downregulation which was blocked by 5-ASA. (D) The effect of EV, WT-PAK1, and KD-PAK1 overexpression on PPARγ was analyzed by WB. α-Tubulin was used as a loading control. Densitometry of PPARγ levels normalized to α-tubulin, data are mean and SD from 2 independent experiments. (E) RTPCR of PAK1 and PPARγ after EV or WT-PAK1 overexpression with or without 5-ASA treatment (24 h). PAK1 overexpression blocked PPARγ at the mRNA level, an effect that was recovered by 5-ASA. Data are representative of 3 independent experiments (t-test, 2 tailed; **p < 0.01). (F) PPAR luciferase reporter assay in PAK1 knock down cells using scrRNA or siPAK1. siPAK1 activated PPAR transcription, and this effect was recovered upon 0.1 μM BADGE pretreatment (12 h), ANOVA, Tukey HSD; *p < 0.05. (G) NF-kB luciferase assay in PAK1 knock down cells using scrRNA or siPAK1. Cells were pretreated with BADGE (12 h) with or without 5-ASA (24 h). BADGE recovered the effect of 5-ASA or siPAK1 on NF-kB. All data are representative of 3 independent experiments ANOVA, Tukey HSD; **p < 0.01, ***p < 0.001.
Pro-inflammatory cytokines, such as TNFα, result in PPARγ degradation [26], therefore we investigated the TNFα-PAK1-PPARγ cascade via immunofluorescence. Control HCEC-1CT cells had low levels of p-PAK1, and PPARγ was present in the nucleus and cytoplasm. TNFα treatment resulted in PAK1 phosphorylation concomitant with an apparent reduction of nuclear and cytoplasmic PPARγ expression (Fig. 4C). 5-ASA blocked activation of PAK1 and retained PPARγ levels in the presence of TNFα. These data suggest that TNFα results in activation of PAK1 and in reduction of PPARγ, both of which can be blocked by 5-ASA.
To better understand the interaction between PAK1 and PPARγ, we overexpressed PAK1 in HCEC-1CT. Total PPARγ protein levels were reduced upon WT-PAK1 but not upon KD-PAK1 transfection (Fig. 4D). At the transcriptional level, 5-ASA inhibited PAK1 while increasing PPARγ mRNA levels (Fig. 4E). WT-PAK1 overexpression decreased PPARγ mRNA levels and 5-ASA recovered PPARγ levels. These data demonstrate that PAK1 activation and overexpression downregulate PPARγ both at the protein and mRNA levels, which can be recovered by 5-ASA.
WT-PAK1 overexpression increased the effect of TNFα on NF-κB transactivation (as shown in Fig. 2E). If this was a result of PPARγ inhibition, PPARγ activation should recover this effect. NF-κB luciferase assay showed that in control cells (EV), Rosi blocked TNFα transactivation of NF-κB (Supplementary Fig. S4A). Rosi also blocked the effect of WT-PAK1 on NF-κB transactivation. The synergistic effect of WT-PAK1 and TNFα on NF-κB transactivation was also reduced upon PPARγ activation by Rosi. These data were further verified by Western blot in which Rosi inhibited nuclear PAK1 and p65 upon TNFα in WT-PAK1 overexpressing HCEC-1CT cells (Supplementary Fig. S4B). Rosi treatment of cells with siPAK1 did not further block transactivation of NF-κB (Supplementary Fig. S4C). These data support that PAK1 activation and overexpression activates NF-κB through inhibition of PPARγ.
Supplementary Fig. S4.
(A–E). PAK1 modulates a PPARγ/p65 cascade. (A) NF-κB luciferase assay of EV and WT-PAK1 overexpressing cells upon TNFα treatment (30 min) with or without Rosi pretreatment (12 h). Rosi recovered both the effect of TNFα and PAK1 overexpression on NF-kB. (B) WT-PAK1 overexpressing cells were pretreated with Rosi (12 h) with or without TNFα (30 min) and a nuclear fraction was harvested and analyzed by WB for PAK1 and p65. Fibrillarin (nuclear) and α-tubulin were used as loading controls. Rosi impeded PAK1 overexpression and reduced the effect of WT-PAK1 and TNFα on p65. (C) NF-κB luciferase assay performed in PAK1 knockdown cells using siPAK1 or scrRNA with or without Rosi. siPAK1 and Rosi blocked NF-kB transcriptional activity, Tukey HSD; ***p < 0.001. (D) RTPCR of PAK1, PPARγ, and CD36, in PAK1 knock down cells using siPAK1 or scrRNA. siPAK1 increased PPARγ transcription and its downstream target CD36. (E) RTPCR of PPARγ and CD36 in WT and PAK1 KO mouse SIO. PAK1 deletion increased mRNA levels of PPARγ and its downstream target CD36.
WT-PAK1 overexpression suppressed PPARγ (Fig. 4D&E), which was recovered upon 5-ASA treatment. If inhibition of PAK1 is required for PPARγ activation, PAK1 knock down should result in PPARγ activation. In fact, siPAK1 increased PPARγ mRNA and its downstream target CD36 (Supplementary Fig. S4D). Moreover, PPARγ and CD36 expression was increased five-fold in PAK1-KO SIO (Supplementary Fig. S4E). siPAK1 also induced PPAR transactivation and the PPARγ antagonist BADGE blocked this effect (Fig. 4F). These data demonstrate that PAK1 inhibition itself is sufficient to activate PPARγ transcriptional activity.
If PAK1 regulates NF-κB signaling through modulation of PPARγ, then PPARγ inhibition should impede the effect of PAK1 inhibition in reducing NF-κB transactivation. As shown in Fig. 3D, 5-ASA, siPAK1, and 5-ASA in combination with siPAK1 inhibited NF-κB transcriptional activity. Inhibition of PPARγ with BADGE reduced the effect of 5-ASA and siPAK1 on NF-κB transactivation (Fig. 4G). Overall these data demonstrate that (1) PAK1 activates NF-κB through suppression of PPARγ, and (2) activation of PPARγ by 5-ASA (PAK1 inhibition) or PAK1 silencing downregulate NF-κB.
4. Discussion
Chronic gut inflammation triggers the development of CAC. Here we demonstrate that in colitis PAK1 is overexpressed in IECs although PAK1 activation is limited to the luminal surface. With advancement to CAC, PAK1 is further overexpressed and phosphorylated throughout the tumor. PAK1 activation by pro-inflammatory cytokines contributes to canonical NF-κB signaling through inhibition of PPARγ. Mechanistically, PAK1 increases p65 stability downstream of IkB, and is required for complete activation of NF-κB by TNFα. 5-ASA, commonly used in the treatment of IBD, downregulated NF-κB transcriptional activity via PAK1–p65 inhibition. Indeed, 5-ASA has been shown to activate PPARγ [22,25] and to inhibit PAK1 [27] and its regular intake reduces the risk of CAC in UC [28]. Altogether, our data reveal a novel role of PAK1 for IEC homeostasis during gut inflammation.
Pro-inflammatory cytokine secretion from immune cells drives inflammation in IBD [21]. Inflammatory cytokines such as TNFα were previously reported to activate PAK1 in fibroblasts, keratinocytes, macrophages, and endothelial cells [13,29–31]. Here we also demonstrate phosphorylation of PAK1 by IL-1β and IFNγ in IEC. Although, we did not investigate the mechanism of PAK1 activation by these cytokines, upstream activators of PAK1 such as the Rho-GTPases Rac1/Cdc42 may be involved [32–34].
In vivo, in the absence of inflammation, PAK1 expression was more robust at the luminal surface epithelium and in small intestinal villi than in the crypts. This was surprising as PAK1 phosphorylates β-catenin, which is predominately located in the proliferative zone at the base of the crypts [35,36]. Interestingly, p65 expression was also localized at the luminal surface, supporting that expression of PAK1 and p65 are associated throughout the intestine. PAK1 expression was more pronounced within SB villi in comparison to the surface of the LB epithelium, which may provide a physiological advantage in modulating cytoskeletal rearrangements required for enterocyte migration along the villus axis. During inflammation, PAK1 triggers p65 nuclear translocation thereby preventing cells from physiological shedding likely through inhibition of apoptosis [12]. This would assist in the restoration of intestinal barrier integrity after intestinal infections. In CAC, PAK1 is both overexpressed and activated. We modeled PAK1 overexpression via WT-PAK1 overexpression in HCEC-1CT, which induced a PAK1–p65 interaction as well as nuclear translocation of p65, and NF-κB transactivation in the absence of TNFα. Such activation of p65 has been shown to delay apoptosis and promote carcinogenesis [8]. Moreover, TNFα-activated NF-κB transactivation was enriched upon overexpression of WT-PAK1 but impeded upon KD-PAK1, suggesting a role for PAK1 kinase activity in TNFα signaling. We observed subtle differences between the transcriptional assay and protein data which may be an effect of the specificity of the two readouts, and a PAK1 specific role for NF-kB activation in the nucleus [37]. Regarding the protein data, KD-PAK1 overexpression could not fully impede nuclear p65 translocation upon TNF-stimulation, possibly due to PAK1 independent pathways. Therefore, it is likely that overexpression of KD-PAK1 reduces NF-kB transactivation more effectively than reducing p65 protein levels. One limitation to our study is that we did not treat PAK1-KO mice with TNFα. However, we utilized SIO from PAK1-KO mice which blocked nuclear translocation of p65 downstream of TNFα. Together, our data support PAK1 as a critical mediator of NF-κB activation downstream of TNFα within IEC and suggest that PAK1 overexpression, activation, and nuclear localization may contribute to tumor development by sustaining NF-κB activity.
TGFβ activated kinase (TAK1) is required for canonical NF-κB activation primarily through phosphorylation of IKKα and through inducing IkB degradation [38]. Neither PAK1 overexpression nor knockdown interfered with the upstream IKKα/IkB cascade, suggesting that PAK1's effect on p65 is through an alternative pathway separate from TAK1 and downstream of IkB.
Besides its role in fatty acid oxidation/transport, and increasing insulin sensitivity [39], PPARγ was recently reported to be an E3 ubiquitin ligase, which induces p65 degradation [23]. 5-ASA acts as a PPARγ agonist and also inhibits PAK1 and NF-κB [22,27,40]. Here we demonstrate that 5-ASA induced degradation of p65 protein (Fig. 3C) indicative of PPARγ's E3 ubiquitin ligase activity. Thus, it was imperative to investigate the PAK1/PPARγ axis. In fact, upon pro-inflammatory signals (as observed in IBD), PAK1 activation suppresses PPARγ and contributes to NF-κB signaling by stabilizing p65. This notion is supported by our observation that total p65 levels are reduced by both 5-ASA and PAK1 inhibition (IPA-3, siPAK1). TNFα initiates PPARγ recruitment and degradation of p65 in mouse embryonic fibroblasts [23]. In our system, TNFα increased PAK1 activation concurrent with PPARγ downregulation (Fig. 4C). As PAK1 overexpression inhibits PPARγ at the protein and transcriptional levels, it is likely that PAK1 itself, or its downstream targets (JNK or ERK) could phosphorylate PPARγ leading to its nuclear export and proteasomal degradation [41]. Moreover, PPARγ is known to regulate its own expression [42], therefore down regulation of PPARγ at the protein level would result in reduced transcription, which we also observed in our system (Fig. 4D&E). PAK1 activation or overexpression could inhibit PPARγ transcription through multiple pathways. In pluripotent mesenchymal stem cells, TAK1 suppresses PPARγ via a NF-κB interacting kinase (NIK) cascade [26]. PAK1 activation of NIK was previously described [43] and may result in PPARγ inhibition. It is plausible that PAK1 activation and subsequent phosphorylation of SMART [44], Snail [45], or the estrogen receptor [46] may downregulate PPARγ [47–49]. Nevertheless, our data implicate PAK1 in modulating a PPARγ/p65 cascade, which has not previously been reported.
We did not study the effect of PAK1 on other PPARs as 5-ASA is a specific agonist for PPARγ [22], and only PPARγ but not PPARα or δ induces p65 degradation [23]. Interestingly, both 5-ASA and Rosiglitazone inhibited PAK1 (Figs. 3C and 4B). One explanation may be upon ligand activation, PPARγ may not only mark p65 but also a PAK1–p65 complex for proteasomal degradation. 5-ASA also inhibits PAK1 at the mRNA level which is suggestive of an additional mechanism independent of PPARγ ligand activation.
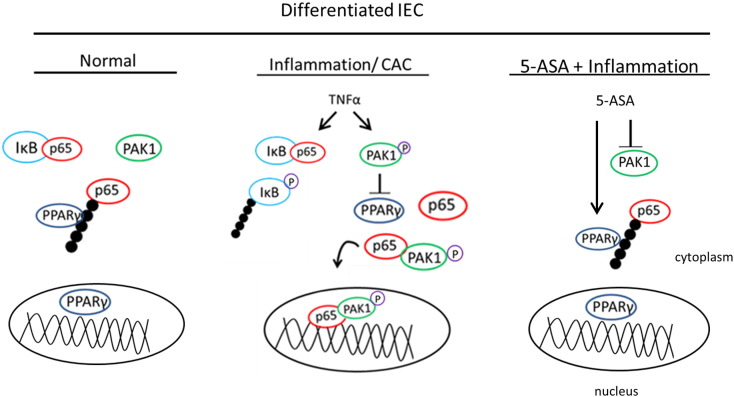
We propose that PAK1 is another player in PPARγ/NF-κB cascade in intestinal epithelial cells (Fig. 5). In differentiated normal IECs, PPARγ regulates NF-κB through proteasomal degradation of p65. However, in the presence of inflammation or CAC, PAK1 is overexpressed and activated, whereas PPARγ is suppressed [50]. PAK1 overexpression or activation blocks PPARγ leading to increased NF-κB transcriptional activation. In addition we further demonstrate that inhibition of PAK1 by 5-ASA also leads to activation of PPARγ's E3 ligase activity.
Fig. 5.
PAK1 modulates a PPARγ/p65 cascade. (Left) Within normal differentiated IEC, PAK1 activity and expression is low while PPARγ is present in both the cytoplasm and nucleus. Cytoplasmic NF-kB is maintained in an inactive state via the sequestration of p65 by IkB. PPARγ further regulates free p65 through E3 ligase activity and proteasomal degradation in the cytoplasm. (Middle) In chronic inflammation and colitis associated cancer (CAC) PAK1 is hyperactivated while PPARγ is downregulated. Pro-inflammatory cytokines such as TNFα induce PAK1 phosphorylation, activation, and nuclear colocalization with p65. Activated PAK1 also blocks PPARγ, further increasing the nuclear accumulation of p65 independently of IkB. (Right) The anti-inflammatory drug 5-ASA inhibits PAK1 thereby restoring PPARγ expression, and increasing its activity in inhibiting free p65 in the cytoplasm.
Overall, these findings support a role for PAK1 in epithelial cell homeostasis in IBD and CAC. Pharmacological inhibition of PAK1 overexpression may recover PPARγ levels and impair the inflammatory response such as NF-kB activation in IEC.
The following are the supplementary data related to this article.
Supplementary tables.
Disclosure of potential conflicts of interest
CG has a research collaboration with Shire Pharmaceuticals and received research support, lecturing or consulting honoraria from Ferring and Dr. Falk Pharma.
Author contributions
KD: conception and design, acquisition of data, analysis and interpretation of data, writing of the manuscript, VK: conception and design, acquisition of data, analysis and interpretation of data and revision of manuscript, ML: analysis and interpretation of data, TC: interpretation of data, proof-reading of the manuscript, development of methodology, FH: analysis and interpretation of data, NG: development of methodology, RE: statistical analysis, JW: acquisition of data, analysis and interpretation of data, MP: interpretation of data, revision of the manuscript, AW: obtained funding, revision of manuscript, CG: obtained funding, conception and design, study supervision, revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The financial support by the Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development is gratefully acknowledged. We acknowledge Dr. Andres I. Roig and Dr. Jerry W. Shay for providing HCEC-1CT cells and Dr. Jonathan Chernoff for the WT-PAK1 overexpression plasmid.
Grant support
The study was supported by Austrian Science Fund (P24121 to Christoph Gasche). The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305564 (SysmedIBD).
References
- 1.Rubin D.T., Cruz-Correa M.R., Gasche C., Jass J.R., Lichtenstein G.R., Montgomery E.A., Riddell R.H., Rutter M.D., Ullman T.A., Velayos F.S., Itzkowitz S., A.S.A.i.C.C.P.M. Group Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: a clinical review and update. Inflamm. Bowel Dis. 2008;14:265–274. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 2.Khare V., Lyakhovich A., Dammann K., Lang M., Borgmann M., Tichy B., Pospisilova S., Luciani G., Campregher C., Evstatiev R., Pflueger M., Hundsberger H., Gasche C. Mesalamine modulates intercellular adhesion through inhibition of p-21 activated kinase-1. Biochem. Pharmacol. 2013;85:234–244. doi: 10.1016/j.bcp.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrini M.C., Lei M., Harrison S.C., Mayer B.J. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell. 2002;9:73–83. doi: 10.1016/s1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R., Gururaj A.E., Barnes C.J. p21-Activated kinases in cancer. Nat. Rev. Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 5.Dammann K., Khare V., Gasche C. Tracing PAKs from GI inflammation to cancer. Gut. 2014;63:1173–1184. doi: 10.1136/gutjnl-2014-306768. [DOI] [PubMed] [Google Scholar]
- 6.Khare V., Dammann K., Asboth M., Krnjic A., Jambrich M., Gasche C. Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm. Bowel Dis. 2015;21:287–296. doi: 10.1097/MIB.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., Kagnoff M.F., Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwitalla S., Fingerle A.A., Cammareri P., Nebelsiek T., Goktuna S.I., Ziegler P.K., Canli O., Heijmans J., Huels D.J., Moreaux G., Rupec R.A., Gerhard M., Schmid R., Barker N., Clevers H., Lang R., Neumann J., Kirchner T., Taketo M.M., van den Brink G.R., Sansom O.J., Arkan M.C., Greten F.R. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Myant K.B., Cammareri P., McGhee E.J., Ridgway R.A., Huels D.J., Cordero J.B., Schwitalla S., Kalna G., Ogg E.L., Athineos D., Timpson P., Vidal M., Murray G.I., Greten F.R., Anderson K.I., Sansom O.J. ROS production and NF-kappaB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoesel B., Schmid J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams J.M., Duckworth C.A., Watson A.J., Frey M.R., Miguel J.C., Burkitt M.D., Sutton R., Hughes K.R., Hall L.J., Caamano J.H., Campbell B.J., Pritchard D.M. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis. Model. Mech. 2013;6:1388–1399. doi: 10.1242/dmm.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost J.A., Swantek J.L., Stippec S., Yin M.J., Gaynor R., Cobb M.H. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J. Biol. Chem. 2000;275:19693–19699. doi: 10.1074/jbc.M909860199. [DOI] [PubMed] [Google Scholar]
- 14.Roig A.I., Eskiocak U., Hight S.K., Kim S.B., Delgado O., Souza R.F., Spechler S.J., Wright W.E., Shay J.W. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology. 2010;138:1012–1021. doi: 10.1053/j.gastro.2009.11.052. (e1011-1015) [DOI] [PubMed] [Google Scholar]
- 15.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 16.Campregher C., Luciani M.G., Gasche C. Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut. 2008;57:780–787. doi: 10.1136/gut.2007.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khare V., Lang M., Dammann K., Campregher C., Lyakhovich A., Gasche C. Modulation of N-glycosylation by mesalamine facilitates membranous E-cadherin expression in colon epithelial cells. Biochem. Pharmacol. 2014;87:312–320. doi: 10.1016/j.bcp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter J.H., Douglass L.E., Deddens J.A., Colligan B.M., Bhatt T.R., Pemberton J.O., Konicek S., Hom J., Marshall M., Graff J.R. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin. Cancer Res. 2004;10:3448–3456. doi: 10.1158/1078-0432.CCR-03-0210. [DOI] [PubMed] [Google Scholar]
- 19.Radu M., Semenova G., Kosoff R., Chernoff J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zenke F.T., King C.C., Bohl B.P., Bokoch G.M. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J. Biol. Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- 21.Neurath M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 22.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J., Metzger D., Wahli W., Desvergne B., Naccari G.C., Chavatte P., Farce A., Bulois P., Cortot A., Colombel J.F., Desreumaux P. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Y., Moreau F., Chadee K. PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65. Nat. Commun. 2012;3:1300. doi: 10.1038/ncomms2270. [DOI] [PubMed] [Google Scholar]
- 24.Chen L., Necela B.M., Su W., Yanagisawa M., Anastasiadis P.Z., Fields A.P., Thompson E.A. Peroxisome proliferator-activated receptor gamma promotes epithelial to mesenchymal transformation by Rho GTPase-dependent activation of ERK1/2. J. Biol. Chem. 2006;281:24575–24587. doi: 10.1074/jbc.M604147200. [DOI] [PubMed] [Google Scholar]
- 25.Rousseaux C., El-Jamal N., Fumery M., Dubuquoy C., Romano O., Chatelain D., Langlois A., Bertin B., Buob D., Colombel J.F., Cortot A., Desreumaux P., Dubuquoy L. The 5-aminosalicylic acid antineoplastic effect in the intestine is mediated by PPARgamma. Carcinogenesis. 2013;34:2580–2586. doi: 10.1093/carcin/bgt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzawa M., Takada I., Yanagisawa J., Ohtake F., Ogawa S., Yamauchi T., Kadowaki T., Takeuchi Y., Shibuya H., Gotoh Y., Matsumoto K., Kato S. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat. Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 27.Dammann K.W., Khare V., Lang M., Granofszky N., Gasche C. Tu1674 PAK1 mediates NF-KB signaling in colitis and colitis-associated cancer. Gastroenterology. 2013;144:S-819. [Google Scholar]
- 28.Velayos F.S., Terdiman J.P., Walsh J.M. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am. J. Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L., Yan C., Gieling R.G., Kida Y., Garner W., Li W., Han Y.P. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15. doi: 10.1186/1471-2172-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu D., Yang Y., Xiao Y., Lin H., Ye Y., Zhan Z., Liang L., Yang X., Sun L., Xu H. Role of p21-activated kinase 1 in regulating the migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Rheumatology (Oxford) 2012;51:1170–1180. doi: 10.1093/rheumatology/kes031. [DOI] [PubMed] [Google Scholar]
- 31.Stockton R.A., Schaefer E., Schwartz M.A. p21-Activated kinase regulates endothelial permeability through modulation of contractility. J. Biol. Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 32.Kant S., Swat W., Zhang S., Zhang Z.Y., Neel B.G., Flavell R.A., Davis R.J. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Genes Dev. 2011;25:2069–2078. doi: 10.1101/gad.17224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojciak-Stothard B., Entwistle A., Garg R., Ridley A.J. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell–cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell. Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Puls A., Eliopoulos A.G., Nobes C.D., Bridges T., Young L.S., Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF(alpha) and IL-1, and by the Epstein–Barr virus transforming protein LMP1. J. Cell Sci. 1999;112(Pt 17):2983–2992. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- 35.Park M.H., Kim D.J., You S.T., Lee C.S., Kim H.K., Park S.M., Shin E.Y., Kim E.G. Phosphorylation of beta-catenin at serine 663 regulates its transcriptional activity. Biochem. Biophys. Res. Commun. 2012;419(3):543–549. doi: 10.1016/j.bbrc.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 36.Zhu G., Wang Y., Huang B., Liang J., Ding Y., Xu A., Wu W. A Rac1/PAK1 cascade controls beta-catenin activation in colon cancer cells. Oncogene. 2012;31:1001–1012. doi: 10.1038/onc.2011.294. [DOI] [PubMed] [Google Scholar]
- 37.Jagadeeshan S., Krishnamoorthy Y.R., Singhal M., Subramanian A., Mavuluri J., Lakshmi A., Roshini A., Baskar G., Ravi M., Joseph L.D., Sadasivan K., Krishnan A., Nair A.S., Venkatraman G., Rayala S.K. Transcriptional regulation of fibronectin by p21-activated kinase-1 modulates pancreatic tumorigenesis. Oncogene. 2015;34:455–464. doi: 10.1038/onc.2013.576. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Hauser S., Adelmant G., Sarraf P., Wright H.M., Mueller E., Spiegelman B.M. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J. Biol. Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 40.Kim H., Jeon H., Kong H., Yang Y., Choi B., Kim Y.M., Neckers L., Jung Y. A molecular mechanism for the anti-inflammatory effect of taurine-conjugated 5-aminosalicylic acid in inflamed colon. Mol. Pharmacol. 2006;69:1405–1412. doi: 10.1124/mol.105.020578. [DOI] [PubMed] [Google Scholar]
- 41.Yin R., Dong Y.G., Li H.L. PPARgamma phosphorylation mediated by JNK MAPK: a potential role in macrophage-derived foam cell formation. Acta Pharmacol. Sin. 2006;27:1146–1152. doi: 10.1111/j.1745-7254.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 42.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 43.Neumann M., Foryst-Ludwig A., Klar S., Schweitzer K., Naumann M. The PAK1 autoregulatory domain is required for interaction with NIK in Helicobacter pylori-induced NF-kappaB activation. Biol. Chem. 2006;387:79–86. doi: 10.1515/BC.2006.011. [DOI] [PubMed] [Google Scholar]
- 44.Vadlamudi R.K., Manavathi B., Singh R.R., Nguyen D., Li F., Kumar R. An essential role of Pak1 phosphorylation of SHARP in Notch signaling. Oncogene. 2005;24:4591–4596. doi: 10.1038/sj.onc.1208672. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z., Rayala S., Nguyen D., Vadlamudi R.K., Chen S., Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 46.Wang R.A., Mazumdar A., Vadlamudi R.K., Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21:5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu C., Markan K., Temple K.A., Deplewski D., Brady M.J., Cohen R.N. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y.H., Kim S.H., Lee Y.J., Kang E.S., Lee B.W., Cha B.S., Kim J.W., Song D.H., Lee H.C. Transcription factor Snail is a novel regulator of adipocyte differentiation via inhibiting the expression of peroxisome proliferator-activated receptor gamma. Cell. Mol. Life Sci. 2013;70:3959–3971. doi: 10.1007/s00018-013-1363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Kilgore M.W. Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol. Cell. Endocrinol. 2002;194:123–133. doi: 10.1016/s0303-7207(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 50.Dubuquoy L., Jansson E.A., Deeb S., Rakotobe S., Karoui M., Colombel J.F., Auwerx J., Pettersson S., Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–1276. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.