Abstract
Background:
Until now, no experimental study has directly assessed the arrhythmogenesis of chronic consumption of anabolic androgenic steroids along with moderate-intensity endurance exercise.
Objectives:
We evaluated the influence of integration of anabolic androgenic steroids along with moderate-intensity endurance exercise on susceptibility to lethal ventricular arrhythmias in rat.
Materials and Methods:
The animal groups were as follows: control group (CTL); exercise group (EX) which were under 6 weeks of treadmill exercise; nandrolone group (Nan) which received 5 mg/kg of nandrolone decanoate twice a week; vehicle group (Arach) which received Arachis oil (solvent of nandrolone); trained vehicle group (Arach + Ex); and trained nandrolone group (Nan + Ex). One day after ending of the intervention period, arrhythmia was inducted by intravenous infusion of aconitine and ventricular arrhythmias were recorded. Then malondialdehyde (MDA) and glutathione peroxidase (GPX) of heart tissue were measured.
Results:
Nandrolone, exercise, and their combination were associated with heart hypertrophy. Exercise could prevent the incremental effect of nandrolone on MDA/GPX ratio. Chronic administration of nandrolone with moderate-intensity endurance exercise had no significant effect on blood pressure, heart rate, and basal electrocardiographic parameters. Combination of nandrolone and exercise significantly increased the incidence of ventricular fibrillation (VF) and reduced the VF latency (P < 0.05).
Conclusions:
The findings suggest that chronic coadministration of nandrolone with moderate-intensity endurance exercise facilitates the VF occurrence in rat. Complementary studies are needed to elucidate the involved mechanisms of this abnormality.
Keywords: Nandrolone Decanoate, Aerobic Exercise, Ventricular Fibrillation
1. Background
Despite some beneficial effects of anabolic androgenic steroids (AAS) compounds in improvement of physical performance, chronic using of high doses of these drugs is associated with numerous adverse effects on the cardiovascular system including hypertension, myocardial infarction, dysrhythmias, hypertrophic cardiomyopathy, and cardiac remodeling (1-4).
Regular courses of exercise are the only practical method that can protect the heart against coronary artery diseases. Only one course of exercise can protect heart against ischemia/reperfusion injury (5). In addition, regular courses of aerobic exercise such as running or swimming can protect heart against the ischemia/reperfusion injury in animal models and can lead to diminution of infarcted area (6, 7). Regarding the cardiovascular adverse effects of AASs, usefulness of exercise training with consumption of high amounts of these drugs is controversial and questionable. Consumption of high doses of anabolic steroids might increase the possibility of cardiac arrhythmias. In animals with cardiac ischemia, acute injection of nandrolone increases the possibility of cardiac arrhythmias (8). It is also reported that high and chronic consumption of nandrolone leads to ventricular repolarization disturbances (9). In addition, there are numerous evidence regarding occurrence of sudden death among athletics using anabolic steroids, especially nandrolone (10-12). However, to our knowledge, thus far no investigation has been directly performed to study the effect of chronic nandrolone consumption along with moderate exercise on the development of lethal cardiac arrhythmia.
2. Objectives
Based on the significance of this issue, we assessed the effects of chronic administration of nandrolone along with moderate-intensity treadmill exercise on susceptibility to lethal ventricular arrhythmias in rat.
3. Materials and Methods
Aconitine and sodium thiopental were purchased from Sigma (England) and Sandoz (Austria), respectively. Nandrolone decanoate and Arachis oil, purchased respectively from Gedeon Richter (Hungary) and Henry Lamotte (Germany), were dedicated by Iran Hormone Company. Animal procedures were performed in accordance with the guidelines for the care and use of laboratory animals (Ethic Committee permission Kerman University of Medical Sciences, Kerman, Iran) and were performed on 48 male Wistar rats weighing 250 to 300 g. The animals were housed in a temperature-controlled room with free access to rat regular diet and water. Before the start of the experiment, all rats were run-tested on a motorized rodent treadmill (3 × 5 minutes, with 15 minutes breaks) for three successive days to habituate them to the training environment and to eliminate rats unwilling to run (13). Then they were randomly assigned to one of the following six experimental groups: 1) sedentary group (CTL group); 2) trained group with aerobic exercise by treadmill training (Ex group); 3) sedentary vehicle-treated (Arach group) (vehicle = 0.2 mL/kg Arachis oil as solvent of nandrolone decanoate twice a week, intramuscularly, for 6 weeks; 4) sedentary nandrolone-treated (Nan group), which received 5 mg/kg of nandrolone decanoate twice a week, intramuscularly, for 6 weeks) (14); 5) trained vehicle-treated (Arach + Ex group) which received Arachis oil and swimming training; and 6) trained nandrolone-treated (Nan + Ex group) which received nandrolone decanoate and swimming training.
3.1. Exercise Training Protocol
Rats in the exercise groups were trained on a rodent treadmill (at 0° slope) five days a week, for 6 weeks. The treadmill speed was 16 m/min during the first week, increased by 4 m/min weekly over the next three weeks, and was kept at 28 m/min (~70 - 75% maximal aerobic capacity (VO2 max)) for the remaining training sessions (13, 15). This protocol is considered as a moderate-intensity endurance training (15).
3.2. Blood Pressure and Electrocardiogram Recording and Arrhythmia Induction
Hemodynamic and electrocardiogram (ECG) recording and arrhythmia induction were performed as described previously (16). A day after the completion of the training session, the animals were anesthetized with intraperitoneal sodium thiopental (50 mg/kg). The right common carotid artery was cannulated and connected to a pressure transducer and a PowerLab system continuously recorded the heart rate (HR) and arterial blood pressure (BP). The trachea was cannulated and animals were artificially ventilated with room air at 50 strokes per minute during arrhythmia induction. Basal ECG (limb lead II) and BP were recorded following recovery time from surgery (15 minutes). Exclusion criteria were the presence of cardiac arrhythmia or a sustained drop in mean arterial BP to < 70 mm Hg during the stabilization period. For arrhythmia induction, aconitine was infused through an angiocatheter (G-24) in the tail vein with a syringe pump at a velocity of 0.1 mL/min (15 µg/mL of saline) for ten minutes. The BP and ECG were simultaneously recorded during the infusion and for another five minutes after the infusion period ended (16).
3.3. Measured and Calculated Parameters
The mean arterial pressure (MAP) was estimated using the following formula: MAP = Pd + (Ps - Pd)/3, where Pd and Ps are respectively the diastolic and systolic arterial pressures. The PR interval (the earliest P-wave onset to the earliest onset of the QRS complex onset) and QT interval (the earliest Q or R-wave onset through the end of T wave) of basal ECG were determined by a mean of two minutes of ECG-recorded strip. In order to obviate the dependence of QT interval on HR, corrected QT interval (QTc) was measured using Bazett’s formula normalized as:
QTcn - B = QT/(RR/f)1/2
Where RR is R-R interval and f = 150 msec (16-18).
The premature ventricular contraction (PVC), ventricular tachycardia (VT), and ventricular fibrillation (VF) were counted during the 15 minutes of the experiment, and the latency and duration of PVC, VT, and VF was measured in seconds. According to the Lambeth conventions, ventricular arrhythmias were defined as follows:
Ventricular premature beat (VPB; equal to PVC) is defined as a ventricular electrical complex (complete electrical event: QRS, RS, QRST, or RST) that is different in shape (voltage and/or duration, i.e. height and/or width) from the preceding (non-VPB) ventricular complex, and is premature in relation to the preceding ventricular complex. VT is a run of four or more consecutive ventricular premature beats. VF is defined as a sequence of a minimum of four consecutive ventricular complexes without intervening diastolic pauses in which the intrinsic shape, the peak-peak interval, and the height vary, and the variation between each is non-progressive (19).
The threshold dose of aconitine required for producing different ventricular arrhythmias (e.g. PVC, VT, or VF) was determined according to the following formula (16):
Threshold (µg/kg) for arrhythmia = 15 µg/mL × 0.1 mL/min × time required for arrhythmia (min)/body weight (BW) (kg) = 1.5 µg/min × time (min)/BW (kg).
In addition, The severity of arrhythmias in the different groups was presented quantitatively by a previous scoring system (18) defined as follows: 0, < 10 PVCs; 1, ≥ 10 PVCs; 2, one to five episodes of VT; 3, > 5 episodes of VT or 1 episode of VF; 4, two to five episodes of VF; 5, > 5 episodes of VF; 6, VT or VF or both with duration > 300 sec.
Finally, the rats were killed and their hearts were removed, rinsed with cold saline, and weighed. The total heart weight (HW, left, and right ventricle) was normalized by total BW of the animal (mg/g). This ratio was considered as an index of cardiac hypertrophy (4, 20). A piece of heart apex dissected, weighed, and homogenized in 5 mL of 0.1% MTris-HCl buffer (pH 7.4) in ice-cold condition. After centrifuging, the clear supernatant solution was taken for the biochemical analysis. Total proteins were measured by using the Lowry et al. method (21). Malondialdehyde (MDA) levels, as an index of lipid peroxidation, was estimated by concentration of thiobarbituric acid reactive substances (TBARS) in heart tissue (22). Glutathione peroxidase (GPX) of heart tissues was determined using relative Randox assay kits (according to the manufacturer’s protocol) (23).
3.4. Statistical Analysis
The data were presented as mean ± standard error of the mean. The normal distribution of quantitative data was confirmed by Kolmogorov-Smirnov test. Comparison of all parameters except arrhythmia scores among different groups were performed using one-way analysis of variance (ANOVA) and Tukey post-hoc test. Arrhythmia scores in the animal groups were compared using nonparametric Kruskal-Wallis test. The data was analyzed by use of SPSS 17 (SPSS Inc., Chicago, Illinois, the United States). P value < 0.05 was considered as statistically significant.
4. Results
4.1. Ventricular Hypertrophy Index
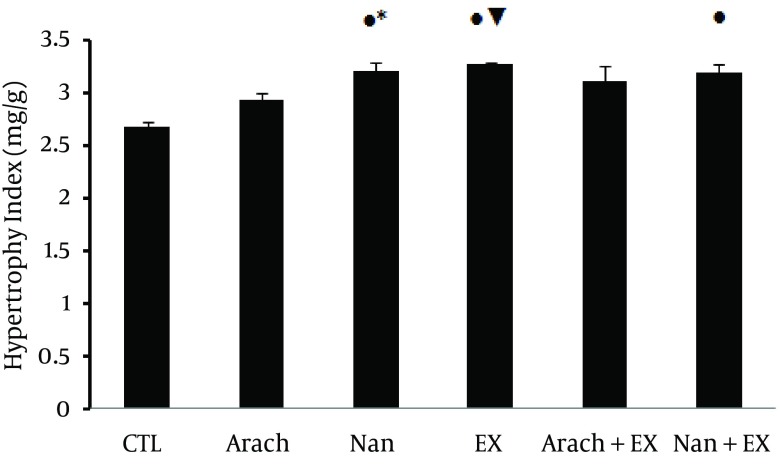
Ventricular hypertrophy index showed significant increase in Nan group (P < 0.01 versus CTL and P < 0.05 versus Arach groups), Ex group (P < 0.01 versus CTL and Arach groups) and in Nan + Ex group (P < 0.01 versus CTL group) (Figure 1).
Figure 1. Mean of Heart Hypertrophy Index in Different Experimental Groups at the End of 6th Week.
Values are presented as mean ± SEM. N = 7 - 9. CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training;*, P < 0.05 compared with Arach group; ●, P < 0.01 compared with CTL group; ▼, P < 0.01 compared with Arach group.
4.2. Redox Indices
Six weeks of nandrolone consumption and moderate intensity exercise with or without nandrolone was associated with non-significant increase in MDA of cardiac tissue. In addition nandrolone-treated group showed insignificant decrease in GPX of heart, so that the ratio of MDA/GPX only was significant in Nan group compared to CTL group (P < 0.05) (Table 1).
Table 1. Glutathione Peroxidase and Malondialdehyde of Heart Tissue in Different Animal Groups a.
| Group b | n | MDA, µmol/mg Protein | GPX, Unit/mg Protein | MDA/GPX |
|---|---|---|---|---|
| CTL | 7 | 1.26 ± 0.267 | 15.98 ± 4.89 | 0.079 ± 0.019 |
| Arach | 7 | 1.305 ± 0.26 | 12.42 ± 1.69 | 0.105 ± 0.018 |
| Nan | 7 | 1.96 ± 0.27 | 12.02 ± 1.45 | 0.163 ± 0.019* |
| EX | 9 | 2.08 ± 0.25 | 23.41 ± 3.51 | 0.089 ± 0.013 |
| Arach + EX | 9 | 2.16 ± 0.34 | 15.82 ± 1.64 | 0.136 ± 0.020 |
| Nan + EX | 8 | 1.8 ± 0.27 | 17.45 ± 2.46 | 0.103 ± 0.017 |
| P value | - | 0.056 | 0.131 | 0.046 |
a Abbreviations: GPX, glutathione peroxidase; MDA, malondialdehyde; and NA, non-applicable.
b CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.
4.3. Blood Pressure and Heart Rate and Basal Electrocardiographic Parameters
A sample strips of arterial BP and lead II of ECG from a rat of the Nan + Ex group, which were simultaneously recorded, are shown in Figure 2 A. Moderate-intensity endurance exercise or nandrolone alone or their combination didn’t have significant effect on BP, HR, and RR, PR, J, QRS complex, and QTc intervals of electrocardiogram (Table 2).
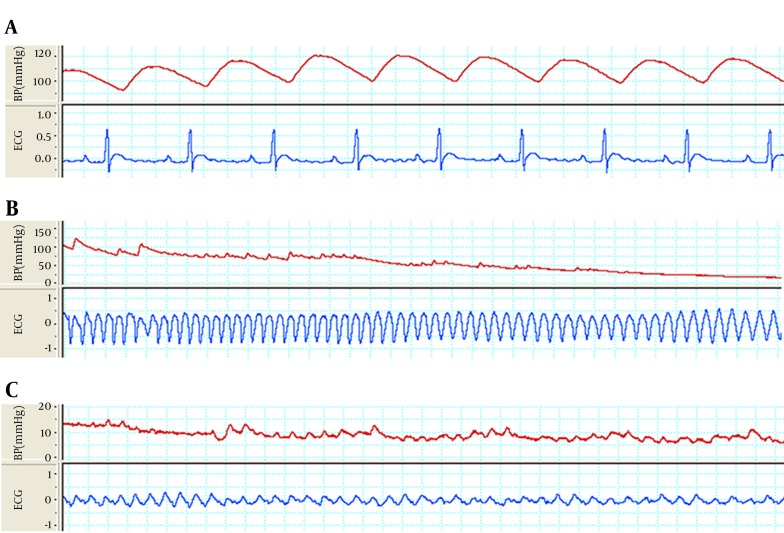
Figure 2. The Strips of Arterial Blood Pressure and Lead II of ECG That Simultaneously was Recorded From an Animal From Nan+ Ex Group in Different Condition.
A, Basal normal sinus rhythm (NSR) and blood pressure of an animal from Nan+ Ex group. B, Sustained ventricular tachycardia (VT) with regular morphology and rate along with blood pressure drop in same animal six minutes after onset of aconitine injection. C, Sustained ventricular fibrillation (VF) along with severe blood pressure drops in same animal 11 minutes after onset of aconitine injection.
Table 2. Mean Arterial Blood Pressure, Heart Rate, and parameters of Electrocardiogram in All Animal Groups a.
| Group b | n | MAP, mmHg | HR, Beat/min | Electrocardiographic Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| RR Interval, msec | PR Interval, msec | QRS Interval, msec | JT Interval, msec | QTc-n Interval, msec | ||||
| CTL | 7 | 117 ± 2.7 | 406 ± 14 | 149 ± 5 | 44.7 ± 1 | 14.9 ± 0.9 | 40 ± 4.2 | 55 ± 4.4 |
| Arach | 8 | 116 ± 5 | 375 ± 36 | 153 ± 4 | 43.7 ± 1 | 16 ± 1 | 38 ± 2.9 | 55 ± 3.5 |
| Nan | 8 | 106 ± 8 | 382 ± 15 | 159 ± 7 | 46.5 ± 1.5 | 15.4 ± 0.8 | 38 ± 2.2 | 52 ± 2.9 |
| EX | 9 | 110 ± 5 | 400 ± 16 | 148 ± 7 | 44.5 ± 1 | 14.2 ± 0.7 | 40 ± 2.3 | 55 ± 2.4 |
| Arach + EX | 9 | 101 ± 9 | 376 ± 11 | 160 ± 4 | 44.5 ± 1.2 | 16.9 ± 1 | 42 ± 4.6 | 57 ± 3.7 |
| Nan + EX | 8 | 104 ± 8 | 395 ± 10 | 153 ± 4 | 42.5 ± 1.7 | 15 ± 0.5 | 43 ± 3.3 | 57 ± 3.4 |
| P value | - | 0.271 | 0.482 | 0.513 | 0.381 | 0.375 | 0.346 | 0.829 |
a Abbreviations: MAP, mean arterial pressure; HR, heart rate; NA, non-applicable.
b CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.
4.4. Susceptibility to Ventricular Arrhythmias
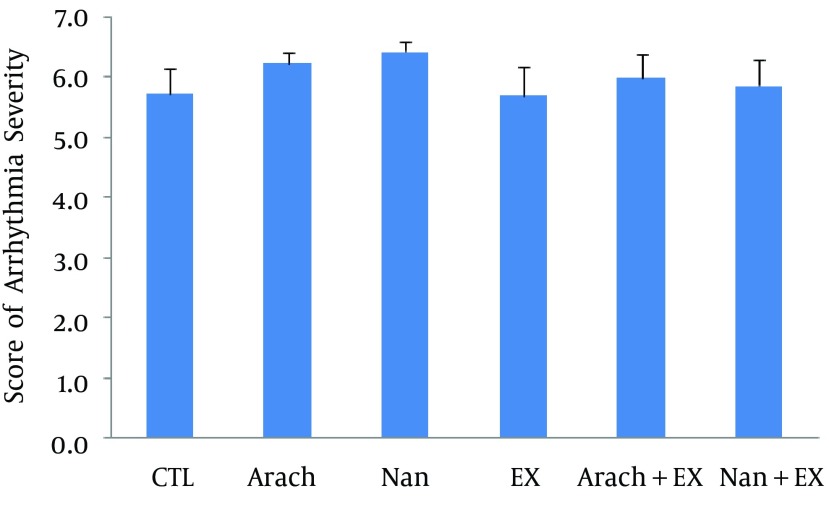
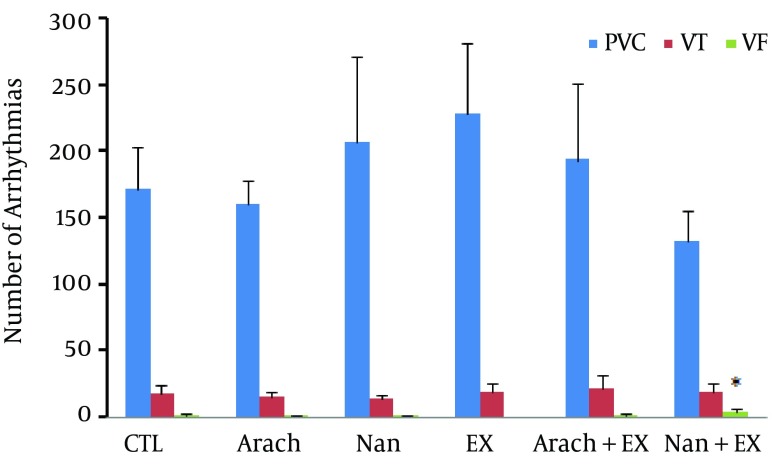
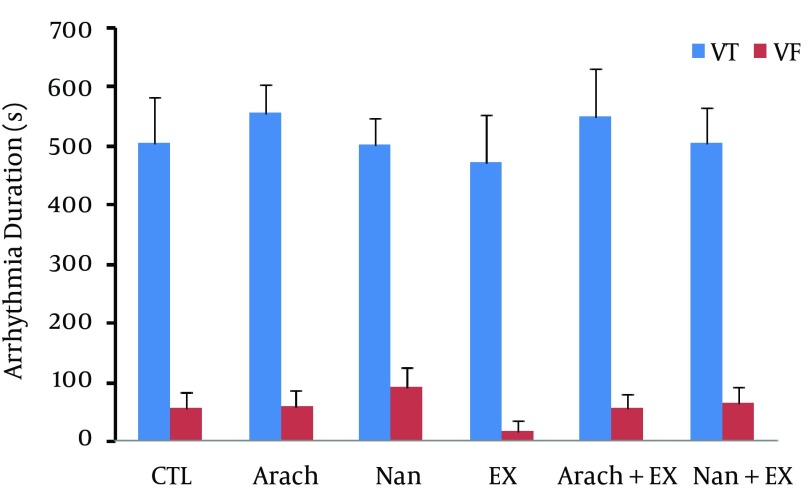
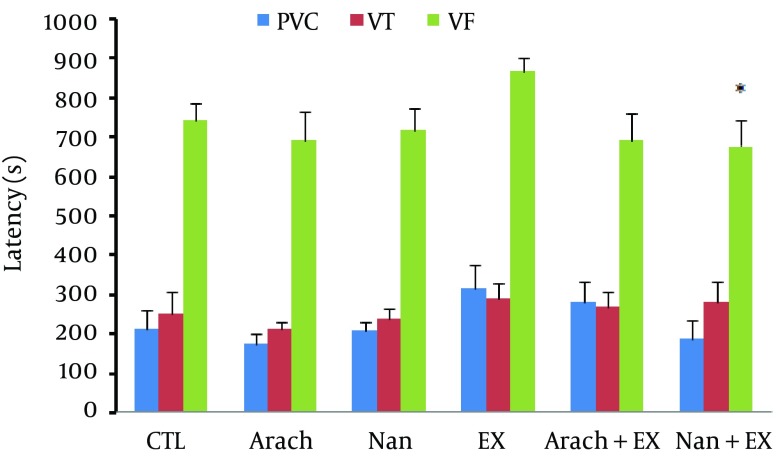
Chronic administration of nandrolone with moderate-intensity endurance exercise did not have significant effect on severity of ventricular arrhythmias (Figure 3) as well as the incidence of PVCs and VT, but increased the incidence of VF when compared with Ex group (P < 0.05) (Figure 4). The duration of VF showed increase in nandrolone group and decrease in Ex group, but did not reach to significant level (Figure 5). The latency of PVC and VT had no significant difference among groups, but latency of VF in Nan + Ex group had a significant reduction compared with Ex group (P < 0.05) (Figure 6). Sample strips of arterial BP and lead II of ECG during VT and VF from a rat of the Nan + Ex group that simultaneously were recorded are shown in Figure 2.
Figure 3. Scores of Arrhythmia Severity in Each Experimental Group.
Values are presented as mean ± SEM. N= 7 - 9. CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.
Figure 4. The Number of Ventricular Arrhythmias in Animal Groups.
Values are presented as mean ± SEM. N= 7 - 9. CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.*, P < 0.05 compared with Ex group.
Figure 5. The Duration of Ventricular Tachycardia and Ventricular Fibrillation Arrhythmias in the Different Groups.
Values are presented as mean ± SEM. N = 7 - 9. CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.
Figure 6. The Latency Periods for Induction of Ventricular Arrhythmias.
Values are presented as mean ± SEM. N= 7 - 9. CTL, control; Arach, animal group which received vehicle (Arachis oil); Nan, animal group which received 5 mg/kg/day of nandrolone decanoate twice a week; Ex, animal group which trained with prolonged moderate intensity exercise; Arach + Ex, animals group which received Arachis oil and exercise training; Nan + Ex, animals group which received nandrolone decanoate and exercise training.*, P < 0.05 compared with Ex group.
5. Discussion
In the present study, the effects of chronic administration of nandrolone decanoate with moderate-intensity endurance exercise on the occurrence of lethal ventricular arrhythmias were investigated in rat. Nandrolone alone significantly increased the ratio of MDA/GPX of cardiac tissue, but moderate-intensity endurance treadmill exercise prevented this adverse effect of nandrolone. In addition, moderate exercise and nandrolone, alone or in combination, were associated with significant increase of the heart hypertrophy index. Nandrolone decanoate alone or along with moderate-intensity endurance exercise did not have significant effects on BP, HR, and basal ECG parameters. Combination of moderate-intensity exercise and nandrolone significantly increased the incidence of VF, the most lethal cardiac arrhythmia, and significantly reduced the VF latency.
In agreement with results of present study, Franquni et.al. (24) showed that chronic administration of nandrolone can lead to significant increase in HW to BW ratio. They showed that activity of angiotensin-converting enzyme during consumption of nandrolone increases and this can lead to increase of angiotensin II release, which is a factor for hypertrophic induction. Some previous animal studies also observed the significant increase of hypertrophy index following exercise training (25-27).
Consistent with our findings, other studies have shown that chronic administration of nandrolone along with exercise is associated with significant increase in heart hypertrophy index (25, 28-30). They showed that renin-angiotensin system activity has increased, which can justify the hypertrophic induction during exercise conditions with consumption of nandrolone. Overall, increase in heart collagen (20) changes the expression of myocardial enzymes (31) and increasing angiotensin-converting enzyme and rennin-angiotensin system activity (20, 24) may be involved in heart hypertrophy following to nandrolone consumption and exercise training.
The MDA is produced from lipid peroxidation, which can be caused by an increase in oxidants. Its production rate is proportional to the breakdown of unsaturated fatty acids. The GPX is an antioxidant enzyme that plays a role in removing free radicals and reducing their damage. In agreement with the findings of present study, others showed that endurance exercise has no significant effect on MDA of cardiac tissue in male rats (23, 32-36). In addition, another study reported that chronic administration of 10 mg/kg of nandrolone for 14 weeks did not have significant effect on GPX level in rats (37). Other studies on male rats showed that endurance exercise causes insignificant increase of GPX activity (35, 36, 38). Similar to findings of the present study, others also reported that exercise and nandrolone, when combined, have no significant effect on cardiac GPX (13, 39). On the other hand, some experiments showed that the treadmill exercise for eight weeks at a rate of 20 m/min significantly increased GPX activity in the male rat (40, 41) and nandrolone administration for 10 weeks at a dose of 10 mg/kg per week non-significantly decreases the GPX; however, nandrolone along with exercise had no effect on the heart GPX (13, 39). In the present study, MDA/GPX in nandrolone group, made up of increased MDA and decreased GPX, in this group was significantly higher than that in the control group; however, moderate-intensity endurance exercise prevented this adverse effect. Therefore, it can be assumed that chronic administration nandrolone induces redox imbalance in favor to oxidants agents and moderate-intensity endurance exercise helps to prevent the destructive effects of nandrolone by balancing redox system.
Regarding the effects of exercise and chronic administration of nandrolone on BP, consistent with the present study, previous animal studies have reported ineffectiveness of endurance treadmill exercise (42, 43) and chronic administration of nandrolone on BP (44). It was also reported that chronic administration of nandrolone and swimming exercise, alone or in combination, have no effect on BP (29, 45). However, previous studies showed that in hypertensive mice, endurance exercise could significantly reduce BP (46, 47). A part of this useful effect of exercise on reduction of BP in hypertensive animals has been attributed to increased plasma-level of atrial natriuretic peptide (ANP) (a vasodilator and reducer of peripheral vascular resistance), reduction of Angiotensin II (a potent vasoconstrictor) and reduction of sympathetic nerve activity during swimming activity (46, 47). The difference between these two studies and ours could be due to examining hypertensive animals in those two studies, while animals were normotensive in our study, and it is evident that treatment protocols such as drugs and exercise can be more effective upon hypertensive animals compared with normotensive ones. Tseng et al. indicated that chronic seven-week administration of 20-mg/kg nandrolone to hypertensive animals can significantly increase BP (48). Of course, in contrast to our study, BP was measured in conscious mode, animals had hypertension, and nandrolone dose in Tseng et al. study was twice as high as our study, all of which justify the difference of their findings with ours.
Regarding the effect of chronic administration of nandrolone and exercise on HR, most of similar studies reported lack of significant effect of exercise (46, 47, 49) or chronic administration of nandrolone (48, 50) on HR of rats. Soares et al. (29) reported insignificant effect of exercise and nandrolone, alone or in combination, on HR of rats. In a human study, Maior et al. (51) also reported the same case as above. Some studies demonstrated contrary findings in which chronic administration of 20-mg/kg nandrolone for ten weeks led to significant reduction of HR (4, 24). In other studies, it was reported that swimming exercise for ten weeks resulted in significant decrease in HR (26, 52). It seems that type, intensity and length of exercise as well as amount of injected nandrolone are the reasons of discrepancy among these findings.
Similar to our results, Phillis et al. (53) demonstrated that chronic administration of 15-mg/kg nandrolone for 17 days had no effect on QTc, QRS, and PR intervals of ECG in male rats. In a human study, Bruder-Nascimento et al. (45) indicated that chronic administration of nandrolone has no effect on QRS. Maior et al. (51) reported that chronic administration of nandrolone results in significant increase of QTc interval in human. The reason of difference between findings of this study and ours is probably the kind of study, i.e., animal vs. human. Achar et al. (54) in a human study showed that chronic administration of nandrolone for five years leads to increase of lethal ventricular arrhythmias such as VF. In another study, Phillis et al. (53) reported that acute administration of nandrolone with a value of 10 to 160 μg/kg/minute during 15 minutes before operation led to significant increase of VF duration while it had no effect on scores of arrhythmias in rat. In the present study, administration of nandrolone and exercise separately were associated with an insignificant increase and decrease in VF duration, respectively. The reason for significance of this parameter in the study of Phillis et al. (53) might be acute intravenous (IV) injection of nandrolone, while injections were intramuscular (IM) in our study that led to much slower absorption of drug and lower serum level peak. Williams and Franklin (55), reported that exercises such as running and walking reduce the risk of ventricular arrhythmias in young males. Other clinical studies on human (mean age, ≥ 40 years) showed that long-term and intense exercises result in increased risk of ventricular arrhythmias (56, 57). They also reported that stimulation of sympathetic system during exercise leads to increase of ventricular automaticity followed by increase the depolarization of Purkinje fibers, which could be a mechanism for VF induction by exercise. The reason of difference of findings in this study and ours could be the kind of study and exercise (i.e., human study and intense treadmill exercise vs. animal study and moderate-intensity endurance exercise).
We showed that moderate-intensity endurance exercise alone caused insignificant decline of VF duration, but exercise along with nandrolone led to significant increase of VF risk as the most dangerous type of ventricular arrhythmia. Because exercise with nandrolone has no significant effect upon ECG parameters, the reason of increase of VF risk in this case cannot probably be changes of basic ECG parameters. The reasons of decline in VF latency and increase in VF incidence in animals undergoing nandrolone plus exercise training might be remodeling of cardiac tissue including structural myocardial modifications, structural abnormality of myofibrils (1, 58), and obstruction of coronary arteries. The latter might originate from increased platelet aggregation due to increase of capacity of thromboxane A2 receptor (59) as well as vascular changes including atherosclerosis, vascular stenosis, and coronary vasospasm during chronic administration of nandrolone and stimulation of sympathetic system during exercise, which leads to rise of ventricular automaticity and increase of phase IV of depolarization of Purkinje fibers (60-62). These conditions may lead to local and widespread ischemia of cardiac tissue; in stress or during administration of arrhythmogenic agents, electrical homogeneity of heart is disrupted more, which provides the condition of re-entry phenomenon and facilitates VF. In addition, it was reported that steroid hormones, chemically related to nandrolone, can acutely inhibit the reuptake of catecholamines into extraneuronal tissues, which could in turn increase catecholamine concentrations at receptor sites. Although normally responsible for reuptake of noradrenaline, the neuronal catecholamine transporter has been shown to be responsible for nonexocytotic release of noradrenaline from sympathetic nerve terminals during ischemia. Increased release of noradrenaline has been implicated in ischemia-induced arrhythmia (63-65). Nandrolone has been shown to cause the release of intracellular calcium in rat primary myotubes, in a manner independent of intracellular androgen receptors but dependent on inositol triphosphate and the extracellular signal-regulated kinase pathway (66). This may be another potential mechanism to explain the increased susceptibility to ventricular arrhythmia in presence of nandrolone and exercise.
It should be noted that because of the limitations of working with animals including ECG recording in a state of consciousness and exercise, the arrhythmia induction performed in anesthetized condition in the present study. The results of this study suggest that chronic administration of nandrolone with long-term moderate-intensity treadmill exercises in male rats can cause significant increase in VF incidence and decrease in VF latency as the most dangerous ventricular arrhythmia. In the present study, this effect was appeared independent of changes of redox system and without vivid changes of ECG parameters. Clarification of involved mechanisms demands complementary studies.
Acknowledgments
The authors express their thanks to Kerman Neuroscience Research Center, Kerman, and Mr. Yaser Masumi from Physiology Research Center of Kerman University for their collaboration. The data presented in this article were derived from a Master of Science thesis (Hamideh Ghorbani Baravati) performed in the Department of Physiology and Physiology Research Center of School of Medicine, Kerman University of Medical Sciences, Kerman, IR Iran.
Footnotes
Authors’ Contributions:Hamideh Ghorbani Baravati and Zeinab Kordestani contributed in the preparation, treatment, and animals training, and helped in experimental procedure and sampling. Siyavash Joukar developed the original idea and the protocol, supervised the study, helped in experimental procedure, data analysis, and wrote the manuscript. Hossein Fathpour contributed as design consultant.
References
- 1.Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41(1):1–15. doi: 10.1016/s0033-0620(98)80019-4. [DOI] [PubMed] [Google Scholar]
- 2.Stergiopoulos K, Brennan JJ, Mathews R, Setaro JF, Kort S. Anabolic steroids, acute myocardial infarction and polycythemia: a case report and review of the literature. Vasc Health Risk Manag. 2008;4(6):1475–80. doi: 10.2147/vhrm.s4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golestani R, Slart RH, Dullaart RP, Glaudemans AW, Zeebregts CJ, Boersma HH, et al. Adverse cardiovascular effects of anabolic steroids: pathophysiology imaging. Eur J Clin Invest. 2012;42(7):795–803. doi: 10.1111/j.1365-2362.2011.02642.x. [DOI] [PubMed] [Google Scholar]
- 4.Pereira-Junior PP, Chaves EA, Costa ER, Masuda MO, de Carvalho AC, Nascimento JH. Cardiac autonomic dysfunction in rats chronically treated with anabolic steroid. Eur J Appl Physiol. 2006;96(5):487–94. doi: 10.1007/s00421-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 5.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol (1985). 2001;91(5):2205–12. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 6.Domenech R, Macho P, Schwarze H, Sanchez G. Exercise induces early and late myocardial preconditioning in dogs. Cardiovasc Res. 2002;55(3):561–6. doi: 10.1016/s0008-6363(02)00334-6. [DOI] [PubMed] [Google Scholar]
- 7.Kloner RA, Simkhovich BZ. Benefit of an exercise program before myocardial infarction. J Am Coll Cardiol. 2005;45(6):939–40. doi: 10.1016/j.jacc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Carmo E, Rosa KT, Koike DC, Fernandes T, Silva Junior N, Mattos KC, et al. Association between anabolic steroids and aerobic physical training leads to cardiac morphological alterations and loss of ventricular function in rats. Revista Brasileira de Medicina do Esporte. 2011;17(2):137–41. [Google Scholar]
- 9.Medei E, Marocolo M, Rodrigues Dde C, Arantes PC, Takiya CM, Silva J, et al. Chronic treatment with anabolic steroids induces ventricular repolarization disturbances: cellular, ionic and molecular mechanism. J Mol Cell Cardiol. 2010;49(2):165–75. doi: 10.1016/j.yjmcc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Luke JL, Farb A, Virmani R, Sample RH. Sudden cardiac death during exercise in a weight lifter using anabolic androgenic steroids: pathological and toxicological findings. J Forensic Sci. 1990;35(6):1441–7. [PubMed] [Google Scholar]
- 11.Fineschi V, Riezzo I, Centini F, Silingardi E, Licata M, Beduschi G, et al. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic findings in two fatal cases of bodybuilders. Int J Legal Med. 2007;121(1):48–53. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 12.Montisci M, El Mazloum R, Cecchetto G, Terranova C, Ferrara SD, Thiene G, et al. Anabolic androgenic steroids abuse and cardiac death in athletes: morphological and toxicological findings in four fatal cases. Forensic Sci Int. 2012;217(1-3):e13–8. doi: 10.1016/j.forsciint.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Sadowska-Krepa E, Klapcinska B, Jagsz S, Sobczak A, Chrapusta SJ, Chalimoniuk M, et al. High-dose testosterone propionate treatment reverses the effects of endurance training on myocardial antioxidant defenses in adolescent male rats. Cardiovasc Toxicol. 2011;11(2):118–27. doi: 10.1007/s12012-011-9105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha TS, Moura MJ, Bernardes CF, Tanno AP, Marcondes FK. Vascular sensitivity to phenylephrine in rats submitted to anaerobic training and nandrolone treatment. Hypertension. 2005;46(4):1010–5. doi: 10.1161/01.HYP.0000174600.51515.e7. [DOI] [PubMed] [Google Scholar]
- 15.Garekani ET, Mohebbi H, Kraemer RR, Fathi R. Exercise training intensity/volume affects plasma and tissue adiponectin concentrations in the male rat. Peptides. 2011;32(5):1008–12. doi: 10.1016/j.peptides.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Joukar S, Ghorbani-Shahrbabaki S, Hajali V, Sheibani V, Naghsh N. Susceptibility to life-threatening ventricular arrhythmias in an animal model of paradoxical sleep deprivation. Sleep Med. 2013;14(12):1277–82. doi: 10.1016/j.sleep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Joukar S, Ghasemipour-Afshar E, Sheibani M, Naghsh N, Bashiri A. Protective effects of saffron (Crocus sativus) against lethal ventricular arrhythmias induced by heart reperfusion in rat: a potential anti-arrhythmic agent. Pharm Biol. 2013;51(7):836–43. doi: 10.3109/13880209.2013.767362. [DOI] [PubMed] [Google Scholar]
- 18.Joukar S, Zarisfi Z, Sepehri G, Bashiri A. Efficacy of Melissa officinalis in suppressing ventricular arrhythmias following ischemia-reperfusion of the heart: a comparison with amiodarone. Med Princ Pract. 2014;23(4):340–5. doi: 10.1159/000363452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, et al. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther. 2013;139(2):213–48. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Rocha FL, Carmo EC, Roque FR, Hashimoto NY, Rossoni LV, Frimm C, et al. Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats. Am J Physiol Heart Circ Physiol. 2007;293(6):H3575–83. doi: 10.1152/ajpheart.01251.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Joukar S, Shahouzehi B, Najafipour H, Gholamhoseinian A, Joukar F. Ameliorative effect of black tea on nicotine induced cardiovascular pathogenesis in rat. EXCLI J. 2012;11:309–17. [PMC free article] [PubMed] [Google Scholar]
- 24.Franquni JV, do Nascimento AM, de Lima EM, Brasil GA, Heringer OA, Cassaro KO, et al. Nandrolone decanoate determines cardiac remodelling and injury by an imbalance in cardiac inflammatory cytokines and ACE activity, blunting of the Bezold-Jarisch reflex, resulting in the development of hypertension. Steroids. 2013;78(3):379–85. doi: 10.1016/j.steroids.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Bayat GR, Hajizadeh S, Javan M, Safari F, Goodarzvand M, Shokri S, et al. Effect of exercise and chronic administration of nandrolone decanoate on expression of rat heart sarcolemmal ATP-sensitive potassium channels. KAUMS Journal (FEYZ). 2012;16(2):102–11. [Google Scholar]
- 26.Fernandes T, Hashimoto NY, Magalhaes FC, Fernandes FB, Casarini DE, Carmona AK, et al. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension. 2011;58(2):182–9. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodiwiss AJ, Norton GR. Exercise-induced cardiac hypertrophy is associated with an increased myocardial compliance. J Appl Physiol (1985). 1995;78(4):1303–11. doi: 10.1152/jappl.1995.78.4.1303. [DOI] [PubMed] [Google Scholar]
- 28.Tanno AP, das Neves VJ, Rosa KT, Cunha TS, Giordano FC, Calil CM, et al. Nandrolone and resistance training induce heart remodeling: role of fetal genes and implications for cardiac pathophysiology. Life Sci. 2011;89(17-18):631–7. doi: 10.1016/j.lfs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Soares MCR, De-Abreu IC, Assenço F, Borges MODR. Decanoato de nandrolona aumenta a parede ventricular esquerda, mas atenua o aumento da cavidade provocado pelo treinamento de natação em ratos. Revista Brasileira de Medicina do Esporte. 2011;17(6):420–4. [Google Scholar]
- 30.Du Toit EF, Rossouw E, Van Rooyen J, Lochner A. Proposed mechanisms for the anabolic steroid-induced increase in myocardial susceptibility to ischaemia/reperfusion injury. Cardiovasc J S Afr. 2005;16(1):21–8. [PubMed] [Google Scholar]
- 31.Penna C, Tullio F, Perrelli MG, Moro F, Abbadessa G, Piccione F, et al. Ischemia/reperfusion injury is increased and cardioprotection by a postconditioning protocol is lost as cardiac hypertrophy develops in nandrolone treated rats. Basic Res Cardiol. 2011;106(3):409–20. doi: 10.1007/s00395-010-0143-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chyu DW, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol (1985). 2000;89(1):21–8. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Osorio RA, Christofani JS, D'Almeida V, Russo AK, Picarro IC. Reactive oxygen species in pregnant rats: effects of exercise and thermal stress. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(1):89–95. doi: 10.1016/s1532-0456(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 34.Ravi Kiran T, Subramanyam MV, Asha Devi S. Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: relationship to swim intensity and duration. Comp Biochem Physiol B Biochem Mol Biol. 2004;137(2):187–96. doi: 10.1016/j.cbpc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Perse M, Injac R, Strukelj B, Cerar A. Effects of high-fat mixed-lipid diet and exercise on the antioxidant system in skeletal and cardiac muscles of rats with colon carcinoma. Pharmacol Rep. 2009;61(5):909–16. doi: 10.1016/s1734-1140(09)70148-3. [DOI] [PubMed] [Google Scholar]
- 36.Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin K, Siktar E, et al. Effects of endurance training and acute exhaustive exercise on antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr Physiol. 2006;143(2):239–45. doi: 10.1016/j.cbpa.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Lubna HT, Noor HM, Hassan IM, Iman MA, Salem RY, Maher YA. Nandrolone decanoate administration to male rats induces oxidative stress, seminiferous tubules abnormalities, and sperm DNA fragmentation. Jord J Biol Sci. 2010;3(4):165–74. [Google Scholar]
- 38.Nakatani K, Komatsu M, Kato T, Yamanaka T, Takekura H, Wagatsuma A, et al. Habitual exercise induced resistance to oxidative stress. Free Radic Res. 2005;39(9):905–11. doi: 10.1080/10715760500183300. [DOI] [PubMed] [Google Scholar]
- 39.Chaves EA, Fortunato RS, Carvalho DP, Nascimento JH, Oliveira MF. Exercise-induced cardioprotection is impaired by anabolic steroid treatment through a redox-dependent mechanism. J Steroid Biochem Mol Biol. 2013;138:267–72. doi: 10.1016/j.jsbmb.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Roshan VD, Assali M, Moghaddam AH, Hosseinzadeh M, Myers J. Exercise training and antioxidants: effects on rat heart tissue exposed to lead acetate. Int J Toxicol. 2011;30(2):190–6. doi: 10.1177/1091581810392809. [DOI] [PubMed] [Google Scholar]
- 41.Oztasan N, Taysi S, Gumustekin K, Altinkaynak K, Aktas O, Timur H, et al. Endurance training attenuates exercise-induced oxidative stress in erythrocytes in rat. Eur J Appl Physiol. 2004;91(5-6):622–7. doi: 10.1007/s00421-003-1029-6. [DOI] [PubMed] [Google Scholar]
- 42.Yeung PK, Dauphinee J, Marcoux T. Effect of acute exercise on cardiovascular hemodynamic and red blood cell concentrations of purine nucleotides in hypertensive compared with normotensives rats. Ther Adv Cardiovasc Dis. 2013;7(2):63–74. doi: 10.1177/1753944712470297. [DOI] [PubMed] [Google Scholar]
- 43.Libonati JR. Cardiac remodeling and function following exercise and angiotensin II receptor antagonism. Eur J Appl Physiol. 2012;112(8):3149–54. doi: 10.1007/s00421-011-2263-y. [DOI] [PubMed] [Google Scholar]
- 44.Andrade TU, Santos MC, Busato VC, Medeiros AR, Abreu GR, Moyses MR, et al. Higher physiological doses of nandrolone decanoate do not influence the Bezold-Jarish reflex control of bradycardia. Arch Med Res. 2008;39(1):27–32. doi: 10.1016/j.arcmed.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Bruder-Nascimento T, Cordellini S. Vascular adaptive responses to physical exercise and to stress are affected differently by nandrolone administration. Brazilian Journal of Medical and Biological Research. 2011;44(4):337–44. doi: 10.1590/s0100-879x2011007500043. [DOI] [PubMed] [Google Scholar]
- 46.Endlich PW, Firmes LB, Goncalves WL, Gouvea SA, Moyses MR, Bissoli NS, et al. Involvement of the atrial natriuretic peptide in the reduction of arterial pressure induced by swimming but not by running training in hypertensive rats. Peptides. 2011;32(8):1706–12. doi: 10.1016/j.peptides.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Zamo FS, Barauna VG, Chiavegatto S, Irigoyen MC, Oliveira EM. The renin-angiotensin system is modulated by swimming training depending on the age of spontaneously hypertensive rats. Life Sci. 2011;89(3-4):93–9. doi: 10.1016/j.lfs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Tseng YT, Rockhold RW, Hoskins B, Ho IK. Cardiovascular toxicities of nandrolone and cocaine in spontaneously hypertensive rats. Fundam Appl Toxicol. 1994;22(1):113–21. doi: 10.1006/faat.1994.1014. [DOI] [PubMed] [Google Scholar]
- 49.Barretti DL, Magalhaes Fde C, Fernandes T, do Carmo EC, Rosa KT, Irigoyen MC, et al. Effects of aerobic exercise training on cardiac renin-angiotensin system in an obese Zucker rat strain. PLoS One. 2012;7(10):e26233. doi: 10.1371/journal.pone.0046114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bissoli NS, Medeiros AR, Santos MC, Busato VC, Jarske RD, Abreu GR, et al. Long-term treatment with supraphysiological doses of nandrolone decanoate reduces the sensitivity of Bezold-Jarisch reflex control of heart rate and blood pressure. Pharmacol Res. 2009;59(6):379–84. doi: 10.1016/j.phrs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Maior AS, Belchior C, Sanches RC, da Silva TO, Leonelli T, Schwingel PA, et al. Chronic Users of Supraphysiological Doses of Anabolic Androgenic Steroids Develop Hematological and Serum Lipoprotein Profiles That Are Characteristic of High Cardiovascular Risk. Int J Spo Exe Sci. 2011;3:27–36. [Google Scholar]
- 52.Tezini GC, Dias DP, Souza HC. Aerobic physical training has little effect on cardiovascular autonomic control in aging rats subjected to early menopause. Exp Gerontol. 2013;48(2):147–53. doi: 10.1016/j.exger.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Phillis BD, Abeywardena MY, Adams MJ, Kennedy JA, Irvine RJ. Nandrolone potentiates arrhythmogenic effects of cardiac ischemia in the rat. Toxicol Sci. 2007;99(2):605–11. doi: 10.1093/toxsci/kfm186. [DOI] [PubMed] [Google Scholar]
- 54.Achar S, Rostamian A, Narayan SM. Cardiac and metabolic effects of anabolic-androgenic steroid abuse on lipids, blood pressure, left ventricular dimensions, and rhythm. Am J Cardiol. 2010;106(6):893–901. doi: 10.1016/j.amjcard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams PT, Franklin BA. Reduced incidence of cardiac arrhythmias in walkers and runners. PLoS One. 2013;8(6):e26233. doi: 10.1371/journal.pone.0065302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23(6):477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 57.Heidbuchel H, Panhuyzen-Goedkoop N, Corrado D, Hoffmann E, Biffi A, Delise P, et al. Recommendations for participation in leisure-time physical activity and competitive sports in patients with arrhythmias and potentially arrhythmogenic conditions Part I: Supraventricular arrhythmias and pacemakers. Eur J Cardiovasc Prev Rehabil. 2006;13(4):475–84. doi: 10.1097/01.hjr.0000216543.54066.72. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy MC, Lawrence C. Anabolic steroid abuse and cardiac death. Med J Aust. 1993;158(5):346–8. doi: 10.5694/j.1326-5377.1993.tb121797.x. [DOI] [PubMed] [Google Scholar]
- 59.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91(11):2742–7. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 60.Goldschlager N, Cake D, Cohn K. Exercise-induced ventricular arrhythmias in patients with coronary artery disease. Their relation to angiographic findings. Am J Cardiol. 1973;31(4):434–40. doi: 10.1016/0002-9149(73)90291-9. [DOI] [PubMed] [Google Scholar]
- 61.Dickerman RD, Schaller F, Prather I, McConathy WJ. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology. 1995;86(2):172–3. doi: 10.1159/000176867. [DOI] [PubMed] [Google Scholar]
- 62.Ferenchick GS, Adelman S. Myocardial infarction associated with anabolic steroid use in a previously healthy 37-year-old weight lifter. Am Heart J. 1992;124(2):507–8. doi: 10.1016/0002-8703(92)90620-b. [DOI] [PubMed] [Google Scholar]
- 63.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 64.Du XJ, Dart AM. Mechanisms of noradrenaline release in the anoxic heart of the rat. Cardiovasc Res. 1993;27(11):2011–5. doi: 10.1093/cvr/27.11.2011. [DOI] [PubMed] [Google Scholar]
- 65.Richardt G, Blessing R, Schomig A. Cardiac noradrenaline release accelerates adenosine formation in the ischemic rat heart: role of neuronal noradrenaline carrier and adrenergic receptors. J Mol Cell Cardiol. 1994;26(10):1321–8. doi: 10.1006/jmcc.1994.1150. [DOI] [PubMed] [Google Scholar]
- 66.Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–97. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]