Abstract
AIM: To determine the feasibility and safety of intraluminal brachytherapy in treatment of malignant obstructive jaundice (MOJ) and to evaluate the clinical effect of intraluminal brachytherapy on stent patency and patient survival.
METHODS: Thirty-four patients with MOJ were included in this study. Having biliary stent placed, all patients were classified into intraluminal brachytherapy group (group A, n = 14) and control group (group B, n = 20) according to their own choice. Intraluminal brachytherapy regimen included: HDR-192Ir was used in the therapy, fractional doses of 4-7 Gy were given every 3-6 d for 3-4 times, and standard points were established at 0.5-1.0 cm. Some patients of both groups received transcatheter arterial chemoembolization (TACE) after stent placement.
RESULTS: In group A, the success rate of intraluminal brachytherapy was 98.0%, RTOG grade 1 acute radiation morbidity occurred in 3 patients, RTOG/EORTC grade 1 late radiation morbidity occurred in 1 patient. Mean stent patency of group A (12.6 mo) was significantly longer than that of group B (8.3 mo) (P < 0.05). There was no significant difference in the mean survival (9.4 mo vs 6.0 mo) between the two groups.
CONCLUSION: HDR-192Ir intraluminal brachytherapy is a safe palliative therapy in treating MOJ, and it may prolong stent patency and has the potentiality of extending survival of patients with MOJ.
INTRODUCTION
Self-expanding metal stent, due to its minimal invasion, low risk of migration and efficient bile drainage, has been widely used for management of malignant obstructive jaundice (MOJ)[1-11]. Although self-expanding metal stent seems to be the first choice in the palliation of MOJ, high rates of stent occlusion, which were mainly caused by tumor ingrowth or overgrowth, usually lead to jaundice recurrence[12,13]. Therefore, treating underlying malignancy is critical to extend stent patency and patient survival. Intraluminal brachytherapy is characterized by superiority in treatment of ductal or periductal tumor, thus preventing tumor from invading or compressing the duct. We designed a prospective study to evaluate the feasibility, safety of intraluminal brachytherapy in the treatment of MOJ and the clinical effect of intraluminal brachytherapy on stent patency and survival of patients.
MATERIALS AND METHODS
Study design
Patients were selected among those admitted to our department between November 2001 and September 2002 with the diagnosis of MOJ. Patients who had percutaneous transhepatic cholangiography and drainage (PTCD) followed by stent placement were included. Exclusion criteria were: (1) patients who were in end-stage MOJ; (2) serum total bilirubin concentration decreased to less than 100 μmol/L or 50% of that before PTCD. After the nature of intraluminal brachytherapy was fully explained, patients were classified into two groups: group A with intraluminal brachytherapy and group B without intraluminal brachytherapy as control group, according to their own choice whether to receive the therapy. The study was performed with the approval of the institutional ethics committees. Written informed consent was obtained from all patients before treatment.
Methods of treatment
Venous blood samples were taken 1-3 d before PTCD for biochemical test. Intraluminal brachytherapy was routinely performed in group A within 1-3 wk after stent placement. Patients in group B did not receive the therapy. Some patients in both groups underwent transcatheter arterial chemoembolization (TACE).
Intraluminal brachytherapy: After stent placement, a 10 F external-internal drainage catheter or a 7 F long vascular sheath was placed, with its distal tip across the stricture. The applicator and sham source were placed through the drainage catheter or the sheath as a whole under fluoroscopic guidance. Irradiated volume was customized to extend 1 cm proximal and distal to the maximum length of the stricture as determined by cholangiography. Dwell positions were recorded, and therapy planning system (TPS) was connected to the remote afterloading microSelectron (Nuclectron. Holland). After the sham source was manually removed, HDR-192Ir (111-370 GBq) was driven by the remote afterloading system to the programmed locations (Figure 1). Fractional dose of 4-7 Gy was prescribed 0.5-1 cm from the source axis. The procedure was performed 3-4 times with an interval of 3-6 d. The drainage catheter or sheath was removed 1 wk later in case there were no complications related to the procedure.
Figure 1.
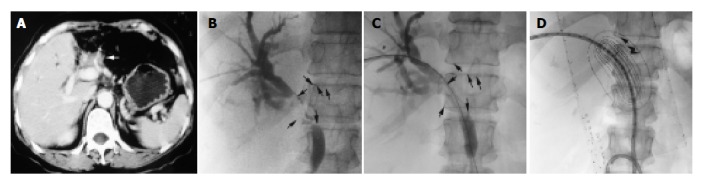
HDR-192Ir (111-370 GBq) driven to the programmed locations. A: In the patient who had undergone left-lobe resection due to cholangiocarcinoma, hilar recurrence with bile duct dilation was shown by portal-phase CT scan (arrow). B: The stricture of bile duct was clearly shown by cholangiography during PTCD (arrow). C: An 8 mm×60 mm SMART stent was deployed across the stricture. D: The applicator and sham source were inserted though a 10 F internal-external drainage catheter. The dose distribution curves were obtained from the TPS (arrow).
Follow-up
The acute morbidity scoring criteria by Radiation Therapy Oncology Group (RTOG) and the late morbidity scoring criteria by Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) were used to evaluate the radiation toxicity[14]. Follow-up of each patient was based on outpatient examinations, telephone interviews, and questionnaires.
In this study, stent patency period was defined as the interval between stent placement and obstructive jaundice recurrence. If occlusion did not occur during a patient’s life time, the patency period was considered equal to the survival period but censored. Jaundice recurrence was defined as the symptom of jaundice recurred after it had subsided and met one of the followings: (1) cholangiography, CT or US demonstrated redilation of bile duct, (2) combined serum bilirubin concentration/total serum bilirubin concentration ≥ 35% (Figure 2).
Figure 2.
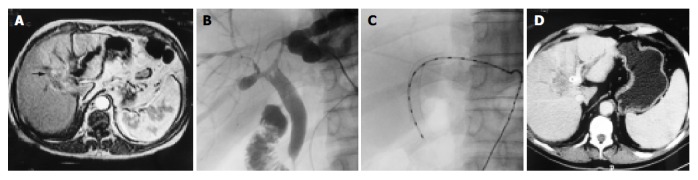
Cholangiography, CT, or US before and after intraluminal brachytherapy. A: Bile duct dilation of left lobe caused by cholangiocarcinoma was shown by CT (arrow). B: Cholangiography showed hilar stricture (arrow). C: Intraluminal brachytherapy (fractional dose 7 Gy, total dose 21 Gy) was performed after an 8 mm×60 mm SMART stent insertion. D: CT follow-up 11 mo after intraluminal brachytherapy showed no progress of the tumor and no redilation of the bile duct (arrow).
Statistical analysis
Comparisons between the two groups were performed with paired-samples t tests for metric data and χ 2 tests for frequencies. Stent patency and survival were evaluated according to the Kaplan-Meier method and compared with the log rank test. For all tests, a P value less than 0.05 was considered statistically significant.
RESULTS
Baseline data of patients
A total of 34 patients were enrolled in this study (18 male and 16 female; age range, 41-87 years; mean age, 62.0 years). Thirty-seven SMART stents (Cordis. Johnson & Johnson. USA) were placed and 28 TACE procedures were performed in these patients, 14 patients underwent intraluminal brachytherapy. In group A (n = 14), the causes of obstruction were cholangiocarcinoma (n = 7), gallbladder carcinoma (n = 2) and metastatic lymphadenopathy (n = 5, from stomach, colorectum or breast). In group B (n = 20), the causes of obstruction were cholangiocarcinoma (n = 9), gallbladder carcinoma (n = 2) and metastatic lymphadenopathy (n = 9, from stomach, colorectum or breast). The diagnosis was based on clinical, radiological findings or histology. There were no differences between the two groups according to gender, mean age, preoperative serum bilirubin, alanine aminotransferase, alkaline phosphatase, albumin, hemoglobin concentration, obstructive duration, obstructive level, and times of TACE performed (Table 1).
Table 1.
Baseline clinical characteristics of patients
| Factor | Group A (n = 14) | Group B (n = 20) | P |
| Mean age (yr) | 57.9 ± 9.2 | 63.3 ± 11.4 | 0.356 |
| Gender | |||
| Male | 8 | 10 | 0.681 |
| Female | 6 | 10 | |
| Mean total serum bilirubin | 318 ± 94 | 314 ± 115 | 0.913 |
| concentration (μmol/L) | |||
| Mean alanine | 71 ± 52 | 106 ± 107 | 0.261 |
| aminotransferase concentration (U/L) | |||
| Mean albumin | 33.6 ± 4.3 | 33.5 ± 3.4 | 0.914 |
| concentration (g/L) | |||
| Mean alkaline | 443 ± 334 | 543 ± 400 | 0.449 |
| phosphatase (U/L) | |||
| Mean hemoglobin | 111 ± 12 | 105 ± 16 | 0.275 |
| concentration (g/L) | |||
| Mean obstructive | 3.7 ± 0.9 | 4.0 ± 1.4 | 0.512 |
| duration (wk) | |||
| Obstructive level | |||
| Hilar | 8 | 12 | 0.868 |
| Common bile duct | 6 | 8 | |
| TACE | |||
| Performed | 9 | 10 | 0.409 |
| Not performed | 5 | 10 |
Intraluminal brachytherapy
A total of 52 procedures were programmed and 51 procedures were successfully performed, achieving the technical success rate of 98.0%. In 1 patient, a drainage catheter kink, which was caused by an acute angle on the drainage passage, resulted in the failure of driving HDR-192Ir to scheduled position in the third procedure. The total dose of these 14 patients was 14-21 Gy. One patient developed nausea, one complained of abdominal upset, one developed diarrhea. One patient, who had undergone bilobectomy 4 mo before stent placement, developed hemobilia for a period of 4 d (Figure 3). The bleeding stopped soon after hemostatic was prescribed without transfusions. According to RTOG acute morbidity scoring criteria, 3 patients were classified into RTOG grade 1 acute morbidity, the others RTOG grade 0 acute morbidity. According to RTOG/EORTC late morbidity scoring criteria, 1 patient was classified into RTOG/EORTC grade 1 late morbidity, the others grade 0 late morbidity.
Figure 3.
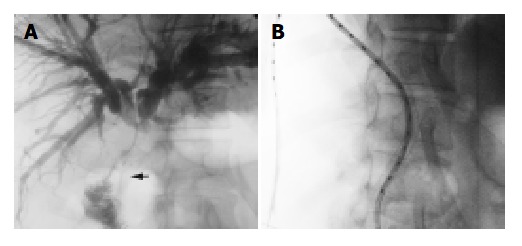
One patient undergoing bilienterostomy and ostomy, which was shown in irradiation region in intraluminal brachy therapy. A: Cholangiography indicated that jejunum was in-vaded by the malignancy (arrow). B: Jejunum and ostomy were in the irradiation region.
Stent patency and patient survival
The median follow-up time was 6.0 mo (range, 1-17 mo). In group B, 1 patient died 1 mo after stent placement of uncontrolled bleeding caused by tumor rupture, another one died 5 mo after stent placement due to cerebrovascular accident. The mean stent patency was 12.6 mo (median, 10.0 mo) with stent occlusion of 21.4% (3 of 14 patients) in group A, whereas the mean stent patency was 8.3 mo (median, 6.0 mo) with stent occlusion of 45.0% (9 of 20 patients) in group B. The stent patency of group A was significantly longer than that of group B (P < 0.05, Figure 4). The mean survival was 9.6 mo (median 7.0 mo) in group A and 6.4 mo (median 6.0 mo) in group B, showing no significant difference.
Figure 4.

Stent patency of the two groups.
DISCUSSION
As a part of brachytherapies, intraluminal brachytherapy is defined as performing brachytherapy by inserting an applicator and radioactive source into a lumen (or cavity) such as nasopharyngeal cavity, uterine cavity, esophagus, rectum and bile duct[15]. The properties of this radiotherapeutic technique include[16]: (1) Small source size enables itself to be very close to or contact with the target tissue. (2) The effective therapeutic range is 0.5-2.0 cm, which has little effect on normal tissue. (3) The absorbed dose is in inverse proportion to the square of the distance from radioactive source. With its irradiation extent confined to ductal wall and tissue adjacent to duct, intraluminal brachytherapy is a proper therapy for malignancy arising from a duct or adjacent to a duct. Because the absorbed dose falls off rapidly with increasing distance from the sources, intraluminal brachytherapy can deal with the malignancy without significantly affecting the adjacent normal tissues. To date, intraluminal brachytherapy not only has been the major therapeutics in treating the carcinoma of nasopharyngeal, uterine cervix, vagina, and endometrium[17-20], but complementary to the treatment of carcinoma of esophagus, lung, and rectum[21-23].
A number of isotopes were available for brachytherapy, including 226Ra, 60Co, 137Cs, 192Ir and 90Sr. With advantages of highly specific activity, relatively short half-life, easy to be shielded, and technical flexibility, 192Ir is the most widely used brachytherapy source in clinical practice[24]. According to the dose delivered per hour, brachytherapy can be classified into low dose rate (LDR, 0.4-2 Gy/h), median dose rate (MDR, 2-12 Gy/h) and high dose rate (HDR, > 12 Gy/h). Among them, LDR and HDR are more frequently used. By comparison with LDR, HDR represents a technologic advance that offers the following advantages[25]: (1) Improved physical dose delivery due to the short treatment time and negligible organ motion. (2) Improved radiation safety and protection with decreased exposure to personnel. (3) Reduced possibility of human error through computerized remote afterloading. (4) Increased efficiency because more patients can be treated with HDR per unit time. Additionally, while treating patients with MOJ by LDR, the applicator and radioactive source must be kept in the drainage catheter for several hours to several days, which may influence bile drainage and increase the risk of infection. The course of HDR therapy only needs several minutes, thus having no effect on bile drainage[26]. For all the reasons mentioned above, we used HDR-192Ir as the radioactive source in our study.
Intraluminal brachytherapy takes use of the drainage passage established during PTCD, making the procedure uncomplicated and inducing less damage. We only failed to complete the therapy in 1 patient in his third procedure. In this case we found an acute angle on the drainage passage. The narrow applicator lumen caused by the kink on the drainage catheter resulted in failure in radioactive source transmission. In patients who are scheduled to receive intraluminal brachytherapy, an acute angle should be avoided on the drainage passage during PTCD. Dose distribution of intraluminal brachytherapy is characterized by “inverse-square law” as we discussed before. In our study it was technically impossible to use instrument such as balloon-catheter to centralize the radioactive source in the bile duct, that is to say, the radioactive source usually located eccentrically in the lumen. The absorbed dose of bile duct wall close to the radioactive source is too high to be calculated by the “inverse-square law”. Brambs[27] investigated the bile duct tolerance in intraluminal brachytherapy in a swine model, and demonstrated that fractional dose of 7.5 Gy only caused slight fibrosis in the bile duct. Because the radiation damage in human is similar to that in swine, fractional dose of 7.5 Gy is deemed to be safe in clinical practice. As to other structures adjacent to bile duct such as hepatic artery, portal vein, duodenum, and pancreas, the distance between radioactive source and those structures makes the “inverse-square law” calculation possible. A tumor causing obstructive jaundice is thought to be at the advanced stage of the disease, the aim of the therapy is palliation rather than cure. Palliative radiation therapy generally follows the regimen[28]: the total dose of 20-30 Gy and the fractional dose of 5-10 Gy, with an intention to alleviate pain, maintain patency, stop bleeding, and reduce morbidity. In consideration of the poor clinical status of patients due to hyperbilirubinemia in our study, we prescribed fractional dose of 4-7 Gy with a total dose of 14-21 Gy, which was certainly tolerable. In this study, only 3 patients developed RTOG grade 1 acute morbidity, and 1 patient developed RTOG/ EORTC grade 1 morbidity. One patient developed hemobilia for a period of 4 d without the need for transfusions. This patient had undergone bilienterostomy and the ostomy, which was shown to be invaded by the malignancy, was in irradiation region in intraluminal brachytherapy. One of the reasons for hemobilia is tumor necrosis. On condition that block of tumor tissue necroses before the supplying arteriole could occlude, tumor bleeding will occur. The other one is ostomy hemorrhage. Jejunum and ostomy are dose-limiting tissues susceptible to irradiation.
MOJ is usually caused by cholangiocarcinoma, gallbladder carcinoma, metastatic lymphadenopathy (from stomach, colorectum, or breast), and pancreatic carcinoma. TACE has limited effect on these malignancies in most cases because of their character of relative avascularity[29]. Cholangiocarcinoma, gallbladder carcinoma, and pancreatic carcinoma have the tendency of invading bile duct, so the rate of stent occlusion due to tumor ingrowth or overgrowth is inevitably high[30-32]. There is an increasing demand for efficient therapy to palliate these malignancies, so to prolong stent patency and patient survival. External-beam irradiation has been used to treat abdominal tumors for nearly a hundred years. Major deterrents to improved results with MOJ are the limited tolerance of the structures (liver, duodenum, stomach, and kidney) around the lesion, lack of clear definition of the lesion’s location, poor clinical status of the patients, and respiration interfering with the irradiation field[33]. Fortunately, intraluminal brachytherapy, with special dosimetric characteristics and accurate location, overcome all the above shortcomings. Intraluminal brachytherapy in MOJ can efficiently palliate the underlying malignancies with fewer morbidities. Dvorak[34,35] and cooperators reported their experiences of HDR-192Ir intraluminal brachytherapy in treatment of 2 groups of patients with MOJ after bile drainage. The causes of MOJ were cholangiocarcinoma and gallbladder carcinoma in one group, cholangiocarcinoma and pancreas carcinoma in the other. The mean survival of both groups was 9 mo. After stent placement and subsequent jaundice subsidence, the underlying malignancy could be controlled by intraluminal brachytherapy, and invasion and compression of the bile duct avoided. Bruha et al[36] reported that patients with MOJ treated with intraluminal brachytherapy using HDR-192Ir, the mean stent patency in patients with cholangiocarcinoma and gallbladder carcinoma were 418 and 220 d, respectively. The result of our study showed that the mean stent patency of intraluminal brachytherapy group (12.6 mo) was significantly longer than the control group (8.3 mo). We attribute the effect on prolonging stent patency to the followings: (1) Prohibit tumor from growing through the mesh of the stent (ingrowth) (2) Prohibit tumor from growing over the edge of the stent (overgrowth) (3) In cases with hilar obstruction, intraluminal brachytherapy in ipsi-lateral duct can prevent tumor tissue from invading contralateral duct. Although not statistically significant, it appeared that the mean survival in group A (9.6 mo) was longer than in group B (6.4 mo). We speculated that the advantage of intraluminal brachytherapy in extending survival be more prominent on condition that the cases were expanded or the follow-up was prolonged.
In conclusion, because the special dosimetric characteristics of HDR-192Ir and the high dose tolerance of bile duct, HDR-192Ir intraluminal brachytherapy is a safe and feasible method in the treatment of MOJ. HDR-192Ir intraluminal brachytherapy may prolong stent patency and has the potentiality of extending survival of patients with MOJ.
Footnotes
Edited by Chen WW and Zhu LH Proofread by Xu FM
References
- 1.Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41–49. doi: 10.1067/mge.2003.292. [DOI] [PubMed] [Google Scholar]
- 2.Mao AW, Gao ZD, Xu JY, Yang RJ, Xiao XS, Jiang TH, Jiang WJ. Treatment of malignant digestive tract obstruction by combined intraluminal stent installation and intra-arterial drug infusion. World J Gastroenterol. 2001;7:587–592. doi: 10.3748/wjg.v7.i4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inal M, Akgül E, Aksungur E, Seydaoğlu G. Percutaneous placement of biliary metallic stents in patients with malignant hilar obstruction: unilobar versus bilobar drainage. J Vasc Interv Radiol. 2003;14:1409–1416. doi: 10.1097/01.rvi.0000096762.74047.a6. [DOI] [PubMed] [Google Scholar]
- 4.Inal M, Aksungur E, Akgül E, Oguz M, Seydaoglu G. Percutaneous placement of metallic stents in malignant biliary obstruction: one-stage or two-stage procedure? Pre-dilate or not? Cardiovasc Intervent Radiol. 2003;26:40–45. doi: 10.1007/s00270-002-2647-9. [DOI] [PubMed] [Google Scholar]
- 5.Shah RJ, Howell DA, Desilets DJ, Sheth SG, Parsons WG, Okolo P, Lehman GA, Sherman S, Baillie J, Branch MS, et al. Multicenter randomized trial of the spiral Z-stent compared with the Wallstent for malignant biliary obstruction. Gastrointest Endosc. 2003;57:830–836. doi: 10.1016/s0016-5107(03)70016-9. [DOI] [PubMed] [Google Scholar]
- 6.Piñol V, Castells A, Bordas JM, Real MI, Llach J, Montañà X, Feu F, Navarro S. Percutaneous self-expanding metal stents versus endoscopic polyethylene endoprostheses for treating malignant biliary obstruction: randomized clinical trial. Radiology. 2002;225:27–34. doi: 10.1148/radiol.2243011517. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad J, Siqueira E, Martin J, Slivka A. Effectiveness of the Ultraflex Diamond stent for the palliation of malignant biliary obstruction. Endoscopy. 2002;34:793–796. doi: 10.1055/s-2002-34269. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Hirai R, Kitagawa M, Takehira Y, Yamada M, Tamakoshi K, Kobayashi Y, Nakamura H, Kanamori M. Treatment of common bile duct obstruction by pancreatic cancer using various stents: single-center experience. Cardiovasc Intervent Radiol. 2002;25:373–380. doi: 10.1007/s00270-002-0426-2. [DOI] [PubMed] [Google Scholar]
- 9.Hatzidakis AA, Tsetis D, Chrysou E, Sanidas E, Petrakis J, Gourtsoyiannis NC. Nitinol stents for palliative treatment of malignant obstructive jaundice: should we stent the sphincter of Oddi in every case? Cardiovasc Intervent Radiol. 2001;24:245–248. doi: 10.1007/s00270-001-0030-x. [DOI] [PubMed] [Google Scholar]
- 10.Ferlitsch A, Oesterreicher C, Dumonceau JM, Deviere J, Leban T, Born P, Rösch T, Suter W, Binek J, Meyenberger C, et al. Diamond stents for palliation of malignant bile duct obstruction: a prospective multicenter evaluation. Endoscopy. 2001;33:645–650. doi: 10.1055/s-2001-16214. [DOI] [PubMed] [Google Scholar]
- 11.Caldicott DG, Ziprin P, Morgan R. Transhepatic insertion of a metallic stent for the relief of malignant afferent loop obstruction. Cardiovasc Intervent Radiol. 2000;23:138–140. doi: 10.1007/s002709910027. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Lee DK, Kim HG, Park JJ, Park SH, Kim JH, Yoo BM, Roe IH, Moon YS, Myung SJ. Features of malignant biliary obstruction affecting the patency of metallic stents: a multicenter study. Gastrointest Endosc. 2002;55:359–365. doi: 10.1067/mge.2002.121603. [DOI] [PubMed] [Google Scholar]
- 13.Eschelman DJ, Shapiro MJ, Bonn J, Sullivan KL, Alden ME, Hovsepian DM, Gardiner GA. Malignant biliary duct obstruction: long-term experience with Gianturco stents and combined-modality radiation therapy. Radiology. 1996;200:717–724. doi: 10.1148/radiology.200.3.8756921. [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 15.Liu TF. Xiandai Fangshe Zhongliuxue. 1sted. Shanghai: Fudan University Pub. 2001:105–153. [Google Scholar]
- 16.Perez CA, Brady LW. Principles and practice of radiation oncology. 3rded. Philadelphia: Lippincott Raven Pub. 1997:405–467. [Google Scholar]
- 17.Hareyama M, Sakata K, Oouchi A, Nagakura H, Shido M, Someya M, Koito K. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix: a randomized trial. Cancer. 2002;94:117–124. doi: 10.1002/cncr.10207. [DOI] [PubMed] [Google Scholar]
- 18.Alektiar KM, McKee A, Venkatraman E, McKee B, Zelefsky MJ, Mychalczak BR, Hoskins WJ, Barakat RR. Intravaginal high-dose-rate brachytherapy for Stage IB (FIGO Grade 1, 2) endometrial cancer. Int J Radiat Oncol Biol Phys. 2002;53:707–713. doi: 10.1016/s0360-3016(02)02792-x. [DOI] [PubMed] [Google Scholar]
- 19.Kong W, Sun J. [Outcome with intracavitary high-dose-rate brachytherapy for primary vaginal cancer] Zhonghua Fuchanke Zazhi. 2002;37:94–96. [PubMed] [Google Scholar]
- 20.Syed AM, Puthawala AA, Damore SJ, Cherlow JM, Austin PA, Sposto R, Ramsinghani NS. Brachytherapy for primary and recurrent nasopharyngeal carcinoma: 20 years' experience at Long Beach Memorial. Int J Radiat Oncol Biol Phys. 2000;47:1311–1321. doi: 10.1016/s0360-3016(00)00520-4. [DOI] [PubMed] [Google Scholar]
- 21.Sharma V, Mahantshetty U, Dinshaw KA, Deshpande R, Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;52:310–315. doi: 10.1016/s0360-3016(01)01822-3. [DOI] [PubMed] [Google Scholar]
- 22.Aumock A, Birnbaum EH, Fleshman JW, Fry RD, Gambacorta MA, Kodner IJ, Malyapa RS, Read TE, Walz BJ, Myerson RJ. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys. 2001;51:363–370. doi: 10.1016/s0360-3016(01)01677-7. [DOI] [PubMed] [Google Scholar]
- 23.Jain SK, Dupuy DE, Cardarelli GA, Zheng Z, DiPetrillo TA. Percutaneous radiofrequency ablation of pulmonary malignancies: combined treatment with brachytherapy. AJR Am J Roentgenol. 2003;181:711–715. doi: 10.2214/ajr.181.3.1810711. [DOI] [PubMed] [Google Scholar]
- 24.Hu YM, Liu Z, She F, Xue WL. 1st ed. Beijing: Atomic Energy Publishing House. 1999:101–102. [Google Scholar]
- 25.Manning MA, Arthur DW, Schmidt-Ullrich RK, Arnfield MR, Amir C, Zwicker RD. Interstitial high-dose-rate brachytherapy boost: the feasibility and cosmetic outcome of a fractionated outpatient delivery scheme. Int J Radiat Oncol Biol Phys. 2000;48:1301–1306. doi: 10.1016/s0360-3016(00)00792-6. [DOI] [PubMed] [Google Scholar]
- 26.Mohan DS, Nori D. Intraluminal brachytherapy in the treatment of pancreas and bile duct carcinoma: regarding Montemaggi et al., IJROBP 32: 437; 1995. Int J Radiat Oncol Biol Phys. 1995;33:773–774. doi: 10.1016/0360-3016(95)90388-7. [DOI] [PubMed] [Google Scholar]
- 27.Brambs HJ, Freund U, Bruggmoser G, Laaff H, Kluger UW, Roth R, Wannenmacher M. [Radiation sensitivity of the normal bile duct during high dose rate afterloading irradiation with Iridium 192. Experimental studies in pigs] Strahlenther Onkol. 1993;169:721–728. [PubMed] [Google Scholar]
- 28.Xu BY, Yu SC, Zeng DW, Chen GX. Modern Tumor Radiotherapy. 1st ed Beijing: People's Malitary Publishing House. 2000:190–199, 23-34. [Google Scholar]
- 29.Kawahara N, Ono M, Taguchi K, Okamoto M, Shimada M, Takenaka K, Hayashi K, Mosher DF, Sugimachi K, Tsuneyoshi M, et al. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512–1517. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- 30.Shirabe K, Shimada M, Harimoto N, Sugimachi K, Yamashita Y, Tsujita E, Aishima S. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131:S159–S164. doi: 10.1067/msy.2002.119498. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, Ha HK. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16:676–684. doi: 10.1002/jmri.10212. [DOI] [PubMed] [Google Scholar]
- 32.Rieber A, Brambs HJ. Metallic stents in malignant biliary obstruction. Cardiovasc Intervent Radiol. 1997;20:43–49. doi: 10.1007/s002709900107. [DOI] [PubMed] [Google Scholar]
- 33.Hayes JK, Sapozink MD, Miller FJ. Definitive radiation therapy in bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1988;15:735–744. doi: 10.1016/0360-3016(88)90319-7. [DOI] [PubMed] [Google Scholar]
- 34.Dvorák J, Petera J, Papík Z, Melichar B, Vanásek T, Hulek P, Jandík P, Mergancová J, Zoul Z, Vacek Z. Transduodenal intraluminal high dose rate brachytherapy in the treatment of carcinomas of the subhepatic region. Hepatogastroenterology. 2002;49:1045–1047. [PubMed] [Google Scholar]
- 35.Dvorák J, Jandík P, Melichar B, Jon B, Mergancová J, Zoul Z, Vacek Z, Petera J. Intraluminal high dose rate brachytherapy in the treatment of bile duct and gallbladder carcinomas. Hepatogastroenterology. 2002;49:916–917. [PubMed] [Google Scholar]
- 36.Bruha R, Petrtyl J, Kubecova M, Marecek Z, Dufek V, Urbanek P, Kodadova J, Chodounsky Z. Intraluminal brachytherapy and selfexpandable stents in nonresectable biliary malignancies--the question of long-term palliation. Hepatogastroenterology. 2001;48:631–637. [PubMed] [Google Scholar]


