Abstract
AIM: To detect the genetic alteration and abnormal expression of cyclin D1 in gastric carcinoma and investigate its clinicopathologic significance in advanced gastric carcinoma.
METHODS: Proteins of cyclin D1 were detected by immunohistochemistry in 42 cases of advanced gastric carcinoma with their follow-up data available, 27 cases of early stage carcinoma, 21 cases of gastric adenoma, 22 cases of hyperplastic polyp and 20 cases of normal mucosa adjacent to adenocarcinomas. Genetic alteration of cyclin D1 was detected by Southern blot and expression of cyclin D1 mRNA was detected by PT-PCR in 42 cases of advanced gastric carcinoma.
RESULTS: Cyclin D1 protein was not expressed in normal mucosa, hyperplastic polyp and gastric adenoma, while it was only positively expressed in gastric carcinoma. The expression rate of cyclin D1 protein in early stage gastric carcinoma, advanced gastric carcinoma and lymph node metastasis was 48.1%, 47.4% and 50.0%, respectively. The amplification of cyclin D1 gene was detected in 16.6% of advanced gastric carcinomas. The overexpression of cyclin D1 mRNA was detected in 40.5% of the samples. There was no significant correlation between cyclin D1 protein expression and age, lymph-node metastasis and histological grading in patients with advanced gastric carcinoma (χ 2 = 0.038,0.059,0.241, P > 0.05). Significant correlation was observed between the expression of cyclin D1 protein and the 5-year survival rate (χ 2 = 3.92, P < 0.05).
CONCLUSION: Detection of cyclin D1 protein by immunohistochemistry may be useful in the diagnosis of early gastric carcinomas. Patients with positive expression of cyclin D1 protein tend to have a worse prognosis.
INTRODUCTION
Hyperplasia is an essential characteristic of malignant tumors. Close attention has been paid to the role of cell-cycle regulators associated with cell proliferation in oncogenesis. Under the stimulation of proliferation signals, cells could produce early stage transcription factors such as c-fos, c-jun. These factors could bind to DNA specifically in nuclei and initiate cell cycles[1]. During the cell cycle, the progression from G1 phase to S phase (DNA synthesis phase) is essential for initiation of the cell cycle[2]. Cyclin D1, a protooncogene identified in recent years, plays a positive-regulation role in the progression[3]. The expression of cyclin D1 is an early event that is stimulated by growth factors or other mitogens .The major targets of cyclin D1-Cdk complexes are the retinoblastoma family of protein Rb[4]. Phosphorylation of Rb in mid-G1 leads to the release of active forms of the E2F family of transcription factors. Free E2F mediates transcription of E2f-dependent genes, including DNA polymerase, thymidine kinase[5]. The role of cyclin D1 in the oncogenesis of gastric carcinoma is worth investigating[6]. So far, there are few reports about the alteration of cyclin D1 in gastric carcinoma in China. In order to understand the alteration of cyclin D1 in gastric carcinoma, the protein expression of cyclin D1 in normal mucosa, hyperplastic polyp, adenoma, early stage carcinoma and advanced carcinoma of stomach was detected by immunohistochemistry. Genetic amplification and mRNA expression of cyclin D1 in advanced gastric carcinoma were detected by Southern blot and PT-PCR. The expression of cyclin D1 was also investigated in combination with other prognostic markers in advanced gastric cancer, such as patient’s age, LN metastasis, histological grade and 5-year survival rate.
MATERIALS AND METHODS
Tissue specimens
Tissues from 132 cases were collected in Qilu Hospital of Shandong University. They concluded 42 cases of advanced gastric carcinoma with their follow-up data available (among them 16 cases had lymph node metastasis), 27 cases of early stage carcinoma (among them 7 cases had carcinomas in situ, the other 20 cases had early infiltrating carcinomas), 21 cases of gastric adenoma, 22 cases of hyperplastic polyp, 20 cases of normal mucosa adjacent to adenocarcinomas. All samples were fixed in 40 g/L formaldehyde and embedded in paraffin for histological diagnosis and immunohistochemistry study. Fresh tissues from advanced gastric carcinoma were obtained and stored in liguid nitrogen for DNA and RNA analyses.
Immunohistochemical staining
Immunohistochemistry was performed by the labeled streptavidin biotin (LSAB) method by using vectastain Elite kit (vector, Burlingame,CA). Monoclonal antibody to cylinD1 was used at 1:100 dilution .The technique was performed as described[6] except for an antigen retrieval step. Sections were placed in plastic coplin jars containing 10 mmol/L citrate buffer and heated in microwave oven for 10 min at 675 W for antigen retrieval. A positive section produced in preparative experiments was used as positive control.
Detection of cylin D1 mRNA by reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from cells was extracted by using acid guanidium-phenol-choroform as described[7] and converted to single-strand cDNA by using random 9 mers .PCR was performed by using a thermal cycler as described[8]. The sequence of primer for cylinD1 was : 5’-CTGGAGCCCGTGAAAAAGAGC-3’, 5’-CTGGAGAGGAAGCGTGTGAGG-3’. A 10 μL of each mixture was analysed by electrophoresis on 20 g/L agarose gel .Each band was quantitated by gel figure analysis system (Alpha, No.IS-1220). β -actin was used as control. The sequence of primer for β -actin was: 5’-CTACAATGAGCTGCGTGTGGC-3’, 5’-CAGGTCCAGACGCAGGATGGC-3’. Cyclin D1 index = cyclin D1 data /β -actin data.
Detection of genetic alteration of cyclin D1 by Southern blot
Nucleic acids were prepared by the guanidinium thyocyanate method as described[9]. Sample DNA (5 µg) was digested with restriction endonuclease EcoR I and fractionated by electrophoresis on 20 g/L agarose gel. The gel was denatured and DNA was transferred to nitrocellulose filters. The probe used for detection of cyclin D1 was 18-mer of oligodexoynucleotide complementary to the initial stretch of bases in cyclin D1 mRNA from - 1 to + 17. The probe was labeled by 32p-dcTP.The filters were prehybridized and hybridized in conditions as described[10], dried and exposed at -70 °C with intensifying screen for various periods of time to XAR5 Kodak films. β -actin was used as control for DNA loading amount, and normal tissue near carcinoma was used as normal control.
Statistical analysis
χ 2 test was used to evaluate the differences between two groups.
RESULTS
Expression of cyclin D1 protein in gastric carcinomas and its correlation with other prognostic fators
The cyclin D1 immunohistochemical signal was located exclusively in nuclei (Figure 1A) and variable in terms of staining intensity and the proportion of positive nuclei among the cells of an individual case. One thousand tumor cells in 10 visual fields chosen randomly were counted for each case. The case was defined as positive if the proportion of stained cells was more than 10% of the tumor cells. In 20 cases of normal mucosa, 22 cases of hyperplastic polyp and 21 cases of gastric adenoma (Figure 1A), only weak to undetectable staining was seen and no one was defined as positive. On the other hand, 13/27 (48.1%) cases of early stage gastric carcinoma (Figure 1B), 20/42 (47.6%) cases of advanced gastric carcinoma (Figure 1C), 8/16 (50.0%) cases of lymph node metastasis were defined as positive. There was no significant difference among the positive rates of them (Table 1, P > 0.05). In the 20 cases of carcinoma with early stage infiltration, there was a good consistency of cyclin D1 expression between the in situ components and infiltration components. Seventeen cases showed the same immunostaining pattern in two components (85.0%). In the 16 cases of carcinoma with lymph node metastasis, 12 cases showed the same cyclin D1 immnostaining pattern in metastasis as in its primary lesion (75.0%).
Figure 1.
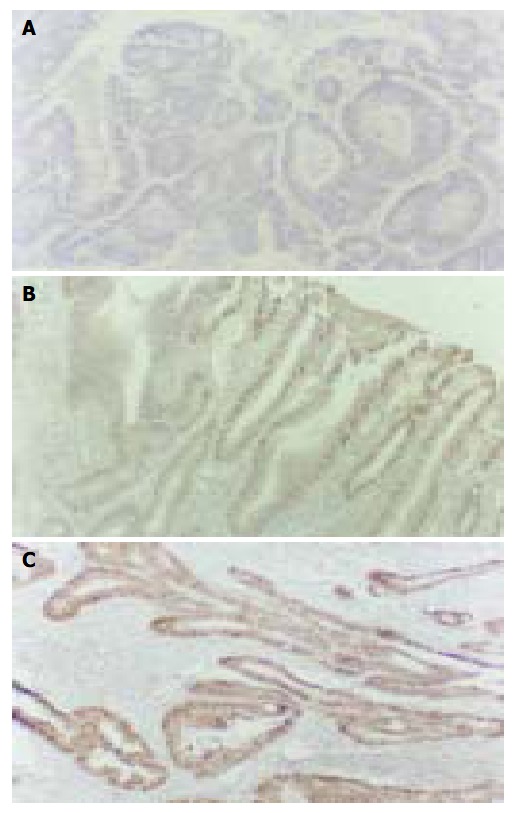
Expression of cyclin D1 protein. LSAB × 200. A: Gas-tric adenoma; B: Early stage gastric carcinoma; C: Advanced gastric carcinoma.
Table 1.
Cyclin D1 expression in early stage gastric carcinoma (EC), advanced gastric carcinoma (AC) and lymph node me-tastasis (LNM) and five-year survival
| Cyclin D1 | EC | AC | LNM |
5-year survival rate |
|
| Alive | Dead | ||||
| Positive | 13 | 20 | 8 | 9 | 11 |
| Negative | 14 | 22 | 8 | 15 | 7 |
| Total | 27 | 42 | 16 | 24 | 18 |
Positive expression of cyclin D1 was observed in 9/24 (37.5%) patients who survived 5 years after operation. While in patients who died within 5 years, 11/18 (61.5%) cases had positive expression. There was a significant difference between the two groups (Table 1, χ 2 = 3.92, P < 0.05). No significant correlation was observed between cyclin D1 protein expression and age, lymph-node metastasis, histological grading in patients with advanced gastric carcinoma (Table 2, χ 2 = 0.038, 0.059, 0.241, P > 0.05).
Table 2.
Relationship between cyclin D1 expression with age, lymph node metastasis (LDM) and histological grades (HG) in patients with advanced gastric carcinoma
| Cyclin D1 |
Age (yr) |
LDM |
HD |
||||
| > 60 | < 60 | Positive | Negative | I | II | III | |
| Positive | 14 | 6 | 8 | 12 | 5 | 11 | 5 |
| Negative | 16 | 6 | 8 | 14 | 4 | 12 | 4 |
Abnormal expression of cyclin D1 mRNA and genetic alteration of cyclin D1 gene in advanced gastric carcinoma
The amplification products of cyclin D1 and β -actin by RT-PCR were 434 bp and 206 bp respectively. Overexpression of cyclin D1 mRNA (Figure 2) was observed in 17/42 cases (40.5%) and all these cases showed positive expression of cyclin D1 protein.
Figure 2.
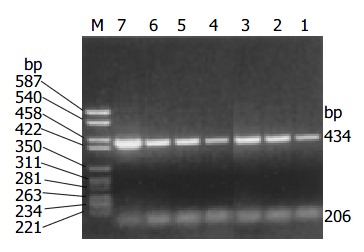
Overexpression of cyclin D1 mRNA detected by RT-PCR. M: marker: 1: Normal control; 3, 6, 7: Overexpression of cyclin D1 mRNA.
The level of amplification was estimated by densitometric tracing of the 4.0 kb cyclin D1 fragments of carcinomas and normal tissue. In this study, 7/42 (16.6%) cases had cyclin D1 gene amplification (2 to 4 fold), which also showed positive expression of cyclin D1 protein and overexpression of cyclin D1 mRNA. No gene rearrangement of cyclin D1 was observed in our study (Figure 3).
Figure 3.
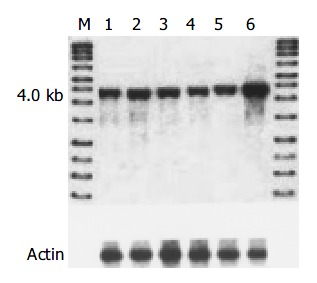
Genetic alteration of cyclin D1 in advanced gastric carcinoma detected by Southern blot. M: DNA marker; 1: Normal control; 6: Cyclin D1 gene amplification.
DISCUSSION
Cyclin D1 gene is located in the 11q13 region. Cyclin D1 protein consists of 295 amino acids, its function in normal cells is to regulate the progression through G1 phrase of the cell cycle in combination with CDKs by phosphorylation of pRb. In normal human tissues, the expression of cyclin D1 protein was rather low and negative by immunohistochemistry[11]. In our study, no one was positive in normal mucosa adjacent to adenocarcinomas. Negative staining was also observed in hyperplastic polyp and gastric adenoma. However, in 33/69 (47.8%) cases of gastric carcinoma, including 42 cases of advanced gastric carcinoma and 27 cases of early stage carcinoma, the expression of cyclin D1 was positive. The detection of cyclin D1 protein by immunohistochemistry is helpful in the diagnosis of early stage gastric carcinoma and could distinguish these cases from hyperplastic polyp. The expression of cell cycle regulators could be used as prognostic markers in some malignant tumors. Some studies showed that the positive expression of cyclin D1 was statistically associated with well-differentiated tumors[12]. In our study, the expression of cyclin D1 protein had no correlation with age, lymph node metastasis and histological grading of the patients. But a significant correlation was observed between cyclin D1 expression and 5-year survival rate. The patients with positive expression of cyclin D1 tended to have a worse prognosis.
Bartkora revealed a very good correlation in the expression of cyclin D1 between the in situ components and the invasive components of carcinoma[13]. Our results were in accordance with it. In our study, the positive rates of cyclin D1 protein in early stage carcinoma, advanced gastric carcinoma and lymph node metastases were similar, with no significant difference among them. A very high degree of consistence was found in the same section of early stage carcinoma between carcinoma in situ and its infiltration component (89.5%).The consistence between primary tumors and lymph node metastases in the same patient with advanced gastric carcinoma was also high (75.0%). These results suggested that positive expression of cyclin D1 protein might be an early event in gastric carcinoma pathogenesis, and it tended to maintain stable throughout the progression of infiltration and metastasis.
Genetic alteration of cyclin D1 was observed in several kinds of human tumors such as breast carcinoma[14], squamous cell carcinoma of the lung[15], endometrial carcinoma[16] and malignant gliomas[17]. Yang et al[18] reported that a characteristic translocation, t (11:14) (q13; q32) involving rearrangement of cyclin D1 gene, was detected in centrocytic lymphoma and could be used in its diagnosis. In this study, cyclin D1 gene amplification accompanying positive expression of cyclin D1 protein and overexpression of cyclin D1 mRNA in advanced gastric carcinoma was observed in 7/42 (16.6%) cases. The expression rate of cyclin D1 protein (47.4%) was significantly higher than that of cyclin D1 gene amplification (16.6%). It was similar to the overexpressiom rate of cyclin D1 mRNA (42.1%). We considered that the expression of cyclin D1 protein was affected more directly by mRNA level rather than gene level. Besides gene amplification, other factors including alteration in cyclin D1 promoter or abnormal regulation by transcription factors could both up- regulate the expression of mRNA and induce more cyclin D1 protein production. The discrepancy between the expression rates of cyclin D1 mRNA and protein might be caused by stroma cells in tumor tissues, which make the overexpression rate of cyclin D1 mRNA lower.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30300124
Edited by Wang XL and Xu CT Proofread by Pan BR and Xu FM
References
- 1.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 2.Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. [PubMed] [Google Scholar]
- 3.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 4.Rafferty MA, Fenton JE, Jones AS. An overview of the role and inter-relationship of epidermal growth factor receptor, cyclin D and retinoblastoma protein on the carcinogenesis of squamous cell carcinoma of the larynx. Clin Otolaryngol Allied Sci. 2001;26:317–320. doi: 10.1046/j.1365-2273.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 5.Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y, Fan DM. Antisense to cyclin D1 reverses the transformed phenotype of human gastric cancer cells. World J Gastroenterol. 1999;5:18–21. doi: 10.3748/wjg.v5.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer. 2001;84:1064–1069. doi: 10.1054/bjoc.2001.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Wan QH, Qian KX, Fang SG. A simple DNA extraction and rapid specific identification technique for single cells and early embryos of two breeds of Bos taurus. Anim Reprod Sci. 2003;77:1–9. doi: 10.1016/s0378-4320(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 10.Renedo M, Martinez-Delgado B, Arranz E, Garcia M, Urioste M, Martinez-Ramirez A, Rivas C, Cigudosa JC, Benitez I. Chromosomal changes pattern and gene amplification in T cell non-Hodgkin's lymphomas. Leukemia. 2001;15:1627–1632. doi: 10.1038/sj.leu.2402248. [DOI] [PubMed] [Google Scholar]
- 11.Turner HE, Nagy Z, Sullivan N, Esiri MM, Wass JA. Expression analysis of cyclins in pituitary adenomas and the normal pituitary gland. Clin Endocrinol (Oxf) 2000;53:337–344. doi: 10.1046/j.1365-2265.2000.01088.x. [DOI] [PubMed] [Google Scholar]
- 12.Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002;9:409–416. [PubMed] [Google Scholar]
- 13.Bartkova J, Lukas J, Müller H, Lützhøft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- 14.Steeg PS, Zhou Q. Cyclins and breast cancer. Breast Cancer Res Treat. 1998;52:17–28. doi: 10.1023/a:1006102916060. [DOI] [PubMed] [Google Scholar]
- 15.Qiuling S, Yuxin Z, Suhua Z, Cheng X, Shuguang L, Fengsheng H. Cyclin D1 gene polymorphism and susceptibility to lung cancer in a Chinese population. Carcinogenesis. 2003;24:1499–1503. doi: 10.1093/carcin/bgg035. [DOI] [PubMed] [Google Scholar]
- 16.Moreno-Bueno G, Rodríguez-Perales S, Sánchez-Estévez C, Hardisson D, Sarrió D, Prat J, Cigudosa JC, Matias-Guiu X, Palacios J. Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene. 2003;22:6115–6118. doi: 10.1038/sj.onc.1206868. [DOI] [PubMed] [Google Scholar]
- 17.Büschges R, Weber RG, Actor B, Lichter P, Collins VP, Reifenberger G. Amplification and expression of cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas. Brain Pathol. 1999;9:435–442; discussion 432-43. doi: 10.1111/j.1750-3639.1999.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang WI, Zukerberg LR, Motokura T, Arnold A, Harris NL. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994;145:86–96. [PMC free article] [PubMed] [Google Scholar]


