Abstract
AIM: To assess the effect of artificial liver support system (ALSS) on patients with severe viral hepatitis, who were divided into treatment group and control group.
METHODS: Four hundred in-hospital patients enrolled during 1995-2003 who received ALSS therapy were studied as the treatment group. Four hundred in-hospital patients enrolled during 1986-1994 who received other medical therapies served as the control group. The methods of ALSS used included plasma exchange, hemoperfusion, hemofiltration, continuous hemodiafiltration (CHDF). The effect of ALSS treatment was studied in patients at different stages of the disease.
RESULTS: The cure rate of acute and subacute severe hepatitis in the treatment group was 78.9% (30/38), and was 11.9% (5/42) in the control group. The improved rate of chronic severe hepatitis in the treatment group was 43.4% (157/362), and was 15.4% (55/358) in the control group. We found that patients treated with ALSS in the early or middle stage of the disease had much higher survival rates than patients in the end stage of the disease.
CONCLUSION: ALSS is an effective and safe therapy for severe viral hepatitis.
INTRODUCTION
Severe viral hepatitis is the main cause of hepatic failure in China because of the great population of hepatitis B patients, which is different from the Western countries where drugs or alcohol usually is the major cause. Despite a combination of all available treatments, the mortality of hepatic failure is more than 70%[1,2].
It is believed that damaged liver has the ability to regenerate and restore normal function of metabolism, synthesis and biotransformation. Liver transplantation remains the only effective therapeutic modality for chronic patients in end-stage [3-5]. There is also a need to develop a liver support system that can serve as a bridge to transplantation[6,7], so that patients can be supported until a liver becomes available or the condition of patients is improved. ALSS has been used to treat hepatic failure and has significantly decreased the mortality[8-11].
We designed an artificial liver support system for severe hepatitis patients. In this report, we described 400 patients with hepatic failure treated with ALSS in our hospital. Data such as concentrations of endotoxin and blood HBV, and serum amino acid spectrum were recorded. The effect of ALSS treatment was also compared in patients at different stages of the disease.
MATERIALS AND METHODS
The treatment group consisting of 400 viral severe hepatitis patients was treated with ALSS at the First Hospital of College of Medicine, Zhejiang University during 1995 to 2003. Two hundred and ninety-five were males and 105 were females. The age ranged from 20 to 64 years, with an average of 34.3 ± 16.5 years. The control group consisting of 400 viral severe hepatitis patients was treated in Department of Internal Medicine at the same hospital during 1986 to 1994, of them 273 were males and 127 were females. The age ranged from 19 to 68 years, with an average of 32.5 ± 18.8 years.
The patients were diagnosed according to the criteria established in the 1995 National Infectious Disease Meeting in Beijing[12]. Type A hepatitis was diagnosed by the identification of HAV-RNA and/or IgM anti-HAV. The diagnosis of hepatitis B was based on positive HBsAg. Acute type B hepatitis was diagnosed by the presence of HBsAg and/or IgM anti-HBc antibody. Three patients among them were negative for HBsAg but had positive IgM anti-HBc and they were diagnosed as type B hepatitis. All were positive for HBV DNA by PCR. An acute exacerbation of HB in a HBV carrier was diagnosed by a history of known HBsAg positivity for more than 6 mo, the presence of HBV-DNA and a markedly elevated IgG anti-HBc level. Type C hepatitis was diagnosed by the presence of either HCV RNA or anti-HCV antibody. Type B + D hepatitis was diagnosed by the presence of HBsAg and HDV-RNA. Type E hepatitis was diagnosed by the identification of IgM anti- HEV antibody.
Three types of severe viral hepatitis have been found in our country: acute, subacute and chronic severe hepatitis. Violent symptoms of acute severe hepatitis occurred within 10 d after the appearance of clinical manifestations, including malignant jaundice, hepatic encephlophathy (above phase II) and prolonged prothrombin time (PTA < 40%). These patients were usually accompanied with shrinking live dullness, rapidly rising blood bilirubin (TB > 171 μmol/L) and obvious abnormal liver functions. Subacute severe hepatitis patients were those who had prolonged prothrombin time (PTA < 40%) after the occurrence of manifestations for more than 10 d, and meanwhile, they had any one of the following symptoms, namely hepatic encephlophathy (above phase IIO), rapid rising of blood bilirubin (blood TB more than 171 μmol/L within several days), severe damage of liver functions, extremely fatigue, loss of appetite, nausea, abdominal distention or hydroperitoneum, sometimes with a tendency to bleed. Chronic severe hepatitis was clinically similar to acute or subacute severe hepatitis, but was distinguished by a known history of HBV carriage, chronic hepatitis or cirrhosis, or by the results of imaging, endoscopy or biopsy showing the existence of chronic hepatitis. Subacute and chronic severe hepatitis was classified into early, middle and end stages. Symptoms of the early stage included fulminant liver failure but without hepatic encephalopathy or ascites. The serum level of bilirubin (TB) was above 171 μmol/L, while the prothrombin time rate (PTA) was less than 40%. Liver biopsy was also taken into account when available. In addition, patients in the middle stage had hepatic encephalopathy (IIOabove), ascites, or a tendency to bleed with a PTA ≤ 30%. End stage patients had severe complications such as hepatorenal syndrome, infection, hepatoencephalopathy (IIOabove), electrolytic disturbance, with a PTA ≤ 20%.
In the treatment group there were 38 cases of acute or subacute severe hepatitis (6 type A, 8 type A + B, 5 type B + E, 17 type B, 2 type E), while the other 362 were cases of chronic severe hepatitis (310 type B, 18 type A + B, 14 type C, 12 type B + D, 7 type B + E, 1 type B + C + D). The average prothrombin time of the patients on admission was 31.8 ± 7.2 s (the normal was 12 s). Sixty-eight cases were treated with ALSS in the early stage, 186 in the middle stage and 146 in the end stage. In the control group there were 42 cases of acute or subacute severe hepatitis (8 type A, 9 type A + B, 3 type B + E,20 type B,1 type E, 1 type B + C + D), while the other 358 were cases of chronic severe hepatitis (313 type B,16 type A + B,10 type C,13 type B + D,5 type B + E,1 type B + C + D) (Table 1). The average prothrombin time of the patients in control group on admission was 30.9 ± 8.4 s. Seventy-four patients were diagnosed as early stage, 168 cases as middle stage and 158 as end stage acute or subacute severe hepatitis, respectively. There were no significant differences in sex, ages, etiology and conditions between two groups.
Table 1.
Types of severe hepatitis in two groups
| Types |
Treatment group |
Control group |
||
| Acute and subacute | Chronic | Acute and subacute | Chronic | |
| A | 6 | 0 | 8 | 0 |
| B | 17 | 310 | 20 | 313 |
| C | 0 | 14 | 0 | 10 |
| E | 2 | 0 | 1 | 0 |
| B + D | 0 | 12 | 0 | 13 |
| A + B | 8 | 18 | 9 | 16 |
| B + E | 5 | 7 | 3 | 5 |
| B + C + D | 0 | 1 | 1 | 1 |
| Total | 38 | 362 | 42 | 358 |
The methods of ALSS included plasma exchange, hemoper-fusion, hemofiltration, continuous hemodiafiltration (CHDF). We chose therapy based on the condition of patients. The ALSS treatment room was thoroughly sterilized by UV light before each treatment. The external circulation system and the separator were connected under sterile condition, washed with 0.9% saline solution at 38 °C to remove the micro bubbles in the line, and then filled with 2 mg/500 mL heparin saline solution. The main parts of ALSS were 160-200 g activated carbon absorber, membrane plasma separator, bilirubin absorbent, dialyser, etc. Plasma exchange was performed by using a membrane separation method marketed as Plasmacure PS-06 (Kuraray Co., Japan) (Figure 1). Fresh frozen plasma (FFP) was supplied by the Hangzhou Blood Center, Chinese Red Cross. Filtration was performed at a flow rate of 4-6 liters/h using a bicarbonate buffer, pH 7.4, having a potassium concentration of 4.0 mmol/L. The volume of substitution fluid was adjusted over a range of 6-30 L, depending on the patient’s response (Figure 2). Dialysis was performed concurrently at a flow rate of 500 mL/min using a conventional acetate buffer.
Figure 1.
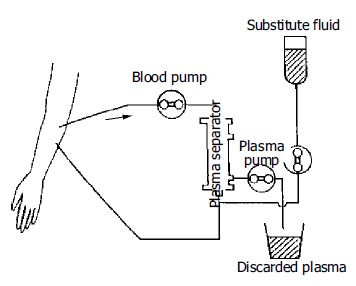
Circuit diagram of plasma exchange.
Figure 2.
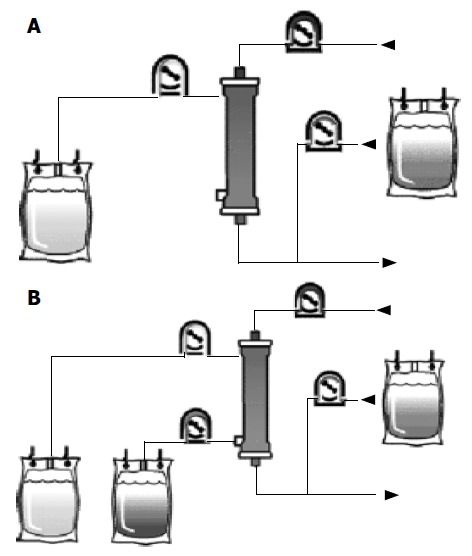
Continuous hemofiltration and hemodiafiltration with aid of a blood pump. A: Continuous hemofiltration with aid of a blood pump; B: Continuous hemodiafiltration with aid of a blood pump.
Before ALSS therapy, blood access was established with a double-lumen catheter inserted into the patient’s jugular or femoral vein, and heparin was then used to block the catheter after each session. The circuit diagrams for plasma exchange and perfusion are shown in Figure 1 and Figure 3. During each session of ALSS therapy which was lasted for 4-6 h, the total volume of exchanged plasma was about 3500 mL, and the exchange rate of plasma was 25-30 mL/min. A total of 3000-4500 mL of fresh frozen plasma and its substitute fluid, and 20-40 g albumin were supplied. The flow rate of blood was adjusted to 60-130 mL/min, and blood pressure and pulse were recorded continuously during treatment. Prophylactic antibiotics were used before and after therapy. Five mg dexamethasone and 10-20 mg heparin were injected routinely before therapy. During the session, the prothrombin time was tested constantly to allow the dose of heparin to be adjusted. A total of 10-108 mg heparin was used for each session. At the end of each ALSS treatment heparin was neutralized by injection of 10-50 mg of protamine sulphate. ALSS therapy was carried out two or three times in the first or second week, then one time each week till the patient’s condition was stable.
Figure 3.
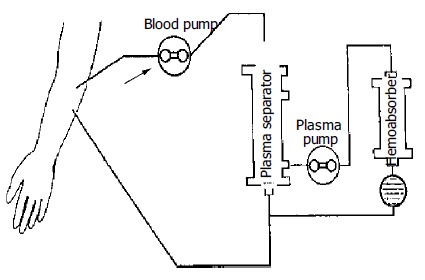
Circuit diagram of plasmapheresis plus plasma absorption. Special absorbers could be used to treat patients with hyperbilirubinemia or hepatic encephalopathy such as bilirubin and carbon absorbers.
Liver function and endotoxin levels were monitored during the therapy. Amino acid spectra were determined for 25 subjects by auto amino acid analyzing system, performed at the Analyzing Center of the Second Medical University in Shanghai. Endotoxin level was measured by quantitative Azo color test. HBV-DNA concentration in 10 cases was measured by chiron branched chain DNA assay, performed at the Virus Center of Luebrek Medical University, Germany.
Statistical analysis
All data were presented as mean ± SE. The data were analyzed by SPSS 10.0. The Student’s t test or Fischer’s exact test was used to determine the level of significance between groups. A P-value < 0.05 was considered statistically significant.
RESULTS
Nearly 90% patients experienced an improvement in symptoms such as fatigue or abdominal distention after each treatment. Results of liver function tests improved significantly in all subjects. The serum ALT, AST and TBA levels declined significantly (P < 0.001), the level of serum bilirubin decreased from 511.36 ± 192.81 μmol/L to 257.38 ± 123.48 μmol/L (P < 0.001), while the prothrombin time decreased from 31.8 ± 7.2 s to 23.6 ± 6.8 s (Table 2). The serum endotoxin levels before therapy in all hepatic failure patients were above the upper limit of normal which was 40 ng/L. After ALSS therapy, serum endotoxin levels declined from 58.2 ± 12.3 ng/L to 32.4 ± 7.8 ng/L ( P < 0.001). In the 10 patients tested, the HBV-DNA concentration declined from an average of 2588 ± 1534 copies/mL to 1815 ± 620 copies/mL ( P < 0.05) as a result of ALSS therapy. The serum level of aromatic amino acids (AAA), such as methionine, tyrosine, phenylalanine, cysteine, arginine, especially methionine declined significantly (P < 0.05) in 25 subjects. Meanwhile, the ratio of branched-chain amino acid/aromatic amino acid (BCAA/AAA) increased significantly (P < 0.05) (Table 3). No significant difference in TNF, rIL-2R and IL-2 levels before and after the ALSS treatment was observed (Table 4). There was no disturbance of electrolytes after ALSS therapy.
Table 2.
Result of liver function test before and after ALSS therapy (mean ± SE)
| Liver function | Pre-treatment | Post-treatment | P |
| ALT (U/L) | 123.35 ± 281.32 | 53.15 ± 94.21 | < 0.001 |
| AST (U/L) | 126.84 ± 115.25 | 63.71 ± 58.45 | < 0.001 |
| ALP (U/L) | 127.97 ± 66.74 | 81.45 ± 39.62 | < 0.001 |
| TBil (μmol/L) | 511.36 ± 192.81 | 257.38 ± 123.48 | < 0.001 |
| ChE (U/L) | 2572.58 ± 2236.95 | 3119.24 ± 1812.62 | 0.001 |
| γ -GT (U/L) | 42.38 ± 53.85 | 20.91 ± 25.58 | < 0.001 |
| TBA (μmol/L) | 256.36 ± 48.69 | 119.42 ± 49.37 | < 0.001 |
| PT (s) | 31.8 ± 7.2 | 23.6 ± 6.8 | < 0.001 |
Table 3.
Serum amino acid levels in 25 cases before and after the first ALSS therapy (mean ± SE)
| Pre-therapy (μmol/L) | After therapy (μmol/L) | |
| Cys | 65.63 ± 41.40 | 53.74 ± 26.86a |
| Phe | 94.78 ± 62.00 | 80.92 ± 40.75a |
| Ala | 256.51 ± 123.73 | 267.84 ± 138.32 |
| Gly | 194.89 ± 83.43 | 212.50 ± 106.07 |
| Glu | 118.50 ± 89.65 | 116.85 ± 91.26 |
| Gln | 494.16 ± 218.91 | 515.84 ± 208.81 |
| Met | 185.85 ± 142.33 | 149.91 ± 134.16b |
| Arg | 170.44 ± 231.69 | 143.18 ± 175.92a |
| Lys | 220.72 ± 168.05 | 190.29 ± 136.28 |
| Tyr | 121.98 ± 82.82 | 104.44 ± 59.94a |
| Leu | 73.73 ± 58.81 | 78.17 ± 43.74 |
| Orn | 113.99 ± 94.58 | 92.84 ± 52.93 |
| Tau | 46.41 ± 27.34 | 43.51 ± 32.90 |
| Ser | 123.78 ± 64.83 | 122.22 ± 61.29 |
| Thr | 165.60 ± 83.34 | 152.94 ± 83.42 |
| Asp | 16.22 ± 10.72 | 13.38 ± 7.87 |
| Asn | 59.40 ± 46.09 | 64.52 ± 49.46 |
| Val | 121.69 ± 75.01 | 126.35 ± 68.72 |
| Ile | 44.86 ± 36.61 | 46.29 ± 25.88 |
| His | 96.18 ± 73.85 | 87.86 ± 51.56 |
| BCAA/AAA | 1.18 ± 0.39 | 1.52 ± 0.77a |
P < 0.05;
P < 0.001 vs pre-therapy group.
Table 4.
Endotoxin, TNF, rIL-2R, IL-2 in patients with hepatic failure before and after ALSS therapy (mean ± SE)
| Pre-treatment | Post-treatment | P | |
| Endotoxin (ng/L) | 58.2 ± 12.3 | 32.4 ± 7.8 | < 0.005 |
| TNF (ng/L) | 3.4 ± 1.2 | 3.2 ± 1.2 | > 0.05 |
| rIL-2R (U/mL) | 1040.1 ± 309.2 | 951.0 ± 285.6 | > 0.05 |
| IL-2 (ng/mL) | 20.1 ± 1.9 | 9.7 ± 1.8 | > 0.05 |
Seventeen candidates for orthotopic liver transplantation (OLT) received 2-3 runs of ALSS and were “bridged” successfully to OLT. After OLT, ALSS was used to replace liver function in non-function period. Twelve of 17 patients were completely recovered and 3 died of infection, acute rejection and abdominal bleeding.
Among the 38 patients with acute or subacute severe hepatitis treated with ALSS 3-5 times, 30 of them (78.9%) survived for at least half a year but 8 ied in 1 mo. One hundred and fifty-seven of 362 patients with chronic severe hepatitis were cured or greatly improved, 107 patients were discharged as their own will, and 98 patients died in the next 3 onths, so the cure rate was 43.4%. In the control group, the cure rate of acute and subacute severe hepatitis was 11.9% (5/42), while cure and improved rate of chronic severe hepatitis was only 15.4% (55/358) (Table 5). There were significantly differences in the cure rate between two groups (P < 0.001). More importantly, in the treatment group, the cure rate of patients in early or middle stage (76.5% and 61.8%, respectively) was much higher than that those in end-stage (13.7%, Table 6).
Table 5.
Prognosis of ALSS treatment group and control group
|
ALSS treatment group |
Control group |
|||||||
|
Acute and subacute severe hepatitis |
Chronic severe hepatitis |
Acute and subacute severe hepatitis |
Chronic severe hepatitis |
|||||
| Cases | Ratio (%) | Cases | Ratio (%) | Cases | Ratio (%) | Cases | Ratio (%) | |
| Cured | 30 | 78.9 | 157 | 43.4 | 5 | 11.9 | 55 | 15.4 |
| Discharged as their own will | 2 | 5.3 | 107 | 29.6 | 16 | 38.1 | 122 | 34.0 |
| Died | 6 | 15.8 | 98 | 27.0 | 21 | 50.0 | 181 | 50.6 |
| Total | 38 | 100 | 362 | 100 | 42 | 100 | 358 | 100 |
Table 6.
Outcome of patients in different stages after ALSS treatment
| Stages |
Cured |
Discharged |
Died |
Total | |||
| Cases | % | Cases | % | Cases | % | ||
| Early stage | 52 | 76.5 | 9 | 13.2 | 7 | 10.3 | 68 |
| Middle stage | 115 | 61.8 | 34 | 18.3 | 37 | 19.9 | 186 |
| End stage | 20 | 13.7 | 68 | 46.6 | 58 | 39.7 | 146 |
| Total | 187 | 46.8 | 111 | 27.8 | 102 | 25.5 | 400 |
Complications occurred during ALSS therapy included skin rash, hypotension, blood coagulation in perfusion apparatus, infection, hyperkalemia, and local bleeding (Figure 4). All patients recovered after management of these complications, except one patient who died of intracranial bleeding.
Figure 4.
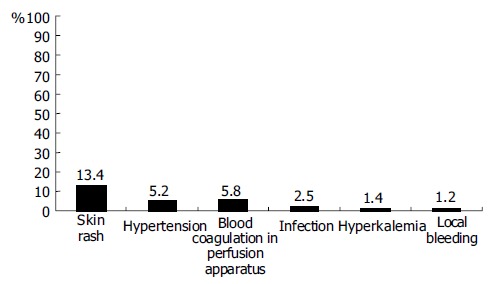
Incidence rate of complications of ALSS therapy.
DISCUSSION
In acute liver failure, the liver parenchyma injured by viral, toxic, or some other insults failed to function sufficiently. Temporary support of normal liver function would promote the damaged liver to restore its normal function. Artificial liver support system could serve this purpose. Moreover, when the liver lost its ability to function normally, then an artificial liver support system might be used as a “bridge” to liver transplantation[6,7]. Although various liver assistant devices have been introduced during the past 40 years, a complete artificial liver system that can support patients with severe hepatic failure till the damaged liver restores its normal function has not yet been developed[8].
We modified the artificial liver support system for treatment of patients with severe hepatic failure. We substituted the usual 50 g activated carbon absorber with 160-200 g one to remove toxic substances of intermediate molecular weight from blood. The 160-200 g carbon absorber proved to be appropriate, as a larger carbon absorber might destroy blood cells and cause side effects. The bilirubin absorbent could absorb excess bilirubin from the plasma, so that the cleaned plasma could be retransmitted to patients directly. The membrane plasma separator could separate and discard plasma containing toxic substances, and supply normal fresh plasma containing albumin and coagulation factors for patients. The volume exchanged utilizing this method was about 3 500 mL each time, much more than that utilizing traditional centrifugation methods, which exchanged only 800-1000 mL each time. Continuous hemodiafiltration (CHDF) could eliminate middle and small molecular toxic substances, and keep the balance of internal environment[13]. These methods were used in different combinations based on the symptoms of patients (Figure 1, Figure 2, Figure 3). For example, when patients had hepatic encephalopathy, we performed plasma exchange in combination with plasma perfusion. For patients with hepatorenal syndrome, we chose plasma exchange and hemodialysis or hemofiltration or CHDF. For those with hyperbilirubinemia, plasma bilirubin absorption in combination with hemoperfusion was used. When the balance of water or electrolytes was disturbed, we used plasma exchange and hemofiltration or CHDF. Sometimes, more than three methods were used together for one patient. In addition, we carefully adjusted the dose of heparin and protamine according to PT, and maintained fluid balance to decrease complications such as bleeding, hemolysis, hypotension. This was crucial to decrease the mortality of hepatic failure.
Changes in the spectrum of amino acids might be another benefit of ALSS therapy. It has been shown that the concentration of aromatic amino acids (AAA) was increased in patients with fulminant hepatitis failure[14,15]. Moreover, the concentration of methionine was a sensitive indicator of liver injury because methionine was released while liver necrosis[16]. Methionine was toxic to the central nervous system, and one of the main causes of hepatic encephalopathy[17]. After ALSS therapy, the serum level of aromatic amino acids, especially methionine declined and the ratio of BCAA/AAA was significantly increased.
In this study, we found that the cure rate of treatment group was significantly higher than that of the control group, indicating that ALSS is an effective therapy in treating patients with severe hepatitis. We also compared the efficiency of ALSS therapy in different stages of severe liver disease and concluded that the cure rate of early or middle stage severe hepatitis was much higher than that of end stage severe hepatitis (76.5%, 61.8% vs 13.7%, respectively). It is possible that ALSS may provide a favorable internal environment for hepatocytes to regenerate and restore normal function in patients at early or middle stage. However, there was massive necrosis of hepatocytes in hepatitis patients at end stage, and liver regeneration was not sufficient to recover liver function. Our data suggested that ALSS therapy might be an effective method to treat hepatitis patients at early stages.
Liver transplantation could provide good results in the treatment of hepatic failure, with a 5-year survival rate of 60%[6,18]. However, its use has been greatly limited because of the lack of sufficient liver donors. Therefore, a system that can serve as a “bridge” to eventual OLT would greatly extend the survival rate[19,20]. Based on our data, the artificial liver support system could serve as an effective “bridge” to OLT. Plasmapheresis was also beneficial, as the levels of coagulation factors and albumin could be increased by several plasma exchanges[19]. Hemoperfusion was effective in improving the neurologic status of patients with hepatic failure[8,21]. Hemodialysis, hemofiltration or CHDF could correct the disturbance of water or electrolytes of patients[13,22]. So ALSS could improve the condition of patients until a donor liver was available. Complications of the transplantation included acid-base imbalance, hyperbilirubinemia, disturbance of electrolytes, dysfunction of blood coagulation, acute rejection, etc.[23]. ALSS treatment could improve the internal environment to support patients in pre- and post-transplantation periods. In addition ALSS could therefore improve the result of OLT in patients with severe liver disease. Further appropriately controlled trials are being initiated to confirm this observation.
Footnotes
Supported by the National High Technology Research and Development Program of China (863 Program), No. 2003AA205015 and the Major Science Foundation of Zhejiang Province, No. 021107689 and No. 021103126 and the Health Foundation of Zhejiang Province, No. 2003A031
Edited by Wang XL Proofread by Chen WW and Xu FM
References
- 1.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 2.Mas A, Rodés J. Fulminant hepatic failure. Lancet. 1997;349:1081–1085. doi: 10.1016/S0140-6736(96)08054-3. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Lee WM. Acute hepatic failure: a Western perspective. J Gastroenterol Hepatol. 2000;15:480–488. doi: 10.1046/j.1440-1746.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 4.van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary versus orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699–705. doi: 10.1016/s0168-8278(99)80202-5. [DOI] [PubMed] [Google Scholar]
- 5.Miwa S, Hashikura Y, Mita A, Kubota T, Chisuwa H, Nakazawa Y, Ikegami T, Terada M, Miyagawa S, Kawasaki S. Living-related liver transplantation for patients with fulminant and subfulminant hepatic failure. Hepatology. 1999;30:1521–1526. doi: 10.1002/hep.510300621. [DOI] [PubMed] [Google Scholar]
- 6.Friedman AL. Why bioartificial liver support remains the Holy Grail. ASAIO J. 1998;44:241–243. [PubMed] [Google Scholar]
- 7.Abouna GM, Ganguly PK, Hamdy HM, Jabur SS, Tweed WA, Costa G. Extracorporeal liver perfusion system for successful hepatic support pending liver regeneration or liver transplantation: a pre-clinical controlled trial. Transplantation. 1999;67:1576–1583. doi: 10.1097/00007890-199906270-00012. [DOI] [PubMed] [Google Scholar]
- 8.Uchino J, Matsushita M. Strategies for the rescue of patients with liver failure. ASAIO J. 1994;40:74–77. [PubMed] [Google Scholar]
- 9.Lanjuan L, Qian Y, Jianrong H, Xiaowei X, Yuemei C, Yagang C, Weihang M, Zhi C, Suzhen F. Severe hepatitis treated with an artificial liver support system. Int J Artif Organs. 2001;24:297–303. [PubMed] [Google Scholar]
- 10.Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18:390–396. doi: 10.1111/j.1525-1594.1994.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Yang Q, Huang J, Xu X, Chen Y, Chen Y, Ma W, Chen Z, Fu S. Treatment of hepatic failure with artificial liver support system. Chin Med J (Engl) 2001;114:941–945. [PubMed] [Google Scholar]
- 12.Si CW, Zhang H, Wang BE. Prevention and cure project of viral hepatitis. Revised Statement 5th National Infectious Disease Meeting, Beijing, 1995. Zhonghua Chuanranbing Zazhi. 1995;13:241–247. [Google Scholar]
- 13.Sadahiro T, Hirasawa H, Oda S, Shiga H, Nakanishi K, Kitamura N, Hirano T. Usefulness of plasma exchange plus continuous hemodiafiltration to reduce adverse effects associated with plasma exchange in patients with acute liver failure. Crit Care Med. 2001;29:1386–1392. doi: 10.1097/00003246-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Kato A, Suzuki K, Sato S. [Imbalance of amino acid metabolism in fulminant hepatitis and its management] Nihon Rinsho. 1992;50:1599–1603. [PubMed] [Google Scholar]
- 15.Takahashi Y. [Evaluation of the special therapies in fulminant viral hepatitis--a multi-institution study] Nihon Shokakibyo Gakkai Zasshi. 1995;92:7–18. [PubMed] [Google Scholar]
- 16.Higashi T. Impaired metabolism of methionine in severe liver diseases. I. Clinical and pathophysiological significance of elevated serum methionine levels. Gastroenterol Jpn. 1982;17:117–124. doi: 10.1007/BF02774550. [DOI] [PubMed] [Google Scholar]
- 17.Toborek M, Kopieczna-Grzebieniak E, Drózdz M, Wieczorek M. Increased lipid peroxidation and antioxidant activity in methionine-induced hepatitis in rabbits. Nutrition. 1996;12:534–537. doi: 10.1016/s0899-9007(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 18.Goss JA, Shackleton CR, Maggard M, Swenson K, Seu P, McDiarmid SV, Busuttil RW. Liver transplantation for fulminant hepatic failure in the pediatric patient. Arch Surg. 1998;133:839–846. doi: 10.1001/archsurg.133.8.839. [DOI] [PubMed] [Google Scholar]
- 19.Agishi T, Nakagawa Y, Teraoka S, Kubo K, Nakazato S, Ota K. Plasma exchange as a rescue strategy for hepatic failure. ASAIO J. 1994;40:77–79. [PubMed] [Google Scholar]
- 20.Larsen FS, Hansen BA, Jørgensen LG, Secher NH, Bondesen S, Linkis P, Hjortrup A, Kirkegaard P, Agerlin N, Kondrup J. Cerebral blood flow velocity during high volume plasmapheresis in fulminant hepatic failure. Int J Artif Organs. 1994;17:353–361. [PubMed] [Google Scholar]
- 21.Ash SR. Hemodiabsorption in the treatment of acute hepatic failure. ASAIO J. 1994;40:80–82. [PubMed] [Google Scholar]
- 22.Kaplan AA, Epstein M. Extracorporeal blood purification in the management of patients with hepatic failure. Semin Nephrol. 1997;17:576–582. [PubMed] [Google Scholar]
- 23.Zhu XF, Chen GH, He XS, Lu MQ, Wang GD, Cai CJ, Yang Y, Huang JF. Liver transplantation and artificial liver support in fulminant hepatic failure. World J Gastroenterol. 2001;7:566–568. doi: 10.3748/wjg.v7.i4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]


