Abstract
AIM: γ -glutamyl transpeptidase (GGT) has been reported as a virulence and colonizing factor of Helicobacter pylori (H pylori). This study examined the effect of GGT on the growth of H pylori.
METHODS: Standard H pylori strain NCTC 11637 and 4 clinical isolates with different levels of GGT activity as measured by an enzymatic assay were used in this study. Growth inhibition and stimulation studies were carried out by culturing H pylori in brain heart infusion broth supplemented with specific GGT inhibitor (L-serine sodium borate complex, SBC) or enhancer (glutathione together with glycyl-glycine), respectively. The growth profiles of H pylori were determined based on viable bacterial count at time interval.
RESULTS: Growth was more profuse for H pylori isolates with higher GGT activity than those present with lower GGT activity. However, in the presence of SBC, growth of H pylori was retarded in a dose dependent manner (P = 0.034). In contrast, higher growth rate was observed when GGT activity was enhanced in the presence of glutathione and glycyl-glycine.
CONCLUSION: Higher GGT activity provides an advantage to the growth of H pylori in vitro. Inhibition of GGT activity by SBC resulted in growth retardation. The study shows that GGT plays an important role on the growth of H pylori.
INTRODUCTION
Helicobacter pylori (H pylori) is a gram-negative spiral bacterium that causes chronic infection of the human stomach in adults[1-5] as well as children[6]. The chronic H pylori infection may lead to peptic ulceration[7-9] and may be a potential risk factor for gastric carcinoma[10-12].
The exact mechanism on how H pylori promotes gastric neoplasia is not known but is hypothesized to have occurred through the production of reactive oxygen species leading to oxidative stress and DNA damage in gastric epithelial cells[13-15]. The level of reduced form of the tripeptide thiol, glutathione (GSH), one of the major endogenous defense mechanisms against oxidative stress, was shown to be decreased in gastric mucosal after H pylori infection[16,17]. Furthermore, it has been well established that GGT plays a major role in glutathione metabolism. This enzyme catalyses transpeptidation reaction in which a γ -glutamyl moiety is transferred from γ -glutamyl compounds, such as glutathione, a non-protein sulphydryl molecule, to amino acids. In addition, GGT can use γ -glutamyl peptides as substrates in the reciprocal hydrolysis reaction, thus playing a role in the synthesis of glutathione[18,19].
It has been reported that GGT activity could be inhibited by the presence of inhibitors like L-serine sodium borate complex (SBC)[20] and acivicin[21]. Although acivicin is a more effective GGT inhibitor, it is nonspecific and inhibits a number of glutamine amino-transferase[21]. In contrast, SBC is a highly specific GGT inhibitor but substantially higher concentration of SBC as compared to acivicin is needed for an effective inhibition of GGT activity[20].
GGT being a constitutive enzyme of H pylori was shown to participate in the colonization of H pylori in Swiss specific pathogen-free mice[22]. However, a later study using a different animal model demonstrated that GGT was not essential for colonization but acted as a virulence factor[23]. The present study examined the effect of GGT on H pylori growth in vitro in the presence of GGT inhibitor as well as enhancer.
MATERIALS AND METHODS
Bacterial strains and culture conditions
A standard H pylori strain NCTC 11637 and 4 clinical isolates with different levels of GGT activity were used in this study. Strains 712 and 1 018 showed high GGT activity ( > 1 U/mg protein) while strains 1082 and 888 had low GGT activity ( < 0.4 U/mg protein).
H pylori was grown for 3 d at 37 °C on chocolate blood agar containing 40 g/L blood agar base No.2 (Oxoid) and 50 mL/L horse blood (Gibco) in a humidified incubator (Forma Scientific) supplied with 50 mL/L CO2[24] The bacterial cells were harvested and washed with PBS buffer (pH 7.4) to give a suspension of ca. 5 × 10 7 CFU/mL (A600 = 0.2) in either PBS or brain heart infusion (BHI, Gibco) broth.
Inhibitory effect of SBC on H pylori GGT activity
A bacterial population of 3 d old 107 CFU/mL H pylori NCTC 11 637 was incubated in BHI broth containing various concentrations (2-10 mmol/L) of SBC (Sigma) at 37 °C for 30 min. GGT activity of H pylori cells was then measured by an enzymatic assay as described by Meister et al[25].
Growth inhibition and stimulation studies
H pylori NCTC 11 637 was suspended in fresh BHI broth at a final cell concentration of approximately 5 × 10 5 CFU per ml. For growth inhibition study, appropriate volumes of filter-sterilized (pore size, 0.2 µm; Nalgene sterile syringe filter) SBC stock solution (100 mmol/L) were added to sterile BHI broth to provide final concentrations of SBC in BHI in the range of 2-10 mmol/L. For growth stimulation study, filter-sterilized glycyl-glycine (Sigma) and GSH (Sigma) were added to BHI broth at a final concentration of 1 mmol/L and 0.1 mmol/L, respectively.
Growth inhibition and stimulation curves were constructed based on viable bacterial count at different time interval.
Growth of various H pylori strains that expressed different levels of GGT activity
Two strains each with high GGT activity (strains 1018 and 712) and low GGT activity (strains 1082 and 888) were grown in BHI broth at 37 °C over a period of 3 wk. The bacterial populations of the various strains were enumerated at time interval.
Statistical analysis
Data was analyzed using one-way ANOVA test (SPSS). A value of P ≤ 0.05 was considered statistically significant.
RESULTS
SBC inhibits GGT activity of H pylori
Figure 1 shows that H pylori strain NCTC 11 637 GGT activity was inhibited in a dose dependent manner upon exposure to a range of SBC concentrations (2-10 mmol/L) for 30 min at 37 °C, where > 90% GGT activity was inhibited by ≥ 4 mmol/L SBC. The maximum inhibitory effect of 96% of GGT activity was achieved at the concentration of 10 mmol/L SBC.
Figure 1.
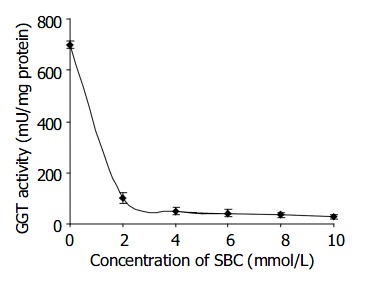
Inhibitory effect of SBC on H pylori GGT activity Inhibition of 85%, 92%, 94%, 95% and 96% were obtained by incubating H pylori in 2, 4, 6, 8 and 10 mmol/L SBC, respectively at 37 for 30 min.
Effect of GGT on H pylori growth
Since SBC has inhibitory effect on H pylori GGT activity, H pylori NCTC 11637 was cultured in BHI broth supplemented with different concentrations of SBC (2-10 mmol/L). Figure 2 shows that the H pylori cultured in the presence of various concentrations of SBC (2-10 mmol/L) over 3 wk displayed marked inhibition on the growth of H pylori in a dose dependent manner (ANOVA, P = 0.034). A > 99% decrease in viability of the bacterial population was observed within 72 h after culturing H pylori in the presence of 10 mmol/L SBC. In contrast, H pylori proliferated at twice the normal growth rate in the presence of GSH and glycyl-glycine with increased GGT activity as compared to the control. And this acceleration in growth lasted over a period of more then two weeks. Similar growth profile was observed with another H pylori strain SS1 (data not shown).
Figure 2.
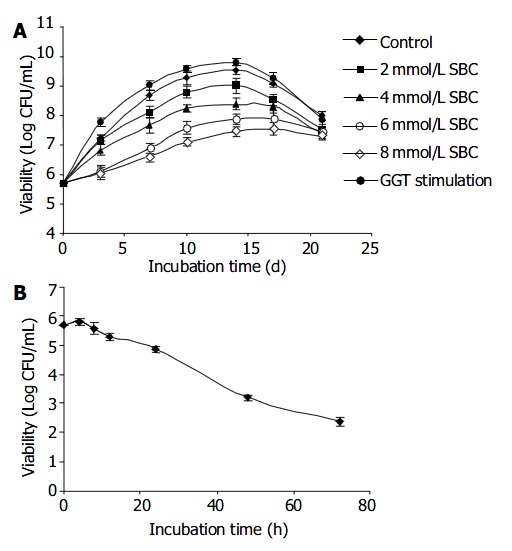
Effect of GGT on the growth of H pylori. H pylori strain NCTC 11 637 was cultured microaerobically in BHI broth medium supplemented with 10% fetal bovine serum at 37 °C nd in the presence of either different concentration of GGT inhibitor or stimulator. A: Effect of 2 mmol/L ( ■square), 4 mmol/L (▲triangle), 6 mmol/L (× ross), 8 mmol/L (* star) SBC and GGT stimulation (0.1 mol/L GSH +1 mol/L glycyl-glycine,●ircle) on NCTC 11637 over 21 d. Control (◆iamond). B: Effect of 10 mmol/L SBC on NCTC 11637 over 72 h. Viability was determined continuously at time intervals. (CFU: colony-forming units).
Growth of different H pylori strains
Growth of the four H pylori strains with disparate GGT activities was followed over a period of 3 wk. Figure 3 shows that H pylori isolates with higher GGT activity (strains 1018 and 712) grew better and more abundant than those with lower GGT activity (strains 1082 and 888) by 10-100 folds depending on the age of culture. However, all 4 cultures showed similar growth rate at 3 wk-old.
Figure 3.
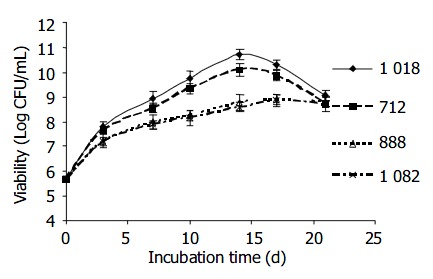
Growth of 4 H pylori strains with different levels of GGT activity H pylori strains 1018 and 712 expressed high GGT activity ( > 1 U/mg protein) while strains 888 and 1082 produced low GGT activity ( < 0.4 U/mg protein). H pylori strains 1018 (diamond), 712 (■square), 888 (△triangle), and 1 082 ( × cross) were cultured in BHI medium microaerobically over 3 wk. Viability was determined continuously at time intervals.
DISCUSSION
Inhibition of GGT activity by SBC was caused by competition with respect to γ -glutamyl substrate, and it was suggested that a serine-borate complex is formed which may bind to the active site of the enzyme by interacting with a carbohydrate residue of the enzyme[20]. Although acivicin is 20 times more effective in inhibiting GGT activity as compared to SBC (data not shown), it was not used for the growth inhibition study because it is a non-specific GGT inhibitor[21]. In this study, more than 90% of GGT activity was suppressed by 4 mmol/L SBC. Inhibition of GGT activity by SBC resulted in retarding the growth of H pylori in a dose dependent manner indicating that GGT is an essential enzyme in the growth of H pylori. In contrast, when physiological concentration of GSH and glycyl-glycine[26,27] were supplemented into the culture medium, growth of H pylori was accelerated by more than tow folds. The results show that the growth of H pylori was enhanced under GGT stimulation condition demonstrating the possibility of such event in in vivo situation.
In this study, it is interesting to note that H pylori with higher GGT activity ( > 1 U/mg protein) grows better than those with lower GGT activity ( < 0.4 U/mg protein). However, in the presence of SBC, a specific GGT inhibitor, the growth of H pylori was shown to be retarded. This finding that GGT is vital for the growth of H pylori could possibly explain why there was a reduction in the recovery of GGT isogenic mutant in the postinfected mice in the in vivo study carried out by McGovern et al[23], while no ggt mutant H pylori were recovered in the animal model of Chevalier et al[22]. The explanation supports the role of GGT activity in the ability of H pylori to proliferate in in vivo condition. However, the variation could also be due to the difference in animal model as suggested by McGovern et al[23], or owing to strain difference in terms of the level of GGT activity expressed as demonstrated in this study.
It has been reported that GGT plays a significant role in H pylori-mediated apoptosis[28]. It was therefore suggested that H pylori induces apoptosis by GGT and that the bacteria gain essential nutrients from the apoptotic cells, thus establishing a permanent colony in vivo[28,29]. The results of earlier studies[22,23,28] indicated that GGT is an important enzyme for the growth and survival of H pylori in vivo while our findings that growth of H pylori is relative to the level of bacterial GGT activity and that higher GGT activity favors its growth in vitro. It is therefore proposed that GGT has a vital and prominent role to play on the growth of H pylori.
Footnotes
Supported by NMRC Grant No. 0415/2000. Gong Min is a National University of Singapore research scholar
Edited by Wang XL Proofread by Xu FM
References
- 1.Israel DA, Peek RM. pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment Pharmacol Ther. 2001;15:1271–1290. doi: 10.1046/j.1365-2036.2001.01052.x. [DOI] [PubMed] [Google Scholar]
- 2.Sanders MK, Peura DA. Helicobacter pylori-Associated Diseases. Curr Gastroenterol Rep. 2002;4:448–454. doi: 10.1007/s11894-002-0019-x. [DOI] [PubMed] [Google Scholar]
- 3.Min K, Hong SM, Kim KR, Ro JY, Park MJ, Kim JS, Kim JM, Jung HC, Yu E. Intramucosal Helicobacter pylori in the human and murine stomach: its relationship to the inflammatory reaction in human Helicobacter pylori gastritis. Pathol Res Pract. 2003;199:1–8. doi: 10.1078/0344-0338-00345. [DOI] [PubMed] [Google Scholar]
- 4.Shibata K, Moriyama M, Fukushima T, Une H, Miyazaki M, Yamaguchi N. Relation of Helicobacter pylori infection and lifestyle to the risk of chronic atrophic gastritis: a cross-sectional study in Japan. J Epidemiol. 2002;12:105–111. doi: 10.2188/jea.12.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cave DR. Chronic gastritis and Helicobacter pylori. Semin Gastrointest Dis. 2001;12:196–202. [PubMed] [Google Scholar]
- 6.Ng BL, Quak SH, Aw M, Goh KT, Ho B. Immune responses to differentiated forms of Helicobacter pylori in children with epigastric pain. Clin Diagn Lab Immunol. 2003;10:866–869. doi: 10.1128/CDLI.10.5.866-869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai YC, Wang TH, Huang SH, Yang SS, Wu CH, Chen TK, Lee CL. Density of Helicobacter pylori may affect the efficacy of eradication therapy and ulcer healing in patients with active duodenal ulcers. World J Gastroenterol. 2003;9:1537–1540. doi: 10.3748/wjg.v9.i7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura AM, Pérez-Pérez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am J Epidemiol. 2002;155:1054–1059. doi: 10.1093/aje/155.11.1054. [DOI] [PubMed] [Google Scholar]
- 9.Stack WA, Atherton JC, Hawkey GM, Logan RF, Hawkey CJ. Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther. 2002;16:497–506. doi: 10.1046/j.1365-2036.2002.01197.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawsey SM, Mark SD, Taylor PR, Limburg PJ. Gastric cancer and H pylori. Gut. 2002;51:457–458. doi: 10.1136/gut.51.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser MJ. Linking Helicobacter pylori to gastric cancer. Nat Med. 2000;6:376–377. doi: 10.1038/74627. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S. Long-term Helicobacter pylori infection and the development of atrophic gastritis and gastric cancer in Japan. J Gastroenterol. 2002;37 Suppl 13:24–27. doi: 10.1007/BF02990095. [DOI] [PubMed] [Google Scholar]
- 13.Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, Blake DR, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111–1115. [PubMed] [Google Scholar]
- 15.Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–356. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beil W, Obst B, Sewing KF, Wagner S. Helicobacter pylori reduces intracellular glutathione in gastric epithelial cells. Dig Dis Sci. 2000;45:1769–1773. doi: 10.1023/a:1005530227603. [DOI] [PubMed] [Google Scholar]
- 17.Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Lett. 2001;164:127–133. doi: 10.1016/s0304-3835(01)00383-4. [DOI] [PubMed] [Google Scholar]
- 18.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 19.Tate SS, Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- 20.Tate SS, Meister A. Serine-borate complex as a transition-state inhibitor of gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1978;75:4806–4809. doi: 10.1073/pnas.75.10.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson ME, Meister A. Inhibition of gamma-glutamyl transpeptidase and induction of glutathionuria by gamma-glutamyl amino acids. Proc Natl Acad Sci U S A. 1986;83:5029–5032. doi: 10.1073/pnas.83.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31:1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 23.McGovern KJ, Blanchard TG, Gutierrez JA, Czinn SJ, Krakowka S, Youngman P. gamma-Glutamyltransferase is a Helicobacter pylori virulence factor but is not essential for colonization. Infect Immun. 2001;69:4168–4173. doi: 10.1128/IAI.69.6.4168-4173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng PY, Hua J, Yeoh KG, Ho B. Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47:18–22. doi: 10.1136/gut.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meister A, Tate SS, Griffith OW. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- 26.Tate SS, Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- 27.Paroni R, De Vecchi E, Cighetti G, Arcelloni C, Fermo I, Grossi A, Bonini P. HPLC with o-phthalaldehyde precolumn derivatization to measure total, oxidized, and protein-bound glutathione in blood, plasma, and tissue. Clin Chem. 1995;41:448–454. [PubMed] [Google Scholar]
- 28.Shibayama K, Kamachi K, Nagata N, Yagi T, Nada T, Doi Y, Shibata N, Yokoyama K, Yamane K, Kato H, et al. A novel apoptosis-inducing protein from Helicobacter pylori. Mol Microbiol. 2003;47:443–451. doi: 10.1046/j.1365-2958.2003.03305.x. [DOI] [PubMed] [Google Scholar]
- 29.Shibayama K, Doi Y, Shibata N, Yagi T, Nada T, Iinuma Y, Arakawa Y. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun. 2001;69:3181–3189. doi: 10.1128/IAI.69.5.3181-3189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


