Abstract
AIM: To study the anti-tumor effect of resveratrol and in combination with 5-FU on murine liver cancer.
METHODS: Transplantable murine hepatoma22 model was used to evaluate the anti-tumor activity of resveratrol (RES) alone or in combination with 5-FU in vivo. H22 cell cycles were analyzed with flow cytometry.
RESULTS: Resveratrol could inhibit the growth of murine hepatoma22, after the mice bearing H22 tumor were treated with 10 mg/kg or 15 mg/kg resveratrol for ten days, and the inhibition rates were 36.3% (n = 10) and 49.3% (n = 9), respectively, which increased obviously compared with that in control group (85 ± 22 vs 68 ± 17, P < 0.01). RES could induce the S phase arrest of H22 cells, and increase the persentage of cells in S phase from 59.1% (n = 9) to 73.5% (n = 9) in a dose-dependent manner (P < 0.05). The enhanced inhibition of tumor growth by 5-FU was also observed in hepatoma22 bearing mice when 5-FU was administered in combination with 10 mg/kg resveratrol. The inhibition rates for 20 mg/kg or 10 mg/kg 5-FU in combination with 10 mg/kg resveratrol were 77.4% and 72.4%, respectively, compared with the group of 20 mg/kg or 10 mg/kg 5-FU alone, in which the inhibition rates were 53.4% and 43.8%, respectively (n = 8). There was a statistical significance between the combination group and 5-FU group.
CONCLUSION: RES could induce the S phase arrest of H22 cells and enhance the anti-tumor effect of 5-FU on murine hepatoma22 and antagonize its toxicity markedly. These results suggest that resveratrol, as a biochemical modulator to enhance the therapeutic effects of 5-FU, may be potentially useful in cancer chemotherapy.
INTRODUCTION
Liver cancer is common in the world, especially in China[1-10]. Since the introduction of 5-FU for the treatment of liver cancer, the prognosis of liver cancer patients has been greatly improved. However, the serious side effects of 5-FU restrict its extensive clinical application. Searching for some new types of drugs to substitute or combine with 5-FU is necessary. Recently, scientists have found that resveratrol (3,4,5-trihydroxy-trans-stilbene, RES), a kind of phytoalexin found in root extract of the weed Polygonum cuaspidatum and in grape skins as well as red wine, has comprehensive pharmacological effects. Studies demonstrated that RES could alter the synthesis and secretion of lipids and lipoproteins by liver cells, block human platelet aggregation and inhibit the synthesis of proaggregatory and proinflammtory eicosanoids by platelets and neutrophils[11-15]. Some reports indicate that RES could prevent tumor growth and metastasis in human lung carcinoma, pancreatic cancer, prostate cancer, bronchial epithelium cancer and breast cancer models[16-20]. The present investigation evaluated the potency of RES and in combination with 5-FU on tumor cell growth and proliferation and on cell cycle distribution in a transplantable murine hepatoma22 model.
MATERIALS AND METHODS
Materials
Resveratrol was purchased from Sigma Co (USA), dissolved and sterilized in dimethyl sulfoxide (DMSO) first and then diluted to the required working concentrations in RPMI 1640 (Gibco, USA) containing 100 mL/L calf serum (Sijiqing Co, Hangzhou, China). Mouse hepatocellular carcinoma cell line H22 was purchased from the Department of Pathology, Fourth Military Medical University. Male BALB/c mice, 6-8 wk old, weighing 20 ± 2 g, were purchased from the Animal Center of Xi’an Jiaotong University.
Suppressive effect of RES on transplanted liver cancer
H22 cells were first subcultured in RPMI 1640 containing 100 mL/L fetal bovine serum, and then washed twice and resuspended in RPMI 1640 culture medium (1 × 10 11/L). About 0.2 mL cell solution (including 2 × 10 7 cells) was taken and injected into the right groin of 5 Balb/c mice. After 14 d, when the tumors of 3-5 mm in diameter formed in the right groin of these mice, they were taken out and cut into small pieces of 1 mm3 under sterile condition. Fifty Balb/c mice were anesthetized using coelio-injection of pentobarbitone (70 mg/kg) and laparotomy was performed. Under sterile condition their middle lobes of liver were punctured to form a 3 mm-long sinus tract and a small piece of tumor tissue was put into each sinus tract. Then these mice were randomly divided into 5 groups: control group, 5-FU group and 3 experimental groups. The experimental groups were injected with RES (dissolved in DMSO and diluted to the working concentration of 25 mmol/L in RPMI 1640 containing 100 mL/L calf serum) at 5, 10 or 15 mg/kg body mass, respectively, while the control group was given the same volume of the solution as for the experimental group without RES and the 5-FU group was injected with 5-FU at 20 mg/kg body mass. Twenty-four hours following liver tumor transplantation, each mouse was injected a corresponding dosage of RES into its abdominal cavity once a day for 10 d. These mice were then sacrificed on the following day after the last injection. After the maximum diameter and transverse length of tumor were measured, hepatocellular carcinoma tissues were sampled. The tumor volume was calculated by using the formula V = 1/2 (maximum diameter × transverse length 2). The suppressive rate of tumor growth was calculated as [(mean V of tumor in control group - mean V of tumor in experimental group)/mean V of tumor in control group] × 100%.
H22 cell cycle in transplanted liver cancer
Fresh hepatocellular carcinoma tissues with a size of 0.5-0.7 cm3 were washed twice in saline and then single cells were isolated from sampled tissues using 21 g/L citric acid 5 g/L Tween 20 according to the method of Otto[21]. After this, cells were first washed with PBS( pH 7.4) three times, then adjusted to the density of 1 × 10 9/L in RPMI 1640, and 0.2 mL cell suspension was taken and stained with 0.2 mL PI compound dye for 20 min at room temperature. Then, cell suspension was centrifuged at 800 r/min for 10 min and washed twice in PBS at pH 7.4. Finally, the cells were added to 1 mL PBS followed by slight shaking at room temperature and cell cycle analysis was performed using flow cytometer (Coulter, Epice Elite, ESP, USA). By using the multicycle software program it was possible to calculate the proportion of H22 cells in S and G2/M phases in tumor.
Synergistic anti-tumor effects of RES and 5-FU
A total of 128 tumor-bearing BALB/c mice were randomly divided into 8 groups: control group, RES group, three 5-FU groups and three experimental groups. The RES group was injected with RES at 10 mg/kg body mass (this dosage was proven to have obvious anti-tumor effect in our preliminary study.) and the 5-FU groups were injected with 5-FU at 5, 10, or 20 mg/kg body mass, respectively, and 3 experimental groups were injected with RES at 10 mg/kg body mass + 5-FU at 5, 10, or 20 mg/kg body mass, respectively. The control group was given the same volume of the solution as for the experimental group without RES and 5-FU. Twenty-four hours after liver tumor transplantation, each mouse was injected with a corresponding drug at respective dosage into its abdominal cavity once a day for 10 d. Half of the mice in each group were then sacrificed on the following day after the last injection and the maximum diameter and transverse length of tumor were measured. The tumor tissues of mice treated with various dosages of 5-FU alone or in combination with RES were observed and photographed with an Olympus BH-I microscope. The rest mice were kept on feeding and their survival time and changes of body mass were recorded and their tumor metastasis conditions in lung or abdominal cavity were observed.
Statistical analysis
Student’s t test was used to evaluate the significance of the difference between experimental groups and control group, between combination groups and corresponding sole drug groups.
RESULTS
Suppressive effect of RES on transplanted liver cancer and distribution of H22 cell cycles
Except 3 Balb/c mice (each in control group, 15 mg/kg RES group and 20.0 mg/kg 5-FU group), all the mice inoculated with hepatocarcinoma cell line H22 were successively transplanted with liver cancer. After treatment of the tumour bearing mice with 5, 10 or 15 mg/kg RES for 10 d, the tumour size was reduced from 134 ± 40 mm 3 in control group to 105 ± 14 mm 3, 85 ± 22 mm 3 and 68 ± 17 mm 3 in three experimental groups, the inhibition rate of tumour growth was 21.6 %, 36.3 % and 49.3 %, respectively. The inhibitory effect on the latter 2 therapeutic groups was significant higher than that on control group (P < 0.01). Though the inhibition rate of tumour growth of 5-FU was rather high (53.0%), its toxicity was serious and the concrete manifestations included poor ingestion, diarrhea, and decrease in body mass, while the mice in RES groups showed no evident toxicity and were alive at the end of treatment (Table 1).
Table 1.
Suppressive effect of resveratrol on murine transplanted liver cancer (mean ± SD)
| Group | Dose (mg/kg) | n | Tumor size (mm3) | Growth inhibitory rate (%) | t value |
| Control | 0.0 | 9 | 134 ± 40 | - | |
| 5-FU | 20.0 | 9 | 63 ± 29 | 53.0 | 4.36b |
| RES | 5.0 | 10 | 105 ± 14 | 21.6 | 2.16a |
| 10.0 | 10 | 85 ± 22 | 36.6 | 3.36b | |
| 15.0 | 9 | 68 ± 17 | 49.3 | 4.33b |
P < 0.05,
P < 0.01, vs control.
The cell cycles were analysed using flow cytometer to calculate the number of H22 cells in each phase in tumor under the action of various dosages of RES (5, 10, or 15 mg/kg body mass). The results showed that the number of H22 cells in S phase increased from 0.60 in control group to 0.75 in 15.0 mg/kg RES group, while the number of H22 cells in G2 phase decreased from 0.11 to 0.00 and the effect was dose-dependent (Table 2, Figure 1).
Table 2.
Effect of RES on the number of H22 cell cycles in transplanted liver cancer of mouse
| Group | Dose (mg/kg) | n |
Number of H22 cell cycles (%) |
||
| G0/G1 | S | G2/M | |||
| Control | 0 | 9 | 0.29 | 0.60 | 0.11 |
| RES | 5.0 | 10 | 0.30 | 0.60 | 0.09 |
| 10.0 | 10 | 0.30 | 0.68a | 0.02a | |
| 15.0 | 9 | 0.25 | 0.75a | 0.00a | |
P < 0.05, vs control.
Figure 1.
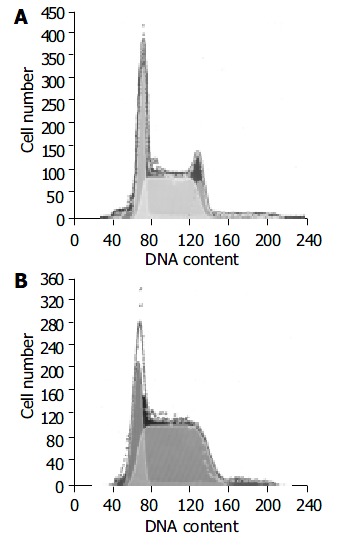
Number of H22 cell cycles in transplanted liver can-cer of mouse treated with RPMI-1640 (A) or 15.0 mg/kg (B).
Suppressive effect of RES in combination with 5-FU on transplanted liver cancer
RES in combination with 5-FU had synergistic suppressive effects on transplanted liver cancer of mouse (Figure 2). When 10 mg/kg RES in combination with 5, 10 or 20 mg/kg 5-FU, the inhibition rate was 50.0%, 72.4%, and 77.4%, respectively. When the group administered 5, 10 or 20 mg/kg 5-FU alone, the inhibition rate was 28.4%, 43.8%, and 53.4%, respectively. There was a statistical significance between the combination group and the 5-FU alone group (Table 3). Morphologic observation showed that more cellular necrosis was found in the combination group than in control group (Figure 3).
Figure 2.
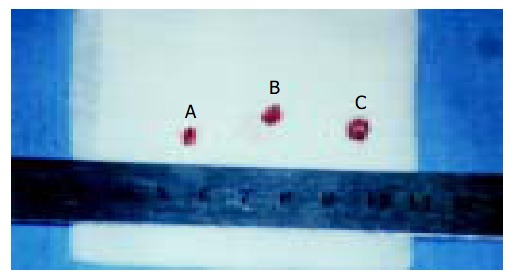
Tumor size of mice treated with 10.0 mg/kg RES + 20.0 mg/kg 5-FU (tumor A: 3.5 × 3.1 × 2.6 mm) and RPMI-1640 (tumor B: 4.8 × 4.7 × 4.2 mm; tumor C: 5.3 × 5.2 × 4.8 mm).
Table 3.
Suppressive effect of RES in combination with 5-FU on murine with transplanted liver cancer (mean ± SD)
| Group | Dose (mg/kg) | n |
Tumor size (mm3) |
t value |
Growth inhibitory rate (%) |
||
| Alone | With 5-FU | Alone | With 5-FU | ||||
| Control | 0 | 8 | 128 ± 33 | ||||
| RES | 10.0 | 8 | 88 ± 21 | 31.5 | |||
| 5-FU | 20.0 | 8 | 60 ± 12 | 29 ± 18 | 4.05b | 53.4 | 77.4 |
| 10.0 | 8 | 72 ± 17 | 35 ± 13 | 4.89b | 43.8 | 72.4 | |
| 5.0 | 8 | 92 ± 19 | 64 ± 22 | 2.72a | 28.4 | 50.0 | |
P < 0.05,
P < 0.01, vs control.
Figure 3.
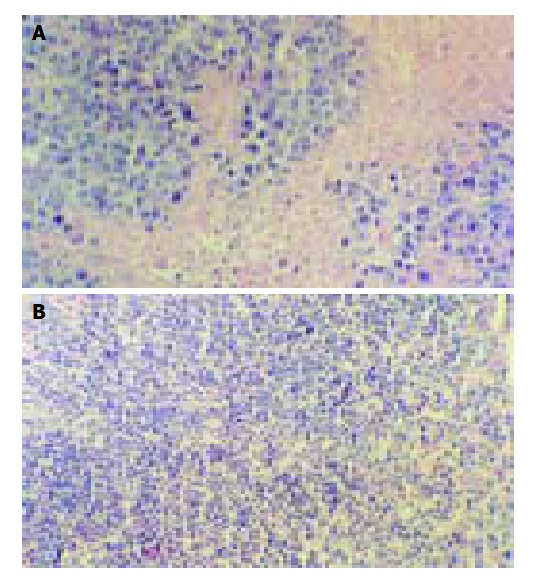
Morphologic observation of tumor tissues of mice treated with RPMI-1640 (A) or 10.0 mg/kg RES + 20.0 mg/kg 5-FU(B).
Effect of RES in combination with 5-FU on survival time and tumor metastasis
When the mice were administered 10 mg/kg RES in combination with 5, 10 or 20 mg/kg 5-FU, the survival time of tumor bearing mouse was 30.6 ± 8.0 d, 36.3 ± 9.4 d, and 44.6 ± 11.6 d, respectively. When group administered 5, 10 or 20 mg/kg 5-FU alone, the survival time was 19.4 ± 4.4 d, 23.9 ± 5.4 d, and 32.1 ± 9.7 d, respectively (Figure 4). There was a statistical significance between the combination group and the 5-FU alone group. In the beginning of the study, there was no statistical difference in the body mass of mouse among various groups. However, at the end of the investigation, the body mass of mouse in combination group was significantly heavier than that in sole drug group (P < 0.01), showing that RES might antagonize the toxicity of 5-FU markedly (Table 4). Two Balb/c mice in control group had lung and celiac lymph node metastases, one in 5 mg/kg 5-FU therapeutic group had celiac lymph node metastasis. Except these 3 groups, all the mice inoculated with hepatocarcinoma cell line H22 showed no signs of tumor metastasis.
Figure 4.
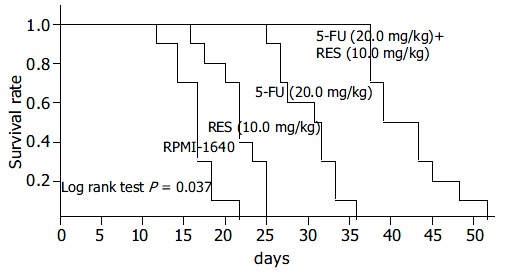
Kaplan-meier curves of survival rates of tumor bearing mice when administered RPMI-1640, 10 mg/kg RES, 20 mg/kg 5-FU, and 5-FU (20.0 mg/kg) + RES (10.0 mg/kg).
Table 4.
Effect of RES in combination with 5-FU on survival time and body mass of tumor bearing mouse (mean ± SD)
| Group | Dose (mg/kg) | n |
Last body mass(g ) |
t value |
Survival time (d ) |
t value | ||
| Alone | With 5-FU | Alone | With 5-FU | |||||
| Control | 0 | 8 | 23.6 ± 2.0 | 17.3 ± 3.3 | ||||
| RES | 10.0 | 8 | 23.8 ± 1.2 | 21.5 ± 5.6 | ||||
| 5-FU | 20.0 | 8 | 16.5 ± 1.8 | 20.3 ± 1.3 | 4.79b | 32.0 ± 9.7 | 44.6 ± 11.6 | 2.34a |
| 10.0 | 8 | 19.6 ± 1.8 | 23.2 ± 2.5 | 3.25b | 23.6 ± 5.4 | 36.3 ± 9.4 | 3.22b | |
| 5.0 | 8 | 21.3 ± 1.7 | 24.5 ± 1.5 | 3.80b | 19.5 ± 4.4 | 30.6 ± 8.0 | 3.48b | |
P < 0.05,
P < 0.01, vs control.
DISCUSSION
Great attention has been paid to the chemopreventive activities and low toxicities of dietary polyphenolic compounds like RES[22]. The function of RES might be mediated via different mechanisms in different cells, and the ability of RES to inhibit cellular events associated with tumor initiation, promotion, and progression might be attributed to its anticyclooxygenase activity, inducing apoptosis of tumor cells, antagonism to mutation, antioxidation and anti-free radical activity and effect on cell cycles[23-28]. Ahmad et al[29] proved that resveratrol treatment of human epidermoid carcinoma A431 cells caused an induction of WAF1/p21 inhibiting cyclin D1/D2-cdk6, cyclin D1/D2-cdk4, and cyclin E-cdk2 complexes, thereby imposing an artificial checkpoint at the G1-S transition of the cell cycle, which resulted in a G1 phase arrest of the cell cycle and subsequent apoptotic death of cancer cells. Our previous studies demonstrated that RES could suppress the growth of murine transplanted liver tumor H22 and the anti-tumor mechanism of RES might prevent mitosis of tumor cells by suppressing the protein expression of cyclin B1and p34cdc2, thus interfering with the process of tumor cells from S stage to G2/M stage[30,31].
One main role of 5-fluorouracil is to affect the biosynthesis of nucleic acids. Inside cells, 5-FU is converted to 5-fluorouracil deoxynucleotide (5F-dUMP) and inhibits the function of deoxythymidylic acid synthetase, blocks the methylation of uracil deoxyribonucleotide into deoxythymidylic acid, thus affecting the synthesis of DNA. As a result, 5-FU can prevent the tumor cells from splitting and proliferating and its cardinal acting period is S phase. Besides that, after the conversion of 5-FU into 5-fluorouracil uridine (5-FUR) in vivo, it also can be added into RNA to interfere with the synthesis of proteins, so it can affect the cells in other phases. Therefore, RES can enhance the anti-tumor effect of 5-FU by inducing the S phase arrest of H22 cells, a stage in which 5-FU can exert its max tumor cell killing function, and this synergism was proved in our in vitro experiments.
In the present investigation, RES was administered into murine abdomen, its potency on growth and proliferation of H22-innoculated tumors and its synergism with 5-FU were evaluated by measuring the size of hepatoma and examining the distributions of H22 cell cycles and observing the survival time of mice. The tumor size was reduced by each dosage of 5, 10 or 15 mg/kg of RES for 10 d. When the larger dosage of RES was applied, the tumor size was significantly reduced, the inhibition rate of tumor growth by 10 or 15 mg/kg reached to 36.3% and 49.3%, respectively (P < 0.01). It was also found that RES could induce the S phase arrest of H22 cells. RES could increase the persentage of cells in S phase from 59.1% to 73.5% in a dose-dependent manner (P < 0.05). The enhanced inhibition of tumor growth by 5-FU was also observed in hepatoma22 bearing mice when 5-FU was administered in combination with 10 mg/kg RES. The inhibition rate of 10 mg/kg or 20 mg/kg 5-FU in combination with 10 mg/kg RES was 72.4% and 77.4%, respectively. The inhibition rate was 43.8% and 53.4%, when the group administered 10 mg/kg or 20 mg/kg 5-FU alone. There was a statistical significance between the combination group and the 5-FU alone group (P < 0.01). In addition to that, when RES was administrated in combination with a smaller dosage of 5-FU, the therapeutic effect was similar to that of a larger dosage of 5-FU but without severe side effects of 5-FU, therefore the survival time of mice was elongated.
In short, the data suggest that RES can induce the S phase arrest of H22 cells and enhance the anti-tumor effect of 5-FU on murine hepatoma22 and antagonize its toxicity markedly. Resveratrol, a biochemical modulator to enhance the therapeutic effects of 5-FU, may be potentially useful in cancer chemotherapy.
Footnotes
Supported by Traditional Chinese Medicine Bureau Foundation of Shaanxi Province, No. 2001-035
Edited by Wang XL and Ren SY Proofread by Xu FM
References
- 1.Kuang SY, Jackson PE, Wang JB, Lu PX, Muñoz A, Qian GS, Kensler TW, Groopman JD. Specific mutations of hepatitis B virus in plasma predict liver cancer development. Proc Natl Acad Sci U S A. 2004;101:3575–3580. doi: 10.1073/pnas.0308232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen HB, Huang Y, Dai DL, Zhang X, Huang ZW, Zhang QK, Wang HH, Zhang JS, Pan G. Therapeutic effect of transcatheter arterial chemoembolization and percutaneous injection of acetic acids on primary liver cancer. Hepatobiliary Pancreat Dis Int. 2004;3:55–57. [PubMed] [Google Scholar]
- 4.Luo W, Birkett NJ, Ugnat AM, Mao Y. Cancer incidence patterns among Chinese immigrant populations in Alberta. J Immigr Health. 2004;6:41–48. doi: 10.1023/B:JOIH.0000014641.68476.2d. [DOI] [PubMed] [Google Scholar]
- 5.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 6.Zou CL, Chen ZJ, Jin WY, Ni SC, Chen BF, Hu YL. [Etiologic fraction and interaction of risk factors for primary hepatocellular carcinoma in Wenzhou, Zhejiang Province] Zhonghua Yufang Yixue Zazhi. 2003;37:355–357. [PubMed] [Google Scholar]
- 7.Tang B, Kruger WD, Chen G, Shen F, Lin WY, Mboup S, London WT, Evans AA. Hepatitis B viremia is associated with increased risk of hepatocellular carcinoma in chronic carriers. J Med Virol. 2004;72:35–40. doi: 10.1002/jmv.10559. [DOI] [PubMed] [Google Scholar]
- 8.Tang ZY. Small hepatocellular carcinoma: current status and prospects. Hepatobiliary Pancreat Dis Int. 2002;1:349–353. [PubMed] [Google Scholar]
- 9.Lu W, Li YH, He XF, Chen Y, Zhao JB. Changes of liver function after transcatheter arterial chemoembolization with use of dif-ferent dose of anticancer drugs in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2004;12:38–41. [Google Scholar]
- 10.Shen BZ, Liu Y, Li RF, Yang G, Yu YT, Dong BW, Liang P. Effects of intraaterial chemoembolization combined with per-cutaneous microwave coagulation on hepatocellular carcinoma: a clinical and experimental study. Shijie Huaren Xiaohua Zazhi. 2003;11:268–271. [Google Scholar]
- 11.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 12.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 13.Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, Bertelli AA, Bertelli A, Das DK. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000;35:263–268. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Roemer K, Mahyar-Roemer M. The basis for the chemopreventive action of resveratrol. Drugs Today (Barc) 2002;38:571–580. doi: 10.1358/dot.2002.38.8.820097. [DOI] [PubMed] [Google Scholar]
- 15.Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med. 2003;11:317–320. [PubMed] [Google Scholar]
- 16.Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr. 2001;131:1844–1849. doi: 10.1093/jn/131.6.1844. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int J Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 18.Ding XZ, Adrian TE. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas. 2002;25:e71–e76. doi: 10.1097/00006676-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Kuo PL, Chiang LC, Lin CC. Resveratrol- induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Life Sci. 2002;72:23–34. doi: 10.1016/s0024-3205(02)02177-x. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 21.Otto FJ. High-resolution analysis of nuclear DNA employing the fluorochrome DAPI. Methods Cell Biol. 1994;41:211–217. doi: 10.1016/s0091-679x(08)61719-7. [DOI] [PubMed] [Google Scholar]
- 22.Narayanan BA, Narayanan NK, Stoner GD, Bullock BP. Interactive gene expression pattern in prostate cancer cells exposed to phenolic antioxidants. Life Sci. 2002;70:1821–1839. doi: 10.1016/s0024-3205(02)01481-9. [DOI] [PubMed] [Google Scholar]
- 23.Young J, Barker M, Fraser L, Walsh MD, Spring K, Biden KG, Hopper JL, Leggett BA, Jass JR. Mutation searching in colorectal cancer studies: experience with a denaturing high-pressure liquid chromatography system for exon-by-exon scanning of tumour suppressor genes. Pathology. 2002;34:529–533. doi: 10.1080/0031302021000035965-1. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Shimomoto T, Miyajima K, Iizuka S, Watanabe T, Yoshida M, Kurokawa Y, Maekawa A. Promotion, but not progression, effects of tamoxifen on uterine carcinogenesis in mice initiated with N-ethyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 2002;23:1549–1555. doi: 10.1093/carcin/23.9.1549. [DOI] [PubMed] [Google Scholar]
- 25.De la Fuente M, Victor VM. Anti-oxidants as modulators of immune function. Immunol Cell Biol. 2000;78:49–54. doi: 10.1046/j.1440-1711.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 26.Falchetti R, Fuggetta MP, Lanzilli G, Tricarico M, Ravagnan G. Effects of resveratrol on human immune cell function. Life Sci. 2001;70:81–96. doi: 10.1016/s0024-3205(01)01367-4. [DOI] [PubMed] [Google Scholar]
- 27.Schneider Y, Duranton B, Gossé F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 28.Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- 30.Holmes-McNary M, Baldwin AS. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 31.Nielsen M, Ruch RJ, Vang O. Resveratrol reverses tumor-promoter-induced inhibition of gap-junctional intercellular communication. Biochem Biophys Res Commun. 2000;275:804–809. doi: 10.1006/bbrc.2000.3378. [DOI] [PubMed] [Google Scholar]


