Abstract
AIM: Cyclooxygenase-2 (COX-2) is one of the rate-limiting enzymes in metabolism of arachidonic acid, and COX-2 inhibitors demonstrate preventive effects on cancer, especially on colorectal cancer. The underlying mechanism remains unclear. The aim of this study was to illustrate the relationship between angiogenesis and COX-2 in carcinogenesis of colorectal cancer.
METHODS: One hundred and seventy patients with colorectal cancer were enrolled in our study from January 1993 to September 2001 in School of Oncology, Peking University. COX-2 and VEGF expression were detected with the immunohistochemistry (IHC) technique. IHC assays were carried out with the aid of tissue microarray (TMA) procedure. Specimens from 35 of these patients were examined with reverse transcriptase PCR (RT-PCR).
RESULTS: COX-2 and VEGF expressions were stronger in colorectal cancer than those in the corresponding normal tissues, at both protein and mRNA levels. One hundred patients were eligible for analysis after IHC assay of COX-2 and VEGF. The positive rate of VEGF was much higher in COX-2 positive group (47/85) than in COX-2 negative group (χ2 = 4.181, P = 0.041). The result was further verified by the result of RT-PCR (χ2 = 8.517, P = 0.003). Correlation coefficient was 0.409 after Spearman correlation analysis (P = 0.015).
CONCLUSION: COX-2 may be involved in the course of tumor angiogenesis of colorectal cancer and acts through VEGF.
INTRODUCTION
Cyclooxygenase (COX) is one of the rate-limiting enzymes in metabolism of arachidonic acid, which catalyzes arachidonic acid into a series of products such as prostaglandins and other eicosanoids. It has two isoforms, COX-1 and COX-2. COX-2 acts as superoxidants and transforms arachidonic acid into PGG2, and then into PGH2. COX-2 is inducibly expressed in many human tissues by cytokines, oncogenes, and tumor promoters[1-3]. Recent clinical epidemiological studies have demonstrated the preventive effect of COX inhibitors on cancer, especially on colorectal cancer[4-6]. Cellular and animal experimental studies have also indicated its relevance to tumor invasion, metastasis, cell apoptosis, cell cycle, and body immunity[7], and the role of COX-2 in the development of colorectal cancer.
Angiogenesis is among the most important characteristics of tumors and plays an important role in the course of cancer invasion and metastasis. Recent studies have confirmed the hypothesis of tumor growth, which is generally dependent on tumor angiogenesis. Any significant increase in tumor mass must be preceded by an increase in vascular supply to deliver nutrients and oxygen to the tumor[8]. Experimental studies from Seed and Tsujii[9,10] suggested that COX-2 might be involved in angiogenesis. Few reports on the role of COX-2 in tumor angiogenesis of colorectal cancer in clinical settings are available.
MATERIALS AND METHODS
Patients and tissues
A total of 170 patients after surgical treatment in School of Oncology, Peking University, from January 1993 to September 2001 were studied retrospectively. Specimens from these patients were prepared as tissue microarray and then underwent immunohistochemical assays. Specimens from thirty-five patients were prepared for reverse transcriptase PCR (RT-PCR), including 22 male patients and 13 female patients.
Tissue array preparation and immunohistochemical staining
Formalin-fixed and paraffin-embedded tissues were subjected to routine sectioning of 3-5 μm thickness and HE staining. Tissue array block was completed for subsequent sectioning according to the predetermined scheme described before[11]. Two-step immunohistochemical staining was used for COX-2 and VEGF detection. The titer of COX-2 (Cayman Chemical, USA) and VEGF (Zhongshan Biological Inc., Beijing, China) antibody were 1:200 and 1:50 respectively. Human colon adenocarcinoma with strong COX-2 staining served as a positive control, whereas PBS instead of antibody served as a negative control. Two pathologists independently reviewed slides with immunohistochemical staining. Microscopically, the slides with no staining in the negative control and the specific dark yellow staining of cytoplasm and neuclear membrane in the positive control were eligible for a further analysis. Semiquantitative scoring system was adopted according to the staining intensity: 0 for no staining, 1 for weak yellow, 2 for dark yellow, and 3 for brown staining with granular distributions. The mean score was used for statistical analysis and the threshold for positivity was 2[11].
Specimen preparation and RT-PCR
Tumor tissues and the corresponding normal mucosae at least 10 cm away were collected 30 min after removal of the specimens. The tissues were then stored under -70 °C for RNA extraction. Total RNA was prepared from the specimens with Trizol reagent (Gibco BRL Co.) according to the manufacturer’s instructions. cDNA synthesis was carried out with 1µg of total RNA. Sense and antisense primers of 0.5 µL were mixed with 11 µL dd H2O, 5 µL PCR buffer, 3 µL MgCl2 (25 mmol/L), 2 µL dNTP (10 mmol/L), 2 µL cDNA, and 0.8 µL Taq DNA polymerase for PCR. The primers for PCR were 5’ TTC AAA TGA GAT TGT GGG AAA AT 3’ (sense primer) and 5’ AGA TCA TCT CTG CCT GAG TAT CTT 3’ (antisense primer). PCR reactions were processed in a PTC 100 thermocycler under the following conditions: at 94 °C for 5 min, at 94 °C for 30 s, extension at 55 °C for 30 s, then at 72 °C for 30 s for 40 cycles, and then at 72 °C for 5 min. The RT-PCR products were then analyzed on 15 g/L agarose gels. Colon cancer cell line HT-29 and β -actin served as a positive and inner control, respectively. PCR reactions without cDNA were used as blank controls.
Statistical analysis
All statistical analyses were carried out with the SPSS software, 10.0, USA. The relationship between COX-2 expression and categorical variables was compared with χ2 test or Fisher two-sided exact test. Continuous variables were analyzed with t test and P < 0.05 was considered statistically significant. Correlation analysis was processed via the Spearman method.
RESULTS
Expression of COX-2 protein in colorectal cancer tissues
Of the 170 colorectal cancer patients, 139 were eligible for analysis after COX-2 immunohistochemical assays. Immunohistochemical assays demonstrated that COX-2 protein was located in cytoplasm and nuclear membrane. The staining was weak yellow, dark yellow, and brown at a low-power field and diffuse or granular staining at a high-power field under a microscope (Figure 1A). A weak staining of COX-2 was observed in normal tissue with a positive rate of 24.1% (7/29). COX-2 expression was much stronger in tumor cells with dark yellow or brown staining with occasional granular distributions than in normal tissues. The positive rate of COX-2 in colorectal cancer was 84.9% (118/139).
Figure 1.
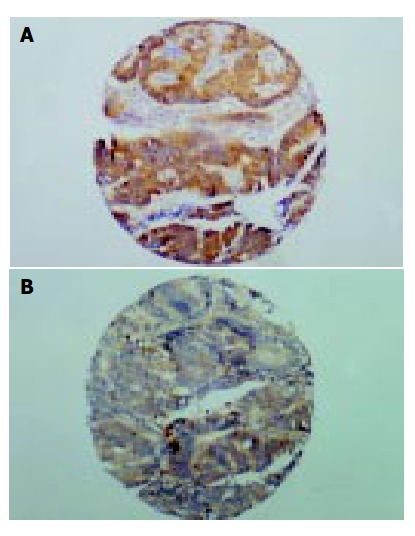
COX-2 and VEGF expressions in colorectal cancer tissues (IHC assay). A: Well-differentiated colonic adenocarcinoma, COX-2 immunostaining, × 100. B: Well-differentiated colonic adenocarcinoma, VEGF immunostaining, × 100.
Expression of VEGF protein in colorectal cancer tissues
For some reasons, only 100 colorectal cancer patients were eligible for further analysis after immunohistochemical assays. VEGF protein was located in cytoplasm of tumor cells and endothelial cells (Figure 1B). About 51.0% (51/100) of the tumors presented with strong VEGF staining, and the rate was much higher than that of normal tissues.
Expression of COX-2 and VEGF mRNA in colorectal cancer tissues
In consistent with the results of IHC assay, RT-PCR revealed stronger expressions of COX-2 and VEGF in tumors than in corresponding normal tissues (Figure 2). Among the 35 paired specimens of colorectal cancer tissues undergoing RT-PCR assays, 27 (77.1%) and 28 (80.0%) were positive with VEGF and COX-2, respectively, compared with 9 (25.7%) and 11 (31.4%) in normal tissues (P < 0.01).
Figure 2.
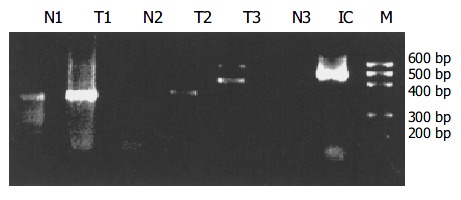
COX-2 and VEGF expressions in colorectal cancer tissues (RT-PCR assay). N: normal T: tumor IC: internal control M: marker. The Arabic number represents the number of samples. The marker has six lanes from 100 bp to 600 bp. The left four lanes stand for the 304 bp products of COX-2 while the right two lanes are the VEGF products of 541 and 408 bp respectively. The internal control of β -actin produced a 496 bp product.
Correlation between COX-2 and VEGF expression in colorectal cancer tissues
For the reason of TMA, a total of 100 cases were eligible for IHC analysis. Among these, 47 COX-2 positive cases presented with a positive VEGF staining (55.3%). The positive rate of VEGF was much higher than that in COX-2 negative group (26.7%). The difference between two groups had a statistical significance (χ2 = 4.181, P = 0.041). Among the 27 cases with COX-2 mRNA expression, 25 had a strong VEGF expression while only 2 in COX-2 negative group (χ2 = 8.517, P = 0.003) (Table 1). COX-2 and VEGF expression demonstrated a tendency to having a positive correlation with correlation coefficient of 0.409 (P = 0.015).
Table 1.
Expression of COX-2 and VEGF in colorectal cancer tissue (RT-PCR/IHC)1
| VEGF expression | COX-2 positive | COX-2 negative | Total |
| Positive | 25/47 | 2/4 | 27/51 |
| Negative | 3/38 | 5/11 | 8/49 |
| Total | 28/85 | 7/15 | 35/100 |
1Fisher double-side exact test (P = 0.003/0.041).
DISCUSSION
It has been documented that COX-2 plays an important role in the development of human tumors[12-16]. COX-2 could promote the growth and invasion of tumors[17,18] and increase invasiveness and potential of tumor metastasis[19,20]. Recent research results indicated that COX-2 could also induce tumor angiogenesis and was correlated with hematogenous metastasis of tumors[10,21]. Tumor angiogenesis is one of the important characteristics of tumors involving their growth and metastasis. The formation of primary or metastatic lesions and even their further development are dependent on tumor angiogenesis. Newly-formed vessels were in need of nutrition and oxygen when the tumor grew to 2-3 mm in size[8].
It had been found many factors are involved in tumor angiogenesis including bFGF, aFGF, PGE1, PGE2, VEGF, TGFα , TGFβ , and TNF[22]. The most important is vessel endothelial growth factor (VEGF), a highly specific mitogen of vessel endothelial cells. The biological functions of VEGF included selective promotion of mitosis of endothelial cells, stimulation of their proliferation and angiogenesis, an increase in vessel transparency and extravasculization of plasma large molecules[23-26]. It was reported that COX-2 might interact with VEGF[27,28]. Therefore, the relationship of COX-2 and angiogenesis (VEGF as the marker of angiogenesis) was then investigated at protein and mRNA levels.
The positive rate of COX-2 mRNA and COX-2 protein were 80% (28/35) and 84.9% in colorectal cancer. Both were much higher than those in normal tissues (P < 0.01), which indicated the role of COX-2 in carcinogenesis of colorectal cancer. The expressions of COX-2 and VEGF were studied through RT-PCR and IHC assays. The result of RT-PCR showed that 25 were VEGF positive among the 28 COX-2 positive patients, and only 2 were VEGF positive in the COX-2 negative group. Correspondingly, of the 85 patients with COX-2 positive staining, 47 (55.3%) expressed VEGF while only 26.7% in the COX-2 negative group, indicating the correlationship between the expressions of COX-2 and VEGF, which was further verified by the Spearman correlation analysis. Cianchi[28] recently found a close correlation between COX-2 and VEGF as well as microvessel density (MVD) in their studies on 31 colorectal cancer patients by Western blot and Northern blot analysis, and immunohistochemical assays, which was consistent with our results.
The mechanism underlying the role of COX-2 in tumor angiogenesis has been unclear[29]. Preliminary results showed that angiogenesis occurred when endothelial cells were cultured with cancer cells over-expressing COX-2[10]. It could be inhibited by selective COX-2 inhibitors in endothelial cells in vitro[29]. It was proved that the activity of COX-2 in interstitial cells was necessary for secretion of VEGF, proliferation of endothelial cells and new vessel formation[30]. These experimental results suggested that such metabolic products might induce the expression of VEGF via pathways of paracrine, autocrine or intracrine, and stimulate proliferation of vessel endothelial cells and formation of tumor vessels[29].
The regulatory effect of COX-2 on VEGF production and tumor angiogenenesis suggested that COX-2 expression might be an upstream event in tumor angiogenesis[10,29]. We also found that COX-2 expression was positively correlated with expression of VEGF, one of the most important factors for tumor angiogenesis. The expression rate of VEGF was high in patients with positive COX-2 expression (25/28) while low in COX-2 negative patients (2/7). In addition, staining intensity of COX-2 was correlated with that of VEGF. Management of the murine animal model of lung and prostate cancers with the gene knockout technique or COX-2 inhibitor resulted in not only tumor shrinkage but also inhibition of tumor angiogenesis. Endothelial cells were scattered without formation of an ordered tubular structure. The MVD of tumor and expression of VEGF were significantly decreased, further suggesting the role of COX-2 on VEGF expression and tumor angiogenesis[31,32].
Great progresses have been made in the research of tumor angiogenesis. Trials on targeted therapy against angiogenesis have been ongoing to block or postpone tumor angiogenesis so as to cure cancer[33,34]. More than twenty drugs are in their phase I or II and even III clinical trials in the United States of America. Though preliminary results were promising, there is still a long way to go. Therefore, as an upper-stream event, COX-2 might be a potential target of the cancer gene therapy worthy of further research.
Footnotes
Edited by Wang XL and Chen ZR Proofread by Xu FM
References
- 1.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 2.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 3.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ, Hennekenn CH. Aspirin and other non-steroidal an-tiinflammatory drugs and the risk of cancer development. In: DeVitt VT, Hellman S, Rosenberg SA. Cancer: principles and practice of oncology. 6th edition. Philadelphia: Lippincott Will-iams Wilkins Press U S A. 2001:601–607. [Google Scholar]
- 5.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 6.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–4569. [PubMed] [Google Scholar]
- 7.Dannenberg AJ, Zakim D. Chemoprevention of colorectal cancer through inhibition of cyclooxygenase-2. Semin Oncol. 1999;26:499–504. [PubMed] [Google Scholar]
- 8.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Seed MP, Brown JR, Freemantle CN, Papworth JL, Colville-Nash PR, Willis D, Somerville KW, Asculai S, Willoughby DA. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625–1629. [PubMed] [Google Scholar]
- 10.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 11.Wu AW, Gu J, Ji JF, Li ZF, Xu GW. Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients' prognosis. World J Gastroenterol. 2003;9:1990–1994. doi: 10.3748/wjg.v9.i9.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 13.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 14.Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923–1931. [PubMed] [Google Scholar]
- 15.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimäki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 16.Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H, et al. Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res. 2000;6:1229–1238. [PubMed] [Google Scholar]
- 17.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000;60:4629–4637. [PubMed] [Google Scholar]
- 19.Tomozawa S, Nagawa H, Tsuno N, Hatano K, Osada T, Kitayama J, Sunami E, Nita ME, Ishihara S, Yano H, et al. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br J Cancer. 1999;81:1274–1279. doi: 10.1038/sj.bjc.6694262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomozawa S, Tsuno NH, Sunami E, Hatano K, Kitayama J, Osada T, Saito S, Tsuruo T, Shibata Y, Nagawa H. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83:324–328. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H, Nagasue N. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–4068. [PubMed] [Google Scholar]
- 22.Fidler IJ. Molecular biology of cancer: invasion and metastasis In: DeVita SH, Rosenberg SA, eds. Cancer: Principles and Prac-tice of Oncology, 5th edition. Philadelphia: Lippincott Raven Publishers. 1997:135–152. [Google Scholar]
- 23.Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 25.Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157–166. doi: 10.1159/000048262. [DOI] [PubMed] [Google Scholar]
- 26.Li CY, Shan S, Huang Q, Dewhirst MW. RESPONSE: re: initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:1445–1446. doi: 10.1093/jnci/92.17.1445-a. [DOI] [PubMed] [Google Scholar]
- 27.Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317–1322. [PubMed] [Google Scholar]
- 28.Cianchi F, Cortesini C, Bechi P, Fantappiè O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–1347. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 29.Prescott SM. Is cyclooxygenase-2 the alpha and the omega in cancer? J Clin Invest. 2000;105:1511–1513. doi: 10.1172/JCI10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 31.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164:820–825. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 33.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, Chang H, Robinson C, Hunter K, Dobrzanski P, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- 34.Ellis LM. A targeted approach for antiangiogenic therapy of metastatic human colon cancer. Am Surg. 2003;69:3–10. [PubMed] [Google Scholar]


