Abstract
The glucagon-like peptide 1 (GLP-1) analog, liraglutide, is a GLP-1 agonist and is used in the treatment of type-2 diabetes mellitus and obesity. From a pharmaceutical perspective, it is important to know the oligomerization state of liraglutide with respect to stability. Compared to GLP-1, liraglutide has an added fatty acid (FA) moiety that causes oligomerization of liraglutide as suggested by small-angle x-ray scattering (SAXS) and multiangle static light scattering (MALS) results. SAXS data suggested a global shape of a hollow elliptical cylinder of size hexa-, hepta-, or octamer, whereas MALS data indicate a hexamer. To elaborate further on the stability of these oligomers and the role of the FA chains, a series of molecular-dynamics simulations were carried out on 11 different hexa-, hepta-, and octameric systems. Our results indicate that interactions of the fatty acid chains contribute noticeably to the stabilization. The simulation results indicate that the heptamer with paired FA chains is the most stable oligomer when compared to the 10 other investigated structures. Theoretical SAXS curves extracted from the simulations qualitatively agree with the experimentally determined SAXS curves supporting the view that liraglutide forms heptamers in solution. In agreement with the SAXS data, the heptamer forms a water-filled oligomer of elliptical cylindrical shape.
Introduction
The glucagon-like peptide 1 (GLP-1) receptor is a well-established therapeutic target for the treatment of type-2 diabetes mellitus (1–3), and extensive research has established the physiologic roles of GLP-1 and its endogenous receptor in regulating glucose homeostasis and energy metabolism (4,5). GLP-1-(7-37) is a 31-amino-acid incretin hormone secreted by the endocrine L cells in the gut wall upon glucose intake (6), and the peptide is secreted in response to the nutrient content of the gastrointestinal tract and thus potentiates insulin exocytosis from pancreatic β-cells in a glucose-dependent manner (6,7). Additionally, GLP-1 suppresses appetite, glucagon secretion, and gastric emptying, all of which contribute to inhibition of the postprandial rise in plasma glucose concentrations (8). GLP-1 is responsible for up to 60% of the postprandial insulin response (9). Recent studies show that GLP-1 is not only a key factor in type-2 diabetes mellitus treatment, but also has potential in the treatment of obesity (1,10) and has shown positive effects on neuroprotection in animal models (11), which can potentially be used for the treatment of Parkinson’s and Alzheimer’s disease (12–15).
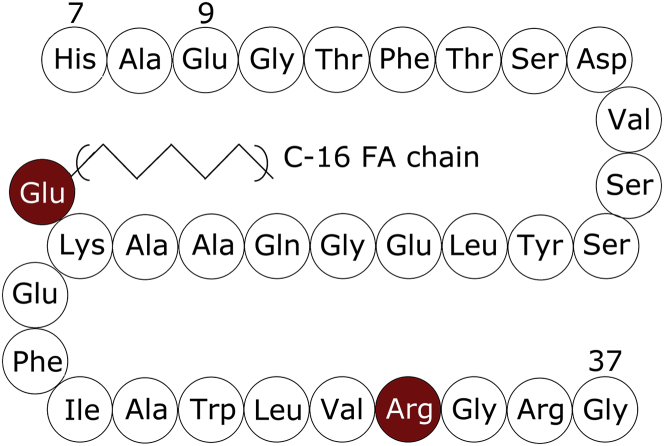
While GLP-1 is interesting for a pharmaceutical application, it cannot be used for routine treatment, because its biological half-life is only a few minutes (16,17). The short insulinotropic action of GLP-1 results from the degradation of the peptide by dipeptidyl-peptidase IV (DPP IV) and rapid renal clearance due to its relatively small size (18–20). DPP IV degrades GLP-1 at the N-terminus by cleaving off the first two amino acids, generating the biologically inactive fragment, GLP-1-(9-37) (9,21). To overcome this shortcoming, different strategies have been used. Those include: 1) incorporation of the peptides in injectable microspheres; 2) fusion with larger carrier molecules like albumin or fragment crystallizable region of immunoglobulin G or polyethylene glycol; and 3) attachment of a fatty acid (FA) directing oligomerization and promoting reversible binding to endogenous human serum albumin (20). All three approaches result in an increased half-life partly due to the increased size of the drug minimizing the renal clearance mechanism. The latter approach, for instance, has been utilized in designing the GLP-1 analog: liraglutide (Victoza; Novo Nordisk A/S, Bagsværd, Denmark) (22,23). An in-depth understanding of the oligomerization state of liraglutide is not only instrumental in the understanding of the increased half-life observed for this molecule but also to ensure a stable oligomeric state, because uncontrolled and extensive oligomerization can drive fibrillation (24). The amino acid sequence of liraglutide is shown in Fig. 1. Compared to GLP-1, two structural modifications have been introduced. A C-16 acyl chain (palmitoyl), is linked to Lys26 via a γ-glutamic acid spacer, and the lysine in position 34 of the native GLP-1 sequence is exchanged with arginine to ensure homogenous palmitoylation at position 26 (25,26). The general understanding is that the acyl chain allows a noncovalent binding to albumin, which delays both proteolytic inactivation by DPP IV and renal clearance, resulting in a biological half-life of ∼13–14 h and allowing once-daily administration (2,23). A further prolongation may also be caused by the fatty acid chain that may sterically hinder DPP IV from degrading liraglutide (5). Furthermore, studies have shown that one way to stabilize GLP-1 is to add a clustering agent that causes the peptide to oligomerize (2), thus, the FA chain in liraglutide could act as a clustering agent.
Figure 1.
Amino-acid sequence of liraglutide. To see this figure in color, go online.
Although the pharmacological efficiency of liraglutide has been established (23,27), there is a lack of a molecular understanding of the solution structure of liraglutide. Using analytical ultracentrifugation, Steensgaard et al. (28) could show that liraglutide oligomerizes in a concentration-independent manner forming, predominately, heptamers in the concentration range of 0.004–4.501 mg/mL. Recently, Wang et al. (29) studied the pH dependence of the size and secondary structure of liraglutide oligomers using light-scattering and circular dichroism, respectively. The authors report a transformation from an octamer to a dodecamer at pH 6.4 and 6.9 with subsequent partial loss of the α-helical structure of liraglutide. Furthermore, it has been shown that the oligomerization of GLP-1 and similar peptide analogs is dependent on the pH and ionic strength (30), and thus different solution structures may exist (3,31).
To get further insight in the solution structure of liraglutide, we have performed a series of molecular-dynamics (MD) simulations, and the simulation results are compared with results from small-angle x-ray scattering (SAXS) and multiangle static light-scattering (MALS) experiments.
Materials and Methods
SAXS
A quantity of 3 mL commercial Victoza (contains 18 mg liraglutide (free-base, anhydrous); Novo Nordisk A/S)) and the inactive ingredients disodium phosphate dihydrate, 1.42 mg; propylene glycol, 14 mg; and phenol, 5.5 mg in aqueous solution (32) were dialyzed against 3 × 1 L buffer containing ∼0.47 mg/mL (0.376 mM) Na2HPO4•2H2O, pH 8.1, over 3 days. Concentration determinations were performed with the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) at 280 nm. The extinction coefficient was calculated to be 6990 cm−1 M−1 with the PROTPARAM (33) tool from ExPASy.org (34) using the primary sequence of the protein.
SAXS measurements were performed at the MAX IV laboratories at beamline I911-SAXS, MAX IV Laboratory, Lund, Sweden (35). The sample detector distance and the direct beam position were calibrated using AgBe (silver behenate). Parameters are shown in Table S2 in the Supporting Material. Measurements on pure water were used to get the data on an absolute scale. Buffers were measured both before and after each sample and averaged before subtraction. The sample size was ∼50 μL injected manually in a flow cell. Measurements were performed on a series of liraglutide samples at the approximate concentrations of 1, 2, and 4.7 mg/mL.
All calibrations and corrections of the SAXS data were done using the in-house software BLI911-4 (35). Buffer averaging and subsequent subtraction before data analysis was done in the program PRIMUS (36). The software package ATSAS, Ver. 2.4 (37), was used for further data analysis. Evaluation of the Guinier region was performed within PRIMUS. The pair distribution function, p(r), was evaluated using the interactive program GNOM (38).
Asymmetric flow field flow fractionation-UV-MALS
Asymmetric flow field flow fractionation (AF4) separation was performed using a Dionex UltiMate 3000 autosampler and pump (Thermo Fisher Scientific, Waltham, MA) connected to an Eclipse AF4 separation system (Wyatt Technology Europe, Dernbach, Germany) followed by a Dionex UltiMate 3000 RS variable wavelength UV detector (Thermo Fisher Scientific) set at 280 nm, and a Dawn Heleos-II 18-angle MALS detector (Wyatt Technology Europe). Separations were performed using a 10-kDa molecular-mass cutoff PES (polyethersulfone) membrane in a 17.5 cm separation channel with an S-350 μm spacer. Samples were introduced to the channel at 0.2 mL/min and subsequently focused at the head of the channel at a focus flow rate of 1.5 mL/min. Samples were eluted over 15 min with a channel flow rate of 1 mL/min and a cross flow gradient of 4.0–2.5 mL/min. Undiluted Victoza (6 mg/mL liraglutide) and 10× diluted Victoza (diluted with eluent) were injected and eluted with 20 mM phosphate, 100 mM NaCl, and 0.05% NaN3, pH 8.1, filtrated through a 0.1 μm filter. Different injection volumes of undiluted and diluted Victoza were used, and the resulting mass loads were 6, 12, and 18 μg liraglutide. The molecular mass of liraglutide was calculated using the software ASTRA, Ver. 6.1.2 (Wyatt Technology Europe) with dn/dc = 0.185 mL/g and UV extinction coefficient (280 nm) = 6990 cm−1 M−1.
MD simulations
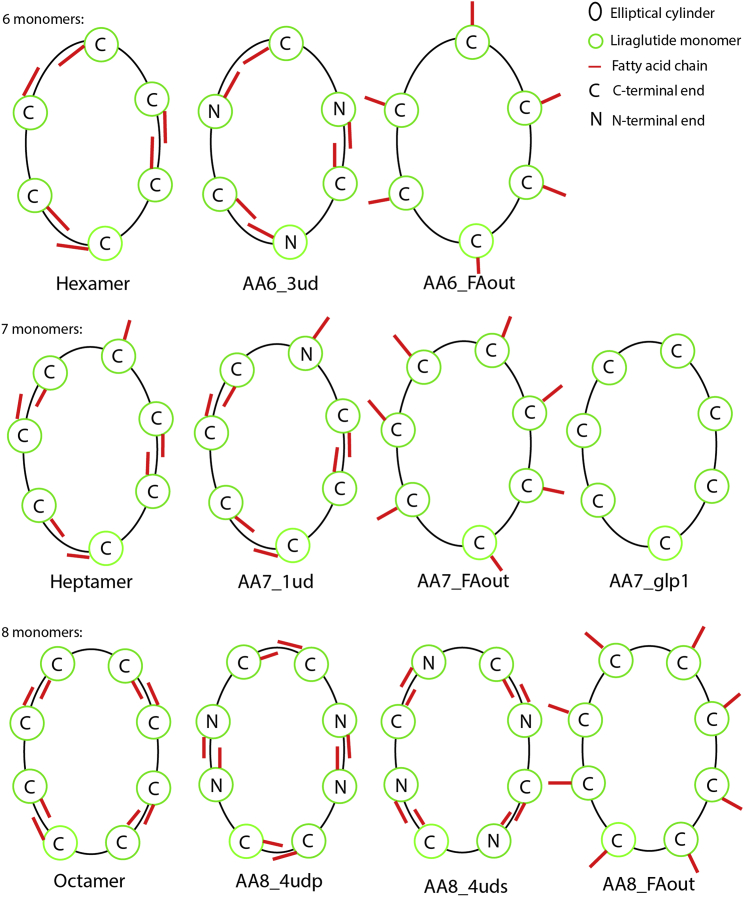
Several orientations and oligomers of liraglutide have been investigated. All are based on the solution NMR structure of liraglutide (PDB: 4APD) obtained from the Protein Data Bank (39). The coordinates from the PDB file were copied, translated, and rotated in a circle with a radius of 20 Å, corresponding to the results from SAXS experiments. This resulted in several oligomers containing six, seven, or eight monomers, respectively. A set of oligomers was created where the monomers were oriented so that two FA chains were paired in the direction of the elliptical cylinder arrangement, which hereafter will be referred to as hexa-, hepta-, and octamer systems (Fig. 2). In the heptamer, one monomer was oriented with the FA chain pointing outward of the elliptical arrangement.
Figure 2.
Representation of the start structures for the 11 oligomeric structures. All structures are shown from a top view. (Red line) Orientation of the FA chain on each monomer; (ellipses) size and shape of the oligomers. To see this figure in color, go online.
Furthermore, another set of oligomers were created where some of the monomers were flipped upside down to see if interactions between the C- and N-terminal charges would stabilize the structures. For this set of oligomers, the hexa- and one octamer were made with every second monomer flipped upside down. In the case of the heptamer, only the monomer with the FA chain pointing outward was flipped upside down. Also, another octameric structure was prepared where every second monomer pair was flipped upside down. These configurations are hereafter referred to as the AA6_3ud, AA7_1ud, AA8_4udp, and AA8_4uds systems where ud, p, and s are short for upside-down, pair, and single, respectively (Fig. 2). To clarify the extent of the stabilizing effect of the FA chain on the structures, another set of oligomers were created. One oligomer is constructed according to the rotation and translation of the first heptamer, but it does not contain any FA chains (hereafter referred to as “AA7_glp1” and represented in Fig. 2). Three other oligomers were also created, namely, a hexa-, hepta-, and octamer where all the monomers are rotated so that the FA chains are pointing outward of the elliptical arrangement. These will hereafter be referred to as the AA6_FAout, AA7_FAout, and AA8_FAout systems (Fig. 2). This gives a total of 11 oligomeric structures.
The structures were solvated using the program SOLVATE from Grubmüller and Groll (40). Water molecules were described by the TIP3 water model (41). Next, the systems were neutralized by adding 3 Na+ ions per monomer. Simulations were performed at an ionic strength of 0.1 M NaCl (see details in Table 1). All simulations were performed using the computer program NAMD (42) with the CHARMM36 force field (43). The same simulation parameters were used as described by Madsen et al. (44). (See the Supporting Material for a detailed description.) Analyses of the trajectories were carried out in the software VMD (45).
Table 1.
System and simulation details of the 11 oligomeric systems
| Structure | No. of Atoms | No. of Waters | No. of NaCl | Initial Box Size (Å) | Simulation Time (ns) |
|---|---|---|---|---|---|
| Hexamer | 140,203 | 45,615 | 86 | 113 × 118 × 115 | 69 |
| Heptamer | 53,774 | 16,665 | 31 | 83 × 88 × 89 | 129 |
| Octamer | 136,414 | 44,000 | 83 | 113 × 115 × 115 | 71 |
| AA6_3ud | 57,419 | 18,055 | 34 | 91 × 88 × 85 | 34 |
| AA7_1ud | 57,090 | 17,769 | 33 | 91 × 87 × 87 | 39 |
| AA8_4udp | 54,814 | 16,834 | 32 | 91 × 85 × 85 | 41 |
| AA8_4uds | 54,928 | 16,872 | 32 | 91 × 85 × 85 | 43 |
| AA6_FAout | 52,289 | 16,247 | 31 | 83 × 90 × 85 | 21 |
| AA7_FAout | 53,114 | 16,445 | 31 | 83 × 88 × 89 | 51 |
| AA8_FAout | 54,937 | 16,875 | 32 | 83 × 90 × 90 | 21 |
| AA7_glp1 | 51,729 | 16,131 | 30 | 83 × 87 × 87 | 69 |
Theoretical SAXS curves
The program CRYSOL (46), which is part of the program package ATSAS, Ver. 2.6 (37), was used to compare the SAXS curves of the structures extracted from the MD simulations with the experimental measured SAXS curve of the oligomer. CRYSOL calculates the scattering intensity based on the atomic coordinates of the protein and adds a hydration layer simplified as a continuous outer envelope (37).
Results
In this section, experimental results from SAXS and AF4-UV-MALS are presented, followed by the computational results.
SAXS
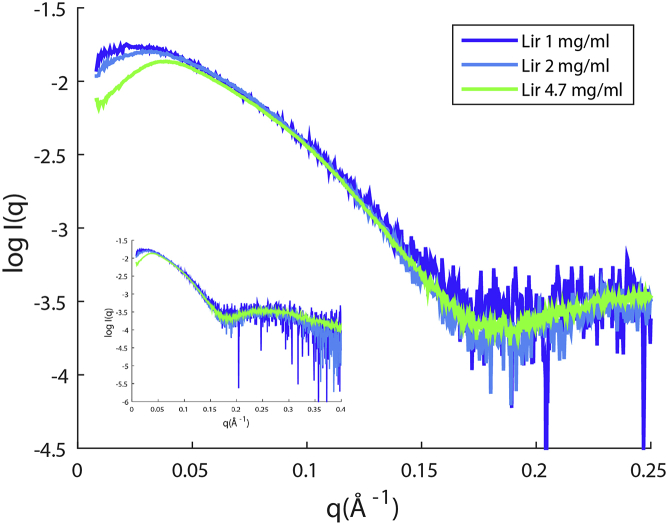
SAXS intensity curves of liraglutide measured at different concentrations are shown in Fig. 3. Repulsion is observed already at 1 mg/mL, while the shape of the curve is consistent over the concentration range, reflecting a similar shape of molecule. This is also reflected in the Kratky plots shown in Fig. S1. Corresponding pair distribution functions are provided in Fig. S2.
Figure 3.
Scattering curves normalized for concentration. Plots at concentrations 1, 2, and 4.7 mg/mL. (Inset) Scattering over entire measured scattering range. (Lir is liraglutide.) To see this figure in color, go online.
Table 2 summarizes the parameters extracted from the SAXS measurements. The radius of gyration (Rg), maximum particle diameter (Dmax) and I(0)/c show a slight decrease with concentration, as expected from the repulsive behavior.
Table 2.
Parameter overview extracted from SAXS measurements
| Concentration (mg/mL) | Rg Guinier (Å) | Rgp(r) (Å) | Dmax (Å) | I(0) Guinier | I(0) p(r) | I(0)/c | Molecular Mass (kDa) |
|---|---|---|---|---|---|---|---|
| 1 | 23.1 | 23.8 | 82.0 | 0.021 | 0.021 | 0.021 | 26 |
| 2 | 21.7 | 22.3 | 75.9 | 0.040 | 0.041 | 0.020 | 25 |
| 4.7 | 22.2 | 22.2 | 74.2 | 0.096 | 0.096 | 0.020 | 25 |
The partial specific volume, ν, used for calculating the molecular mass is chosen to match pure protein and is set to the average value of 0.73 cm3/g. To compare the experimental results with the model structures extracted from the simulations, the experimental data were extrapolated to q = 0 to avoid interparticle repulsion.
AF4-UV-MALS
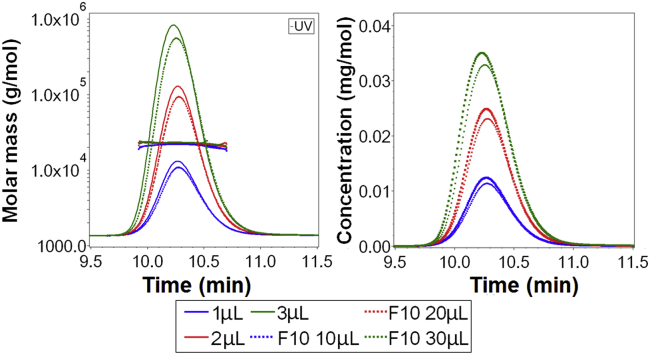
Analysis of liraglutide showed a single peak and a uniform molecular weight across the peak in all analyses (Fig. 4, left) indicating that repulsive behavior is negligible at the concentration range found in the detector. The average molecular mass across the peak was 22 kDa, which corresponds to a hexamer assuming a monomer molecular mass of 3.7 kDa. Undiluted Victoza (6 mg/mL liraglutide) and 10× diluted Victoza were analyzed and different injection volumes were used, which resulted in mass loads of 6, 12, and 18 μg liraglutide. Liraglutide is diluted during analysis by the eluate, and the resulting liraglutide concentration was quantified in the eluate passing the UV detector (Fig. 4, right). Peak liraglutide concentrations of 0.012, 0.024, and 0.036 mg/mL were observed.
Figure 4.
AF4-UV-MALS analysis of undiluted Victoza (6 mg/mL liraglutide) and 10× diluted Victoza (F10). Different injection volumes were tested. All analyses showed a single peak in the UV chromatogram and a uniform molar mass of ∼22 kDa across the peak (left). No other peaks were observed in the chromatogram. The liraglutide concentration in the eluate passing the UV detector is shown in the right graph. To see this figure in color, go online.
MD simulations
Simulations were performed on several sets consisting of hexa-, hepta-, and octamer oligomers, to study the structural arrangement and stability of the oligomers including the role of the FA in promoting the stability of the oligomers. The last structures taken from the simulations are shown in Figs. S3–S5.
Figs. S3–S5 show that although the internal structures for all 11 oligomers are highly distorted compared to the start structures, all of them but AA6_3ud (Fig. S4 a) maintain a tunnel-like structure which is, however, more or less flattened and resembling an elliptical shape.
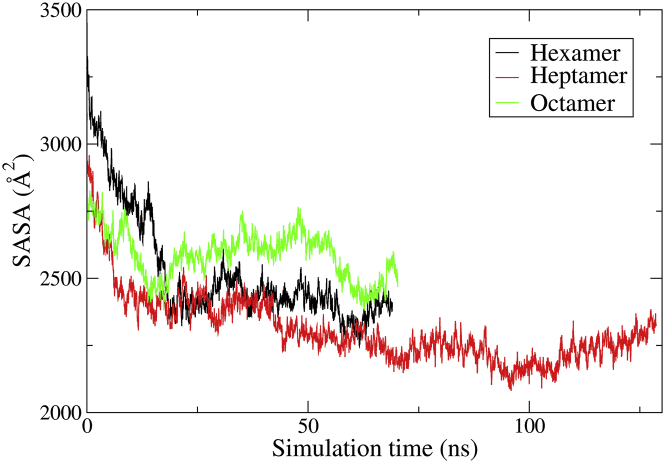
The solvent-accessible surface area (SASA) of the oligomers seen in Figs. 5, S6, and S7 relates to the packing of the monomers.
Figure 5.
SASA as a function of time for the hexamer, heptamer, and octamer. The area has been normalized according to the number of monomers. SASA was calculated every 50 ps along the trajectory using a van der Waals radius of 1.4 Å. To see this figure in color, go online.
Overall, the packing of the heptamers appears to be more prominent than the octamers throughout the simulations, indicated by the lower SASA. The AA6_3ud structure has a significant lower packing than the AA7_1ud, AA8_4udp, and AA8_4uds structures, which most likely is a result of a complete opening of the elliptical structure as seen in Fig S4 a. The relatively high SASA for the AA6_FAout oligomer could be due to the elongation of some of the monomers, which appear to unfold from the helix structure (Fig. S5 a).
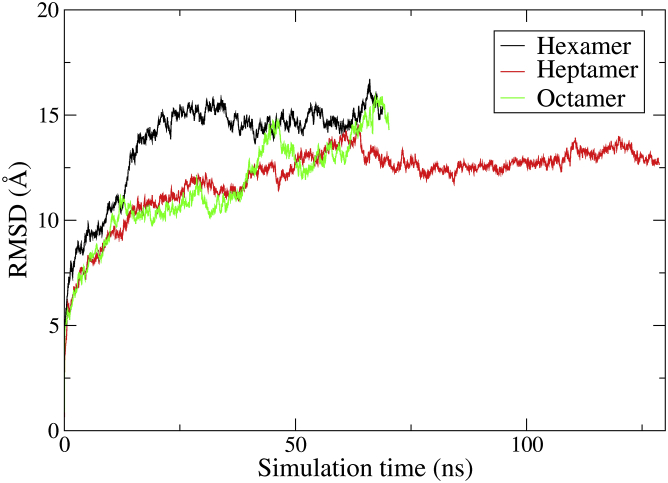
The time evolution of the root-mean-square deviation (RMSD) is shown for the 11 structures in Figs. 6, S8, and S9.
Figure 6.
RMSD of the entire oligomeric structure for the hexa-, hepta-, and octamer. Structures were aligned to the first frame (t = 0), and deviations are determined for the backbone chains. To see this figure in color, go online.
From the initial steep increase in RMSD, it is evident that the oligomers rearrange to some extent within the first 6 ns. RMSD converges for the hexamer, AA6_3ud and heptamer, AA7_1ud, AA8_4udp, AA8_4uds, AA8_FAout, and AA7_glp1 conformations. These structures appear to be stable when it comes to the overall movement of the systems. The octamer, AA6_FAout and AA7_FAout, however, do not converge. Furthermore, the three hexameric systems and AA7_FAout present a significantly higher RMSD value compared to the other systems.
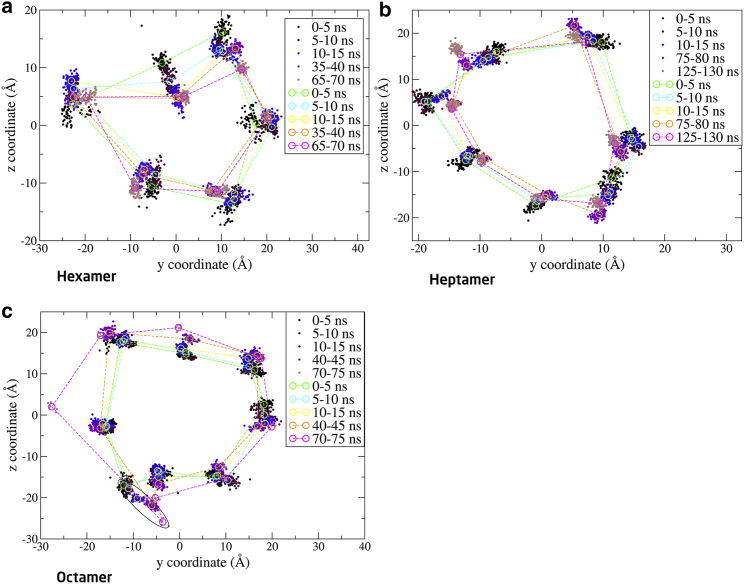
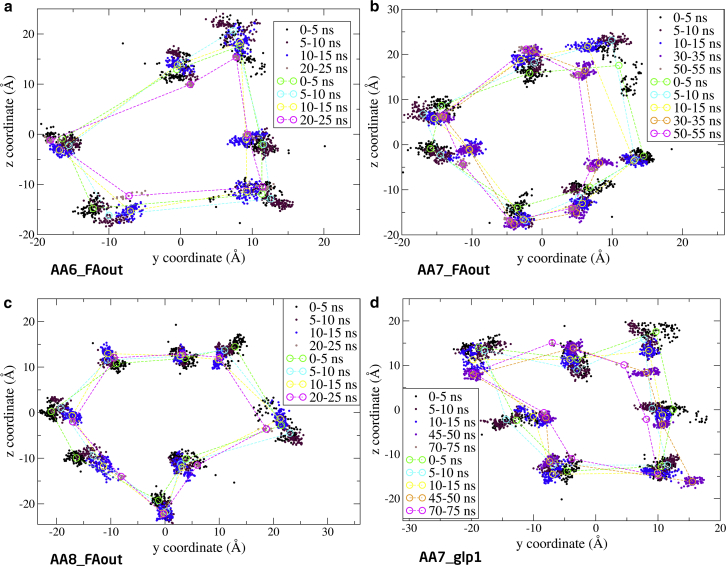
To further monitor the movement of the monomers, the two-dimensional positions of the α-carbon in the FA-Lys linker (Fig. S11) for all 11 oligomer conformations, projected onto the yz plane (the monomers are translated and rotated around the x axis), are shown as a function of simulation time in Figs. 7, 8, and 9.
Figure 7.
Position of α-carbon in the FA-Lys linker of the hexamer (a), heptamer (b), and octamer (c), as a function of simulation time. One monomer is highlighted (circle) in the octamer (c), to indicate the possible disintegration of this structure. Coordinates taken from the first 15 ns, 5 ns in the middle of the simulation, and then the last ∼5 ns, are shown. (Circles and dashed lines) Average structure within the 5-ns intervals (dots). The yz α-carbon atom coordinates for the FA-Lys linker were plotted in intervals of 50 ps. To see this figure in color, go online.
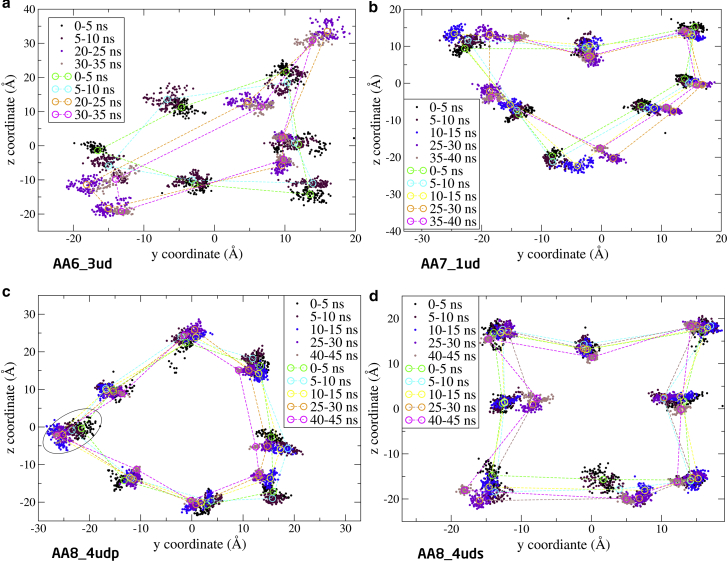
Figure 8.
Position of α-carbon in the FA-Lys linker of the AA6_3ud (a), AA7_1ud (b), AA8_4udp (c), and AA8_4uds (d) as a function of simulation time. One monomer is highlighted in a circumference in AA8_4udp (c), to indicate the possible disintegration of the AA8_4udp system. See Fig. 7 legend for details. To see this figure in color, go online.
Figure 9.
Position of α-carbon in the FA-Lys linker of the AA6_FAout (a), AA7_FAout (b), AA8_FAout (c), and AA7_glp1 (d), as a function of simulation time. See Fig. 7 legend for details. To see this figure in color, go online.
From Fig. 7, we can conclude that the spread of the hexamer is larger than for the heptamer and the octamer throughout the simulation. Furthermore, the hexamer is squeezed to give a more flattened shape. For the octamer, it appears that the elliptical structure is unstable because one monomer seems to migrate from the oligomeric structure. In the case of the heptamer, Fig. 7b, it is evident that the position of the α-carbon is rather dense throughout the simulation and that the structure resembles an ellipse.
Fig. 8a shows that the AA6_3ud structure flattens drastically, which corresponds to the more open structure seen in Fig. S4 a. The AA8_4udp structure maintains a more elliptical arrangement, even though one monomer seems to be leaving the structure (indicated by the circumference). The AA8_4uds structure is rather mobile and gains a squared shape. The AA7_1ud structure is, like the hexamer in Fig. 7a, squeezed so that the elliptical structure is destroyed.
In AA7_glp1, Fig. 9d, monomers are more mobile than compared with the other structures resulting in a disordered (unstable) structure. In Fig. 9b, the AA7_FAout structure appears to maintain an elliptical structure, but the movement of the α-carbons is very spread out. Fig. 9c shows that the α-carbon movement of the AA8_FAout system is rather centered on the starting position throughout the simulation, which indicates a stable system. However, as seen for the octamer and AA8_4udp systems, one monomer escapes from the elliptical arrangement. The AA6_FAout system (Fig. 9a) moves significantly throughout the simulation, and this movement results in a flattened structure.
Mean energies based on the structures taken at every 50 ps throughout the simulations are given in Table 3. The energies calculated for the oligomeric system (peptide-peptide, P-P) and the oligomer-water interactions (P-W) are normalized to the number of monomers in each oligomer to make comparison of the different systems possible. From these, it can be seen that the total P-P van der Waals (vdW) energy for the heptamer is lower than for any of the other oligomers. Considering the P-W interactions, the energy for the heptamer system is less negative than found for the other systems. In general, all the hexameric systems of liraglutide (hexamer, AA6_3ud, AA6_FAout) are higher in P-P vdW energy, which might indicate that these structures are less stable than the hepta- and octameric liraglutide oligomers. The energies show relatively large fluctuations (data not shown) which is also reflected by the relatively large standard deviations. This indicates that internally the 11 oligomeric systems are flexible structures.
Table 3.
Mean energies and corresponding standard error of the mean (for P-P and P-W) and standard deviation (for S-S) for the 11 systems are calculated from the simulations
| Structure | P-P vdW (kcal/mol) | P-W vdW (kcal/mol) | S-S vdW (kcal/mol) | S-S FA vdW (kcal/mol) | S-S FA out vdW (kcal/mol) |
|---|---|---|---|---|---|
| Hexamer | −188 ± 18.9 | −84.7 ± 21.1 | −20 ± 4.5 | −44 ± 6.1 | — |
| Heptamer | − 200 ± 14.8 | −68.1 ± 19.3 | −37 ± 5.6 | −56 ± 4.7 | −23 ± 3.8 |
| Octamer | −187 ± 13.7 | −84.4 ± 18.0 | −21 ± 3.8 | −62 ± 4.1 | — |
| AA6_3ud | −185 ± 16.4 | −88.1 ± 22.1 | −4.6 ± 2.2 | −52 ± 7.1 | — |
| AA7_1ud | −191 ± 19.8 | −83.0 ± 22.4 | −12 ± 4.6 | −50 ± 4.7 | −38 ± 4.8 |
| AA8_4udp | −189 ± 15.1 | −86.8 ± 18.9 | −18 ± 3.1 | −55 ± 3.5 | — |
| AA8_4uds | −190 ± 16.5 | −85.3 ± 21.1 | −24 ± 3.6 | −54 ± 4.4 | — |
| AA7_glp1 | −160 ± 15.1 | −84.9 ± 19.0 | −23 ± 5.1 | −26 ± 5.0 | −29 ± 5.2 |
| Structure | P-P vdW (kcal/mol) | P-W vdW (kcal/mol) | S-S all FA out vdW (kcal/mol) | ||
| AA6_FAout | −171 ± 13.4 | −104 ± 21.5 | −23 ± 3.0 | ||
| AA7_FAout | −187 ± 18.9 | −86.5 ± 25.3 | −33 ± 3.0 | ||
| AA8_FAout | −176 ± 13.1 | −98.6 ± 22.8 | −35 ± 3.0 | ||
The vdW energies for the peptide-peptide (P-P), peptide-water (P-W), segment-segment (seg-seg) with FA pairs (S-S FA), seg-seg without FA pairs interacting (S-S), and—for the heptamer—seg-seg interactions for the monomer pairs including the monomer with the FA pointing outward (S-S FA out), are calculated. Energies are also calculated for the systems where all FA chains are pointing outward (S-S all FA out). The energies for the P-P and P-W interactions are normalized according to the number of monomers in the structure. MDENERGY from the program NAMD (http://www.ks.uiuc.edu/Research/namd/) was used to calculate the energies in intervals of 50 ps.
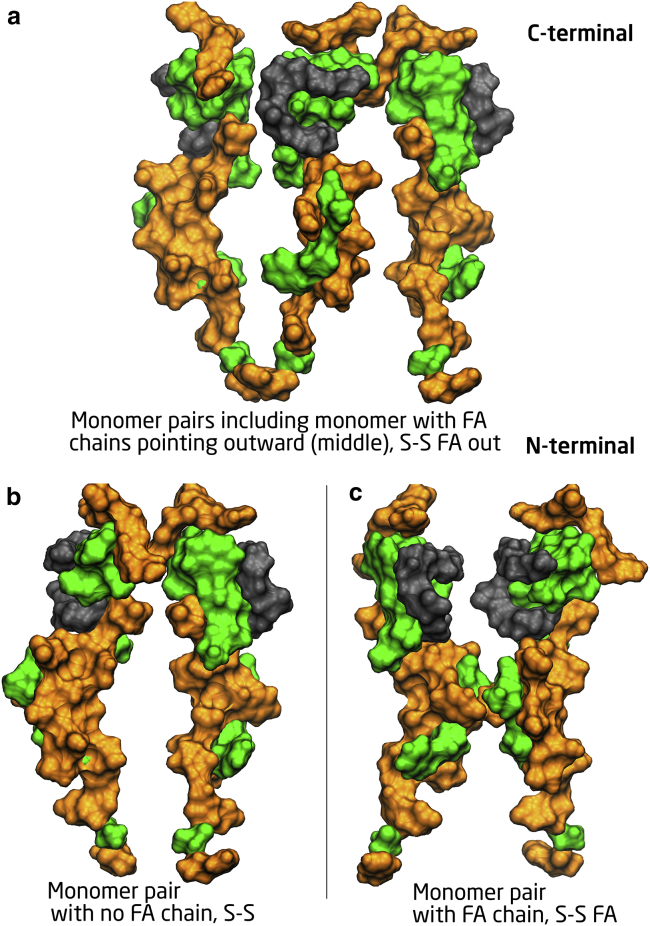
The analyses were done for segment-segment interactions where FA chains are facing each other (S-S FA), no FA chains are between them (S-S), one FA chain pointing outward (S-S FA out), and all FA chains pointing outward (S-S all FA out). See Figs. 10 and 2 for illustration of S-S, S-S FA, S-S FA out, and S-S all FA out (AA6/7/8_FAout structures). The two last analyses were only done for the heptamer, AA7_1ud, and for the oligomer, AA6_FAout, AA7_FAout, and AA8_FAout because these were the only conformations relevant for such investigation. Results for the AA7_glp1 structure (with no Fa chains attached) are also reported since the monomers in the AA7_glp1 structure are rotated the same way as those in the heptamer with paired FA chains. It can be seen that for the structures with FA pairs, the energies are significantly lower for the monomer pairs that have FA chains facing each other than for those where no FA chains are between them. This supports the view that interactions of the FA chains contribute to the stabilization of the oligomers of liraglutide (2,5).
Figure 10.
A surface plot of the liraglutide monomer showing the seg-seg orientations. (Green) Hydrophobic; (orange) hydrophilic; (gray) FA chain. (a–c) Different hydrophobicities on either side of the monomer. To see this figure in color, go online.
Discussion
The experimental SAXS curves (Fig. 3) show that even at the lowest measured concentration of 1 mg/mL, repulsive interactions between liraglutide oligomers are present, which increases with increasing concentration. Repulsive interactions can lead to an underestimation of the molecular weight. The SAXS data suggest that the solution structure of liraglutide is a hexa-, hepta-, or octamer. The uncertainty of the structure arises from uncertainties related to the measured concentration and the estimated partial specific volume (v) (and hence the number of monomers in the oligomer). To our knowledge, there is no value of ν for liraglutide available in the literature. In this study, v = 0.73 cm3/g (corresponding to an average value for pure proteins) was used. The FA chain could contribute to an increase in the partial specific volume, but to which extent is difficult to estimate. Using v = 0.74 cm3/g as also reported by Mylonas and Svergun (47), the molecular weight increases to that resembling an octamer. From the SAXS measurements, it can only be concluded that the solution structure of liraglutide is an oligomer of approximately heptameric molecular size, with a consistent shape of an elliptical cylinder, and that this oligomerization is concentration-independent within the measured concentration range.
The MALS results indicate a hexameric solution structure of liraglutide. In contrast to the SAXS data, no repulsion interactions between oligomers were observed in the concentration range of 0.012–0.036 mg/mL. Note that the SAXS data were measured in the concentration range of 1.0–4.7 mg/mL, where 1 mg/mL corresponds to the lowest concentration that can be measured.
Simulation results suggest that the hexamer is an unfavorable arrangement for the monomers as seen from the structural deviation of the oligomers, given by the RMSD (Fig. 6) and the two-dimensional plot of the α-carbon in the FA-Lys linker (Fig. 7). However, the packing of the structure is relatively tight with a low SASA (Fig. 5), which indicates that more interactions are obtainable. This is also supported by the relatively low P-P energy of the hexameric system compared to the others (Table 3). The heptamer seems to present a very favorable arrangement with a tight packing as indicated by the low SASA (Fig. 5), little displacement given by the low and converging RMSD (Fig. 6), and the overall elliptical shape seen in the two-dimensional plot of the α-carbon in the FA-Lys linker (Fig. 7). This is also the structure with the overall lowest P-P energy of ∼−200 kcal/mol. The octamer, like the hexamer, also appears to present an unfavorable arrangement with a slight opening and disintegration of the elliptical structure as seen in the end structure (Fig. S3), the two-dimensional plot of the α-carbon (Fig. 7), and the high SASA (Fig. 5). The AA6_3ud presents the worst monomer arrangement out of the 11 investigated structures based on the end structure (Fig. S4) that opens completely, resulting in an unstable conformation as also seen from the relatively large SASA (Fig. S6), increasing RMSD (Fig. S8), and the scattered α-carbon position (Fig. 8). The AA7_1ud, AA8_4udp, and AA8_4uds systems all maintain a relatively well-arranged end structure (Fig. S4), even though one monomer appears to be leaving the general elliptical structure of AA8_4up. In contrast to this, the overall packing of the three systems is relatively tight (Fig. S6), but the overall movement is scattered and very spread out as seen in the two-dimensional plot of the α-carbon (Fig. 8). Thus, it seems like interactions between the C- and N-termini are not contributing to the energy, hence, liraglutide is most likely not to arrange like the flipped structures (AA6_3ud, AA7_1ud, AA8_4udp, and AA8_4uds). The AA6/7/8_FAout structures also present unfavorable arrangements of the monomers, as can be seen by the large scattering of the individual monomers in the two-dimensional plot of the α-carbon (Fig. 9) and large overall displacement represented by the RMSD (Fig. S9).
The most important analysis result for these structures is, however, that the S-S all FAout energies for all three systems are less negative than those of the segment interactions in systems where the FA chains are pointing toward each other (Table 3). This amplifies the hypothesis of liraglutide oligomer structures that give rise to FA interactions. However, when the FA chains are pointing outward they could, in theory, wrap around the elliptical structure in such a manner that they interact. This appears not to be the case, based on the energy calculations (Table 3) and the fact that all the FA chains in all the three structures seem to be randomly laying on the surface of the oligomers (Fig. S5). This arrangement could promote clustering of oligomers. However, this is not the case because SAXS and MALS data indicate the presence of one defined oligomeric species. The AA7_glp1 was made as a reference structure when considering the role of the FA chains. The results show that the movement of the individual monomers of this system (Fig. 9) is rather large. This emphasizes the stabilization effect of the FA chains. The system seems to have a tight packing (Fig. S7); however, this fact is more likely to be a result of the missing FA chains in the structure, and hence, less surface area. The S-S energies bear witness to a great lack in possible interactions because these energies are significantly less negative (∼−29 kcal/mol) than for those systems with FA chains present (S-S FA average energy ∼−54 kcal/mol). This corresponds well with the hydrophobic/hydrophilic areas of the monomer shown in Fig. 10, where it is evident that there is a difference in the hydrophobicity around the monomeric structure. All in all, it shows that the FA chains are important in stabilizing the liraglutide oligomer.
It thus appears that the heptamer is the most favorable arrangement, which is further supported by the comparison between the experimentally determined SAXS (SAXSexp) curve and curves extracted from the simulations. The theoretical SAXS (SAXScomp) curves along with the experimentally determined curve are shown in Fig. S10. SAXScomp curves were calculated from the last structure of the 11 simulations.
The discrepancies (χ2 values) for the 11 curves compared to the experimentally obtained SAXS curve are, with the radii of gyration, given in Table S1. Besides the radius of gyration for the octamer, the radii of the systems lie very close to those found from the experimental data seen in Table 2. Also, the discrepancies given in Table S1 do not present one specific candidate with the best fit, as several of the systems have low discrepancies between their end structures and the experimentally obtained data.
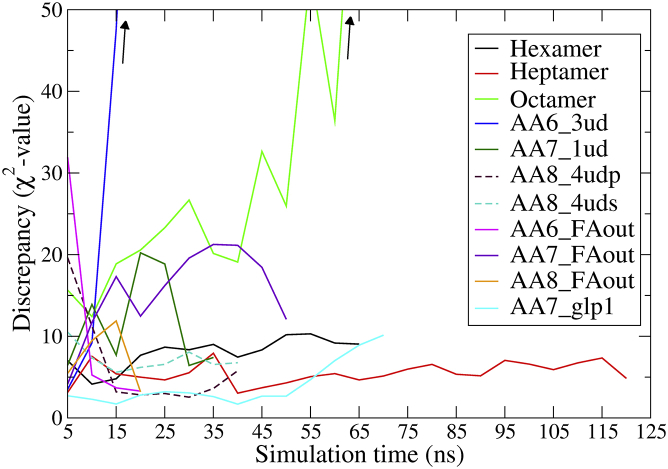
To clarify the best fit further, we show the time evolution of the discrepancies between SAXScomp and SAXSexp for the 11 oligomers in Fig. 11.
Figure 11.
Discrepancies calculated between the SAXScomp curves and the experimentally determined SAXS curve of liraglutide as a function of simulation time, calculated every 5 ns. (Arrows) χ2 values continuously increase with simulation time. To see this figure in color, go online.
The discrepancy of the heptamer is rather stable and low throughout the simulation. So is χ2 of the hexamer, AA8_4udp, AA8_4uds, and the AA7_glp1 systems. On the contrary, the discrepancy of the octamer and AA6_3ud is very high and increases with simulation time. That of the AA6_FAout, AA7_FAout, AA8_FAout, and AA7_1ud systems fluctuate significantly throughout the simulations. The results show that the global structure and size seems to be correct, especially for the heptamer and hexamer, but none of our simulated structures captures the precise shape of SAXSexp.
Summarizing and combining all the results (see Table 4 for a combined scoring chart), it appears that the most likely solution structure of liraglutide is a heptamer where the monomers are oriented in such a way that the attached FA chains can interact in three pairs in the direction of the elliptical arrangement, and with the remaining monomer oriented so that the FA chain is pointing out.
Table 4.
Scoring chart of the 11 oligomeric systems
| Structure | SASA | RMSD | 2D Projection | Energy |
SAXS |
Total Score | ||
|---|---|---|---|---|---|---|---|---|
| P-P vdW | P-W vdW | χ2 Value | χ2 Value Fluctuation | |||||
| Hexamer | 2 | 8 | 3 | 5 | 5 | 7 | 4 | 4.9 |
| Heptamer | 1 | 4 | 1 | 1 | 1 | 3 | 2 | 1.9 |
| Octamer | 7 | 10 | 6 | 6 | 3 | 11 | 10 | 7.6 |
| AA6_3ud | 10 | 11 | 11 | 8 | 9 | 10 | 11 | 10 |
| AA7_1ud | 4 | 5 | 10 | 2 | 2 | 6 | 7 | 5.1 |
| AA8_4udp | 6 | 3 | 5 | 3 | 8 | 5 | 6 | 5.1 |
| AA8_4uds | 5 | 2 | 8 | 4 | 7 | 4 | 3 | 4.7 |
| AA6_FAout | 11 | 9 | 2 | 10 | 11 | 2 | 8 | 7.6 |
| AA7_FAout | 8 | 7 | 9 | 7 | 4 | 9 | 9 | 7.6 |
| AA8_FAout | 9 | 1 | 4 | 9 | 10 | 1 | 5 | 5.6 |
| AA7_glp1 | 3 | 6 | 7 | 11 | 6 | 8 | 1 | 6.0 |
Each system is evaluated and compared to each of the others in these categories: lowest and most stable SASA, lowest and most stable RMSD, most stable two-dimensional (2D) projection and elliptical shape, lowest P-P vdW energy, highest P-W vdW energy, lowest discrepancy from measured SAXS curve taken for the end structure, and lowest mean and standard deviation of the discrepancy throughout the simulation (data taken from Fig. 11). The SASA, RMSD, and 2D projection plots are inspected visually; 1 is the best score and 11 is the worst. The total score is normalized.
The heptamer, with the above-mentioned conformation, presents the best model with a total score of 1.9 being the best scoring structure in four out of seven categories and landing either a second, third, or fourth place in the remaining categories. It presents the best energy interactions as well as the least structural deviation of the monomers and highest packing. The hexamer presents the second-best solution, but does not score best in any category. The AA6_3ud structure presents the worst arrangement of the monomers, with a total score of 10.
Conclusions
From a pharmaceutical perspective, it is important to know the oligomerization state of liraglutide with respect to stability, because uncontrolled and extensive oligomerization can drive fibrillation. Hence, an important criterion for a stable formulation and, in turn, long shelf-life in the liquid form is the solution structure. To gain further insight in the solution structure of liraglutide, we performed a series of MD simulations accompanied by MALS and SAXS experiments. MALS provides information about the mass, and SAXS provides information on mass, radius of gyration, and shape. The SAXS curves indicate that liraglutide undergoes concentration-independent oligomerization. Depending on the partial specific volume used for deducing the molecular weight and in turn the number of monomers in an oligomer, liraglutide may form hexa-, hepta-, or octamers in solution. In contrast, the MALS results suggest that liraglutide forms hexamers in solution. The experimental results are not conclusive with respect to the size of the oligomers. No information can be deduced from these measurements regarding the orientation and stabilizing role of the acyl chains in the oligomers.
We, therefore, performed MD simulations on several oligomeric sets consisting of hexa-, hepta-, and octamers. Our simulation results indicate that interactions between the FA chains contribute to the stabilization of the structure and that the heptamer presents the best representation of the investigated liraglutide oligomers. Furthermore, comparing the experimentally determined SAXS curve with the SAXS curves determined from the structures extracted from the simulations shows qualitative agreement for the overall size and shape. This indicates that liraglutide in solution is most likely to form heptamers in a hollow, water-filled, elliptical cylindrical-shaped structure where the monomers are oriented in such a way that the FA chains can interact pairwise. From the simulations, as of this writing, we are not able to identify the absolute position of the FA chains in the heptamer, but it is clear that interactions between them are significant.
Author Contributions
T.M.F. wrote the article and performed and analyzed simulations; P.S. conducted and analyzed SAXS experiments, and contributed in writing the article; L.A.R. performed preliminary simulations and contributed to the article; P.H. conducted SAXS experiments and contributed in writing the article; J.T.B. conducted SAXS experiments and contributed in writing the article; A.M.S.-P. conducted and analyzed AF4-MALS experiments, and contributed in writing the article; M.N.E.-L. performed preliminary simulations; and G.H.P. performed and analyzed simulations, and contributed in writing the article.
Acknowledgments
Simulations were performed at the Danish Center for Scientific Computing at the Technical University of Denmark. MAXIV Synchrotron is acknowledged for providing beamtime and for support during the experiments. VMD (45) was used for all graphical representations of liraglutide.
The research leading to these results has received funding from the European Community’s Seventh Framework Program (grant No. FP7/2007-2013) BioStruct-X, under grant agreement No. 283570. DANSCATT (the Danish Agency for Science, Technology and Innovation) is acknowledged for financial support.
Editor: Ivet Bahar.
Footnotes
Supporting Materials and Methods, eleven figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)00815-2.
Contributor Information
Pernille Harris, Email: ghp@kemi.dtu.dk.
Günther H. Peters, Email: ph@kemi.dtu.dk.
Supporting Citations
References (48–50) appear in the Supporting Material.
Supporting Material
References
- 1.Kaspar A.A., Reichert J.M. Future directions for peptide therapeutics development. Drug Discov. Today. 2013;18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Li Y., Shao M., Gong M. Self-assembling peptides improve the stability of glucagon-like peptide-1 by forming a stable and sustained complex. Mol. Pharm. 2013;10:3356–3365. doi: 10.1021/mp4001734. [DOI] [PubMed] [Google Scholar]
- 3.Chang X., Keller D., Led J.J. NMR studies of the aggregation of glucagon-like peptide-1: formation of a symmetric helical dimer. FEBS Lett. 2002;515:165–170. doi: 10.1016/s0014-5793(02)02466-3. [DOI] [PubMed] [Google Scholar]
- 4.Willard F.S., Sloop K.W. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Exp. Diabetes Res. 2012;2012:470851. doi: 10.1155/2012/470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauck M.A. Liraglutide, a once-daily human GLP-1 analogue. Br. J. Diabetes Vasc. Dis. 2008;8:S26–S33. [Google Scholar]
- 6.Jang H.-J., Kokrashvili Z., Egan J.M. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran-Ramos S., Tovar A.R., Torres N. Diet, friend or foe of enteroendocrine cells: how it interacts with enteroendocrine cells. Adv. Nutr. Int. Rev. J. 2012;3:8–20. doi: 10.3945/an.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai K., Lee E.Y., Miki T. Glucagon-like peptide-1 secretion by direct stimulation of L cells with luminal sugar vs non-nutritive sweetener. J. Diabetes Investig. 2012;3:156–163. doi: 10.1111/j.2040-1124.2011.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle M.E., Egan J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsbad S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014;16:9–21. doi: 10.1111/dom.12119. [DOI] [PubMed] [Google Scholar]
- 11.Briyal S., Shah S., Gulati A. Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience. 2014;281C:269–281. doi: 10.1016/j.neuroscience.2014.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Talbot K., Wang H.Y. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 2014;10(Suppl 1):S12–S25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velmurugan K., Bouchard R., Pugazhenthi S. Neuroprotective actions of glucagon-like peptide-1 in differentiated human neuroprogenitor cells. J. Neurochem. 2012;123:919–931. doi: 10.1111/jnc.12036. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L., Xu J., Fang Y. Protective effect of rhGLP-1 (7-36) on brain ischemia/reperfusion damage in diabetic rats. Brain Res. 2015;1602:153–159. doi: 10.1016/j.brainres.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 15.McClean P.L., Hölscher C. Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology. 2014;76(Pt A):57–67. doi: 10.1016/j.neuropharm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Deacon C.F. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53:2181–2189. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- 17.Tasyurek H.M., Altunbas H.A., Sanlioglu S. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab. Res. Rev. 2014;30:354–371. doi: 10.1002/dmrr.2501. [DOI] [PubMed] [Google Scholar]
- 18.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 19.Orskov C., Wettergren A., Holst J.J. Biological effects and metabolic rates of glucagonlike peptide-1 7-36 amide and glucagonlike peptide-1 7-37 in healthy subjects are indistinguishable. Diabetes. 1993;42:658–661. doi: 10.2337/diab.42.5.658. [DOI] [PubMed] [Google Scholar]
- 20.Lund A., Knop F.K., Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur. J. Intern. Med. 2014;25:407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Gallwitz B., Ropeter T., Schmidt W.E. GLP-1-analogues resistant to degradation by dipeptidyl-peptidase IV in vitro. Regul. Pept. 2000;86:103–111. doi: 10.1016/s0167-0115(99)00095-6. [DOI] [PubMed] [Google Scholar]
- 22.Ahrén B. GLP-1 for type 2 diabetes. Exp. Cell Res. 2011;317:1239–1245. doi: 10.1016/j.yexcr.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Ladenheim E.E. Liraglutide and obesity: a review of the data so far. Drug Des. Devel. Ther. 2015;9:1867–1875. doi: 10.2147/DDDT.S58459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bemporad F., Chiti F. Protein misfolded oligomers: experimental approaches, mechanism of formation, and structure-toxicity relationships. Chem. Biol. 2012;19:315–327. doi: 10.1016/j.chembiol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol. Cell. Endocrinol. 2009;297:137–140. doi: 10.1016/j.mce.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen L.B., Nielsen P.F., Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 27.Pabreja K., Mohd M.A., Furness S.G.B. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharmacol. 2014;171:1114–1128. doi: 10.1111/bph.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steensgaard D.B., Thomsen J.K., Knudsen L.B. The molecular basis for the delayed absorption of the once-daily Human GLP-1 analogue, liraglutide. Diabetes. 2008;57(Suppl 1):A164. [Google Scholar]
- 29.Wang Y., Lomakin A., Benedek G.B. Transformation of oligomers of lipidated peptide induced by change in pH. Mol. Pharm. 2015;12:411–419. doi: 10.1021/mp500519s. [DOI] [PubMed] [Google Scholar]
- 30.Trier S., Linderoth L., Rahbek U.L. Acylation of Glucagon-like peptide-2: interaction with lipid membranes and in vitro intestinal permeability. PLoS One. 2014;9:e109939. doi: 10.1371/journal.pone.0109939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X., Keller D., Led J.J. Structure and folding of glucagon-like peptide-1-(7-36)-amide in aqueous trifluoroethanol studied by NMR spectroscopy. Magn. Reson. Chem. 2001;39:477–483. [Google Scholar]
- 32.RxList, The Internet Drug Index. 2015. Victoza. http://www.rxlist.com/victoza-drug.htm. Accessed July 5, 2015.
- 33.Gasteiger E., Hoogland C., Bairoch A. The Proteomics Protocols Handbook. Humana Press; New York: 2005. Protein identification and analysis tools in the ExPASy server; pp. 571–607. [Google Scholar]
- 34.Gasteiger E., Gattiker A., Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labrador A., Cerenius Y., Plivelic T. The yellow mini-hutch for SAXS experiments at MAX IV Laboratory. J. Phys. Conf. Ser. 2013;425:72019. [Google Scholar]
- 36.Konarev P.V., Volkov V.V., Svergun D.I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003;36:1277–1282. [Google Scholar]
- 37.Petoukhov M.V., Franke D., Svergun D.I. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Cryst. 2012;45:342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenyuk A.V., Svergun D.I. GNOM. A program package for small-angle scattering data processing. J. Appl. Cryst. 1991;24:537–540. [Google Scholar]
- 39.Bernstein F.C., Koetzle T.F., Tasumi M. The Protein Data Bank. A computer-based archival file for macromolecular structures. Eur. J. Biochem. 1977;80:319–324. doi: 10.1111/j.1432-1033.1977.tb11885.x. [DOI] [PubMed] [Google Scholar]
- 40.Grubmüller, H., and V. Groll. 2015. SOLVATE. Biophysical Chemistry Department, Max Planck Institute of Biophysics, Frankfurt, Germany. http://www.mpibpc.mpg.de/grubmueller/solvate. Accessed February 28, 2015.
- 41.Jorgensen W.L., Chandrasekhar J., Klein M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926. [Google Scholar]
- 42.Nelson M.T., Humphrey W., Schulten K. NAMD: a parallel, object-oriented molecular dynamics program. Int. J. High Perform. Comput. Appl. 1996;10:251–268. [Google Scholar]
- 43.MacKerell A.D., Bashford D., Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 44.Madsen J.J., Linderoth L., Peters G.H. Secretory phospholipase A2 activity toward diverse substrates. J. Phys. Chem. B. 2011;115:6853–6861. doi: 10.1021/jp112137b. [DOI] [PubMed] [Google Scholar]
- 45.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. 27–28. [DOI] [PubMed] [Google Scholar]
- 46.Svergun D., Barberato C., Koch M.H. CRYSOL—a program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Cryst. 1995;28:768–773. [Google Scholar]
- 47.Mylonas E., Svergun D.I. Accuracy of molecular mass determination of proteins in solution by small-angle x-ray scattering. J. Appl. Cryst. 2007;40:s245–s249. [Google Scholar]
- 48.Darden T., York D., Pedersen L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 49.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 50.Feller S.E., Zhang Y., Brooks B.R. Constant-pressure molecular-dynamics simulation—the Langevin piston method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.