Abstract
Objective:
To determine whether patients with idiopathic and symptomatic RBD were sleepier than controls, and if sleepiness in idiopathic RBD predicted earlier conversion to Parkinson disease.
Methods:
The Epworth Sleepiness Scale (ESS) and its determinants were compared at the time of a video-polysomnography for an RBD diagnosis in patients with idiopathic RBD, in patients with Parkinson disease, and in controls. Whether sleepiness at time of RBD diagnosis predicted an earlier conversion to neurodegenerative diseases was retrospectively analyzed in the followed-up patients.
Results:
The 75 patients with idiopathic RBD were sleepier (ESS: 7.8 ± 4.6) at the time of RBD diagnosis than 74 age- and sex-matched controls (ESS: 5.0 ± 3.6, P < 0.0001). They reached the levels of 114 patients with Parkinson disease (ESS: 8.7 ± 4.8), whether they had (n = 78) or did not have (n = 36) concomitant RBD. The severity of sleepiness in idiopathic RBD correlated with younger age, but not with sleep measures. Among the 69 patients with idiopathic RBD who were followed up for a median 3 years (1–15 years), 16 (23.2%) developed parkinsonism (n = 6), dementia (n = 6), dementia plus parkinsonism (n = 2), and multiple system atrophy (n = 2). An ESS greater than 8 at time of RBD diagnosis predicted a shorter time to phenoconversion to parkinsonism and dementia, from RBD onset, and from RBD diagnosis (when adjusted for age and time between RBD onset and diagnosis).
Conclusions:
Sleepiness is associated with idiopathic REM sleep behavior disorder and predicts more rapid conversion to parkinsonism and dementia, suggesting it is an early marker of neuronal loss in brainstem arousal systems.
Citation:
Arnulf I, Neutel D, Herlin B, Golmard JL, Leu-Semenescu S, Cochen de Cock V, Vidailhet M. Sleepiness in idiopathic REM sleep behavior disorder and Parkinson disease. SLEEP 2015;38(10):1529–1535.
Keywords: hypersomnolence, synucleinopathy, dementia, parkinsonism
INTRODUCTION
Around one-third of patients with Parkinson disease (PD), dementia with Lewy bodies, and multiple system atrophy suffer from excessive daytime sleepiness, with an incidence of 6% per year in PD and an increased risk of driving accidents.1–5 The mechanism of sleepiness in these synucleinopathies is multifactorial, including non-restorative sleep, sedative effects of treatment, and lesions in arousal systems.6 It is possible that sleepiness precedes PD onset, as sleepy adults in a longitudinal study were 3.3 times more likely than non-sleepy adults to develop PD later in life.7 In 4,894 elderly subjects, the subjects who felt sleepy during daytime had a 1.26 risk ratio of cognitive decline 8 years later.8 These results suggest that sleepiness may be partly related to neuronal loss in arousal systems and that this loss may precede the onset of parkinsonism or dementia.
In addition to sleepiness, most patients with PD, dementia with Lewy bodies and multiple system atrophy exhibit REM sleep behavior disorder (RBD),9 a disorder characterized by violent nightmare enactment. Notably, idiopathic RBD may precede the onset of these diseases,10 with a risk of 44% within 5 years after RBD diagnosis and 82% within 10 years.11–13 Patients with idiopathic RBD present non-motor symptoms and signs also found in PD and dementia with Lewy bodies, including a mild cognitive impairment, altered olfaction and color vision, and dysautonomia.9 However, whether they have higher levels of sleepiness than controls and whether sleepiness predicts a more rapid conversion towards parkinsonism/ dementia has not been studied. We therefore examined the association between sleepiness and RBD in patients with idiopathic RBD, controls, and patients with PD.
METHODS
Participants
The medical and sleep files of the patients who received a diagnosis of idiopathic RBD between 1998 and 2013 in our university hospital and those of patients with PD who were consecutively studied in two different sleep studies were collected.14,15 Patients with PD met UK Brain Bank criteria for definite idiopathic PD16 and had no significant cognitive impairment, with a score at Mini-mental state examination (MMSE) > 26.17 The RBD was defined using the International Classification of Sleep Disorders, Second Edition,18 modified to include the definition of muscle tone enhancement19 and normative criteria for REM sleep without atonia: (1) a history of dream enactment with injurious or potentially injurious movements, plus the presence of enhanced (≥ 3 times superior to its activity during NREM sleep) tonic chin muscle tone during > 50% of each epoch of REM sleep (REM sleep without atonia), with REM sleep without atonia representing > 18% of REM sleep time,20 or (2) the presence of abnormal complex behaviors during REM sleep on video-polysomnography. Idiopathic RBD was defined after a complete interview and neurological and cognitive examination by neurologists, by the absence of definite criteria for parkinsonism,16 for dementia (score on the MMSE > 26 or score on the Montreal Cognitive Assessment > 23) and for other neurodegenerative disorders. The control group was composed of 74 subjects without any neurological disease, including 46 healthy paid controls and 28 hospital controls. The healthy controls were gathered from 2 different studies.21,15They had been recruited by public announcement, and among the friends and families of patients and investigators. The hospital controls were gathered from another research program including subjects with a suspicion of pulmonary embolism, later disconfirmed. They were considered to be free of any neurological disease after clinical examination. There was no limit on sleepiness score to take part in these studies. For the present study, controls were selected in these three lists to match with patients with idiopathic RBD for age and sex. All participants signed an agreement to take part in their proper study that allowed re-using their measures for further studies. The study was approved by the local ethics committee (Comité de Protection des Personnes Ile de France 06).
Investigations
The participants were interviewed (symptoms, drugs) and examined by sleep neurologists at time of polysomnography diagnosis. The onset of RBD symptoms was determined by patients and their spouse at time of RBD diagnosis. At the same time, they completed the Epworth Sleepiness Scale (ESS), a subjective scale evaluating within the preceding month the propensity (0: none; 1: mild; 2: moderate; 3: severe) to doze during 8 conditions of daily life, including reading, watching TV, as a passenger, in a meeting or a waiting room, sitting after lunch, lying after lunch, speaking, and sitting in a car stopped in traffic. The total score ranges from 0 to 24 and is considered abnormal when > 10.22,23 The scale has excellent clinimetric properties in PD.24 Patients with idiopathic RBD underwent an MMSE between 1998 and 2008, and a Montreal Cognitive Assessment between 2009 and 2013. Due to this change in method for assessing cognition, it was not possible to correlate sleepiness with cognitive scores. The cognitive tests were not valid in 7 patients (analphabetism, low education, or no fluent French). The drug regimen was collected, and the levodopa and dopamine agonist daily doses were transformed into levodopa-equivalent doses following previously published methods.25
All participants underwent an attended video-polysomnography including EEG, EOG, chin (levator menti) and legs (left and right tibialis anterior) EMG, nasal pressure, oro-pharyngeal sounds via a tracheal microphone, chest and abdominal efforts via belts, pulse oximetry, EKG, and synchronized video and audio monitoring. The sleep stages, arousals, periodic leg movements, respiratory events, and chin EMG activities (after excluding snoring artifacts) were scored according to international criteria.19 Video recordings were carefully examined to detect any movement during REM sleep.26
The patients with idiopathic RBD were contacted by phone in 2013 or followed and examined every year to determine whether they had developed parkinsonism or dementia, and the year of diagnosis. In addition, we used the hospital software tracking patient trajectories. The medical files were obtained in the neurology department to clarify the diagnosis and time in the patients not followed up at the sleep center. The patients were diagnosed as affected by parkinsonism if they met the corresponding criteria of the UK brain bank, as demented if they had an MMSE < 26, or a score on the Montreal cognitive assessment after educational adjustment < 22,27,28 and as suffering from multiple system atrophy.28 The date of last news was collected.
Statistical Analysis
Measures were compared between all groups using ANOVAs, followed by Tukey-Kramer pair-wise comparisons when significant for quantitative variables and using logistic models for qualitative ones. The determinants of sleepiness (ESS) in the idiopathic RBD group were determined by Spearman rank correlation coefficient tests for continuous variables and Wilcoxon tests for qualitative ones. The median times of conversion from RBD onset or RBD diagnosis to parkinsonism/ dementia were calculated using a Kaplan-Meier survival curve. These conversion times were examined for clinical and sleep covariates using Cox proportional hazard model with a univariate analysis, unadjusted and adjusted for age for time between RBD onset and parkinsonism/dementia, and unadjusted and adjusted for age and time between RBD onset and diagnosis for time from RBD diagnosis to parkinsonism/dementia. The time-to-event functions were compared with log rank tests between subgroups. The statistical analysis was performed using the SAS V9 statistical package (SAS Institute, Cary, NC).
RESULTS
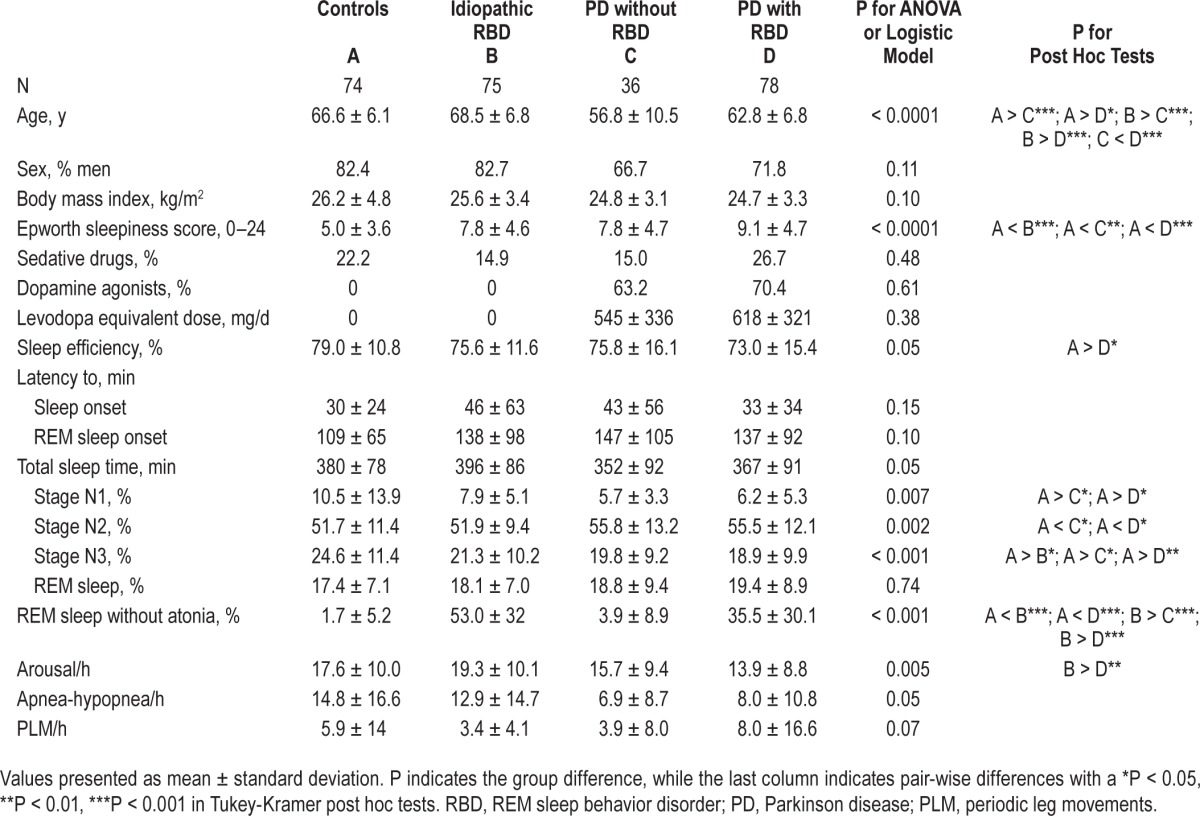
The 75 patients had idiopathic RBD for a mean of 6 ± 5.2 years (range: 0–30) at the time of RBD diagnosis and ESS measurement and were predominantly male. They all had complex behaviors during REM sleep. As expected by age- and sex-matching, there was no difference for age and sex between patients with idiopathic RBD and controls, but the latter were more frequently male and older than patients with PD (Table 1). Patients with PD and no RBD were younger than patients with PD plus RBD.
Table 1.
Clinical and sleep characteristics of patients with idiopathic REM sleep behavior disorder, controls, and patients with Parkinson disease with and without REM sleep behavior disorder.
Sleepiness Severity and Nighttime Sleep
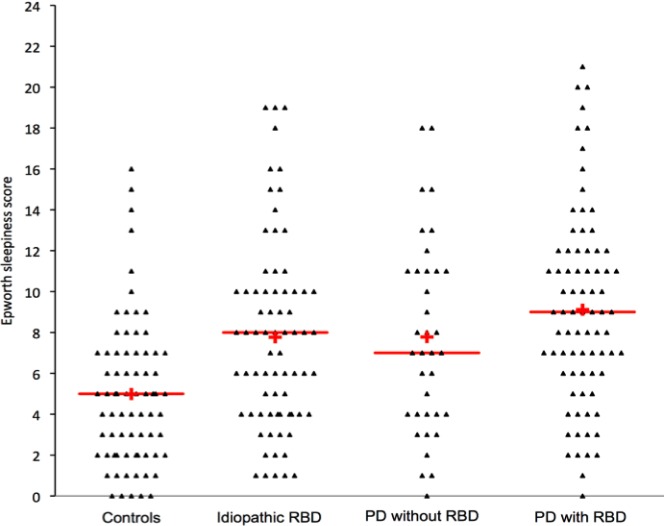
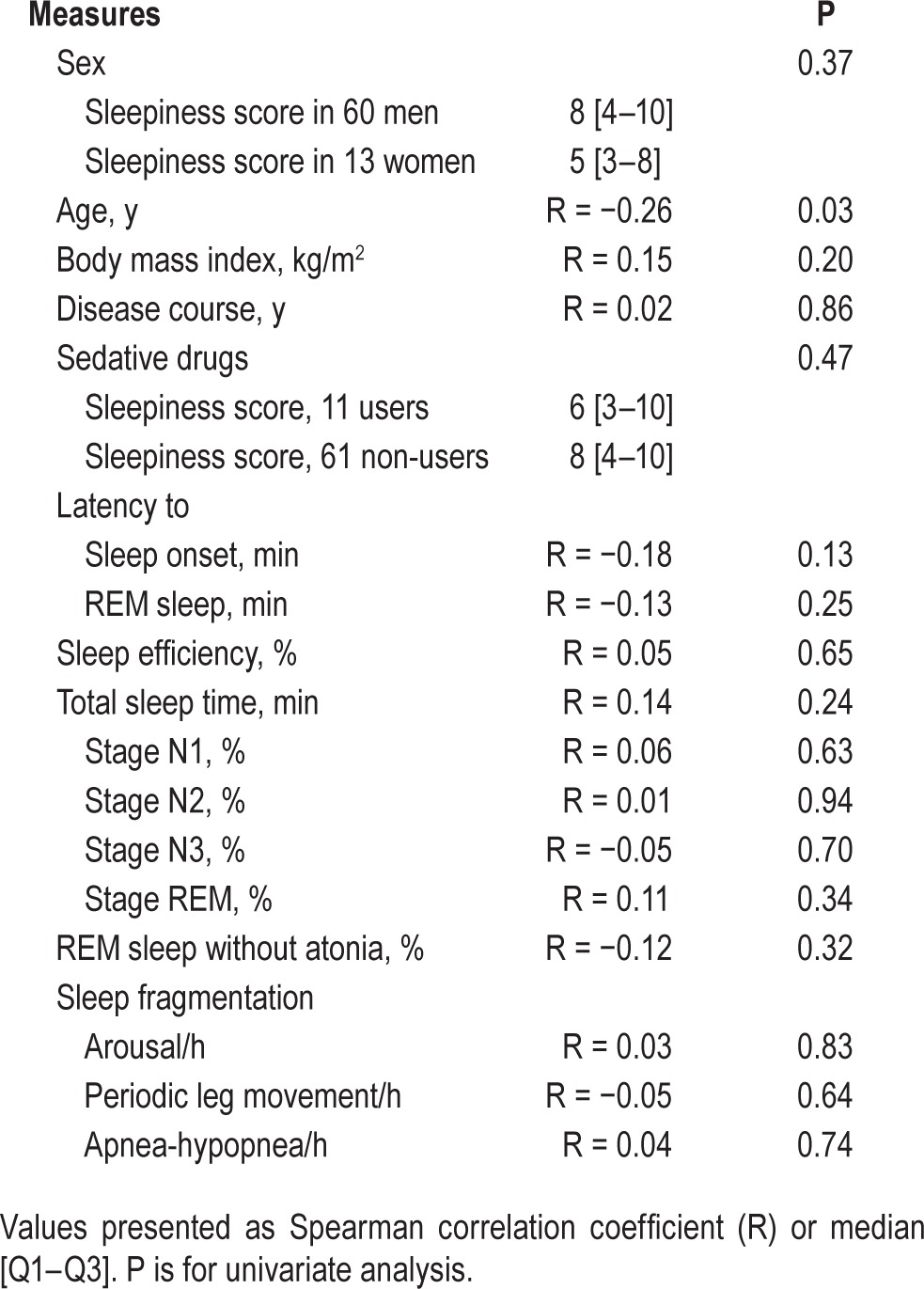
The ESS score was higher in the idiopathic RBD, than in the control group but not higher than in the PD groups (ESS: 8.7 ± 4.8) and not different in the PD with RBD and PD without RBD subgroups (Figure 1). Fifteen (20%) patients with idiopathic RBD (and 15% of patients with PD) vs. 5 controls (5%, P = 0.02) had an ESS score > 10, a threshold considered as abnormal. There were no differences for any sleep measures between idiopathic RBD and control groups, except for a lower percentage of N3 sleep and a higher percentage of REM sleep without atonia in the patients with idiopathic RBD (Table 1, Figure S1, supplemental material). The patients with idiopathic RBD had sleep measures similar to those of patients with PD and with and without RBD. Within the PD group, the patients with RBD had a higher percentage of REM sleep without atonia than the patients without RBD; there were no other differences. Within the idiopathic RBD group, none of the demographic (including sex) or sleep measures had any impact on daytime sleepiness except age (Table 2), as the ESS mildly decreased with age (r = −0.26, P = 0.03). In the PD group, none of the demographic, clinical (including the levodopa-equivalent daily dose), or sleep measures had any impact on daytime sleepiness, except that the level of sleepiness was higher when the patients were treated with a dopamine agonist (ESS: 9.8 ± 4.8) than when they were not (ESS: 6.1 ± 4.2, P = 0.01).
Figure 1.
Sleepiness in idiopathic REM sleep behavior disorder (RBD), controls, and Parkinson disease. The triangles correspond to the score of each patient at the Epworth Sleepiness Scale (mean: red horizontal line, median: red cross).
Table 2.
Determinants of daytime sleepiness (evaluated by the score on the Epworth sleepiness scale) in patients with idiopathic REM sleep behavior disorder.
Evolution toward Parkinsonism and Dementia
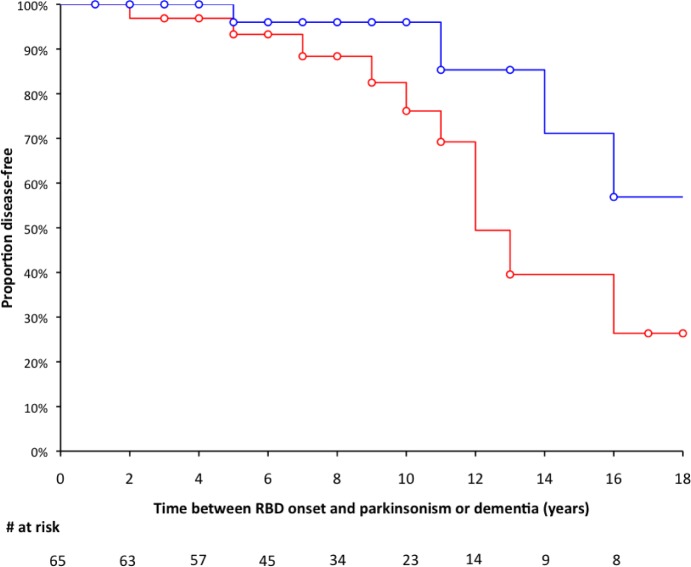
There was valid information in 2013 on the medical status of 69 of the 75 patients with idiopathic RBD (Figure 2). The 69 patients (56 men, 13 women) had been followed up for a median 3 y (range 1–15 y). Among the 69 patients followed up, 36 were examined in person by IA and SLS, 9 by another neurologist in the same hospital (files found in the neurological department), and 24 answered by phone that they were in good shape and had no motor or memory problems. From 1998 to 2013, 16 (15 men and 1 woman, 23.2%) patients had converted toward a disease, including parkinsonism (n = 6 men, 37.5% of the converted group, converted after a mean 9 years from RBD onset and 3 years from RBD diagnosis), dementia (n = 6, 5 men and 1 woman, 37.5%, converted after a mean 11 years from RBD onset and 2 years from RBD diagnosis), parkinsonism plus dementia (n = 2 men, 12.5%, converted after a mean 21 years from RBD onset and 1 year from RBD diagnosis), and multiple system atrophy (n = 2 men, 12.5%, converted after a mean 13 years from RBD onset and 3 years from RBD diagnosis); 53 patients remained disease-free at the time of last news. The median time from RBD onset to parkinsonism/dementia was 16 years (mean 19.5 y). In univariate analysis with Cox proportional hazard models, no clinical (age, sex, body mass index) and sleep measures (including total sleep time, sleep efficiency, sleep onset and REM sleep onset latencies, N1, N2, N3, and REM sleep percentages, but also the percentage of REM sleep without atonia, as well as arousal index, periodic leg movement index, and apnea-hypopnea index) except ESS were associated with a shorter time from RBD onset or RBD diagnosis to parkinsonism and dementia. These hazard functions remained nonsignificant when adjusted for age (for conversion time from RBD onset) and on age and time between RBD onset and diagnosis (for conversion time from RBD diagnosis, Tables S1–S4, supplemental material). In contrast, the Cox proportional hazard models indicated that an ESS > 8 (the median of the group) at time of RBD diagnosis predicted a shorter neurodegenerative conversion from RBD onset (unadjusted hazard ratio [HR]: 3.530; CI [1.163, 10.715], P = 0.026; adjusted HR: 4.232 [1.308–13.689], P = 0.016, Figure 3), and from RBD diagnosis, but this last one only after adjustment on age and particularly on time between RBD onset and diagnosis (unadjusted HR: 1.395 [0.517–3.761], P = 0.51; adjusted HR: 3.616 [1.061–12.330], P = 0.04). The ESS as a continuous variable shortened the conversion risk (adjusted HR: 1.119 [1.001–1.250], P = 0.048) from RBD diagnosis to neuro-degenerative disease. An ESS score > 10 at time of RBD diagnosis was not associated with a higher risk of developing parkinsonism or dementia.
Figure 2.
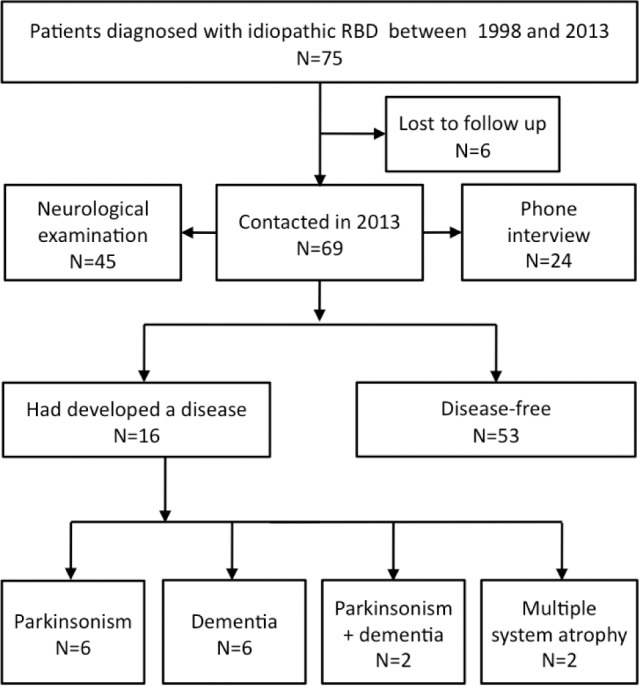
Flow chart of conversion towards parkinsonism and dementia in the patients with idiopathic RBD.
Figure 3.
The rate of conversion from idiopathic RBD to parkinsonism/dementia onset is influenced by initial sleepiness. Survival curve of patients with idiopathic RBD, as the time from the onset RBD of symptoms to the onset of parkinsonism or dementia, in patients scoring ≥ 8 (in red) or < 8 (in blue) on the Epworth Sleepiness Scale at the time of RBD diagnosis. Censored measures are represented as circles.
DISCUSSION
The 75 patients with idiopathic RBD were sleepier at time of RBD diagnosis than age- and sex-matched controls. They reached the values observed in patients with PD. The severity of sleepiness correlated only with younger age, but not with use of sedative drugs or sleep measures. An ESS greater than 8 at time of RBD diagnosis (but no other sleep measures) predicted a shorter time from RBD onset and RBD diagnosis to parkinsonism/ dementia (but this last one only after adjustment on age and particularly on time between RBD onset and diagnosis).
The patients with idiopathic RBD scored nearly 3 points higher on the ESS than the controls, highlighting the importance of the difference. In addition, 20% of them exceeded the usually abnormal threshold of 11 or greater on the ESS, suggesting a clinical impact of sleepiness in a subsample of patients with idiopathic RBD. Note, however, that the majority of patients with idiopathic RBD were not abnormally sleepy. Sleepiness in PD can cause driving accidents, with a higher risk when ESS is 7 or higher.1 In the PD group here, the patients taking a dopamine agonist were sleepier than those treated with levodopa alone, as already shown in several previous reports.29–31 One may note that patients with idiopathic RBD, despite not taking a dopamine agonist, had levels of sleepiness similar to those of patients with PD treated with levodopa and dopamine agonists. As the mean level of sleepiness was similar in idiopathic RBD and in patients with PD whether they had RBD or not, this suggests that sleepiness is a marker of PD rather than of RBD. Notably, sleepiness was higher in patients with than without probable RBD and early PD in a recent large series.32 Patients with early PD plus RBD probably share several common features with patients with idiopathic RBD.
The causes of sleepiness in individuals are various and may include non-restorative night-time sleep (when it is shortened or fragmented by arousals and respiratory events), use of sedative drugs, and lesions in arousal systems. In patients with idiopathic RBD, the sleep structure was similar to that of matched controls, except for lower percentage of N3 sleep (which was also observed in the PD groups and did not correlate with sleepiness) and higher chin muscle tone during REM sleep (which is one of the markers of RBD). Enacted dreams did not reduce REM sleep time or increase sleep fragmentation. Plus, none of the sleep measures correlated with the ESS. The use of sedative drugs was rare in all groups, and unrelated to sleepiness severity, suggesting that these drugs did not contribute to the ESS difference with controls. All in all, higher sleepiness in idiopathic RBD was unrelated to nocturnal sleep, sex, obesity, and use of sedative drugs. The inverse link between sleepiness and age (the older patients having the lower ESS value) should be viewed with caution, as the correlation coefficient is low.
One may imagine that idiopathic RBD is associated with concomitant lesions in arousal systems, as observed in PD. As patients with idiopathic RBD eventually develop PD, dementia with Lewy bodies and multiple system atrophy,33,34 one may compare the lesions in arousal systems known in these diseases. Neuronal loss has been found in arousal systems in the brainstem (locus coeruleus, raphe, periaqueductal gray matter, pedunculopontine nucleus) and in the hypothalamus (hypo-cretin-1/orexin-A neurons) in PD brains.35 In 3 patients with idiopathic RBD who developed dementia with Lewy bodies and PD, marked neuronal loss and α-synuclein deposits were found in several arousal systems, including the coeruleus/ subcoeruleus complex, the raphe nucleus, the pedunculopontine nucleus, and the hypothalamus.34 Of interest, the raphe and the coeruleus/subcoeruleus complex are affected early by α-synuclein deposits in preclinical PD stages.36 These data suggest that patients with idiopathic RBD have concomitant lesions in the pontine structures responsible for atonia during REM sleep and in those containing arousal systems. Alternatively, idiopathic RBD may signal a systemic loss of cholinergic neurons in the subcoeruleus complex (causing REM sleep without atonia), in the pedunculopontine nucleus (causing sleepiness), and in the cortex (causing cognitive impairment, not measured here). It has recently been shown that RBD in PD was associated with relative neocortical, limbic cortical, and thalamic cholinergic denervation but not with differential serotoninergic or nigrostriatal dopaminergic denervation.37
Eventually, patients with a higher ESS at time of RBD diagnosis were more likely to develop parkinsonism/dementia earlier in our cohort. As a consequence, an ESS greater than 8 should be viewed as a red flag for earlier conversion to parkinsonism/dementia in patients with idiopathic RBD, especially if the patients are old and have RBD symptoms for a long time. This result reinforces the findings that excessive sleepiness in elderly subjects leads more frequently to the development of PD or dementia in several longitudinal cohorts.7,8
This study has several limitations. It was based on a retrospective examination of medical files and was not constructed as a longitudinal, controlled study with yearly endpoints. A similar design was however successfully used in previous studies that also tracked patient trajectories in neurological departments,38,39 and is statistically founded.40 Only 8% of the sample was, however, lost to follow-up here. Depression, which can contribute to subjective sleepiness and may precede PD onset, was not measured here. Dementia may alter the quality of scoring the ESS, but patients with idiopathic RBD were not demented at time of completing the ESS. Mild cognitive impairment may impact on ESS, but this aspect could not be studied in our cohort. In absence of present cognitive tests in the 24 patients contacted by phone who declared no specific motor or cognitive complaint, we may miss some mild cognitive impairment that would indicate a higher risk of future Lewy body dementia. Eventually, the conversion from RBD onset is only measured in patients referred for idiopathic RBD, not in patients with PD who later reported that RBD symptoms preceded parkinsonism. Consequently, the rate of conversion towards parkinsonism/dementia is here an underestimate. The study design did not allow us to follow up on the ESS as a marker across time. The ESS is a subjective measure of sleepiness, whereas objective measures such as multiple sleep latency tests and maintenance of wakefulness tests would complete the measure of the whole sleepiness spectrum, and do not correlate well with the ESS.41 Notably, some patients with PD underscore their level of sleepiness on the ESS, while they fall asleep rapidly during the multiple sleep latency tests.42 The ESS had however excellent psychomotor properties in PD24 and is however recommended to evaluate sleepiness in PD.43 It is widely used, cheap, and valid, with results that are easily transposable in clinical practice. As the measure of ESS at time of polysomnography is a routine procedure in most sleep units, it would be interesting to check this association in the longitudinal cohorts of idiopathic RBD performed in other countries.
We found that sleepiness was more severe in patients with idiopathic RBD than in controls and that an ESS greater than 8 at the time of RBD diagnosis predicted a more rapid development of parkinsonism/dementia. These results suggest that sleepiness is an early marker of neuronal loss and α-synuclein deposits in brainstem arousal systems during the preclinical PD, dementia, and multiple system atrophy stages.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was funded in part by the NRJ12-Institut of France prize to Dr. Arnulf, the Inserm grant Nucleipark to Dr. Vidaihet, and the Institute of Neurosciences of Pitié-Salpêtrière, Paris. Dr. Arnulf received honoraria from UCB Pharma for speaking engagement and consultancy. Dr. Leu-Semenescu and Dr. Vidailhet had a paid speaking engagement from UCB Pharma. Dr. Cochen de Cock received funding for a trip by UCB Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Centre d'Investigation Clinique (CIC-Paris Est) for organizing the study of the patients with PD and for part of the recruitment of normal controls. Author contributions: Drs. Arnulf, Cochen de Cock, Leu-Semenescu, and Vidailhet included participants and performed the investigations. Dr. Neutel and Bastien Herlin collected measures in the participants' files. Dr. Golmard performed the statistical analyses. Drs. Arnulf and Neutel wrote the manuscript, which was co-edited by all other authors. Drs. Arnulf and Golmard have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had access to the data generated in the study including the statistical analysis and decided to submit the paper for publication.
ABBREVIATIONS
- EEG
electroencephalography
- EOG
electro-oculography
- EMG
electromyography
- ESS
Epworth Sleepiness Scale
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- PLM
periodic leg movements
- RBD
REM sleep behavior disorder
SUPPLEMENTAL MATERIAL
REM sleep without atonia in idiopathic RBD, controls, and PD patients with and without RBD. The dots correspond to the percentage of enhanced chin tonic activity during REM sleep in each patient (mean: red horizontal line, median: red cross)
Hazard ratio for clinical and sleep factors measured at RBD diagnosis, as predictors of conversion time from RBD symptom onset (unadjusted model).
Hazard ratio for clinical and sleep factors measured at time of RBD diagnosis, as predictors of conversion time from RBD symptom onset (model adjusted for age).
Hazard ratio for clinical and sleep measures at RBD diagnosis as predictors of conversion time from RBD diagnosis (unadjusted model).
Hazard ratio for clinical and sleep measures at RBD diagnosis as predictors of conversion time from RBD diagnosis (model adjusted for age and for time between RBD onset and RBD diagnosis).
REFERENCES
- 1.Hobson D, Lang A, Wayne Martin W, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson Disease. A survey by the Canadian Movement Disorder Group. JAMA. 2002;287:455–63. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 2.Arnulf I, Leu-Semenescu S. Sleepiness in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S101–4. doi: 10.1016/S1353-8020(09)70792-8. [DOI] [PubMed] [Google Scholar]
- 3.Moreno-Lopez C, Santamaria J, Salamero M, et al. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA Study) Arch Neurol. 2011;68:223–30. doi: 10.1001/archneurol.2010.359. [DOI] [PubMed] [Google Scholar]
- 4.Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc. 2010;58:480–6. doi: 10.1111/j.1532-5415.2010.02733.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77:875–82. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson's disease and sleepiness: an integral part of PD. Neurology. 2002;58:1019–24. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–6. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 8.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–7. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 2012;27:677–89. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- 10.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 11.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): 16 year update on a previously reported series. Sleep Med. 2013;14:744–48. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo A, Fernandez-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Cock VC, Vidailhet M, Leu S, et al. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007;130:450–6. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, et al. The locus coeruleus/subcoeruleus complex in rapid eye movement sleep behavior disorders in Parkinson's disease: a 3T MRI study. Brain. 2013;136:2120–9. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes A, Daniel S, Kilford L, Lees A. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. The International Classification of Sleep Disorders. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1rst Ed. Westchester, IL: American Academy of Sleep Medecine; 2007. [Google Scholar]
- 20.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35:835–47. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32:752–9. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johns MH. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 24.Hagell P, Broman JE. Measurement properties and hierarchical item structure of the Epworth Sleepiness Scale in Parkinson's disease. J Sleep Res. 2007;16:102–9. doi: 10.1111/j.1365-2869.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 25.Razmy A, Lang AE, Shapiro CM. Predictors of impaired daytime sleep and wakefulness in patients with Parkinson's disease treated with older (ergot) vs. newer (nonergot) dopamine agonist. Arch Neurol. 2004;61:97–102. doi: 10.1001/archneur.61.1.97. [DOI] [PubMed] [Google Scholar]
- 26.Oudiette D, Leu-Semenescu S, Roze E, et al. A motor signature of REM sleep behavior disorder. Mov Disord. 2012;27:428–31. doi: 10.1002/mds.24044. [DOI] [PubMed] [Google Scholar]
- 27.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson's disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304–8. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology. 2001;57:1392–6. doi: 10.1212/wnl.57.8.1392. [DOI] [PubMed] [Google Scholar]
- 30.Avorn J, Schneeweiss S, Sudarsky LR, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol. 2005;62:1242–8. doi: 10.1001/archneur.62.8.1242. [DOI] [PubMed] [Google Scholar]
- 31.O'Suilleabhain P, Dewey R. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson's disease. Arch Neurol. 2002;59:986–9. doi: 10.1001/archneur.59.6.986. [DOI] [PubMed] [Google Scholar]
- 32.Rolinski M, Szewczyk-Krolikowski K, Tomlinson PR, et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2014;85:560–6. doi: 10.1136/jnnp-2013-306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14:754–62. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 35.De Cock VC, Vidailhet M, Arnulf I. Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol. 2008;4:254–66. doi: 10.1038/ncpneuro0775. [DOI] [PubMed] [Google Scholar]
- 36.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 37.Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71:560–8. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 39.Toft M, Lilleeng B, Ramm-Pettersen, et al. Long-term efficacy and mortality in Parkinson's disease patients treated with subthalamic stimulation. Mov Dis. 2011:1931–4. doi: 10.1002/mds.23817. [DOI] [PubMed] [Google Scholar]
- 40.Copas A, Farewell V. Incorporating retrospective data into an analyisis of time to illness. Biostatistics. 2001;2:1–12. doi: 10.1093/biostatistics/2.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Arnulf I. Excessive daytime sleepiness and parkinsonism. Sleep Med Rev. 2005;9:185–200. doi: 10.1016/j.smrv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Merino-Andreu M, Arnulf I, Konofal E, Derenne JP, Agid Y. Unawareness of naps in Parkinson's disease and in disorders with excessive daytime sleepiness. Neurology. 2003;60:1553–4. doi: 10.1212/01.wnl.0000058905.71369.97. [DOI] [PubMed] [Google Scholar]
- 43.Hogl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov Disord. 2010;25:2704–16. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
REM sleep without atonia in idiopathic RBD, controls, and PD patients with and without RBD. The dots correspond to the percentage of enhanced chin tonic activity during REM sleep in each patient (mean: red horizontal line, median: red cross)
Hazard ratio for clinical and sleep factors measured at RBD diagnosis, as predictors of conversion time from RBD symptom onset (unadjusted model).
Hazard ratio for clinical and sleep factors measured at time of RBD diagnosis, as predictors of conversion time from RBD symptom onset (model adjusted for age).
Hazard ratio for clinical and sleep measures at RBD diagnosis as predictors of conversion time from RBD diagnosis (unadjusted model).
Hazard ratio for clinical and sleep measures at RBD diagnosis as predictors of conversion time from RBD diagnosis (model adjusted for age and for time between RBD onset and RBD diagnosis).