Abstract
Study Objectives:
Many people with Parkinson disease experience “sleep benefit”: temporarily improved mobility upon awakening. Here we used quantitative motor tasks to assess the influence of sleep on motor functioning in Parkinson disease.
Design:
Eighteen Parkinson patients with and 20 without subjective sleep benefit and 20 healthy controls participated. Before and directly after a regular night sleep and an afternoon nap, subjects performed the timed pegboard dexterity task and quantified finger tapping task. Subjective ratings of motor functioning and mood/vigilange were included. Sleep was monitored using polysomnography.
Results:
On both tasks, patients were overall slower than healthy controls (night: F2,55 = 16.938, P < 0.001; nap: F2,55 = 15.331, P < 0.001). On the pegboard task, there was a small overall effect of night sleep (F1,55 = 9.695, P = 0.003); both patients and controls were on average slightly slower in the morning. However, in both tasks there was no sleep*group interaction for nighttime sleep nor for afternoon nap. There was a modest correlation between the score on the pegboard task and self-rated motor symptoms among patients (rho = 0.233, P = 0.004). No correlations in task performance and mood/vigilance or sleep time/efficiency were found.
Conclusions:
A positive effect of sleep on motor function is commonly reported by Parkinson patients. Here we show that the subjective experience of sleep benefit is not paralleled by an actual improvement in motor functioning. Sleep benefit therefore appears to be a subjective phenomenon and not a Parkinson-specific reduction in symptoms.
Citation:
van Gilst MM, van Mierlo P, Bloem BR, Overeem S. Quantitative Motor Performance and Sleep Benefit in Parkinson Disease. SLEEP 2015;38(10):1567–1573.
Keywords: sleep, Parkinson disease, sleep benefit, pegboard task, finger tapping, nap
INTRODUCTION
Sleep disorders are highly prevalent among patients with Parkinson disease. However, there are also reports of Parkinson patients experiencing a beneficial effect of sleep. Upon awaking in the morning, many patients experience an improved mobility as if they are in a medication-induced “on” state, contrary to what would be expected after a night without medication. This intriguing phenomenon is known as “sleep benefit.”1 Some Parkinson patients are even able to delay or skip their morning dose of medication because of this sleep benefit.2 According to questionnaire studies, the prevalence of sleep benefit is consistently reported to be quite high, with 33% to 55% of Parkinson patients reporting to experience sleep benefit.2,3
Studies using objective measures of sleep benefit are limited in number so far. A few studies assessed motor performance using the Unified Parkinson's Disease Rating Scale (UPDRS) motor scale.4,5 In the Högl study, there was no difference in morning function between patients with and without subjective sleep benefit. However, comparisons between evening and morning status showed an improvement in motor function in those patients reporting sleep benefit, and a deterioration in patients without.5
Although widely used, the UPDRS strongly depends on the observer and is time-consuming. Attractive alternative measures for motor fuction are available, such as the pegboard dexterity test, which is a fast and sensitive instrument to objectively and quantitatively evaluate motor dysfunction in Parkinson disease.6–8 Additionally, bradykinesia can be quantified using an alternating finger tapping task (digitography).9 Both tests correlate well with the UPDRS III.7,10 Therefore, the pegboard dexterity test and finger tapping task appear to be excellent tools to assess the motor correlates of sleep benefit. In this study, we used these quantitative motor tasks to assess possible changes in motor function after a period of sleep in Parkinson patients. We compare and describe sleep-related changes in motor function in patients that report to experience sleep benefit, those who do not and in healthy elderly controls. We focus not only on nighttime sleep but also on daytime naps, and assess the quality and characteristics of the preceding period of sleep (using polysomnography) and subjective ratings of motor function and mood/vigilance to examine whether these are related to changes in motor functioning.
METHODS
Patients
Patients were recruited from a cohort of 240 Parkinson patients who completed both a questionnaire and a diary on sleep benefit. Patients filled out a daily symptom diary before and directly after sleep for 7 consecutive days, in which they subjectively rated different aspects of their motor functioning (see measures section for more details). A night with a positive change of at least one point (better functioning in the morning than in the evening) was regarded as a “sleep benefit-night.” The same approach was used for daytime naps. Patients were classified as having sleep benefit, when sleep benefit was present after ≥ 2 nights or 2 naps during the 7-day period. We alo used a questionnaire that asked patients whether or not they experienced any sleep benefit, based on the recently revised definition of sleep benefit, as follows: “Sleep benefit is the experience of a temporary decrease in Parkinson's symptoms upon awakening after a period of sleep (night or daytime), before drug intake; the patient is feeling as good as ‘on’ (or better).”11 More details on both the diary and questionnaire can be found in the paper describing the results of these instruments.12
We estimated a required number of subjects based on an effect size of one-third of the effect size to differentiate between healthy controls and early diagnosed Parkinson patients.8 With a correlation between consecutive pegboard test results of 0.93,7 an α value of 0.05 and β of 0.8, this yielded a required group size of approximately 17 evaluable subjects. We were able to include 18 Parkinson patients who clearly reported to experience a subjective improvement in motor functioning after night and/or daytime sleep. In addition, we selected 20 patients who reported no improvement at all after sleep.
We included patients with idiopathic Parkinson disease, defined according to the UK Brain Bank criteria, Hoehn & Yahr stage I-III. Exclusion criteria included current major psychiatric diagnosis, deep brain surgery for Parkinson disease, neurological disease other than Parkinson disease and chronic daily use of hypnotics. All patients were used to taking an afternoon nap, so they felt able to sleep during the nap period in the experiment.
Healthy Controls
Twenty healthy controls participated. These controls were recruited in the older healthy population (40–75 years old). Controls were not necessarily habitual nappers; however, they all indicated that they were able to sleep during the day. Exclusion criteria were current neurologic or psychiatric diagnosis and chronic daily use of hypnotics.
Consent
The study was approved by the institutional medical ethical committee. All participants gave their written informed consent before participating. Participants received a financial compensation for their participation in this study.
Study Design
Procedure
Figure 1 shows the study design. In the early evening, subjects arrived at the sleep lab. First they were trained extensively in the pegboard dexterity task and finger tapping task. Before bedtime, a test session was completed (test 1). All test sessions included the pegboard dexterity task, finger tapping task, and subjective rating scales on motor functioning and mood/vigilance, described below. The order of administration was counterbalanced across subjects.
Figure 1.
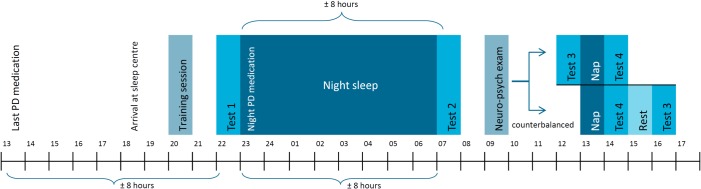
Schematic overview of the study design. At every test session the pegboard task, task switching task and motor and mood/vigilance rating scales were obtained. During the night and afternoon nap, sleep was monitored by polysomnography and video. During the neuropsychological examination the Mini mental state examination, Frontal assessment battery, Dutch adult reading test, and Beck depression inventory were obtained.
Subjects spent the night at the sleep lab. On awakening in the morning, a test session was completed (test 2). For all post-sleep measures, the tasks were administered 15 minutes after awakening to reduce the possible influence of sleep inertia. Later in the morning, a neuropsychological test battery was completed.
In the early afternoon, all subjects took an afternoon nap directly followed by a test session after awakening (test 4). We applied a crossover design, in which the reference examination (test 3) was done either before the nap or after an additional period of active wakefulness, when possible sleep benefit effects had disappeared. The order of examination was randomized and counterbalanced in all groups. The results of test 3 did not differ, either when this test was performed before the nap or after rest. Therefore, these data were pooled in the analyses.
Night Sleep
Subjects went to bed at 23:00 and slept until they woke up spontaneously to reduce the possible effects of residual sleepiness (sleep inertia) on morning testing. However, when not yet awake at 07:30, they were awakened by the experimenter. After 06:00, when a subject woke up and did not fall asleep again within 15 minutes, the night sleep period was terminated and post sleep tasks were started.
Nap
For the afternoon nap, subjects had a 90-min nap opportunity. The subjects slept until they woke up spontaneously or were awakened by the experimenter when the 90 min were over. When a subject slept a cumulative minimum of 15 min and did not fall asleep again within 15 min, the nap period was terminated and post sleep tasks were started.
Rest Period
During the rest period, subjects could perform their normal afternoon activities for 90 min. They were, for example, allowed to talk to the experimenter, read a book, or take a short walk, but they had to stay at the lab. Subjects were not allowed to sleep during the rest period.
Medication
On the day of arrival in the sleep lab for the night of the study, patients did not take their Parkinson medication after 13:00. When they arrived in the sleep lab, they were thus already in the “off'' state. After the pre-night test sessions, patients took their normal bedtime dose of Parkinson medication before the night (23:00), to increase the probability of a normal night of sleep. When patients did not take medication at bedtime, they postponed their evening medication (i.e., dinner time) to the night. This was the case for 10 patients (55.5%) with sleep benefit and 6 (30%) patients without sleep benefit. All dopaminergic medication was converted into the L-dopa equivalent dose (LED), using the formula described by Tomlinson et al.13 In the sleep benefit group, 16 patients were using levodopa and 9 patients (also) used dopamine agonists. In the group without sleep benefit, 19 patients used levodopa and 9 patients (also) used dopamine agonists. Both patients and controls were allowed to take other prescribed drugs. During the stay at the sleep lab, subjects were not allowed to drink any caffeine or alcohol containing drinks.
Measures
Motor Tasks
In the modified Perdue pegboard task, the time needed to place eight pegs from one hole to the next using one hand was measured. In the finger tapping task, the number of taps in 30 sec and the number of errors (pressing the 2 keys simultaneously) were registered. These finger tapping scores were automatically converted into a number of cycles (Hz). Both tasks were performed with both hands separately using a computer-based device (part of the At-Home Testing Device, Intel, courtesy of the Kinetics Foundation).9 Subjects practiced the tasks, alternating both hands, until scores reach asymptotic levels, with a minimum of 4 practice trials.
Subjective Assessment of Functioning after Sleep
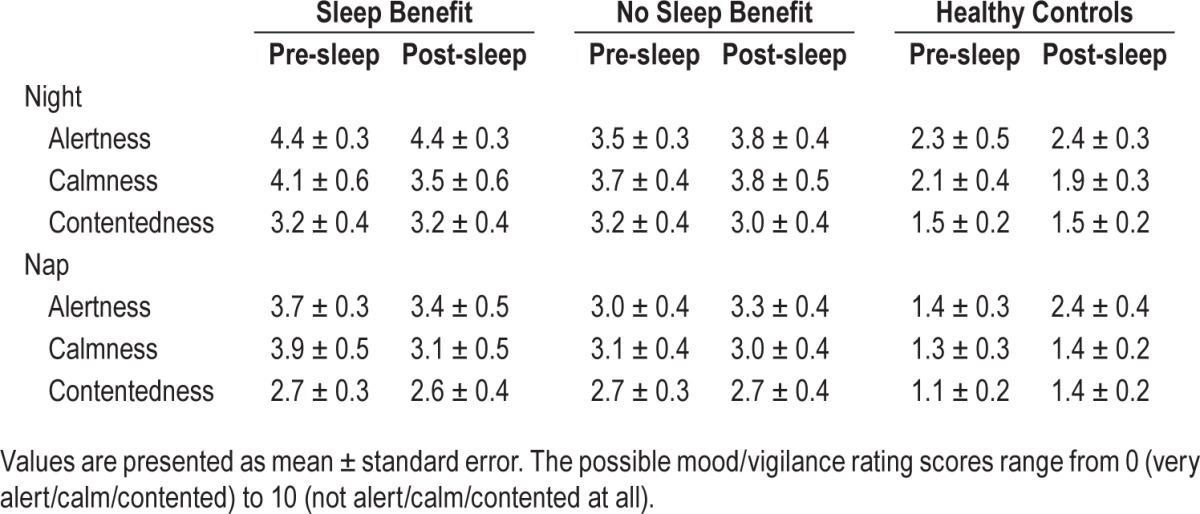
The symptom diary was based on the SCOPA Diary Card (SCOPA DC),14 a validated instrument to assess changes in motor functioning during the day. Patients indicated on a 4-point Likert scale how well they could perform 3 activities, i.e., walking, changing position, and using their hands, a higher score indicating more difficulties in performance. These activities were proven to give a reliable indication of general motor functioning.14 The scores on different items were added to obtain one subjective motor score. In addition, the question on sleep quality from the SCOPA DC was used and we added an extra question on feeling rested upon awakening. Mood/vigilance ratings were obtained using a 16-item visual analogue scale containing 3 factors: alertness, calmness, and contentedness.15
Neuropsychological Tasks
To asses baseline neuropsychological functions, a battery of tasks was used: Beck Depression Inventory (BDI),16 Mini-Mental State Examination (MMSE),17 Frontal Assessment Battery (FAB)18; and premorbid intelligence levels were assessed using the Dutch version of the National Adult Reading Test (DART).19,20
Polysomnography
All sleep was polysomnographically monitored using a dedicated recording system (Compumedics Grael PSG, Compumedics, USA). PSG registration included standard 6-channel electroencephalography and electromyography of m. submentalis. Sleep stages were scored according to the AASM 2007 criteria21 using ProFusion software (ProFusion sleep 3 [build 392], Compumedics, USA). The main sleep outcomes were total sleep time, sleep efficiency, and sleep latency.
Analyses
All analyses were performed separately for night sleep and afternoon nap. The motor tasks and subjective measures on motor function and mood/vigilance were analyzed using repeated measures ANOVA, with the between-subject factor “group” (subjective sleep benefit, no sleep benefit, healthy controls) and one within-subject factor “sleep” (before sleep vs. after sleep). Sleep benefit was defined as a group by sleep interaction in these ANOVA models. Correlation analyses were performed using partial correlations controlled for “group.” Alpha was set to 0.01 in all analyses regarding multiple comparisons. All analyses were performed in SPSS version 20 for Windows.
RESULTS
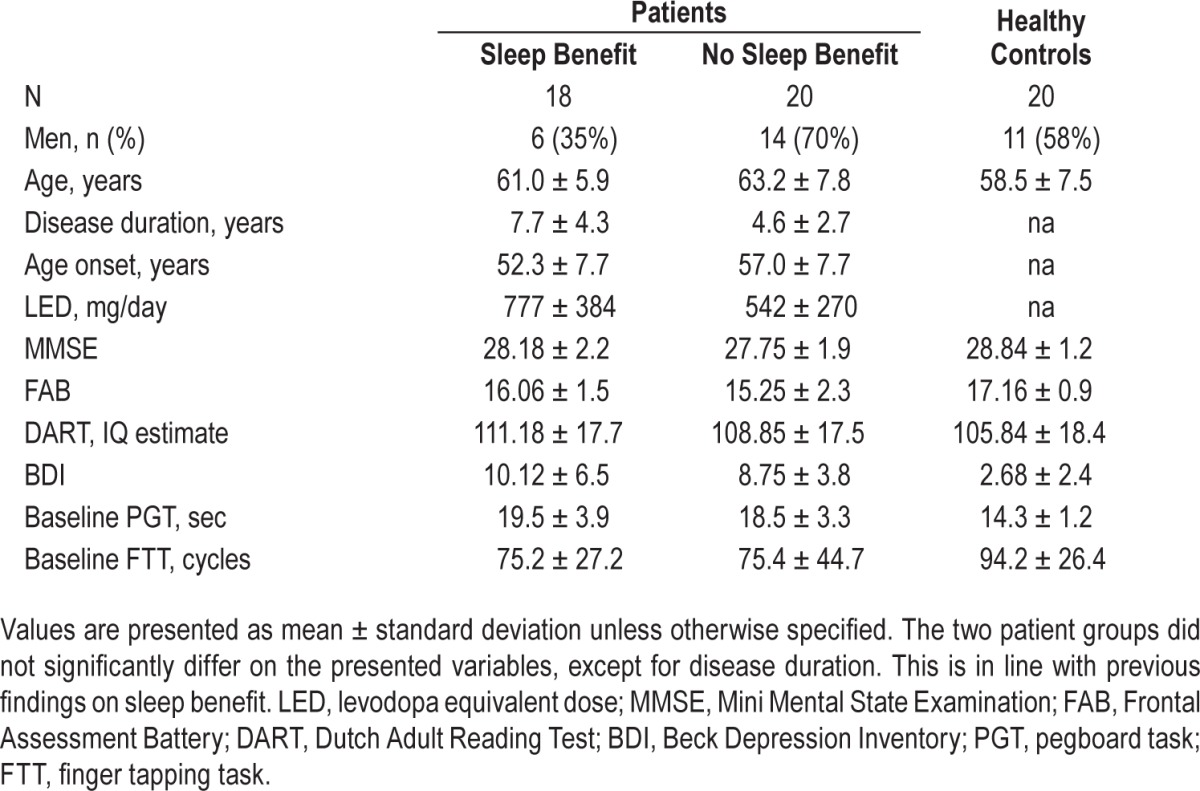
Characteristics of the participants, baseline motor scores, and scores on the neuropsychological examination can be found in Table 1. The patient groups did not differ from each other, except that patients with sleep benefit tended to have longer disease duration (t = 2.56, P = 0.015). There was a trend towards higher LED in the sleep benefit group (t = 2.046, P = 0.05). However, there were no between group differences in the proportions of patients using extended release L-dopa, dopamine agonists, or COMT inhibitors. Patients had higher scores on the Beck depression inventory than the healthy controls (healthy controls vs. sleep benefit t = 4.84, P < 0.001, healthy controls vs. no-sleep benefit t = 6.18, P < 0.001). The no-sleep benefit group scored slightly lower on the Frontal Assessment Battery (FAB) than healthy controls (t = −3.33, P = 0.002); however, the group reporting sleep benefit showed no significant differences on the FAB when compared to healthy controls.
Table 1.
Descriptive demographics and clinical disease characteristics of study sample.
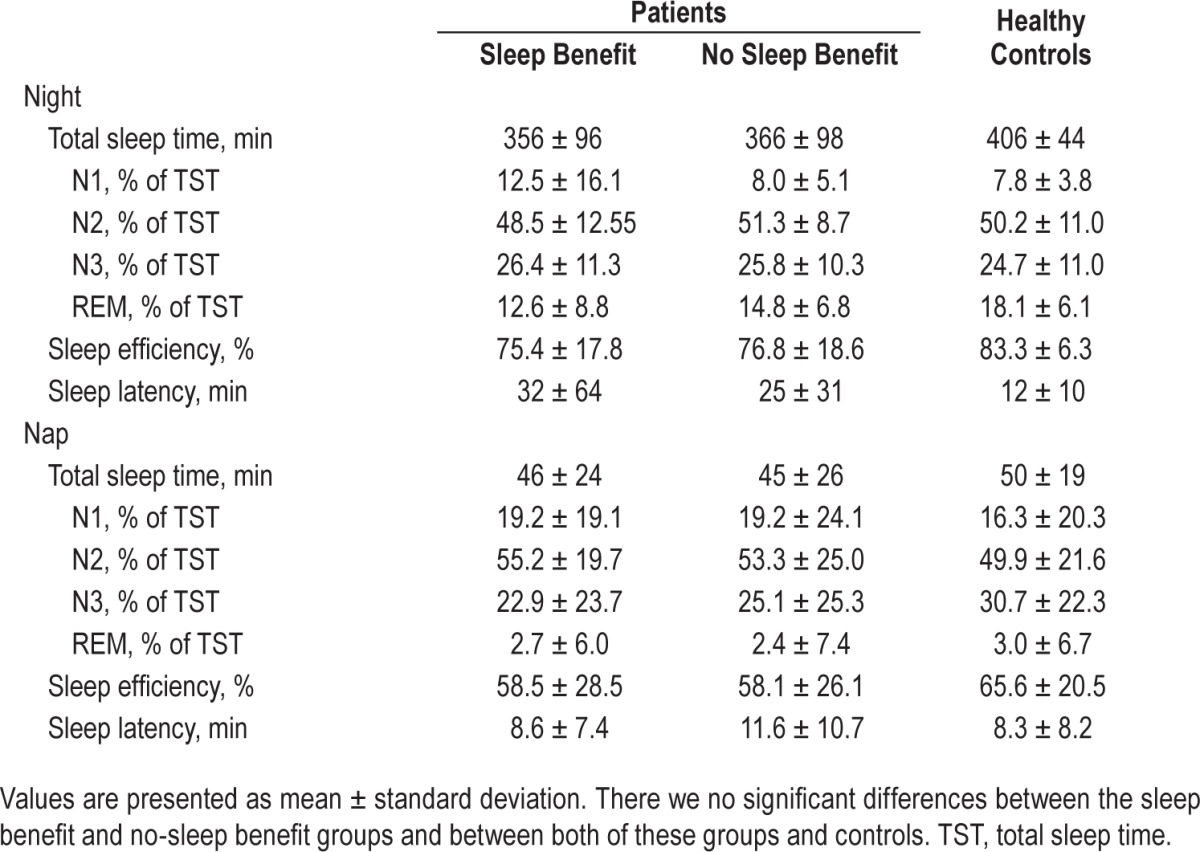
There were no between group differences in total sleep time, sleep stages, sleep efficiency, or sleep latency for night sleep nor afternoon nap (Table 2). There were also no differences in subjective ratings of sleep quality and feeling rested after sleep (night sleep and nap).
Table 2.
Sleep parameters.
Motor Tasks
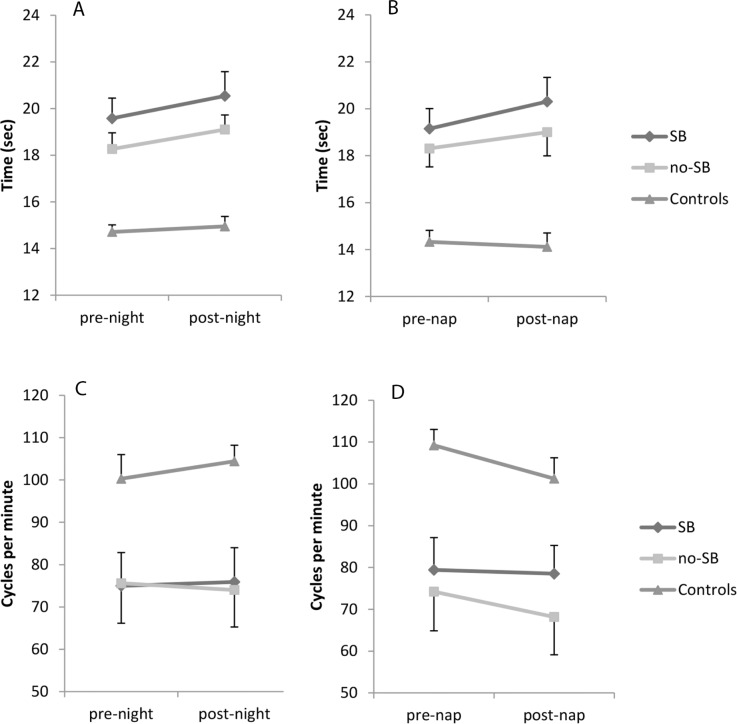
The results of the motor tasks can be found in Figure 2. There was a small overall effect of night sleep on the pegboard task (F1,55 = 9.695, P = 0.003); subjects were on average slower in the morning. However, for the finger tapping tasks, there was no overall difference in evening and morning performance. Patients were slower than healthy controls on both tasks (Pegboard: F2,55 = 16.938, P < 0.001, Finger tapping: F2,55 = 406.5, P < 0.001). For both tasks the overnight change in performance did not differ between the groups (no group*sleep interaction).
Figure 2.
Motor task scores. (A) Pegboard task night. (B) Pegboard task nap. (C) Finger tapping night. (D) Finger tapping nap. SB, sleep benefit.
For the afternoon nap, patients were again slower than healthy controls (Pegboard: F2,55 = 15.331, P < 0.001, Finger tapping: F2,55 = 450.3, P = 0.002). There was no significant overall effect of sleep on both tasks, nor was there a group difference in the change in performance (no group*sleep interaction). There was no correlation between sleep time or efficiency and the difference in scores on either of the motor tasks, for both night sleep and the afternoon nap, nor did any of the sleep architecture measures show such an effect.
We did a subgroup analysis with only the 11 patients that reported subjective sleep benefit in both the sleep benefit questionnaire and symptom diary, hypothesizing that these patients had the clearest subjective sleep benefit. However, when comparing this subgroup with patients without subjective sleep benefit and healthy controls, this yielded comparable results.
Subjective Measures
Motor Symptom Diary
Patients had higher (worse) scores in the motor symptom diary than healthy controls (night: F2,55 = 22.4, P < 0.001; healthy controls vs. sleep benefit P < 0.001, healthy controls vs no-sleep benefit P = 0.001; nap: F2,55 = 15.2, P < 0.001; healthy controls vs. sleep benefit P < 0.001, healthy controls vs. no-sleep benefit P = 0.01, see also Figure 3). Patients in the sleep benefit group tended to have higher (worse) diary scores than patients in the no sleep benefit group (night: P = 0.012 nap: P = 0.031, Figure 3). We did not find a main effect of sleep on subjective motor scores after either night sleep or afternoon nap. There was also no group difference in the over-sleep change in subjective motor function (no group*sleep interaction). The correlation between the subjective motor score and the pegboard test, calculated using all available timepoints (before and after night and daytime sleep) was rp = 0.43 (P < 0.001) and between the subjective motor score and the finger tapping test was rp = −0.30 (P < 0.0001).
Figure 3.
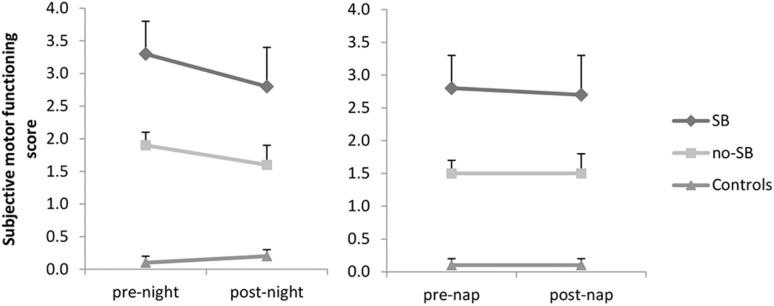
Subjective symptom diary scores. SB, sleep benefit.
Mood/Vigilance Rating Scales
There was no effect of sleep on the mood/vigilance scales for night sleep or afternoon nap. There was also no group difference in the over-sleep change in mood/vigilance (no group*sleep interaction). The healthy controls felt better than both patient groups (P < 0.005 for all scales, see also Table 3); however, the patient groups did not differ. The mood/vigilance rating scores did not correlate with the scores on the motor tasks.
Table 3.
Subjective measures.
DISCUSSION
The existence of subjective sleep benefit in Parkinson disease has been reported repeatedly in the literature.1–4,22–25 However, only few studies assessed whether sleep benefit is also associated with an objective improvement in motor symptoms. Here we used two quantitative motor tasks to assess sleep benefit in Parkinson patients. We showed that there was no objective sleep-related improvement in Parkinson signs in patients that declared to experience subjective sleep benefit. As expected, patients with and without subjective sleep benefit were slower on the quantified motor tasks than healthy controls. However, patients with or without sleep benefit did not differ in task performance after sleep.
We examined whether the lack of objective sleep benefit could be explained by the quality of the intervening sleep episodes. If “sleep benefit” is a real phenomenon, qualitatively better sleep should result in greater benefit for patients. Objective and subjective sleep quality did not differ between groups, and both nighttime sleep and afternoon naps were similar among groups. This shows that all groups achieved a good amount of sleep, excluding the possibility that lack of actual sleep explained the negative findings. Some studies suggested that sleep benefit might be related to poorer sleep.2,5,26 However, we found no support for this hypothesis. Taken together, our results suggest that the quality of the preceding period of sleep does not determine the occurrence of sleep benefit either way, at least not for the range of sleep qualities achieved in this experimental setting.
Patients with subjective sleep benefit may also simply feel better after sleep. However, we did not find differences in subjective mood/vigilance and subjective sleep quality among patient groups. Although there was no change in subjective motor scores after sleep in patients with or without subjective sleep benefit, patients with sleep benefit tended to rate their motor signs as worse than patients without sleep benefit. So, the diary scores were not simply a reflection of mood/vigilance at the moment of testing. Moreover, the quantitative motor performance correlated with the subjective motor evaluation whereas the other subjective measures did not correlate with quatitative motor performance.
During the study, patients were not allowed to take Parkinson medication, so they were in the “off” state. We chose this approach because medication could mask possible sleep benefit. Furthermore, the exact influence of medication on motor functioning is difficult to define. Because of the large variability in medication we decided to test all patients without medication. There was a trend towards more medication use in patients with sleep benefit, so they possibly experienced a greater medication withdrawal effect. However, this was not reflected by the quantitative motor scores, nor by the the mood/ vigilance scores.
The leading hypothesis on the mechanism of sleep benefit states that dopamine storages in nigral neuronal terminals are replenished during sleep.2,5,11 According to this hypothesis, the presence of sleep benefit should have been more clear without medication, as its effect would not be masked by dopaminergic medication. As we did not find an objective sleep benefit effect, this hypothesis seems less likely. It could also be that sleep benefit is an overnight medication effect, for example caused by extended release L-dopa or longer acting dopamine agonists. In our patients, there was a trend toward more daily L-dopa equivalent medication use in the sleep benefit group, but the use of different types of medication did not differ between patient groups. However, we acknowledge that our study was designed to assess the effect of sleep and not the effect of medication. Therefore, the exact influence of medication on (fluctuations in) motor function remains to be studied formally using a design dedicated to diurnal medication effects.
As sleep benefit is a temporary phenomenon,2,4,11 we decided not to use an additional clinical examination to assess Parkinson signs. When performing an extensive examination such as the UPDRS, possible sleep benefit effects could have disappeared before finishing the complete test. As the aim of this study was to assess differences in objective motor performance, we chose to use two quick, quantitative motor tests, which do correlate well with the UPDRS.7,10
We evaluated only one night of sleep and one daytime nap. There could be some day-to-day variation in the occurrence of sleep benefit. None of the patients reported improvement every night in the 7-day screening diary used for recruitment. So, even the patients with clear subjective sleep benefit had nights without improvement in the symptom diary. A longitudinal study including more nights and naps could account for this variation. There was some variability in results; in both patient groups there were patients who improved and patients who worsened on the motor tasks. However, the individual differences in performance over sleep were small, and even in the healthy control group there were subjects who improved and subjects who deteriorated a similar amount after a period of sleep. So the observed differences in performance are probably not Parkinson-specific, but represent a naturally occurring effect throughout the population.
It was shown before that patients with subjective sleep benefit sometimes seem to misperceive their motor functioning at awakening. Högl et al. reported that “several patients with sleep benefit praised their morning mobility as unrestrained […] although their overall appearance and objective evaluation using UPDRS were indicative of being in the “off” state.”5 Our results also showed that the subjective experience of sleep benefit did not match actual improvement in motor functioning. Our results combined with these previous findings offer further evidence that sleep benefit is mainly a subjective phenomenon that is not related to a Parkinson-specific improvement in motor function. Nevertheless, subjective sleep benefit is reported in the literature by a consistently high number of patients. The origin and underlying mechanism of this subjective sleep benefit should be the focus of future research.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a VIDI research grant to Dr. Overeem from the Netherlands Organization for Scientific Research (grant no. 016.116.371). Dr. Bloem has received research support from the Netherlands Organization for Scientific Research, Prinses Beatrix Fonds, Stichting Internationaal Parkinson Fonds, Alke-made Keuls Fonds, and Michael J Fox foundation (all unrelated to this study), and has consulted for Danone, Glaxo-Smith-Kleine, and UCB Pharma (all unrelated to this study). Dr. Overeem has received research support, consulted for, been on the speaker's bureau of, and received a travel grant from UCB Pharma (all unrelated to this study) The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Marsden CD. “On-off” phenomena in Parkinson's disease. In: Rinne U, Klinger M, Stamm G, editors. Parkinson's disease: current progress, problems and management. Amsterdam: Elsevier; 1980. pp. 241–54. [Google Scholar]
- 2.Merello M, Hughes A, Colosimo C, Hoffman M, Starkstein S, Leiguarda R. Sleep benefit in Parkinson's disease. Mov Disord. 1997;12:506–8. doi: 10.1002/mds.870120405. [DOI] [PubMed] [Google Scholar]
- 3.Currie LJ, Bennett JP, Jr., Harrison MB, Trugman JM, Wooten GF. Clinical correlates of sleep benefit in Parkinson's disease. Neurology. 1997;48:1115–7. doi: 10.1212/wnl.48.4.1115. [DOI] [PubMed] [Google Scholar]
- 4.Bateman DE, Levett K, Marsden CD. Sleep benefit in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:384–5. doi: 10.1136/jnnp.67.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Högl BE, Gomez-Arevalo G, Garcia S, et al. A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson's disease. Neurology. 1998;50:1332–9. doi: 10.1212/wnl.50.5.1332. [DOI] [PubMed] [Google Scholar]
- 6.Earhart GM, Cavanaugh JT, Ellis T, Ford MP, Foreman KB, Dibble L. The 9-hole PEG test of upper extremity function: average values, test-retest reliability, and factors contributing to performance in people with Parkinson disease. J Neurol Phys Ther. 2011;35:157–63. doi: 10.1097/NPT.0b013e318235da08. [DOI] [PubMed] [Google Scholar]
- 7.Haaxma CA, Bloem BR, Borm GF, Horstink MW. Comparison of a timed motor test battery to the Unified Parkinson's Disease Rating Scale-III in Parkinson's disease. Mov Disord. 2008;23:1707–17. doi: 10.1002/mds.22197. [DOI] [PubMed] [Google Scholar]
- 8.Haaxma CA, Bloem BR, Overeem S, Borm GF, Horstink MW. Timed motor tests can detect subtle motor dysfunction in early Parkinson's disease. Mov Disord. 2010;25:1150–6. doi: 10.1002/mds.23100. [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Stebbins GT, Wolff D, et al. Testing objective measures of motor impairment in early Parkinson's disease: feasibility study of an at-home testing device. Mov Disord. 2009;24:551–6. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor Tavares AL, Jefferis GS, Koop M, et al. Quantitative measurements of alternating finger tapping in Parkinson's disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord. 2005;20:1286–98. doi: 10.1002/mds.20556. [DOI] [PubMed] [Google Scholar]
- 11.van Gilst MM, Bloem BR, Overeem S. “Sleep benefit” in Parkinson's disease: a systematic review. Parkinsonism Relat Disord. 2013;19:654–9. doi: 10.1016/j.parkreldis.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 12.van Gilst MM, Bloem BR, Overeem S. Prospective assessment of subjective sleep benefit in Parkinson's disease. BMC Neurol. 2015;15:2. doi: 10.1186/s12883-014-0256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 14.Marinus J, Visser M, Stiggelbout AM, et al. Activity-based diary for Parkinson's disease. Clin Neuropharmacol. 2002;25:43–50. doi: 10.1097/00002826-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Bond A, Lader M. Use of Analog Scales in Rating Subjective Feelings. Brit J Med Psychol. 1974;47:211–8. [Google Scholar]
- 16.Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, Mchugh PR. Mini-Mental State-practical method for grading cognitive state of patients for clinician. J Psychiat Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB--a frontal assessment battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 19.Nelson HE, Oconnell A. Dementia--estimation of premorbid intelligence levels using new adult reading test. Cortex. 1978;14:234–44. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- 20.Schmand B, Bakker D, Saan R, Louman J. [The Dutch Reading Test for Adults: a measure of premorbid intelligence level] Tijdschr Gerontol Geriatr. 1991;22:15–9. [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Comella CL. Sleep disorders in Parkinson's disease: an overview. Mov Disord. 2007;22(Suppl 17):S367–73. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
- 23.Tandberg E, Larsen JP, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community-based study. Mov Disord. 1999;14:922–7. doi: 10.1002/1531-8257(199911)14:6<922::aid-mds1003>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.van Gilst MM, Louter M, Baumann CR, Bloem BR, Overeem S. Sleep benefit in Parkinson's disease: time to revive an enigma? J Parkinsons Dis. 2012;2:167–70. doi: 10.3233/JPD-2012-12087. [DOI] [PubMed] [Google Scholar]
- 25.Factor SA, Weiner WJ. ‘Sleep benefit’ in Parkinson's disease. Neurology. 1998;50:1514–5. doi: 10.1212/wnl.50.5.1514-b. [DOI] [PubMed] [Google Scholar]
- 26.Sherif E, Valko PO, Overeem S, Baumann CR. Sleep benefit in Parkinson's disease is associated with short sleep times. Parkinsonism Relat Disord. 2014;20:116–8. doi: 10.1016/j.parkreldis.2013.09.005. [DOI] [PubMed] [Google Scholar]