Abstract
Study Objectives:
The objectives of this study were to quantify the (1) sensitivity and specificity of nocturnal PSG SOREMP (REM latency ≤ 15 min) for narcolepsy in those being evaluated for hypersomnolence and (2) prevalence and predictors of SOREMP during baseline PSG for patients being evaluated for various sleep disorders.
Design:
This was a retrospective analysis of a large repository of de-identified PSG and MSLT test results from 2007 to 2013.
Setting and Patients:
Patient records were retrieved from a repository of studies completed at a variety of sleep laboratories across the USA. Included in the analyses were 79,651 general sleep clinic patients (without an MSLT; 48% male; 72% Caucasian) and an additional 3,059 patients (31.3% male; 72% Caucasian) being evaluated for hypersomnolence (with a consecutive MSLT).
Interventions:
NA.
Measurements and Results:
For patients being evaluated for hypersomnolence, the prevalence of PSG SOREMP increased in a dose-response fashion with the number of REM onsets that occurred on a consecutive MSLT (0.5% for no MSLT SOREMPs to > 33.0% for those with 5 MSLT SOREMPs). Overall, having a PSG SOREMP was highly specific (99.5%; 95% CI: 99.1–99.7%) but not sensitive (6.7%; 95% CI: 4.7–9.2%) for narcolepsy. The prevalence of PSG SOREMP for patients in the general sleep clinic sample (i.e., not being evaluated by a consecutive MSLT) was 0.8% and was much higher in those that work night/swing shift. In adjusted models, African American race contributed to the most variance in PSG SOREMP.
Conclusions:
A short onset rapid eye movement (REM) latency occurs rarely in general sleep clinic samples (< 1.0%), but is highly specific for the diagnosis of narcolepsy. Although rare, the prevalence of the phenomenon is much higher than the estimated prevalence of narcolepsy and may provide a critical opportunity for practitioners to identify narcolepsy in sleep clinic patients. These data also suggest that the utility of polysomnography (PSG) short onset REM peroid (SOREMP) for the diagnosis of narcolepsy may be altered by a history of shift/night work and/ or other factors that may allow for a rebound of REM sleep (e.g., undergoing a positive airway pressure titration), supporting published guidelines that other sleep disorders and insufficient and/or poorly timed sleep should be ruled out and/or adequately controlled for prior to conducting sleep testing. Further research is needed to understand racial differences in PSG SOREMP and narcolepsy. This study was limited in that data on cataplexy (with exception to that in final diagnosis) and habitual sleep duration were not available.
Citation:
Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. SLEEP 2015;38(10):1575–1581.
Keywords: narcolepsy, SOREMP, REM, PSG, MSLT
INTRODUCTION
Narcolepsy is a heterogenous disorder characterized by unstable sleep/wake regulation, excessive sleepiness, fragmented nocturnal sleep, and in some cases, REM-sleep intrusion into wakefulness (cataplexy, hypnogogic/hypnopompic hallucinations, and sleep paralysis1). Nocturnal symptoms include short sleep onset latency, reduced sleep efficiency and quality, and increased state transitions.2 When cataplexy is present, the disease is typically caused by the destruction of neuropeptide hypocretin neurons and is tightly linked to HLA DQB1*06:02 positivity.3,4 The prevalence of narcolepsy with and without cataplexy is rare (between 0.02% and 0.05% and approximately 0.2%, respectively5,6) and is associated with increased morbidity, reduced quality of life, and substantial medical costs.7,8 Narcolepsy without cataplexy is less well understood than narcolepsy with cataplexy and is often misdiagnosed as other sleep/wake conditions.9
Until recently, the diagnosis of narcolepsy in the absence of cataplexy as per the International Classification of Sleep Disorders, Second Edition (ICSD-2)9 has required a positive result on the (daytime) multiple sleep latency test (MSLT) with a mean sleep latency ≤ 8 minutes and ≥ 2 sleep onset REM periods (SOREMPs). However, mounting data has linked SOREMP on nocturnal polysomnography (PSG) to narcolepsy. This phenomenon was first quantified by Rechtschaffen and colleagues in 1963, when they found approximately 50% of narcoleptics had a PSG REM latency ≤ 10 minutes.10 More recently, Andlauer and colleagues11 found the prevalence of PSG SOREMP (defined as a REM latency ≤ 15 min) in patients with confirmed narcolepsy cases with cataplexy or hypocretin deficiency to range between 35.7% and 57.4%. These statistics are in stark contrast to the < 0.5% found in patients without narcolepsy.6,11 Moreover, data suggest that a PSG SOREMP is more specific (99.2% vs. 71.2%) and has a higher positive predictive value (92.1% vs. 75.0%) for confirmed narcolepsy cases with hypocretin deficiency than the MSLT.11
These data have prompted a nosology change for the diagnosis of narcolepsy where International Classification of Sleep Disorders, Third Edition (ICSD-3)12 allows for a SOREMP during PSG to count toward one of the ≥ 2 SOREMPs required on the subsequent MSLT. Even further, the DSM-5 suggests that a SOREMP during PSG is sufficient to confirm the diagnosis of narcolepsy without a series of MSLT naps.13 Although data have shown that a PSG SOREMP is highly specific for confirmed narcolepsy with hypocretin deficiency, information on prevalence and clinical correlates in other populations, such as clinic and general populations, is limited due to the sheer rarity of the phenomenon (4 in every 1,000 people).6,11 A recent study by Goldbart and colleagues of the Wisconsin Sleep Cohort (n = 4,866) found the prevalence of SOREMP during PSG to be only modestly associated with the previous night's sleep duration, but suggested that adequate power was not achieved due to a small sample size.6
This study had two objectives (1) to quantify the prevalence, sensitivity, and specificity of PSG SOREMP (defined as a REM latency ≤ 15 min) for narcolepsy in those being evaluated for hypersomnolence via a consecutive MSLT, and (2) to quantify the prevalence and predictors of SOREMP on baseline diagnostic PSG in a large clinical sample of patients being tested for a variety of sleep disorders.
METHODS
Sample Selection
Patient records of nocturnal PSGs completed between 2007 and 2013 were retrieved from SleepMed's repository of scored and physician interpreted records. Patients with a consecutive MSLT14 will hereafter be referred to as the “suspected hyper-somnia group” to distinguish from the “general clinic sample,” who were patients being evaluated for a wide range of sleep disorders (without a consecutive MSLT). If a patient had a repeat PSG and/or positive airway pressure (PAP) titration (12.3% of the sample), the earlier of the 2 (or more) PSGs was chosen for analysis to avoid multiple observations. For the purposes of this study, we excluded pediatric records (age < 18), as SOREMPs can be found in healthy infants and adolescents15 and may not represent sleep pathology, per se. Data were originally acquired using a variety of native (to the sleep center) sleep systems across the United States and were converted to European data format16 to allow for signal processing (see signal processing below) in a format that is independent of the acquisition system. The study protocol was approved by Schulman Associates IRB for the protection of human subjects.
Signal Processing
Raw sleep study data were processed using Morpheus, a technologist-edited automated signal processing software. The Morpheus system analyzes all biological signals acquired during a sleep study including electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), pressure and thermal airflow, thoracoabdominal movement, pulse oximetry, body position, and snoring. Morpheus decomposes EEG data into a 4-frequency state model (high frequency, low-frequency, and mixed frequency [low or high energy]) using adaptive segmentation with fuzzy clustering and feature extraction.17 Membership in the high-frequency domain is typical when the person is awake, whereas low-frequency typically occurs during N3. High energy mixed frequency is typical during N2 sleep. Low energy mixed frequency in the presence of relatively high EMG is scored as N1 whereas it is scored as REM if EMG tone is low. The EEG sleep stage scoring algorithm has shown good agreement compared to manual scoring of sleep staging (κ = 0.61–0.67) and fair agreement for REM (intraclass correlation coefficient; ICC = 0.72–0.76).17 However, because Morpheus, like other autoscoring techniques,18 has demonstrated difficulty when detecting REM onset latency (ICC = 0.43–0.46),17 technologists are instructed to verify the timing of the first REM period for each study as per standard.
Nocturnal sleep onset was defined as the first of 3 consecutive epochs of N1 or the first of any other stage of sleep. Diurnal sleep onset (MSLT) was defined as the first of any epoch of sleep.14 Latency to REM sleep was defined as the duration from sleep onset to the first epoch of REM. Respiratory events and limb movements were also autoscored using Morpheus and technologist edited as per a standard protocol.17 The apnea hypopnea index (AHI) was calculated as the average number of apneas and hypopneas (4% desaturation) per hour of sleep. The respiratory disturbance index (RDI) was calculated as the average number of apneas, hypopneas (4%), and RERAs (reduction in flow that terminated in an arousal or a 3% desaturation) per hour of sleep.
Statistical Analyses
Analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL). Descriptive analyses were completed to analyze the shape, central tendency, and dispersion of all variables. Differences in continuous variables between SOREMP and No-SOREMP groups were analyzed using univariate analysis of variance (ANOVA) with eta squared (η2) to estimate effect size. Differences in categorical variables were analyzed using χ2 analysis with phi coefficient (Φ) to estimate effect size. Variables that reached statistical significance (P < 0.05) in univariate analyses were further analyzed using an adjusted logistic regression analysis with Nagelkerke R2 to evaluate effect size. Sensitivity was defined as how often a PSG SOREMP indicated narcolepsy when the disease was truly present (calculated as TP × 100 / TP + FN), whereas specificity referred to how often a normal REM onset latency was observed when narcolepsy was indeed not present (calculated as TN × 100 / TN + FP). Positive predictive value was defined as the probability of having narcolepsy when PSG SOREMPs were present (calculated as TP × 100 / TP + FP) and negative predictive value referred to the probability of not having narcolepsy when a normal REM onset latency (> 15 min) was observed (calculated as TP × 100 / TP + FP).
RESULTS
Sample Differences and Confounders of PSG SOREMP
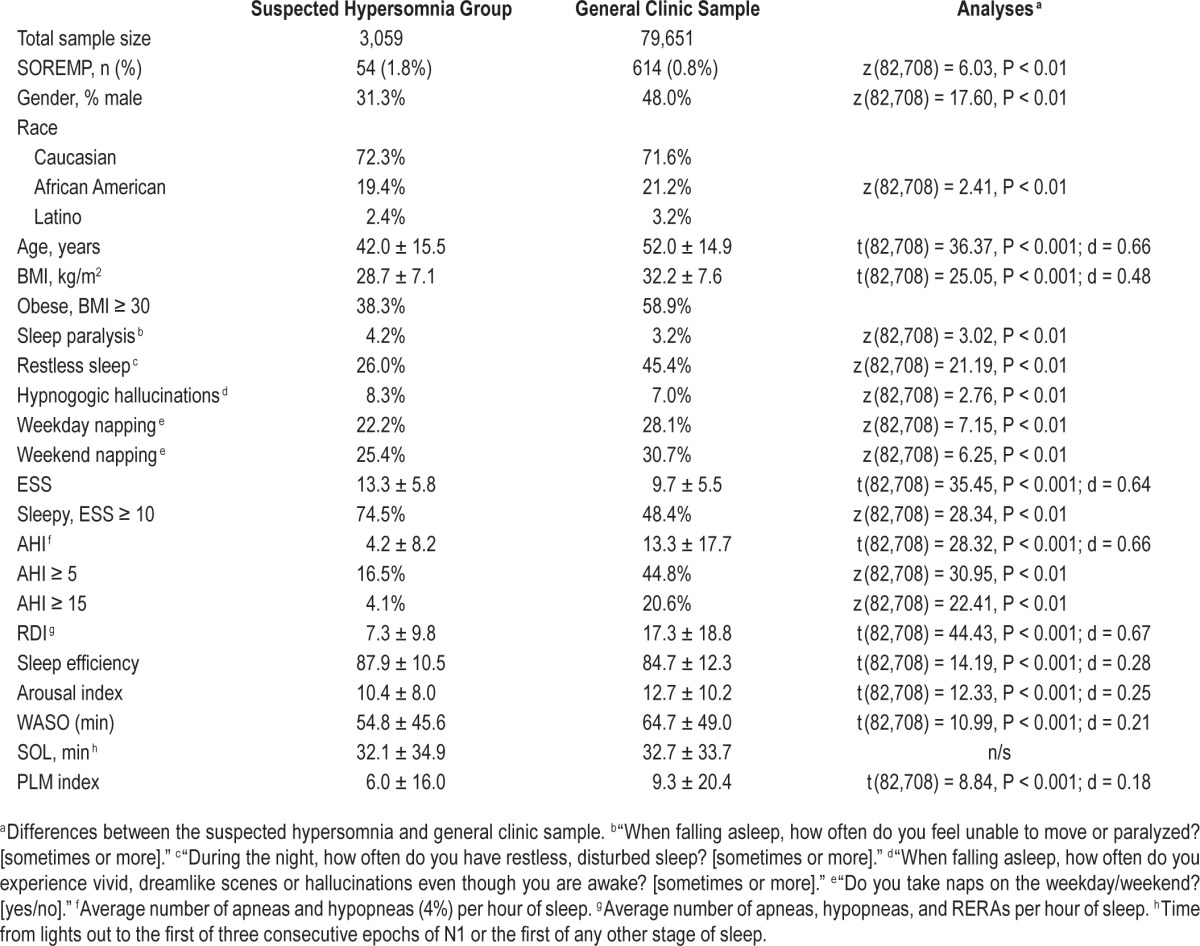
As Table 1 illustrates, patients in the suspected hypersomnia group (n = 3,059) were younger and leaner (BMI) compared to the general sleep clinic sample. The suspected hypersomnia sample was also comprised of mostly females, where there was a more even sex distribution in the general clinic sample. Also, not surprisingly, patients in the suspected hypersomnia group were less likely to have an indication of OSA or PLM. These sample differences should be kept in mind when interpreting the results as adjustment for age, sex, and BMI was not possible due to the nature of the datasets.
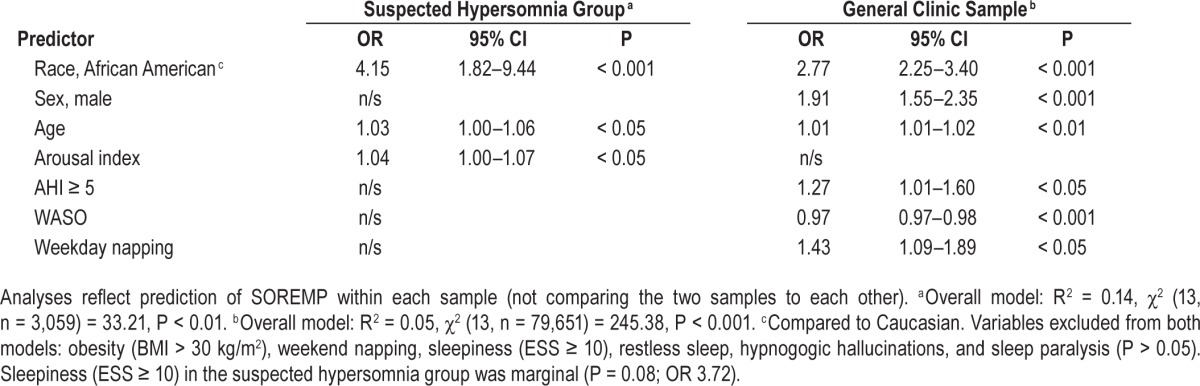
Table 1.
Demographics, self-report data, and study outcomes for patients with suspected hypersomnia and general sleep clinic patients.
The prevalence of a PSG SOREMP was much higher in those who worked nights and/or shift work compared to those who worked during the day. This finding was consistent for both the suspected hypersomnia sample (7.5% vs. 1.7%; OR: 4.4 [95% CI: 1.6–11.8]; χ2 (8, 3,520) = 32.8, P < 0.001) and the general clinic sample (2.1% vs. 0.9%; OR: 2.0 [95% CI: 1.6–2.7]; χ2 (1, 156,654) = 28.5, P < 0.001). Likewise, the prevalence of a PSG SOREMP was higher in patients undergoing a PAP titration in the general clinic sample (1.2% vs. 0.8%; OR: 1.3 [95% CI: 1.2–1.5]; χ2 (8, 156,654) = 19.7, P < 0.001). Based on these findings, patients with any of the above were excluded from final analyses to reduce confounding.
Objective 1: To quantify the prevalence, sensitivity, and specificity of PSG SOREMP (≤ 15 min) for narcolepsy
Overall, the prevalence of PSG SOREMP for those undergoing an MSLT the following morning was 1.8% (n = 54) and increased proportionally with the number of REM onsets on a consecutive MSLT (from < 0.5% for no MSLT REM to > 33.0% for those with 5 MSLT REMs; Figure 1). Of 54 patients with a PSG SOREMP, final diagnosis was available for only 48 patients. Of these 48 patients, 37 (73%) received a diagnosis of narcolepsy. The majority of narcolepsy diagnoses were without cataplexy (n = 33; 89%); only 4 patients were diagnosed with narcolepsy with cataplexy. Overall, having a PSG SOREMP was highly specific (99.5%; [95% CI: 99.1%–99.7%]) but not sensitive (6.7%; [95% CI: 4.7%–9.2%]) for narcolepsy. Positive and negative predictive values for PSG SOREMP were 77.9% (95% CI: 58.2%–84.7%) and 83.7% (95% CI: 82.4%–85.0%), respectively. Eight of the 48 patients with a PSG SOREMP received a diagnosis of hypersomnia, 3 of whom had only 1 REM onset on the MSLT (and hence may have potentially qualified for narcolepsy as per the ICSD-3).
Figure 1.
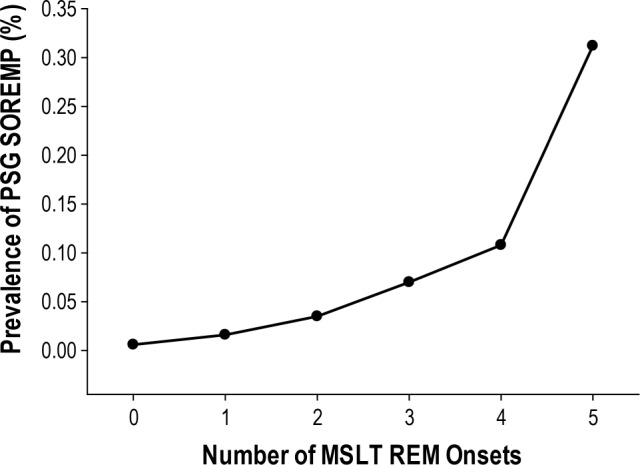
Prevalence of a short onset REM period (SOREMP) on PSG (latency ≤ 15 min) for those with 1, 2, 3, 4, and 5 REM onsets during consecutive MSLT naps
Compared to those with a normal REM latency, those with a PSG SOREMP were older, sleepier (as assessed by the ESS and sleep onset latency), had more indications of disturbed sleep (subjective and objective), and were more likely to have moderate to severe OSA (Table 2). Curiously, however, patients with a PSG SOREMP were not more likely to report some of the associated features of narcolepsy, including hypnogogic hallucinations, sleep paralysis, and increased WASO. The most robust predictor of a PSG SOREMP was African American race. Compared to Caucasians, African Americans were over 4 times as likely to have a PSG SOREMP, controlling for other significant variables in the model (Table 3). In addition to race, age and arousal index also explained unique variance, but effect sizes were generally small. In sum, age, race, and arousal index explained 12.0% of the variance in PSG SOREMP.
Table 2.
Univariate comparisons between SOREMP and No-SOREMP groups: patients with suspected hypersomnia and general sleep clinic patients.
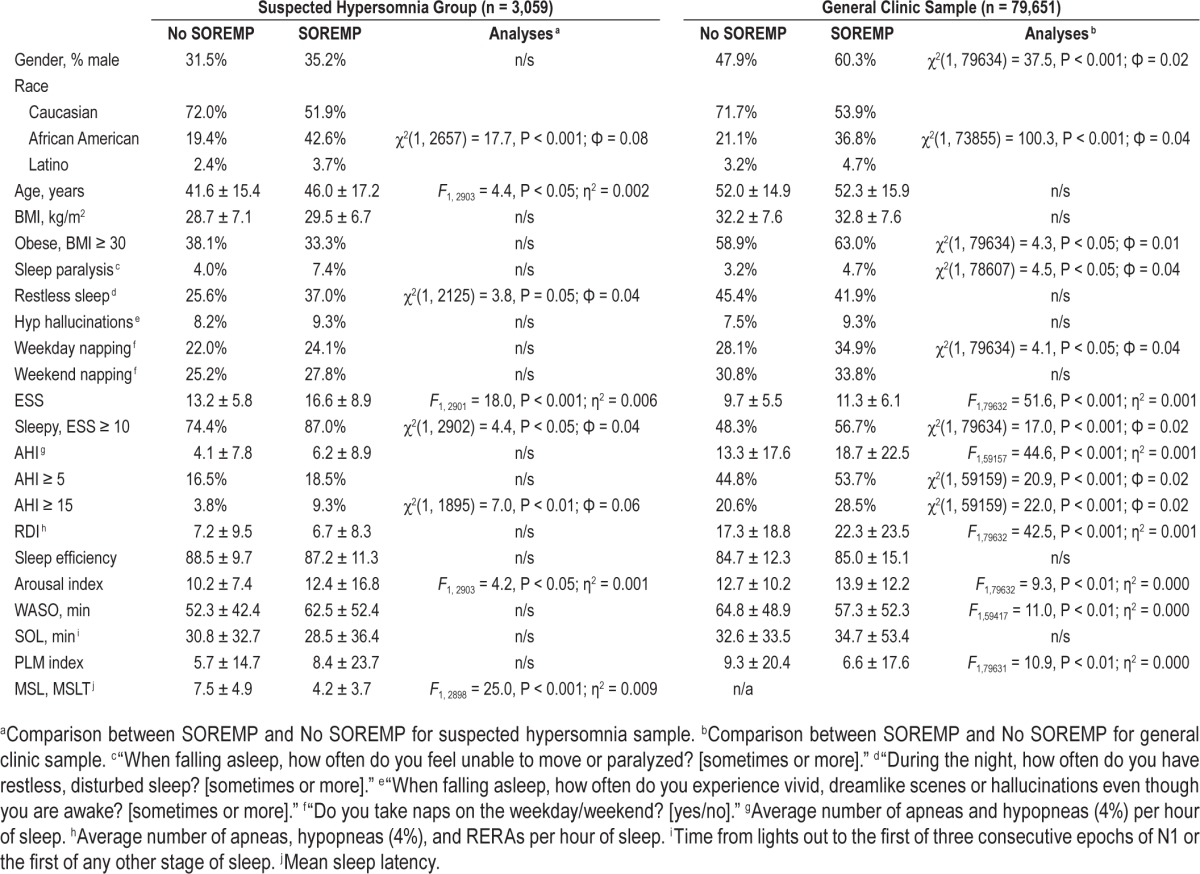
Table 3.
Adjusted logistic multiple regression predicting PSG SOREMP.
Objective 2: To quantify the prevalence and predictors of SOREMPs on baseline diagnostic PSG in patients being evaluated for a variety of sleep disorders
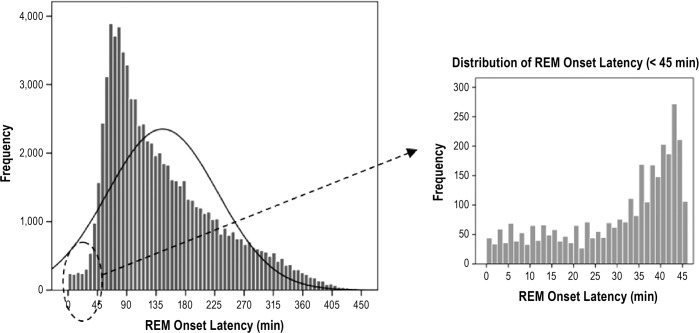
On average, REM occurred 145.1 ± 84.5 min after sleep onset with the bulk of patients having REM between 60 and 90 min after sleep onset (Figure 2). The prevalence of PSG SOREMP in this sample was 0.8% (Table 1). Similar to those in the suspected hypersomnia sample, those with a PSG SOREMP in the general clinic sample were sleepier (as assessed by the Epworth Sleepiness Scale) and had an elevated arousal index (Table 2). Additionally, in this sample, patients with a PSG SOREMP were also more likely to report regular weekday napping and sleep paralysis compared to those with a normal REM onset latency. Similar to the suspected hyper-somnia group, the most robust predictor of a PSG SOREMP was African American race. Compared to Caucasians, African Americans were 2.8 times more likely have a PSG SOREMP, controlling for other significant variables in the model (Table 3). Also, in this sample, male sex, napping, WASO, and OSA accounted for unique variance; however, total variance explained was small at 5%.
Figure 2.
Histogram of REM onset latency (ROL) in 79,651 patients being evaluated by diagnostic PSG for various sleep disorders. Mean ROL was 145.1 ± 84.5 minutes
DISCUSSION
A short onset REM latency, defined as 15 minutes or less, occurs rarely in general sleep clinic samples (< 1.0%), but is highly specific for narcolepsy, supporting previous data by Andlauer and colleagues.11 Although rare, the prevalence of the phenomenon is much higher than the estimated prevalence of narcolepsy and may provide a critical opportunity for practitioners to identify narcolepsy in sleep clinic patients. These data also suggest that the utility of PSG SOREMPs for the diagnosis of narcolepsy may be altered by a history of shift/ night work and/or other factors that may allow for a rebound of REM sleep (e.g., undergoing a PAP titration). It has been well established that those working shifts have an increased false positive rate on the MSLT6,19 and is intuitive, as shift/ night workers are generally chronically sleep restricted due to circadian misalignment and irregular work vs. non-work sleep schedules. This supports published guidelines that other sleep disorders and insufficient and/or poorly timed sleep should be ruled out or adequately controlled for prior to conducting a sleep study.14
For patients being evaluated for suspected hypersomnia, the specificity of a PSG SOREMP for narcolepsy was very high at 99.5%. This statistic is almost identical to that found in a sample of 516 patients with confirmed narcolepsy (99.2%).11 These findings, coupled with the discovery that the prevalence of PSG SOREMPs increased in proportion to the frequency of REM onsets on a consecutive MSLT and that adjusted predictive models only explained a small amount of variance in PSG SOREMP (i.e., something else appeared to be explaining the phenomenon), support the salience of PSG SOREMP in the detection of narcolepsy. Further, the rate of false positives was low at 0.7%, suggesting that it is unlikely for a patient with a PSG SOREMP not to be diagnosed with narcolepsy. The sensitivity of PSG SOREMP for narcolepsy, however, is very low, suggesting that patients with narcolepsy often have a normal latency to REM; i.e. ≥ 93% of narcolepsy cases would be “missed” if using only PSG SOREMPs as a diagnostic indicator. It is important to note, however, that the sensitivity of a PSG SOREMP in our sample (6.7%) was much lower than that found in Andlauer et al. (50.6%) and may be due to differences in scoring techniques (automated/human-supervised vs. manual as in Andlauer et al.), or, more likely, sample characteristics. Most notably, our clinic-based narcolepsy sample was comprised of a majority of cases without cataplexy (for example only 8.3% of the suspected hypersomnia sample with a SOREMP at night had documented cataplexy), while the sample of Andlauer et al. included confirmed narcolepsy cases with cataplexy or documented hypocretin deficiency.
Interestingly, some but not all characteristics of narcolepsy were present in patients with a PSG SOREMP, including some indications of disturbed nocturnal sleep (shortened sleep onset, increased WASO, and reduced sleep efficiency). These findings are curious as narcolepsy has been characterized by an incomplete sleep-wake switch resulting in rapid state transitions and poor sleep continuity.2 These data warrant further exploration, but may be due to a variety of reasons like the scoring method used for these data, sample heterogeneity, sample comparator, and statistical power. Generally speaking, all comparisons were in the “expected direction,” but failed to reach statistical significance. This could be because the PSG SOREMP sample was comprised of a heterogeneous mix of patients with narcolepsy with and without cataplexy, hypersomnolence, and other sleep/wake and health conditions. Likewise, statistical comparisons were made to patients with varying degrees of sleep/wake and medical conditions including sleep apnea and other sleep related breathing disorders, which can also interrupt sleep continuity. Lastly, it is also possible that our autoscoring technique was not sensitive enough to detect frequent stage shifts. Other clinical indicators that failed to yield consistent predictive results included symptoms of hypnogogic hallucinations and sleep paralysis. This may be due to a variety of reasons, including those cited above, as well as measure-related limitations including how the questions assessed such symptoms and/or how the question was dichotomized (“at least sometimes”). It is also important to note that although hypnogogic hallucinations and sleep paralysis are associated features of narcolepsy, they do not occur in all patients with narcolepsy. In fact, hypnogogic hallucinations have been estimated to occur in only 28% of patients with narcolepsy without cataplexy.20
Special attention should be given to the profound racial differences in the prevalence of PSG SOREMPs. Controlling for other factors that were shown to influence SOREMPs, African Americans in both samples were approximately 3 to 4 times more likely to have a PSG SOREMP than their Caucasian counterparts. Ultimately, these differences require further investigation as there may be racial differences in narcolepsy21–23 and could offer an important opportunity for narcolepsy to be diagnosed where it may have otherwise gone undetected. For example, data have suggested that African Americans with narcolepsy are generally less likely to present with cataplexy and may be more likely to present with “atypical” cataplexy symptoms (i.e., cataplexy in response to negative as opposed to positive emotions) despite being at a 20-fold higher risk for low CSF hypocretin than Caucasians.24,25 However, our findings could also be due to other confounding variables not assessed in this study, such as differences in sleep timing and/or habitual sleep duration.6,24 For example, a large national sample of 29,818 adults suggested that African Americans were at a 35% increased risk for “extreme” sleep durations—both short (≤ 5 h) and long (≥ 9 h).24 Similar racial differences have even been found in young children.26
All conclusions should be made in consideration of the study's limitations. A main limitation included not having access to information on cataplexy (the best clinical indicator of hypocretin deficiency), with the exception of that which was available in the final diagnosis. Second, another significant limitation was that data on habitual sleep duration was not available. It is well known that inadequate and/or poorly timed sleep can increase the likelihood of premature REM onsets, both during diurnal sleep27 and nocturnally.6 For example, according to a study by Golbart and colleagues, every hour decrease in sleep the night prior was associated with a 34% increase in odds of having a nocturnal SOREMP.6 Also, it is important to note that because very little variance was accounted for by adjusted analyses suggests that “something else” was driving the phenotype. Of course, this could be the true pathology of narcolepsy or it could be other variables, like habitual sleep duration/ timing, medication, and/or other unknown factors. Narcolepsy is often comorbid with several health and psychiatric conditions,28 and medications for the aforementioned conditions often have REM-suppressing and/or other sleep architecture altering effects.29,30 Also, it is possible that some of these patients were using wake promoting therapeutics or stimulants, which can alter sleep architecture. Lastly, there were limitations associated with our scoring of sleep data. Because these data were processed using a human-edited autoscoring technique, it is not impossible that a sleep onset REM period, especially if it is brief and in the absence of atonia (which is not uncommon for patients with narcolepsy31), may have been missed altogether. These data support the importance of clinical correlation and patient follow-up with a practitioner trained in sleep medicine for patients being evaluated for all sleep disorders, especially hypersomnolence and narcolepsy.
DISCLOSURE STATEMENT
This study was funded by Jazz Pharmaceuticals, Inc. Drs. Bogan and Cairns have received research support from Jazz Pharmaceuticals and are employed by SleepMed, Inc. Dr. Bogan also serves on the speakers bureau for and is a consultant to Jazz Pharmaceuticals. Study Institution: SleepMed, Inc., Columbia, SC.
REFERENCES
- 1.Guillemault C, Cao MT. Narcolepsy: diagnosis and management. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5th ed. St. Louis, MO: Elsevier,; 2011. pp. 957–67. [Google Scholar]
- 2.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9:955–65. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human Narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 4.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 5.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5th ed. St. Louis, MO: Elsevier; 2011. pp. 694–715. [Google Scholar]
- 6.Goldbart A, Peppard P, Finn L, et al. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep. 2014;37:1043–51. doi: 10.5665/sleep.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignatelli L, Plazzi G, Peschechera F, Delaj L, D'Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12:19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Jennum P, Knudsen S, Kjellberg J. The economic consequences of narcolepsy. J Clin Sleep Med. 2009;5:240–5. [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. [Google Scholar]
- 10.Rechtschaffen A, Wolpert E, Dement W, Mitchell S, Fisher C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neurophysiol. 1963;15:599–609. doi: 10.1016/0013-4694(63)90032-4. [DOI] [PubMed] [Google Scholar]
- 11.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70:891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing Inc.; 2014. [Google Scholar]
- 14.Littner M, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 16.Kemp B, Värri A, Rosa AC, Nielsen KD, Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr Clin Neurophysiol. 1992;82:391–3. doi: 10.1016/0013-4694(92)90009-7. [DOI] [PubMed] [Google Scholar]
- 17.Pittman SD, MacDonald MM, Fogel RB, et al. Assessment of automated scoring of polysomnographic recordings in a population with suspected sleep-disordered breathing. Sleep. 2004;27:1394–403. doi: 10.1093/sleep/27.7.1394. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra A, Younes M, Kuna ST, et al. Performance of an automated polysomnography scoring system versus computer-assisted manual scoring. Sleep. 2013;36:573–82. doi: 10.5665/sleep.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain. 2006;129:1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 20.Baumann CR, Mignot E, Lammers GJ, et al. Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. Sleep. 2014;37:1035–42. doi: 10.5665/sleep.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longstreth W, Than Ton G, Koepsell T, Gersuk V, Hendrickson A, Velde S. Prevalence of Narcolepsy in King County, Washington, USA. Sleep Med. 2009;10:422–6. doi: 10.1016/j.sleep.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andlauer O, Moore H, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–55. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koepsell TD, Longstreth WT, Ton TG. Medical exposures in youth and the frequency of narcolepsy with cataplexy: a population-based case-control study in genetically predisposed people. J Sleep Res. 2010;19:80–6. doi: 10.1111/j.1365-2869.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the national health interview survey. J Natl Med Assoc. 2008;100:317–22. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 25.Okun M, Lin L, Pelin Z, Hong S, Mignot E. Clinical aspects of narcolepsy-cataplexy across ethnic groups. Sleep. 2002;25:27–35. doi: 10.1093/sleep/25.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115:225–32. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marti I, Valko PO, Khatami R, Bassetti CL, Baumann CR. Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 2009;10:1146–50. doi: 10.1016/j.sleep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Ohayon M. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–92. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Pagel JF, Parnes BL. Medications for the treatment of sleep disorders: an overview. Prim Care Companion J Clin Psychiatry. 2001;3:118–25. doi: 10.4088/pcc.v03n0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayers A, Baldwin D. Antidepressants and their effect on sleep. Hum Psychopharmacol. 2005;20:533–59. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
- 31.Dauvilliers Y, Rompré S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]