Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with the progression of nonalcoholic fatty liver disease (NAFLD). We hypothesized that the hypoxia of OSA increases hepatic production of lysyl oxidase (LOX), an enzyme that cross-links collagen, and that LOX may serve as a biomarker of hepatic fibrosis.
Design:
Thirty-five patients with severe obesity underwent liver biopsy, polysomnography, and serum LOX testing. A separate group with severe OSA had serum LOX measured before and after 3 mo of CPAP or no therapy, as did age-matched controls. LOX expression and secretion were measured in mouse hepatocytes following exposure to hypoxia.
Setting:
The Johns Hopkins Bayview Sleep Disorders Center, and the Hypertension Unit of the Heart Institute at the University of São Paulo Medical School.
Measurements and Results:
In the bariatric cohort, the apnea-hypopnea index was higher in patients with hepatic fibrosis than in those without fibrosis (42.7 ± 30.2 events/h, versus 16.2 ± 15.5 events/h; P = 0.002), as was serum LOX (84.64 ± 29.71 ng/mL, versus 45.46 ± 17.16 ng/mL; P < 0.001). In the sleep clinic sample, patients with severe OSA had higher baseline LOX than healthy controls (70.75 ng/mL versus 52.36 ng/mL, P = 0.046), and serum LOX decreased in patients with OSA on CPAP (mean decrease 20.49 ng/mL) but not in untreated patients (mean decrease 0.19 ng/mL). Hypoxic mouse hepatocytes demonstrated 5.9-fold increased LOX transcription (P = 0.046), and enhanced LOX protein secretion.
Conclusions:
The hypoxic stress of obstructive sleep apnea may increase circulating lysyl oxidase (LOX) levels. LOX may serve as a biomarker of liver fibrosis in patients with severe obesity and nonalcoholic fatty liver disease.
Citation:
Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, Torbenson MS, Pedrosa RP, Lorenzi-Filho G, Steele KE, Schweitzer MA, Magnuson TH, Lidor AO, Schwartz AR, Polotsky VY. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. SLEEP 2015;38(10):1583–1591.
Keywords: hepatocyte, hypoxia, nonalcoholic fatty liver disease, sleep disordered breathing
INTRODUCTION
Obstructive sleep apnea (OSA) is a disease of recurrent upper airway closure during sleep. One cardinal manifestation of OSA is chronic intermittent hypoxia (IH).1 It has been suggested that the IH of OSA may play a role in the progression of nonalcoholic fatty liver disease (NAFLD).2 NAFLD is a disease of the liver related to insulin resistance and the metabolic syndrome, and is characterized by hepatic steatosis without an identifiable secondary cause such as drug toxicity or chronic alcohol use.3 In some patients with NAFLD, the disease phenotype will progress to one of liver injury, inflammation, and fibrosis, termed nonalcoholic steatohepatitis (NASH). Several associative studies have noted that markers of liver injury and fibrosis are more pronounced in patients with more severe OSA, and that this worsening appears related to the burden of nocturnal hypoxia experienced by a patient with OSA.4–8 Moreover, chronic IH mimicking the oxygen profile of patients with severe OSA in mice on a high-fat diet also induces progression of hepatic steatosis to liver fibrosis and worsened hepatocellular injury.9,10
Despite the prevalence of NAFLD and the risks posed by its progression, there is no single noninvasive diagnostic test to identify early hepatic fibrosis among patients with hepatic steatosis. Several serum biomarkers, when used in concert, have been validated to detect advanced liver fibrosis,11 but none has been linked to the pathogenesis of liver fibrosis.12 There is also little understanding of the mechanisms that trigger the evolution of NAFLD and its relation to OSA.
A number of recent publications have demonstrated that tissue hypoxia, among other stimuli, increases expression of the fibrogenic enzyme lysyl oxidase (LOX),13,14 regulated by hypoxia inducible factor-1 (HIF-1).13,15,16 LOX is a secreted amine oxidase that catalyzes the formation of covalent cross-links between collagen fibers in the extracellular matrix.17 Several lines of evidence suggest that IH and hepatic steatosis may interact to increase LOX. Fatty liver in mice leads to liver tissue hypoxia, which is then exacerbated by IH.18 Fatty liver is also associated with increased expression of HIF-1α, the oxygen sensitive subunit of HIF-1.19,20 Furthermore, we have previously shown that IH exposure results in liver fibrosis in mice on a high-fat diet.10
Based on these observations, we hypothesized that the hypoxic stress of OSA might contribute to the progression of NAFLD to liver fibrosis by increasing LOX levels. To test our hypothesis, we performed a set of experiments. First, we obtained polysomnograms, examined intraoperative liver biopsies, and measured serum and liver LOX levels in bariatric patients with severe obesity who routinely undergo liver biopsy during the surgery. Second, we examined whether OSA can independently affect LOX by measuring serum levels of this enzyme in patients with severe OSA who underwent a randomized clinical trial of CPAP versus no treatment for 3 mo. Finally, we employed a mouse model of IH and primary cell culture of hepatocytes, and examined LOX expression and activity in response to hypoxia.
METHODS
Enrollment and Evaluation of the Bariatric Cohort
Thirty-five consecutive patients of the Johns Hopkins Bay-view Medical Center Bariatric Surgery Clinic (BMI > 35 kg/m2) were prospectively enrolled from August 2005 through May 2010, as in one of our previous studies.7 Participants underwent intraoperative liver biopsy at the time of bariatric surgery, and had a polysomnogram and pulmonary function tests 1–2 mo prior to surgery date. Exclusion criteria included a known diagnosis of OSA or chronic obstructive pulmonary disease; use of nasal continuous positive airway pressure (CPAP); recent weight loss of 10% body weight or more; diabetes mellitus requiring insulin; history of alcohol abuse, any known liver disease, or human immunodeficiency virus; current use of systemic steroids; unstable cardiovascular disease; or supplemental oxygen use. This study was approved by the Johns Hopkins University Institutional Review Board (protocol number NA_00048965), and written informed consent was obtained from each study participant as a prerequisite of study enrollment. Polysomnography was performed at the Center for Interdisciplinary Sleep Research and Education at the Johns Hopkins Bayview Medical Center, as described previously.7 Disordered breathing events were scored by apnea-hypopnea index (AHI) according to criteria from the American Academy of Sleep Medicine,21 where apnea was defined as a cessation of airflow for at least 10 sec, and hypopnea was defined as a significant decrease in flow as measured by nasal pressure monitor with either a ≥ 3% oxygen desaturation or a respiratory arousal. At the termination of the sleep study, a fasting venous blood sample was obtained. Pulmonary function testing was completed at the Johns Hopkins University Asthma and Allergy Center.
Evaluation of the Sleep Clinic Group
We retrospectively analyzed fasting samples of serum, which had been stored at −80°C, from among male patients who had been previously enrolled in a randomized trial comparing the effect of CPAP versus no therapy on blood pressure.22 Briefly, all patients recruited for this previous study were men with severe OSA (n = 15, AHI > 30 events/h). We also used stored samples of fasting serum from a subset of healthy controls without OSA (n = 7, AHI < 5 events/h) involved in a similar study.23 Patients were excluded from the studies if they had hypertension; diabetes mellitus; cardiovascular disease including any history of heart failure, valvular heart disease, or arrhythmias; renal failure; morbid obesity (body mass index [BMI] > 40 kg/m2); any history of smoking; a history of regular alcohol intake; or were taking any medications at all. All patients with OSA were age matched to control patients without OSA, were naïve to treatment, and were randomized to receive therapy with CPAP for 3 mo (n = 8) or no therapy (n = 7) for 3 mo. Healthy controls had baseline levels of LOX and glucose measured, and patients with OSA had these same data measured both before and after 3 mo of either no therapy or CPAP. CPAP adherence was objectively assessed by downloading stored data from each patient's machine.
Serum Markers and Liver Biopsy
Insulin and LOX levels were checked using enzyme-linked immunosorbent assay kits for human insulin (Alpco, Salem, NH, USA) and LOX (USCN Life Science, Wuhan, China) per manufacturer guidelines. Liver tissue was sampled intraoperatively with wedge biopsy, and part of each liver biopsy specimen was quick frozen in liquid nitrogen and stored at −80°C for later messenger RNA (mRNA) extraction. Biopsy specimens were stained with hematoxylin and eosin, and Masson trichrome stain, and were analyzed by a liver pathologist (M.S.T.) in a blinded fashion according to the NASH Clinical Research Network Scoring System, based on the NAFLD activity score (NAS), the sum of individual scores for steatosis, lobular inflammation, and ballooning. Fibrosis was scored per published guidelines24 on a scale ranging from F0 (none) to F4 (cirrhosis).
Mice
For hepatocyte isolation, 8-w-old C57BL/6 mice fed a chow diet were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Animal studies were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine (permit number MO12M309). All mouse surgery and euthanasia was performed with isoflurane anesthesia (1–2%), and all efforts were made to minimize suffering.
Hepatocyte Isolation and Hypoxia Exposure
Hepatocytes were isolated from adult mice (n = 3) using a two-step perfusion process,25 then plated onto dishes with Dulbecco Modified Eagle Medium containing high glucose, L-glutamine, and phenol red (Life Technologies, Grand Island, NY, USA), with 10% fetal bovine serum. The media was replaced with serum-free media the following day, with or without 100 μM of β-aminopropionitrile (BAPN, Sigma-Aldrich, St. Louis, MO, USA), an irreversible LOX inhibitor. The cells were placed for 24 h into airtight chambers containing 5% CO2 and either 1% or 16% O2. After exposure, cell media was collected and cells were recovered and stained with 0.4% Trypan Blue solution (Life Technologies, Rockville, MD, USA) to quantify hepatocyte survival.
Reverse Transcription Polymerase Chain Reaction and Western Blot
Total RNA was isolated from hepatocytes exposed to hypoxia and from historic liver samples of IH-exposed B6129PF2/J mice fed a chow diet for 4 w, as previously described.26 [Briefly, mice 6–8 w of age were exposed to IH, with oxygen concentrations cycling from 20.9% to 6.5% (or 20.9% to 20.9% for the control intermittent air group) 60 times/h during the light phase, 09:00 to 21:00.] RNA was extracted from livers using Trizol reagent (Life Technologies, Rockville, MD, USA), and complementary DNA was synthesized using an Advantage RT for PCR kit (Clontech, Palo Alto, CA, USA). Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed with primers from Invitrogen (Carlsbad, CA, USA), and Taqman probes from Applied Biosystems (Foster City, CA, USA). LOX mRNA level was normalized to 18s ribosomal RNA, using the formula: LOX/18s = 2 Ct(18s) – Ct(LOX). Western blotting on in vitro samples was performed with the primary antibody to LOX (rabbit polyclonal, 1:2000, Novus Biologicals, Littleton, CO, USA).
Collagen Assay
A stock solution of type I rat tail collagen at 3 mg/mL (Life Technologies, Grand Island, NY) was diluted to a final concentration of 1.2 mg/mL with 300 μL of concentrated culture media from hepatocytes and double distilled water at neutral pH, then plated onto coverslip-bottom plastic dishes (MatTek Corp., Ashland, MA, USA), and incubated at 37°C for 16 h. The resulting collagen matrix was examined using confocal reflection microscopy with a Leica TCS SP5 microscope and the accompanying Leica Application Suite software (Wetzlar, Germany).
Statistical Analysis
Patient characteristics in the bariatric cohort were compared based on the presence (histologic stage F1 and above) or absence (histologic stage F0) of hepatic fibrosis, by calculating P values for differences using t-tests for continuous variables and χ2 tests for categorical variables. Serum LOX was compared between groups using t-tests, in both patient groups. Results were confirmed using unadjusted logistic regression, with the outcome being presence of fibrosis and the exposure of interest being serum LOX level, scaled by 10 for ease of interpretation. Odds ratios and 95% confidence intervals were calculated, and then repeated after adjusting for BMI or age and waist circumference, where appropriate. Receiver operator characteristic (ROC) curves were constructed, and the area under the curve was calculated to understand the discriminative performance of serum LOX as a true predictor of fibrosis based on an optimum cutoff for reported sensitivity and specificity. Differences in the mean hepatic LOX mRNA were calculated between groups using t-tests. The difference in AHI between groups was assessed using t-tests, and confirmed using logistic regression with risk of fibrosis as the outcome and the exposure of interest being AHI scaled by 5, both un-adjusted and adjusted for BMI or age and waist circumference, where appropriate. T-tests were used to compare differences in experimental values (hepatic LOX mRNA expression in mice, and hepatocyte mRNA expression in isolated cells). For supplemental analyses of serum LOX versus NAS and hepatic fibrosis stage, P values for trend were calculated using unadjusted linear regression analysis with serum LOX as the outcome and NAS or hepatic fibrosis as the categorical variable of interest. For all statistical comparisons, P < 0.05 was the threshold used for statistical significance; however, we report numeric P values throughout this manuscript unless very small (i.e., below 0.001). Data are reported as mean ± standard deviation unless otherwise noted. Stata 12 software (StataCorp, College Station, TX, USA) was used for all analyses.
RESULTS
Bariatric Patient Characteristics
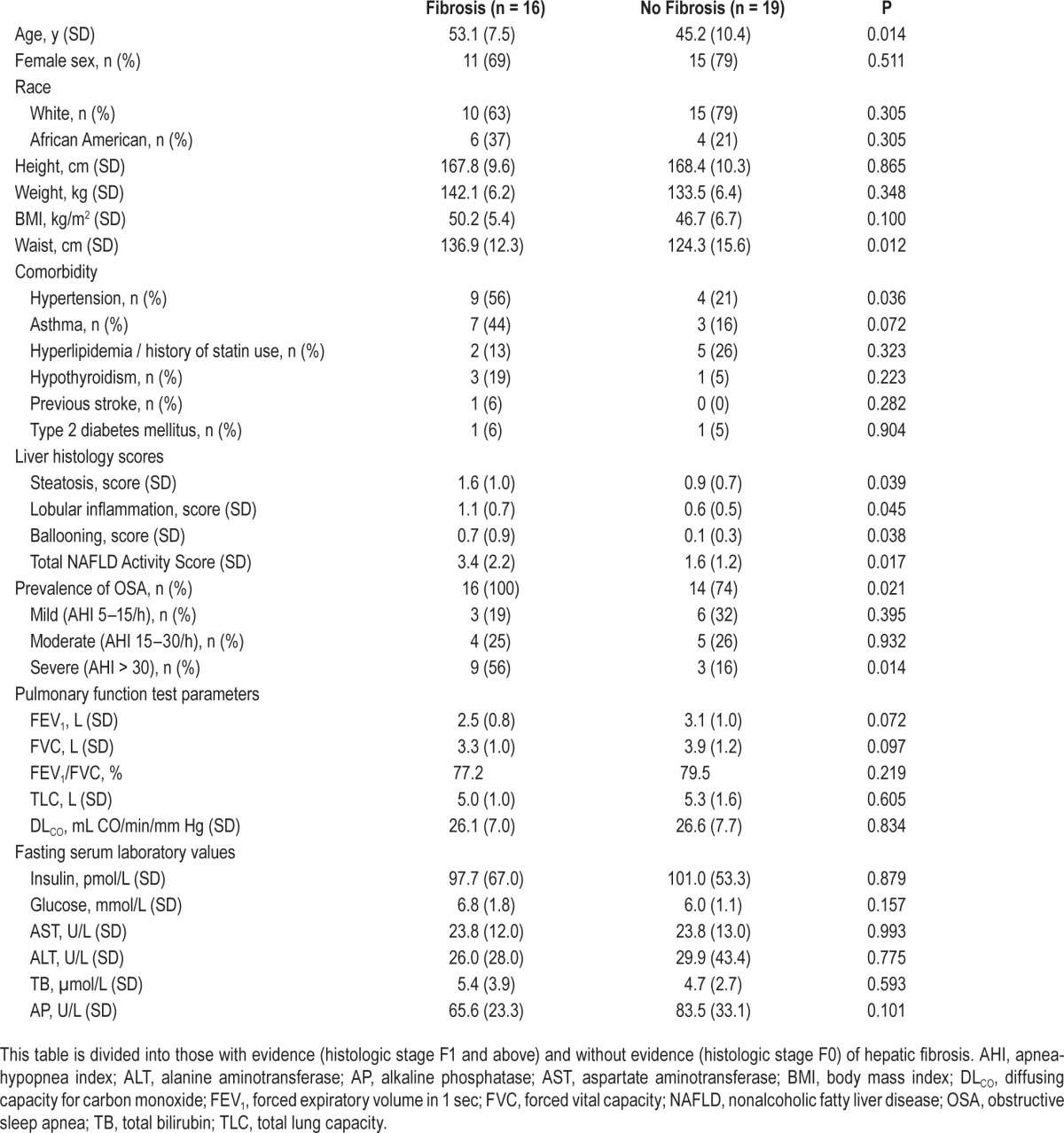
Patient characteristics from the bariatric cohort are presented in Table 1. Among those with hepatic fibrosis (n = 16), most patients (81%) had histologic stage F1 (six with stage 1a, three with stage 1b, and four with stage 1c), with the remainder having stage F2 or F3. There was a higher prevalence of OSA, and evidence of more severe disease, among those with fibrosis. Pulmonary function parameters were not different between groups. There was no difference in liver enzymes or insulin levels between the two groups. Patients with hepatic fibrosis had more steatosis, ballooning, and lobular inflammation, each contributing to a higher NAS. There was no difference in BMI, but those with fibrosis were older and had larger waist circumference. There were no differences in the prevalence of self-reported illnesses except for hypertension.
Table 1.
Clinical characteristics of the bariatric patient cohort.
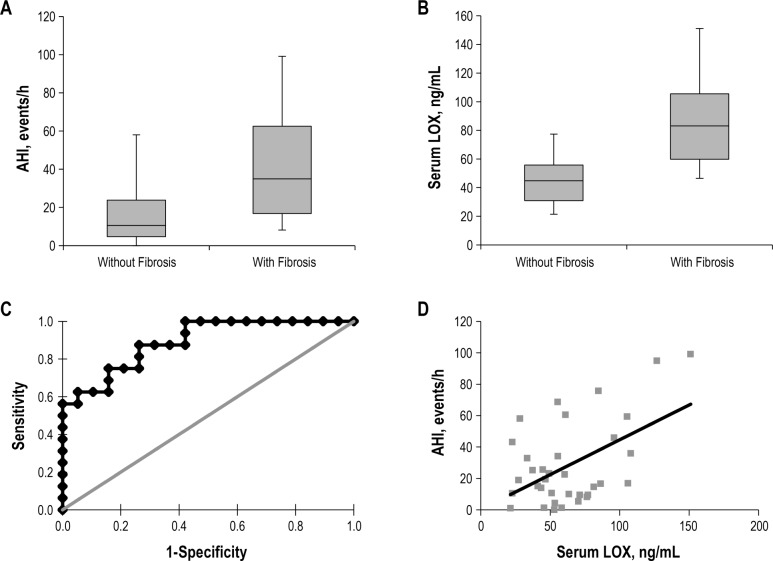
AHI Correlates with the Prevalence of Hepatic Fibrosis
AHI was higher in patients with fibrosis (42.7 ± 30.2 events/h, versus 16.2 ± 15.5 events/h, P = 0.002, Figure 1A). Each increase in AHI of 5 events/h was associated with a 30% increased risk of hepatic fibrosis (odds ratio [OR] 1.30, 95% confidence interval [CI] 1.06–1.60, P = 0.012), which was unchanged when corrected for age and waist circumference (OR 1.31, 95% CI 1.01–1.71, P = 0.041). In sensitivity analysis, we confirmed that the association was robust regardless of the measure of obesity used for adjustment (BMI versus waist circumference), and also after additionally adjusting for sex (Table S1, supplemental material). In the entire cohort, there was no difference in the mean nocturnal oxygen saturation, the nadir oxygen saturation, the percentage of sleep time with oxygen saturation less than 90%, or the oxygen desaturation index (using a cutoff value of either 3% or 4% from baseline) between groups. However, when patients were instead dichotomized by mean nadir SpO2 during episodes of apnea or hypopnea (≤ 90%, n = 13; or > 90%, n = 22), we found that hepatic fibrosis was more prevalent (69%) among those with more severe oxygen desatu-ration, compared to those who exhibited milder desaturations (32% in this group, P = 0.035). There was no difference in BMI or sex between these two groups, but waist circumference was higher in patients with more severe nadir oxygen desaturations (137.2 ± 11.7 cm versus 125.8 ± 16.0 cm, P = 0.032), and these patients were also older (53.5 ± 8.9 y, versus 46.1 ± 9.6 y, P = 0.030).
Figure 1.
(A) Box plots of apnea-hypoxia index (AHI) in patients with or without hepatic fibrosis. Upper and lower tick marks in each group represent high and low values among each patient cohort; lower and upper bounds on boxes represent first and third quartiles, and midline represents median values. (B) Box plots of serum lysyl oxidase (LOX) in patients with or without histologic evidence of hepatic fibrosis. (C) Receiver operating characteristic (ROC) curve demonstrating the performance of serum LOX. The area under the ROC curve when serum LOX is used as a biomarker of hepatic fibrosis in patients with severe obesity is 0.891 (95% confidence interval, 0.789–0.994). (D) Correlation between AHI and serum LOX levels among all patients studied. R = 0.51, P = 0.002.
Performance of Serum LOX as a Biomarker of Fibrosis
Serum LOX levels were higher in patients with evidence of hepatic fibrosis (84.64 ± 29.71 ng/mL versus 45.46 ± 17.16 ng/ mL in those without, P < 0.001; Figure 1B), and serum LOX increased with worsening hepatic fibrosis (P < 0.001 for the trend, Figure S1, supplemental material). Each 10 ng/mL increase in LOX level was associated with a 2.3-fold increased risk of hepatic fibrosis (OR 2.30, 95% CI 1.31–4.05, P = 0.004), and this association held after adjustment for waist circumference and age (OR 2.44, 95% CI 1.16–5.12, P = 0.018). Again, these associations held regardless of the measure of obesity used for adjustment (BMI versus waist circumference), and after additionally adjusting for sex (Table S2, supplemental material). Serum LOX levels did not correlate with the NAS or any of the individual components of this score (Table S3, supplemental material), with BMI, or with liver enzyme levels. Figure 1C shows the ROC curve in which serum LOX level was used to detect hepatic fibrosis in our patient cohort. The area under the ROC curve is 0.891 (95% CI, 0.789–0.994; P < 0.001). AHI correlated with serum LOX (Figure 1D), and each increase in LOX of 10 ng/mL was associated with an increase in AHI of 4.44 events/h (95% CI 1.79–7.09, P = 0.002).
Hepatic LOX is Increased in Fibrosis and is Associated with Serum LOX
Hepatic LOX mRNA levels were 6.7-fold higher in patients with evidence of fibrosis (26.2 ± 37.4 × 10−8/18s versus 3.9 ± 11.5 × 10−8/18s, P = 0.019). Fourteen of the 16 patients (88%) with hepatic fibrosis had detectable hepatic LOX levels, whereas only four of the 19 patients (21%) without fibrosis had detectable levels. Serum LOX levels also correlated with hepatic LOX mRNA levels (R = 0.485, P = 0.003).
Serum LOX is Increased in Patients with OSA
Patient characteristics of the sleep clinic sample are shown in Table 2. Patients with OSA were age matched to control patients, although control patients had slightly lower BMI than those with OSA (27.4 ± 2.5 kg/m2 in the control group versus 30.5 ± 2.6 kg/m2 in the OSA group, P = 0.020). Patients without OSA had a mean serum LOX of 52.36 ± 18.26 ng/mL, and those with OSA had a mean serum LOX of 70.75 ± 17.79 ng/mL (P = 0.046). Patients with OSA who used CPAP had an average nightly use of 5.2 ± 0.6 h, at a mean pressure of 10.2 ± 1.0 cm H2O. Among OSA patients, initial serum LOX was not different between those who received CPAP and those who did not. Patients with OSA who did not receive CPAP saw no significant change in their serum LOX over 3 mo (75.78 ± 17.77 ng/mL to 72.38 ± 18.81 ng/mL, P = 0.726), whereas patients with OSA who received CPAP had decreased serum LOX over the duration of therapy (65.73 ± 17.44 ng/mL to 45.24 ± 10.38 ng/mL, P = 0.015, Figure 2A). Patients with OSA who received CPAP, as a group, had a mean decline in serum LOX of 20.49 ng/mL during study duration, whereas patients with OSA not on CPAP had a decline of only 0.19 ng/mL (P = 0.088). Among those patients receiving CPAP, there was not a statistically significant correlation between mean CPAP use and net decrease in serum LOX over the study duration (R = 0.64, P = 0.084, Figure 2B).
Table 2.
Clinical characteristics of the sleep clinic patient cohort.
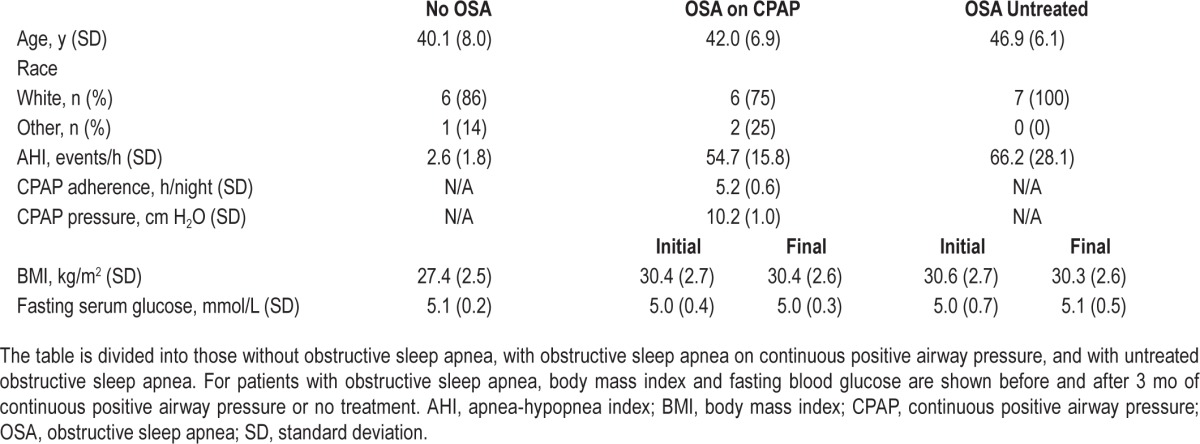
Figure 2.
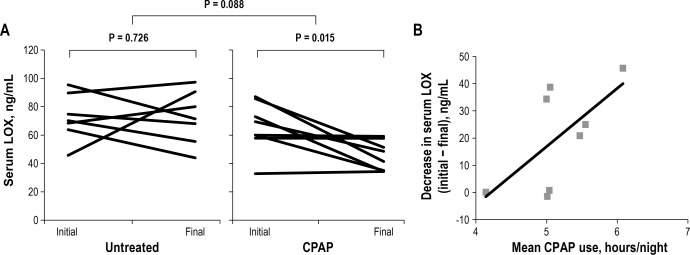
(A) Comparison of initial and final serum lysyl oxidase (LOX) values in patients with severe obstructive sleep apnea (OSA) who went untreated (left) and received continuous positive airway pressure (CPAP) therapy (right). Patients who received CPAP had a decline in serum LOX. Mean differences between initial and final values were 20.49 ng/mL (CPAP) and 0.19 ng/mL (control). (B) Correlation between mean nightly CPAP use and decrease in serum LOX among patients with OSA who received CPAP. R = 0.64, P = 0.084.
IH in B6129PF2/J Mice Induces Hepatic LOX Expression
To test whether IH exposure increases transcription of LOX in vivo, we retrospectively examined LOX mRNA levels from a previous experiment in which B6129PF2/J mice on a regular chow diet were exposed to IH or intermittent air for 4 w.26 Under these conditions, hepatic LOX was increased 5.2-fold in mice exposed to IH (P = 0.031, Figure 3).
Figure 3.
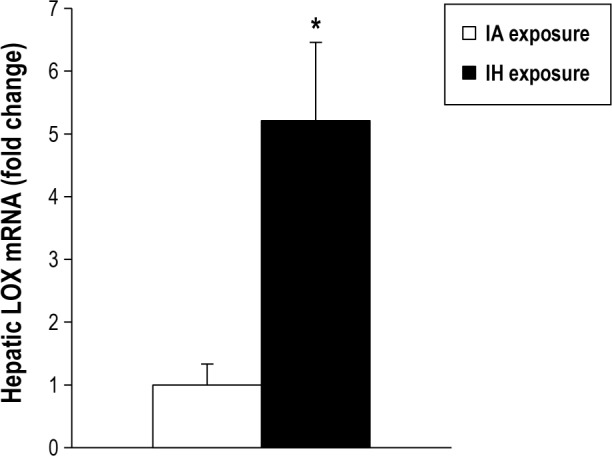
Hepatic mRNA expression of lysyl oxidase (LOX) in B6129PF2/J mice. Mice were fed a chow diet, exposed either to intermittent hypoxia (IH) or IA for 4 w. LOX is increased 5.2-fold in IH. *P = 0.031 between groups.
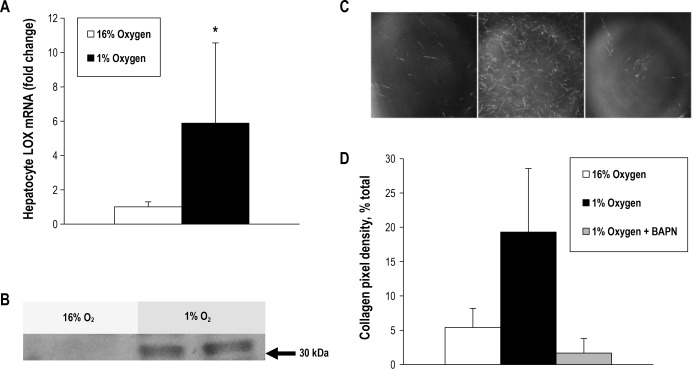
Hypoxia Induces LOX Expression and Secretion in Mouse Hepatocytes, and Collagen Cross-links
To test whether hypoxia directly increases LOX transcription in vitro, we exposed isolated mouse hepatocytes to hypoxia (1% O2) or normoxia (16% O2) for 24 h. Hypoxia increased LOX mRNA expression 5.9-fold in isolated hepatocytes (P = 0.046, Figure 4A), and resulted in secretion of LOX to the media, which was undetectable in normoxia (Figure 4B). Moreover, culture media from hepatocytes exposed to hypoxia caused more robust cross-linking of type I rat tail collagen, whereas minimal cross-linking was observed when media from normoxic hepatocytes was used (Figures 4C and 4D). The hypoxia-induced increase in cross-linking was prevented by BAPN, a LOX inhibitor. There was no significant change in hepatocyte survival with hypoxia or BAPN administration (69.3% survival in normoxia, 63.7% survival in hypoxia, and 60.7% survival in hypoxia with BAPN, P = 0.831 for the group comparison).
Figure 4.
Profile of isolated hepatocytes exposed to hypoxia and normoxia. Hepatocytes isolated from C57BL/6 mice express and secrete lysyl oxidase (LOX) in response to hypoxia, resulting in more active collagen cross-linking. (A) Results of reverse-transcriptase polymerase chain reaction demonstrating that expression of LOX messenger RNA from hepatocytes exposed to hypoxia for 24 h is increased (5.9 times normoxia value; *P = 0.046). (B) Results of Western blot showing that LOX is secreted into culture media by hepatocytes exposed to hypoxia for 24 h. (C) Confocal reflection microscopy images of collagen matrices incorporating fixed amounts of concentrated culture media from cells exposed to 16% O2 (left panel), 1% O2 (middle panel), and 1% O2 plus β-aminopropionitrile (BAPN), a LOX inhibitor (right panel). A denser network of precipitated, cross-linked collagen fibers is seen in hypoxia without BAPN. (D) Mean pixel density of collagen from images obtained during the collagen cross-linking experiment in C. P < 0.001 in all group comparisons.
DISCUSSION
In this study, we examined the mechanism by which OSA modulates the progression of NAFLD to liver fibrosis. We found that (1) OSA severity correlated with liver fibrosis, corroborating several earlier studies; (2) serum LOX was a sensitive and specific biomarker of liver fibrosis in patients with severe obesity and sleep apnea; and (3) LOX is elevated in OSA and is reduced with CPAP use. We also demonstrated that in vivo or in vitro hypoxia increases hepatocyte LOX mRNA expression in mice, thereby accelerating collagen cross-linking in vitro. Thus, we provide a biologically plausible mechanism by which OSA might contribute to the development of liver fibrosis in NAFLD.
Several studies have implicated LOX in the development of liver fibrosis of various etiologies. More than 30 y ago, Siegel et al. showed that hepatic LOX activity was increased in a rat model of hepatic fibrosis,27 and LOX activity was also increased in a cohort of human patients with chronic active hepatitis, cirrhosis, and hepatocellular carcinoma.28 More recently, hepatic LOX expression was found to be increased in patients with primary biliary cirrhosis and Wilson disease.29 In rats, hepatic LOX was upregulated prior to the development of liver fibrosis.30 However, to our knowledge, no previous study has examined the role of LOX as a serum biomarker of hepatic fibrosis in any liver disease.
LOX may have particular utility in detecting liver fibrosis in NAFLD associated with OSA. Our mouse data suggest that hepatic LOX is induced by hypoxia. There is additional evidence to support this hypothesis in various tissue types. The LOX promoter region has a hypoxia response element to which HIF-1 binds,13 and LOX is known to be highly regulated by HIF-1 in embryonic kidney cells.31 LOX is also an important regulator of hypoxia-induced tumor progression, via HIF-1.13,14 Constitutive expression of HIF-1α in adipose tissue caused LOX overexpression and tissue fibrosis.32 Expression of LOXL2, another in the family of lysyl oxidases, was increased in renal interstitial fibrosis, and HIF-1 was crucial in this development.14 Additionally, in the current study, CPAP decreased serum LOX in patients with OSA. Thus, it is conceivable that the systemic hypoxia of OSA may augment liver tissue hypoxia in NAFLD to increase hepatic LOX, leading to the progression of liver fibrosis. We have also shown that hypoxia increased LOX expression and secretion by mouse hepatocytes, and led to more robust collagen cross-linking. However, the roles of hypoxia and HIF-1 in the progression of hepatic fibrosis in humans have not been proven.
Our study suggests that LOX may be useful as a biomarker of liver fibrosis in sleep apnea. Liver biopsy remains the diagnostic gold standard, but other noninvasive tests exist to detect hepatic fibrosis. Transient elastography is a method by which ultrasound or magnetic resonance imaging is used to characterize liver tissue stiffness by gauging response to the application of a mechanical vibration.33 FibroSure and similar panels comprise serum biomarkers that predict hepatic fibrosis in chronic liver disease.12,34 Although LOX appears to have promise as a serum biomarker in this limited trial, and may also provide a mechanistic link to the pathogenesis of liver fibrosis, further testing will be required to validate its use in the routine management of patients with NAFLD.
In our bariatric patient group, the precise origin of the excess serum LOX among patients with fibrosis is unknown. LOX can be overexpressed in many tissue types. Halberg et al. found that LOX is overexpressed in adipose tissue in mice with obesity, and treatment with BAPN led to an improved metabolic phenotype and less adipose fibrosis.35 LOX expression is also increased by hypoxia in malignancy36 and hypoxia-induced metastasis,13 in renal tubular epithelial cells,14 and in other cell types. There are data in our study, however, to hint that this excess may be hepatic in origin: Patients with fibrosis had higher hepatic LOX mRNA levels, which correlated with higher serum LOX levels. Also, we also observed increased expression of LOX in hypoxic mouse hepatocytes in our in vitro model, suggesting that hepatocytes may account for some of the elevation in serum LOX among bariatric patients with fibrosis. Even if the liver is implicated as the source of some of the LOX overexpression in the bariatric cohort, this does not necessarily single out the hepatocyte as the sole site of origin. Hepatic LOX levels in our patients were determined in homogenates of liver tissue. Hepatic stellate cells, pericytes that undergo transdifferentiation to myofibroblast-like cells, are known to express LOX and may contribute to the increased hepatic LOX mRNA levels.37 Further complicating the issue is the fact that among our sleep clinic patients, we saw increased LOX in those with severe OSA but substantially lower BMI; these patients were unlikely to have NAFLD or significant liver fibrosis. We speculate that hypoxia—whether systemic as in IH and OSA, or tissue specific as in obesity—causes increased LOX, at least partly due to overexpression in hepatocytes, but this paradigm is not yet proven.
There are several limitations to our findings. The first and main limitation of our studies revolves around small sample sizes. Our bariatric patient cohort was small and comprised solely patients with severe obesity, but this population was chosen for two reasons. A major practical advantage is that liver biopsy specimens are easily obtained during bariatric surgery. Moreover, in understanding the validity of a serum biomarker of liver fibrosis, it seems appropriate to select a population in which the pretest probability of NAFLD and OSA are both high, as in those with severe obesity. Nonetheless, the generalizability of our findings showing a correlation between liver fibrosis and increased serum LOX remains to be demonstrated. Indeed, a recent study has shown that an independent association between nocturnal hypoxia and markers of liver injury was present only in patients with severe obesity.38 Our sleep clinic cohort was also small and comprised only male patients, and so the same limitation of generalizability applies here. However, we are intrigued by certain findings that did not meet statistical significance, such as the change in serum LOX as a function of mean CPAP use among our sleep clinic patients. Small sample sizes in the current study may also have hindered our ability to replicate findings that others have reported, such as a clear association between hypoxic burden and hepatic fibrosis. Clearly in this context we would advocate cautious interpretation of our data and further study of these findings in larger cohorts.
A second limitation is that our bariatric cohort study is cross-sectional and therefore cannot establish causal links between IH, LOX, and liver fibrosis, although we attempted an initial investigation of this with retrospective analysis of the sleep clinic patient sample. Third, our data in the bariatric cohort provide only a single measure of the enzyme level, so we lack insight about the time course of LOX change. LOX is known to cross-link only freshly made collagen fibers,39 so the observation of higher LOX levels in patients with fibrosis suggests active collagen cross-linking. Fourth, data from our sleep clinic cohort showing a serum LOX decrease with CPAP use does not necessarily imply that LOX is increased in OSA due to systemic hypoxia, as CPAP is known to have myriad effects. Fifth, our cell culture system relies on sustained hypoxic exposure, which does not precisely mimic the chronic IH that is characteristic of OSA. However, we have previously shown in an animal model that obesity resulting in fatty liver causes sustained liver hypoxia, which is exacerbated with IH exposure.18 Centrilobular hepatocytes from mice with obesity demonstrated positive pimonidazole staining, indicating severe hypoxia (≤ 10 mm Hg) in this zone of the hepatic lobule. Thus, the hypoxic exposure to the liver during OSA may be quite severe, and our cell culture system probably recapitulates this effect to a large degree. Finally, in our sleep cohort we did not investigate the presence of liver disease. Based on the lower BMI of these patients, we would estimate that the prevalence of NAFLD in this group would be considerably less than in our bariatric cohort.40 The purpose of retrospective analysis of LOX levels in the sleep clinic cohort was primarily to lend support to the idea that OSA can independently increase serum LOX, and to investigate the effect of CPAP on serum LOX levels. However, despite our initial investigations in our clinic cohorts, a true definition of “normal” serum LOX levels remains unknown.
In conclusion, our findings suggest that hepatic fibrosis and serum LOX are related to the severity of OSA in patients with severe obesity, and that serum LOX may predict hepatic fibrosis in this population. We also provide evidence that OSA increases serum LOX levels, and that this increase is reversible by CPAP. Finally, our animal and in vitro data demonstrate that IH causes hepatic LOX overexpression and that sustained hypoxia induces LOX secretion by hepatocytes, resulting in collagen cross-linking, a crucial step in the progression of fibrosis. We propose that LOX should be tested as a biomarker of liver fibrosis in a larger cohort of patients with NAFLD and OSA, across a range of BMI, and that the effect of OSA treatment on the progression of liver fibrosis should be assessed.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the following grants: NIH R01 HL080105; R01 HL050381; T32 HL007534; T32 HL110952; ResMed Foundation grant 90048207; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Young Investigator Award (2012/02953-2). No drugs were used in the study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr Gregg Semenza for the collagen cross-linking protocol, Dr Zhiping Li for helpful discussions, and Ms Irina Hawks for database management.
SUPPLEMENTAL MATERIAL
Sensitivity analysis for apnea-hypopnea index (scaled by 5) and risk of fibrosis models.
Sensitivity analysis for serum LOX (scaled by 10) and risk of fibrosis models.
Number of patients and mean serum lysyl oxidase measures in patients with each histologic stage of steatosis, lobular inflammation, and ballooning, and in patients without or with nonalcoholic steatohepatitis (NASH) (as determined by nonalcoholic fatty liver disease (NAFLD) activity score < 5 or ≥ 5, respectively).
Serum lysyl oxidase (LOX) for each stage of hepatic fibrosis among all bariatric patients. P < 0.001 for the trend in serum LOX.
REFERENCES
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev. 2013;22:353–64. doi: 10.1183/09059180.00003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 4.Aron-Wisnewsky J, Minville C, Tordjman J, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–33. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Tanne F, Gagnadoux F, Chazouilleres O, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–6. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 6.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41:918–21. doi: 10.1097/01.mcg.0000225692.62121.55. [DOI] [PubMed] [Google Scholar]
- 7.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–34. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nobili V, Cutrera R, Liccardo D, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med. 2014;189:66–76. doi: 10.1164/rccm.201307-1339OC. [DOI] [PubMed] [Google Scholar]
- 9.Savransky V, Nanayakkara A, Vivero A, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007–13. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 10.Savransky V, Bevans S, Nanayakkara A, et al. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–7. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26:802–9. doi: 10.1111/j.1440-1746.2010.06612.x. [DOI] [PubMed] [Google Scholar]
- 12.Poynard T, Lassailly G, Diaz E, et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS One. 2012;7:e30325. doi: 10.1371/journal.pone.0030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 14.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–20. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong CC, Gilkes DM, Zhang H, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108:16369–74. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CC, Zhang H, Gilkes DM, et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl) 2012;90:803–15. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 18.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–90. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carabelli J, Burgueno AL, Rosselli MS, et al. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med. 2011;15:1329–38. doi: 10.1111/j.1582-4934.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiai D, Goda N, Hishiki T, et al. Disruption of HIF-1alpha in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415:445–9. doi: 10.1016/j.bbrc.2011.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry R, Brooks R, Gamaldo C, et al. Darien, IL: American Academy of Sleep Medicine; 2012. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0. www.aasmnet.org. [Google Scholar]
- 22.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–55. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 23.Drager LF, Diegues-Silva L, Diniz PM, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–54. doi: 10.1038/ajh.2009.246. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Li WC, Ralphs KL, Tosh D. Isolation and culture of adult mouse hepatocytes. Methods Mol Biol. 2010;633:185–96. doi: 10.1007/978-1-59745-019-5_13. [DOI] [PubMed] [Google Scholar]
- 26.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–8. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel RC, Chen KH, Greenspan JS, Aguiar JM. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc Natl Acad Sci USA. 1978;75:2945–9. doi: 10.1073/pnas.75.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murawaki Y, Kusakabe Y, Hirayama C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology. 1991;14:1167–73. [PubMed] [Google Scholar]
- 29.Vadasz Z, Kessler O, Akiri G, et al. Abnormal deposition of collagen around hepatocytes in Wilson's disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol. 2005;43:499–507. doi: 10.1016/j.jhep.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Georges PC, Hui JJ, Gombos Z, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–54. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 31.Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 32.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei L, Song X-R, Sun J-J, Wang X-W, Xie L, Lv L-Y. Lysyl oxidase may play a critical role in hypoxia-induced NSCLC cells invasion and migration. Cancer Biother Radiopharm. 2012;27:672–7. doi: 10.1089/cbr.2012.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perepelyuk M, Terajima M, Wang AY, et al. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605–14. doi: 10.1152/ajpgi.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minville C, Hilleret M-N, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest. 2014;145:525–33. doi: 10.1378/chest.13-0938. [DOI] [PubMed] [Google Scholar]
- 39.Siegel RC, Fu JC. Collagen cross-linking. Purification and substrate specificity of lysyl oxidase. J Biol Chem. 1976;251:5779–85. [PubMed] [Google Scholar]
- 40.Church TS, Kuk JL, Ross R, Priest EL, Biltoff E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–30. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis for apnea-hypopnea index (scaled by 5) and risk of fibrosis models.
Sensitivity analysis for serum LOX (scaled by 10) and risk of fibrosis models.
Number of patients and mean serum lysyl oxidase measures in patients with each histologic stage of steatosis, lobular inflammation, and ballooning, and in patients without or with nonalcoholic steatohepatitis (NASH) (as determined by nonalcoholic fatty liver disease (NAFLD) activity score < 5 or ≥ 5, respectively).
Serum lysyl oxidase (LOX) for each stage of hepatic fibrosis among all bariatric patients. P < 0.001 for the trend in serum LOX.