Abstract
Study Objectives:
To assess the direction of the relationship and degree of shared associations between symptoms of depression and difficulty initiating sleep (DIS) from early adolescence to early adulthood.
Design:
Cross-sectional and longitudinal assessment of the symptoms of depression-DIS association from early adolescence (age 13 y) to early adulthood (age 23 y).
Setting:
Hordaland, Norway.
Participants:
There were 1,105 individuals (55% male) who took part in the Norwegian Longitudinal Health Behaviour Study (NLHB) and participated at least once across seven data collection waves during the years 1990–2000.
Interventions:
N/A.
Measurements and Results:
Characteristic data were obtained during the first assessment. Symptoms of depression and instances of DIS were assessed during each data collection wave. Symptoms of depression and DIS were associated in all data waves, and one-step cross-lagged bivariate correlations were significant and comparatively high for both factors. Structural equation modelling indicated that DIS and symptoms of depression at wave 1 remain relatively stable across waves (all P < 0.001), and a significant and consistent unidirectional cross-lagged effect was noted running from symptoms of depression to DIS from early adolescence to early adulthood. DIS is only marginally and inconsistently associated with the lagged symptoms of depression score across waves.
Conclusions:
These results suggest that symptoms of depression established in early adolescence are a moderate predictor of difficulty initiating sleep (DIS) in early adulthood, whereas the reverse association of DIS predicting depression was not convincingly supported. These findings are in contrast to previous findings that suggest sleep problems as a risk factor for the later development of depression.
Citation:
Hayley AC, Skogen JC, Sivertsen B, Wold B, Berk M, Pasco JA, Øverland S. Symptoms of depression and difficulty initiating sleep from early adolescence to early adulthood: a longitudinal study.SLEEP 2015;38(10):1599–1606.
Keywords: adolescence, adulthood, cross-sectional, depression, etiology, longitudinal, risk factor, sleep
INTRODUCTION
A number of cross-sectional and longitudinal studies have demonstrated that both individual symptomatic components of insomnia, such as sleep onset difficulties1,2 as well as defined clinical instances of insomnia are closely related to depressive illness.3,4 Prospective studies have shown that instances of insomnia in early adulthood predict the later development of depressive symptoms,5 and that depressive symptoms can precede the later onset of sleep problems.1 Despite this, there is lack of specificity in regard to the age of onset of these symptoms, the developmental course of these symptoms across time, and whether each condition is differently represented with respect to directionality of the association, particularly among population-based cohorts.
Mechanistically, it has been suggested that the reciprocal association between depression and insomnia is primarily driven by shared neurobiological and behavioral deficits with regard to the sleep-wake regulatory dysfunction, which in turn acts to mediate deficits in emotional reactivity.6 Indeed, successful amelioration of sleep difficulties among depressed individuals demonstrates improvements in self-reported mood states as well as higher remission rates among these individuals.7 As such, the causal association of underlying sleep pathology leading to the subsequent development of depressive illness is perhaps the most prominent theoretical perspective.8 Many of the available epidemiological studies assessing sleep pathology at baseline and the subsequent development of depressive illness have yielded range risk estimates of between 3.8–6.7.9,10 Meta-analyses have similarly demonstrated that depressive illness and sleep problems are often observed to naturally co-occur8; a finding shown to be relatively consistent among cohorts of children, adults, and older individuals.8
The reverse model, whereby depression predicts the later development of sleep problems has also been explored, albeit in less detail and with less specificity.5 To our knowledge, there are only a few prospective studies that have examined, and found, depression to be a risk factor for the future development of general sleep problems1,11 or clinically defined insomnia.12–14 Some studies utilizing retrospective analysis design have reported no association between depressive symptoms at baseline and the subsequent development of sleep problems at follow-up.9 Other studies assessing the depressive symptoms-sleep problems relationship often feature sleep as a function of underlying physical disease,15 and thus any confirmative conclusions regarding the strength of the observed relationship are equivocal. Compared to the amount of literature focusing on sleep difficulties as a proxy for the later development of depressive symptoms, the proposed evidence supporting the reverse path is less clear, with the implication that sleep disturbance is an emergent symptom of a depressive phenotype. Evidence supporting the depression-to-sleep problem association has been primarily derived from clinical treatment studies, of which have demonstrated improvements in insomnia symptomology following the application of cognitive behavioral therapy for insomnia (CBT-I) or pharmacotherapy targeting depressive symptoms.7
Although a significant amount of data has demonstrated a link between sleep problems and depressive illness, particularly among adults, several areas of investigation remain. Indeed, much of the available literature spans only brief periods of early adolescence or adulthood,1 includes only a limited number of sampling phases,15 or fails to accurately describe the strength of these associations and role of possible peripheral confounders. Consistent assessment of these factors over several time points will allow for greater specificity in regard to subtle changes over time, and assist in describing the natural course of these associations. Moreover, there is a paucity of information assessing the specific role of sleep onset difficul-ties with regard to depressive symptomology, as the available literature typically focuses on instances of general insomnia complaints only. Currently, there is a lack of empirical evidence that accurately quantifies the developmental trajectory of these associations from the time of early adolescence to early adulthood. Such investigations may lend evidence to help delineate whether this relationship can be considered causal, or if it instead represents an epiphenomenon or occurs as a result of peripheral factors.
METHOD
For the current study, we used data from the Norwegian Longitudinal Health Behaviour Study (NLHB). The NLHB is a nine-wave, cluster-sample research study that followed a cohort of adolescents from age 13 y (initial testing period 1990) to 30 y (final follow-up in 2007). As part of the initial wave in 1990, a total of 1,195 13-y-olds and their parents were invited to participate in the study. As the data collection was conducted during school hours in the first three waves, 47 students who joined the sampled school classes after the first wave were invited to participate during the two subsequent waves, resulting in a total sample of 1,242. Inclusion for the study required consent from both the adolescent and the parent/ guardian, which resulted in a total of 1,105 students (55% male, 89% of the total invited sample).
Six hundred twenty-seven of the 1,105 students responded in the eighth wave (at age 23 y), indicating a retention rate of 57% (and representing 50% of the original sample). A more detailed description of the sampling procedures and data collection methods used in the NLHB-study can be found elsewhere.16,17 Actual participation rates across data waves are presented in Table 1.
Table 1.
Age and number of participants included for analyses for each data collection wave.

Study questionnaires were distributed at the participants' schools at the time of the first few years of the study (age 13–15 y), and were later distributed by mail to the respondents' home addresses at the time of each follow-up assessment wave. This paper presents data from the years 1990, 1991, 1992, 1993, 1996, 1998, and 2000 (that is, at ages 13, 14, 15, 16, 19, 21, and 23 y). Data from the fifth wave (age 18 y) was not included because no sleep item was included in this survey. To use the longitudinal data in an optimal way, full information maximum likelihood (FIML) were used in order to obtain a likelihood function for each individual based on the variables that were present for each data wave (n = 1,076).
Written informed consent was obtained for each study participant and the study has been approved by the Norwegian Data Inspectorate. The study was approved by The Regional Committee for Medical Research Ethics in Western Norway.
Variables
Sex was first reported in the initial data collection wave, and was noted at the time of each follow-up in order to assess sex distribution for each wave. Symptoms of depression were assessed using the seven-item depression inventory originally proposed by Alsaker et al.18 Inventory items include statements that relate to hopelessness, sadness, and depressive feelings. Assessment of the internal consistency yielded acceptable values, with Cronbach α being high across all seven waves, ranging from 0.82 to 0.92, and also Guttman Split-half correlations were comparably acceptable (ranging from 0.79 to 0.92 across all seven waves). Test–retest reliability was assessed during the data assessment wave 1991 among 80 respondents. Results indicated an acceptable temporal stability between total scores (Guttman Split-half correlations 0.87); however, this was variable for assessment of single items (between 0.52 and 0.80). Questionnaire items included the following statements; “I often feel depressed without knowing why,” “Sometimes I think everything is so hopeless that I don't feel like doing anything,” “I don't think I have anything to look forward to,” “Sometimes I am just so depressed that I feel like staying in bed for the whole day,” “I am often sad without seeing any reason for it,” “I think my life is mostly miserable” and “Sometimes I think my life is not worth living.” Response options were presented on a six-point Likert-style scale, and included the statements “Does not apply at all,” “Does not apply well,” “Applies somewhat,” “Applies fairly well,” “Applies well,” and “Applies exactly” (scored as 0–5, respectively).
Difficulties initiating sleep (DIS) at all seven waves (at ages 13, 14, 15, 16, 18, 21, and 23 y) were measured using the following item ”Have you had difficulties falling asleep during the last 3 months?” For the first three waves (ages 13, 14, and 15 y) participants scored this item on a four-point Likert-scale, with the following response option: “Seldom or never,” “Sometimes,” and “1–3 nights per week” and “4 nights per week or more often.” For the last four waves (ages 16, 18, 21, and 23 y) the response options were “Seldom or never,” “Sometimes,” and “Very often (weekly).” To enable comparisons across all seven waves, the four response options in the first three waves were reduced to three by combining “1–3 nights per week” and “4 nights per week or more often“ into “Very often (weekly).”
Statistics/Analytic Strategy
Pearson product-moment correlation coefficient (Pearson r) analyses were performed to determine how symptoms of depression were associated with DIS within each data collection wave as well as between the seven time points, for the entire group. A Wald test of parameter constraints was used to compare the autocorrelations of DIS and depression scores across time. To examine possible causal models of the relationship between symptoms of depression and difficulty initiating sleep, we employed structural equation modeling (SEM), and cross-lagged longitudinal models were tested in Mplus version 7 using full information maximum-likelihood and MLR as estimator.19 The cross-lagged longitudinal approach was chosen to perform an analysis including both variables (symptoms of depression and DIS) across multiple time points. The approach enables the simultaneous modeling of within-concept cross-time effects (for example symptoms of depression at t1, t 2, and so on), cross-concept within-time relations (for example, symptoms of depression and DIS at t2) and the cross-time cross-concept effects (for example symptoms of depression at t 1 influencing DIS at t2). By employing SEM instead of other, more simpler models (such as regression analysis), a complex and simultaneous investigation of how symptoms of depression and DIS affect each other across multiple time-points was possible, and it is possible to shed light on the temporal relationship between the two phenomena.
Four different models were investigated, from the simplest to the most complex model. In M1, autoregressions within each time span for both symptoms of depression and DIS were included (for example symptoms of depression at t2 regressed on symptoms of depression at t1), and the cross-lagged effects of both symptoms of depression and DIS (for example DIS at t2 regressed on depression at t1). For both symptoms of depression and DIS, the variables at t1 were exogenous, whereas they were endogenous at all other time points, meaning that the former is not thought to be caused by other variables in the model, whereas the latter is thought to be caused by other variables (here depression and DIS at t1) in the model. M2 expanded on M1 by allowing for the long-term effects within concepts to be estimated (for example, symptoms depression at t 3 regressed on t1). M3 also expanded on M1 by also including cross-concept cross-time effects (correlation of residual variance between symptoms of depression and problems initiating sleep at t2 and onward). M4 was the most complex model, and included symptoms of depression and DIS as exogenous at t1, but also included intraconceptual autoregressive paths, the long-term within-concept coefficients, cross-lagged paths, and cross-concept cross-time effects.
RESULTS
Bivariate Cross-sectional and Longitudinal Analyses
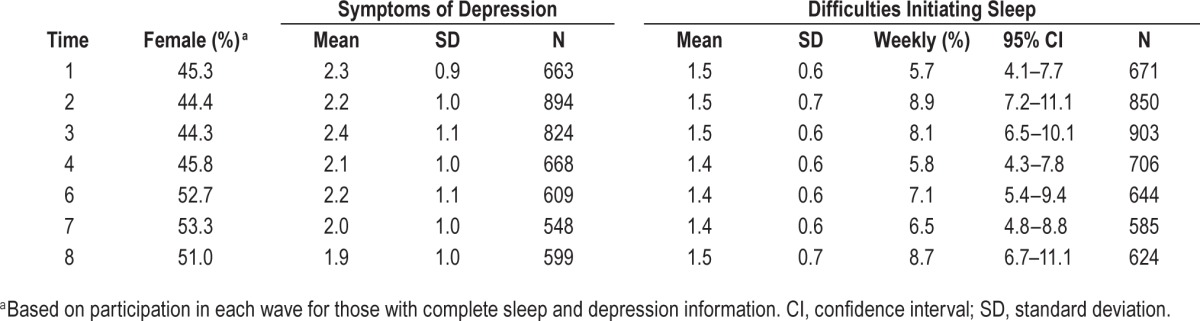
Descriptive statistics for sex, DIS, and symptoms of depression across all time points are presented in Table 2. Sex distribution is comparable across waves; however, a higher proportion of males compared to females were seen in the first four data collection waves, and vice versa in the final three data collection waves. Mean depression scores were relatively stable over time; however, an incremental reduction in mean depression scale scores was noted with increasing age. Mean DIS scores were also noted to be relatively stable over time, but were incrementally lower during the later data collection waves 4, 6, and 7 (ages 16, 19 and 21 y, respectively) (F(6,1584) = 3.063, P = 0.006).
Table 2.
Descriptive statistics for sex, symptoms of depression and difficulties initiating sleep across time.
Comparisons of baseline (age 13 y) variables between responders (individuals who completed all waves) and nonresponders (those who dropped out at any of the waves following baseline) showed that there were no significant differences between responders and nonresponders on either DIS or symptoms of depression, nor on reports of smoking, alcohol use, and body mass index (BMI). Moreover, no differences were found for parental, occupational, or cohabitant status. However, significantly more girls completed all waves (48% compared to boys, 32%, P < 0.001).
In all of the seven waves, DIS was consistently highly correlated with symptoms of depression, and the strength of these associations was noted to increase incrementally with advancing age (see Table 3). This association was strongest at data collection wave 8 (age 23 y), and weakest at data collection wave 1 (age 13 y).
Table 3.
Cross-conceptual bivariate cross-sectional correlations for symptoms of depression and difficulties initiating sleep across time points.
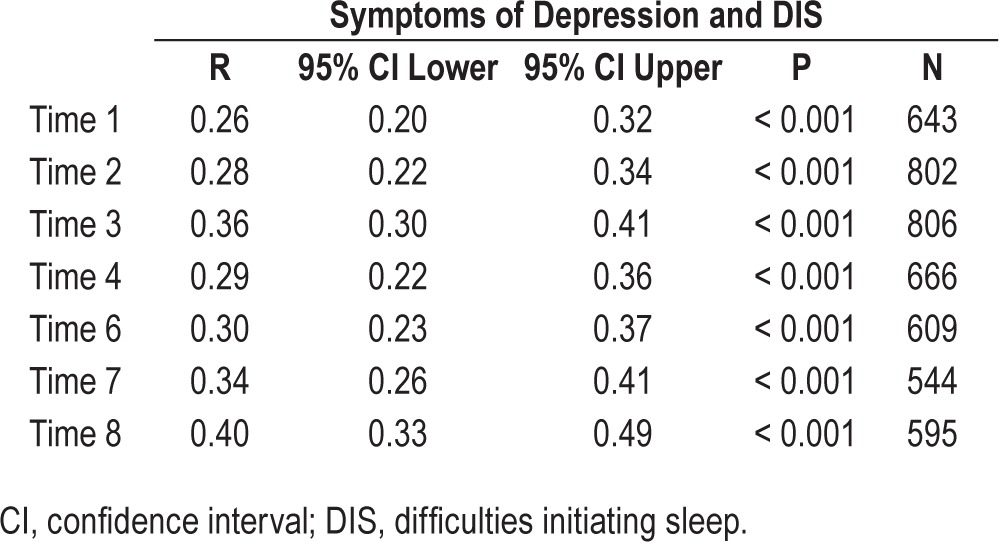
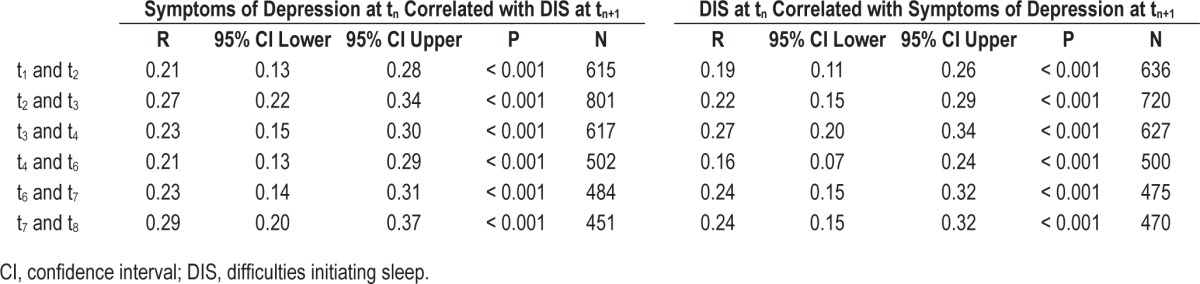
Table 4 shows the one-step cross-lagged bivariate correlations between DIS and symptoms of depression across time points. Associations between DIS and symptoms of depression were comparably high across time; however, the association between symptoms of depression at tn with DIS at tn+1 were slightly stronger than the associations of DIS at tn with symptoms of depression at tn+1, with the exception of t3–t4 and t6–t7.
Table 4.
Cross-conceptual bivariate cross-lagged correlations (tn correlated with tn+1) for symptoms of depression and difficulties initiating sleep across time points.
SEM: Cross-lagged Longitudinal Model
Table 5 describes the standardized coefficients of the initial correlation, autoregressive and cross-lagged paths for insomnia and depression from wave 1 to wave 8. The initial correlation between DIS and symptoms of depression indicated a moderate positive association (r = 0.27, P < 0.001).
Table 5.
Standardized coefficients of the correlations, autoregressive and cross-lagged paths for symptoms of depression and difficulties initiating sleep across time points (n = 1,076).
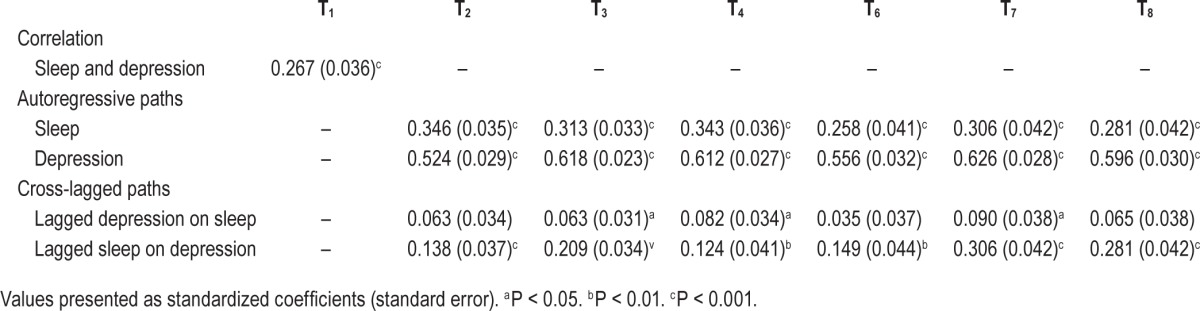
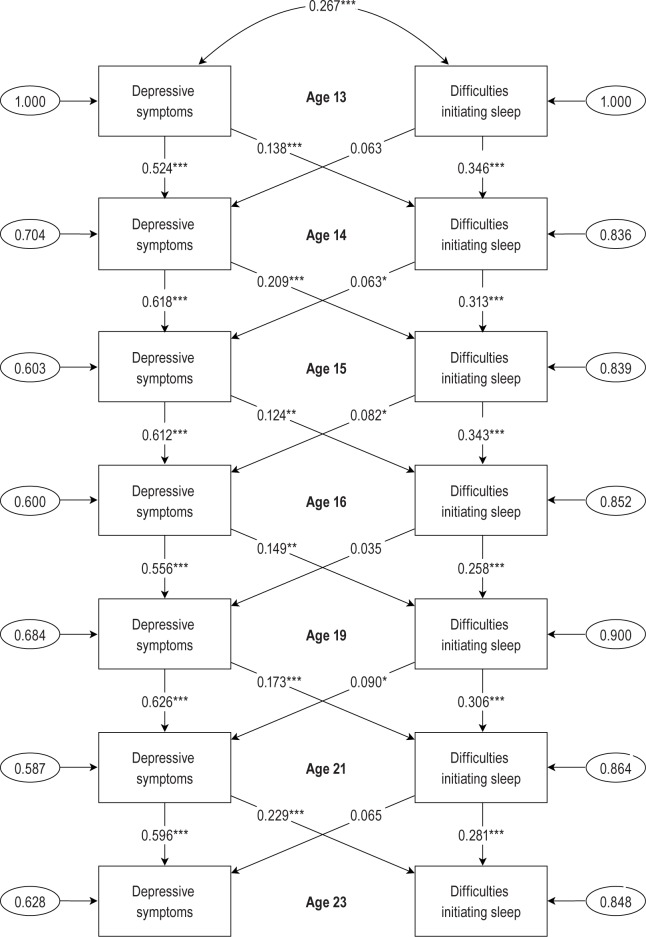
Autoregressive analyses were used to assess the stability of time for both DIS and symptoms of depression. Results indicated that both DIS and symptoms of depression at wave 1 remain relatively stable across waves (all P < 0.001). The autocorrelations for DIS were, however, smaller across time than the autocorrelations for symptoms of depression. In order to compare the autocorrelations of DIS and depression scores across time, we performed a series of Wald tests of parameter constraints. There was strong statistical evidence that the autocorrelations for depression scores were stronger than DIS, both when tested sequentially (all P < 0.001) and collectively (P < 0.001).
The main purpose of the current study was to explore the mutual relations between symptoms of depression and DIS across time. Cross-lagged paths were used to assess the cross-concept effects of both symptoms of depression and DIS. It was demonstrated that symptoms of depression are substantially and consistently associated with the lagged DIS scores across all waves (all P < 0.01), however DIS is only marginally and inconsistently associated with the lagged symptoms of depression score across waves, with significant associations only seen in waves 3, 4, and 7 (all P < 0.05) (see Table 5).
Considering model fit, there were differences between the models:
M1, where both symptoms of depression and DIS were entered as exogenous at t1, had a moderate fit [root mean square error of approximation (RMSEA): 0.069 (CI90% 0.063–0.076), comparative fit index (CFI): 0.826, Tucker-Lewis Index (TLI): 0.762)].
M2, which assumed both symptoms of depression and DIS were entered as exogenous at t1, but also includes the long-term within-concept coefficients, demonstrated similar fit indices [(RMSEA): 0.068 (CI90% 0.062–0.072), (CFI): 0.843, (TLI): 0.768)].
M3, where both symptoms of depression and DIS were entered as exogenous at t1, but also includes the cross-concept cross-time effects demonstrated an improved fit [(RMSEA): 0.053 (CI90% 0.046–0.060), (CFI): 0.907, (TLI): 0.860)].
M4, which assumes that symptoms of depression and DIS are exogenous at t1, but also includes intra-conceptual autoregressive paths, the long-term within-concept coefficients, cross-lagged paths and cross-concept cross-time effects demonstrated the best fit; [(RMSEA): 0.045 (CI90% 0.038–0.053), (CFI): 0.943, (TLI): 0.898)].
The differences between the four models in relation to the path coefficients of interest in the current study (auto-regressive and cross-lagged paths) were not substantial. We therefore chose to present the results from M1 because this was the less complex model (see Figure 1).
Figure 1.
The longitudinal relationship between symptoms of depression and DIS. Auto-regressive and cross-lagged paths.
DISCUSSION
The results of the current study suggest that there is a strong cross-sectional association between symptoms of depression and DIS, and that the strength of this relationship is similarly observed from early adolescence to early adulthood. Longitudinally, we report that when assessed individually, symptoms of depression at baseline remain relatively and comparably stable from early adolescence to early adulthood. For DIS, this association is somewhat less stable across time. Notably, we report that although symptoms of depression are substantially and consistently associated with the lagged sleep score, DIS is only marginally and inconsistently associated with the lagged depression score across time.
The results of our cross-sectional analyses are consistent with previous research which has demonstrated a strong association between depressive symptoms and sleep problems.20,21 We report that the strength of this association was sustained in every data collection wave from early adolescence through to early adulthood, which is consistent with, and builds on, previous research that has demonstrated a close association between these factors during this age period. Markedly, our research is the first to describe that these associations remain significant across such an extended period of time, as much of the available literature is typically focused on periods of childhood,21 brief period of adolescence, or periods of late adolescence/early adulthood.22
Considering the longitudinal analyses, several interesting findings were noted. First, we report that when assessed cross-sectionally, DIS and symptoms of depression are moderately correlated. Moreover, when assessed individually at baseline, symptoms of depression demonstrated a moderate correlation but a high internal stability across data assessment waves over time from early adolescence to adulthood. Furthermore, we report a decline in severity of depressive symptoms and DIS at ages 13 and 23 y, a time when depression and insomnia are typically observed to increase in prevalence. Previous longitudinal assessments have demonstrated that depressive symptoms and depressive disorders that appear during periods of early adolescence have a lifetime prevalence of between 3.7%–11.2%,23,24 suggesting that a sensitive developmental period may exist which influences the natural development of these symptoms over time and thus help to maintain them throughout the lifespan. Thus, it is possible that these endogenous factors may in fact have a greater influence on the expression of depressive symptoms among young adolescence than external factors per se. For DIS, descriptions of the natural development of these symptoms across the lifespan and the lifetime prevalence rates are less clear. Some studies have found that the development of sleep problems during periods of adolescence are often associated with a number of biological, physiological, and social transitions that often characterize adolescence and affect sleep/ wake regulation during this time.25 It is therefore plausible that these symptoms are similarly developed during critical periods of early adolescence, and subsequently maintained throughout the lifespan via continuous feedback from a variety of behavioral, physiological, and biological inputs associated with these developmental periods during early adulthood. Such explanations may, in part, help to explain our reported findings, and lend argument to the often high lifetime prevalence and disability observed among those who report chronic insomnia syndrome and often low rates of disease remission.26
We describe a significant and consistent unidirectional effect running from symptoms of depression to DIS from the time of early adolescence to early adulthood. A substantial portion of longitudinal studies suggest that the reverse is true, that sleep problems precede the onset of later mood disturbances8,9; however, it must be noted that the assessment framework used is often confined to specific developmental stages, such as defined periods of adolescence9 or adulthood.13 Research investigating depression as a predictor of the later development of sleep problems are also limited, and are similarly restricted to periods of adolescence1 or older age,11 or focus on specific clinical populations only.14 Currently, comprehensive and inclusive assessments of these relationships from periods of adolescence through to adulthood are inadequate, and thus assumptions regarding the underlying mechanistic assumptions are incomplete. Therefore, it is possible that the expression of these associations when evaluated during these transitional periods of adolescence/adulthood presents a hitherto unassessed characteristic in the depressive-sleep matrix. Thus, our findings lend weight to the small number of previous studies that have shown depressive symptoms to predict later sleep problems and to our knowledge, present the first research that evaluates these relationships across these critical developmental periods.12 Additionally, they build on previous studies that demonstrate the incidence, but do not adequately describe the persistence of comorbid sleep and psychiatric illness over time.12 When considering these findings, it is important to note that the reported longitudinal associations are of moderate strength. However, given that the long-term within-concept coefficients, cross-concept cross-time and autoregressive associations were controlled for in the development of the models, the cross-lagged associations should be weaker than in analyses in which no such adjustments are made. Despite this, the observed strength of the association was preserved and the model was robust, and thus the simplest model was chosen. Given these considerations, it is therefore unlikely that the observed associations are attributable to erroneous variance or error; rather, it is likely that these associations represent a true and persistent relationship between symptoms of depression and DIS scores across time.
Unlike several studies assessing these factors, we did not find clear evidence for a bidirectional relationship between symptoms of depression and DIS, as DIS at age 13 y was not a significant risk factor for symptoms of depression at age 23 y. Conceptually, it is possible that this relationship is represented only among complete diagnoses of insomnia and depressive syndrome, as was shown in previous studies,13 and that typical methodological considerations and statistical analyses used are insufficient at identifying more subtle aspects of individual symptoms of both conditions.
Sex distribution is comparable across waves; however, a higher proportion of females compared to males participated in the last three data collection waves. These values are reflective of participation trends and retention rates for the overall study.16 Several population-based cross-sectional and longitudinal analyses have highlighted that female sex represents a significant and independent risk factor for both insomnia and depressive illness,27,28 and that these symptoms are consistently more prevalent among women.29,30 We did not stratify these data by sex for cross-sectional analyses; however, sex stratification techniques were initially employed for longitudinal models (data not shown). No effect for sex was identified, and thus these techniques were not maintained in the development of the final model. It is possible that sex is not an attenuating factor when assessing individual symptom expression of these factors, as is often observed when assessing the entire syndrome. The primary strengths of the current study were the use of a longitudinal study design, which allowed for detailed assessment of the relative association between the target variables across all assessment periods, as well as the extended duration of total data collection from early adolescence to early adulthood (10 y). Such methods provided a detailed description of the strength of these associations across the transition period of adolescence to adulthood, thus addressing the limitations of some past studies. Indeed, in the available comparable literature, there is a lack of studies tracking the natural development of these associations over such an extended testing period. Often, the longitudinal associations between symptoms of depression and sleep problems are ascribed following a brief total assessment period, or are based on retrospective analysis.9
There are still some limitations within the design of the current study. First, because depressive symptomology was assessed using a validated, but not widely used inventory, it is possible that the prevalence of these symptoms were underrepresented among this cohort. We also concede that the use of the relatively unknown depression scale in the current study also limits the generalizability of the reported findings. Despite this, other longitudinal studies using the NLHB have used similar methodology,31 and psychometric assessment of the inventory have yielded similar indices to other, more well-known depression scales that use frequency indicators, such as the Centre for Epidemiological Studies Depression Scale (CES-D).32–34 Moreover, as only a single sleep item assessing sleep onset difficulties was used in this study, we are unable to generalize these findings to other individual subtypes of sleep problems often characteristic of insomnia disorder, such as sleep maintenance problems or complaints of early morning awakenings. Despite this, the purpose of the current study was to assess this singular insomnia symptom, and not the whole syndrome, per se. Indeed, other, comparable studies have used similar sleep metrics when assessing these factors among cohort of children and adolescents.35,36We assessed the relative distribution and contribution of several health and lifestyle factors between responders (individuals who completed all waves) and non-responders (those who dropped out at any wave following baseline). We did not find any differences between groups with regard to characteristic factors and DIS or symptoms of depression, and thus these factors were not included in the development of our subsequent analytic strategy. It is acknowledged, however, that assessment of confounders was limited to those available, which differs from the list of theoretically possible, and thus analysis was limited to the data available and these factors may have influenced the reported findings. Despite this, the comprehensive assessment of these peripheral factors was not a primary aim of the current study, and as no differences were revealed at initial assessment, we do not anticipate that these factors significantly attenuated our reported findings. Finally, the changes in sex distribution and participant attrition rate over the course of the data collection waves may also restrict the generalizability of the findings.
In conclusion, this study confirms cross-sectional and longitudinal findings showing that a strong relationship exists between symptoms of depression and DIS, and contributes more information suggesting the role of early depressive symptoms as a predictor of later sleep difficulties. Longitudinal assessment of symptoms of depression and DIS during the time of early adolescence is advantageous, as increasing evidence has suggested that these factors, which may indicate proxy markers for depressive illness and insomnia, develop early, and often remain stable across the lifespan. As both depressive illness and sleep problems represent significant areas of morbidity and disability among both adolescents and adults, assessing and describing the developmental origins of these conditions are important for public health initiatives.
DISCLOSURE STATEMENT
This was not an industry supported study. The Norwegian Research Council has been the main funding source of the study. Dr. Berk has received Grant/Research Support from the NIH, Simons Foundation, CRC for Mental Health, Stanley Medical Research Institute, MBF, NHMRC, Beyond Blue, Gee-long Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Organon, Novartis, Mayne Pharma, Servier and Astra Zeneca. He has been a paid consultant for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo Smith-Kline, Janssen Cilag, Lundbeck and Pfizer and a paid speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo Smith-Kline, Janssen Cilag, Lundbeck, Organon, Pfizer, Sanofi Synthelabo, Solvay, and Wyeth. He is supported by a NHMRC Senior Principal Research Fellowship (1059660). Amie C. Hayley is supported by NHMRC grant (APP1065576). The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Norwegian Longitudinal Health Behaviour study was initiated and conducted from the Department of Health Promotion and Development, Faculty of Psychology, University of Bergen. The founder of the study was professor Knut-Inge Klepp.
REFERENCES
- 1.Patten CA, Choi WS, Gillin JC, Pierce JP. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatr. 2000;106:E23. doi: 10.1542/peds.106.2.e23. [DOI] [PubMed] [Google Scholar]
- 2.Szklo-Coxe M, Young T, Peppard PE, Finn LA, Benca RM. Prospective associations of insomnia markers and symptoms with depression. Am J Epidemiol. 2010;171:709–20. doi: 10.1093/aje/kwp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9:S3–9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 4.Hayley AC, Williams LJ, Venugopal K, Kennedy GA, Berk M, Pasco JA. The relationships between insomnia, sleep apnoea and depression: findings from the American National Health and Nutrition Examination Survey, 2005–2008. Aust N Z J Psychiat. 2014 doi: 10.1177/0004867414546700. 0004867414546700. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66–71. doi: 10.1016/j.jad.2012.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40:700–8. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Sivertsen B, Salo P, Mykletun A, et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med. 2012;74:758–65. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 11.Rodin J, McAvay G, Timko C. A longitudinal study of depressed mood and sleep disturbances in elderly adults. J Gerontol. 1988;43:45–53. doi: 10.1093/geronj/43.2.p45. [DOI] [PubMed] [Google Scholar]
- 12.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 13.Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64:443–9. doi: 10.1016/j.jpsychores.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75:37–44. doi: 10.1016/j.biopsycho.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen R. Stages of progression in noncoital sexual interactions among young adolescents: an application of the Mokken scale analysis. Int J Behav Dev. 1997;21:537–53. [Google Scholar]
- 17.Birkeland MS, Torsheim T, Wold B. A longitudinal study of the relationship between leisure-time physical activity and depressed mood among adolescents. Psych Sport Exerc. 2009;10:25–34. [Google Scholar]
- 18.Alsaker FD, Dundas I, Olweus D. A growth curve approach to the study of parental relations and depression in adolescence. Paper presented in a symposium at the Biennial Meeting of the Society for Research on Child Development; 18–20 April 1991; Seattle, WA. [Google Scholar]
- 19.Muthén LK, Muthén BO. Mplus User's Guide. 7th ed. Los Angeles, CA: 1998–2012. [Google Scholar]
- 20.Manni R, Ratti M, Marchioni E, et al. Poor sleep in adolescents: a study of 869 17-year-old Italian secondary school students. J Sleep Res. 1997;6:44–9. doi: 10.1046/j.1365-2869.1997.00025.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson EO, Chilcoat HD, Breslau N. Trouble sleeping and anxiety/ depression in childhood. Psychiatr Res. 2000;94:93–102. doi: 10.1016/s0165-1781(00)00145-1. [DOI] [PubMed] [Google Scholar]
- 22.Ohayon MM, Roberts RE, Zulley J, Smirnes S, Priest RG. Prevalence and patterns of problematic sleep among older adolescents. J Am Acad Child Adolesc Psychiatr. 2000;39:1549–56. doi: 10.1097/00004583-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Merikangas KR, He J-P, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatr. 2010;125:75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merikangas KR, He J-p, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatr. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002;31:175–84. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 26.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 27.Leng Y, Wainwright NW, Cappuccio FP, et al. Self-reported sleep patterns in a British population cohort. Sleep Med. 2014;15:295–302. doi: 10.1016/j.sleep.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groeger JA, Zijlstra F, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J Sleep Res. 2004;13:359–71. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 29.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccinelli M, Wilkinson G. Gender differences in depression Critical review. Brit J Psychiatr. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 31.Tjora T, Hetland J, Aarø LE, Wold B, Wiium N, Øverland S. The association between smoking and depression from adolescence to adulthood. Addiction. 2014;109:1022–30. doi: 10.1111/add.12522. [DOI] [PubMed] [Google Scholar]
- 32.Holsen I, Kraft P, Vittersø J. Stability in depressed mood in adolescence: results from a 6-year longitudinal panel study. J Youth Adolesc. 2000;29:61–78. [Google Scholar]
- 33.Holsen I, Kraft P, Røysamb E. The relationship between body image and depressed mood in adolescence: a 5-year longitudinal panel study. J Health Psychol. 2001;6:613–27. doi: 10.1177/135910530100600601. [DOI] [PubMed] [Google Scholar]
- 34.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. App Psychol Measure. 1977;1:385–401. [Google Scholar]
- 35.Blader JC, Koplewicz HS, Abikoff H, Foley C. Sleep problems of elementary school children: a community survey. Arch Pediatr Adolesc Med. 1997;151:473–80. doi: 10.1001/archpedi.1997.02170420043007. [DOI] [PubMed] [Google Scholar]
- 36.Fricke-Oerkermann L, Plück J, Schredl M, et al. Prevalence and course of sleep problems in childhood. Sleep. 2007;30:1371–7. doi: 10.1093/sleep/30.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]