Abstract
Study Objectives:
Sleep restriction (SR) has been hypothesized to sensitize the pain system. The current study determined whether experimental sleep restriction had an effect on experimentally induced pain and pain-elicited electroencephalographic (EEG) responses.
Design:
A paired crossover study.
Intervention:
Pain testing was performed after 2 nights of 50% SR and after 2 nights with habitual sleep (HS).
Setting:
Laboratory experiment at research center.
Participants:
Self-reported healthy volunteers (n = 21, age range: 18–31 y).
Measurements and Results:
Brief high-density electrical stimuli to the forearm skin produced pinprick-like pain. Subjective pain ratings increased after SR, but only in response to the highest stimulus intensity (P = 0.018). SR increased the magnitude of the pain-elicited EEG response analyzed in the time-frequency domain (P = 0.021). Habituation across blocks did not differ between HS and SR. Event-related desynchronization (ERD) was reduced after SR (P = 0.039). Pressure pain threshold of the trapezius muscle region also decreased after SR (P = 0.017).
Conclusion:
Sleep restriction (SR) increased the sensitivity to pressure pain and to electrically induced pain of moderate, but not low, intensity. The increased electrical pain could not be explained by a difference in habituation. Increased response magnitude is possibly related to reduced processing within the somatosensory cortex after partial SR.
Citation:
Matre D, Hu L, Viken LA, Hjelle IB, Wigemyr M, Knardahl S, Sand T, Nilsen KB. Experimental sleep restriction facilitates pain and electrically induced cortical responses. SLEEP 2015;38(10):1607–1617.
Keywords: EEG, event-related desynchronization (ERD), event-related potential (ERP), pain, pressure pain threshold (PPT), time-frequency analysis
INTRODUCTION
Poor sleep is a major health problem and symptoms of insomnia are reported by 7% to 15% of the general population in Norway.1,2 The bidirectional association of sleep and pain has been investigated in a large number of studies. According to a recent review, sleep impairments seem to be a stronger, more reliable predictor of pain than the reverse.3 Two prospective studies indicate that sleep disturbances are associated with up to twofold risk for developing chronic musculoskeletal pain.4,5 However, there are examples of longitudinal studies reporting that pain leads to sleep impairments; e.g., a recent study showed that headache and chronic musculoskeletal disorders predicted insomnia 11 y later.6
In order to understand the mechanisms that account for the associations between sleep and pain, experimental studies are needed. The combined results from such studies leaves little doubt about the hyperalgesic effect of restricted or disturbed sleep.7 Sleep restriction (SR) varies between total sleep deprivation, sleep restriction for parts of the night, sleep fragmentation, and deprivation of specific sleep stages.8–21 The majority of these studies evaluated changes in pain by subjective psychophysical measures, such as pain threshold and pain-intensity ratings of suprathreshold stimuli across different types of experimental pain stimuli.
Three sleep restriction studies measured neural correlates of pain, focusing on cortical mechanisms.14,22,23 In these studies, subjective ratings of laser-induced pain were unchanged23 or increased14,22 after sleep restriction, whereas sleep restriction was accompanied by reduced amplitude of the laser-evoked potentials. The seemingly paradoxical changes between psychophysical and neurophysiological outcome measures following reduced sleep needs attention.
Conventional time-domain averaging makes a considerable part of the information present in single-trial electroencephalographic (EEG) responses undetectable, primarily because time-domain averaging may miss potentials that are not perfectly time- and phase-locked to the stimulus.24 Decomposing the EEG into both time and frequency domain (time-frequency domain averaging) accounts for these potential differences in response latency (jitter). Time-frequency analysis is also able to detect modulations of the ongoing EEG oscillatory activity that may appear either as a transient increase (event-related synchronization, ERS) or as a transient decrease (event-related desynchronization, ERD).24,25 Therefore, time-frequency analysis could reveal more of the information that code for subjective pain than does time-domain analysis.24,26 We wanted to assess the contribution of sensorimotor cortex on pain processing and therefore analyzed ERD from contralateral central electrodes (C3/C4).27,28 Thus, the current study addressed the associations between sleep restriction and elicited brain responses by expanding the EEG analysis to the time-frequency (TF) domain, in addition to the more conventional time-domain averaging.
Two types of experimental pain stimuli were included in the current study: muscular pressure pain and electrically induced cutaneous pain. Muscular pressure pain sensitivity was tested at a shoulder muscle, a common region for musculoskeletal pain disorders and an area in which the effect of SR on pain sensitivity has not previously been tested. In order to excite nociceptors, high-density electrical stimulation of free nerve endings located in the superficial skin is an alternative to the laser.29 The current study induced pain by high-density electrical stimulation of the skin by a punctuate electrode similar to the electrode described by Inui and coworkers.30 Special attention was given to the role of habituation to the painful test stimuli, as attenuation of habituation to stimuli is one potential mechanism for sleep restriction-related hyperalgesia. Attention was also given to the role of stimulus intensity, because a recent study found that reduced sleep affect pain of high intensity only.21
The aim of the current study was to determine the associations between experimental SR and both subjective pain and electrically elicited brain potentials in the laboratory. Specific aims were (1) to determine whether habituation to repeated blocks of painful electrical stimulation varied between sleep conditions, (2) to determine whether sleep condition affected electrical painful stimulation at different intensities, and (3) to determine whether SR would affect modulations of the ongoing EEG oscillatory activity after painful electrical stimulation. Finally, (4) whether the muscular pressure-pain threshold varied between sleep conditions was determined. A crossover design was chosen, comparing the effects of 2 nights of habitual sleep (HS) with 2 nights of partial SR.
METHODS
Subjects
Subjects were recruited through advertisement and flyers posted at the university and colleges in Oslo and by advertisement at the homepage of the National Institute of Occupational Health, Norway. Exclusion criteria were self-reported history of pain, neurological or psychiatric disease, or the use of prescription drugs (female subjects could use contraceptives). All volunteers were self-reported healthy.
Twenty-one subjects (mean age 23.4 ± 3.7 y, range: 18–31 y; 13 female, 8 male) participated in the study. The subjects were instructed not to drink alcohol and to discontinue use of any over-the-counter analgesics the least 24 h before the experiment. Subjects were blinded for the study's hypothesis. All participants received written information and signed an informed consent form. The study was approved by the Norwegian Regional Committee for Medical Research Ethics (approval number 2012/199).
Sleep Restriction
The SR condition consisted of 2 nights with 50 % sleep restriction, self-administered in the subject's home. The subject received the following instruction 2 d before the laboratory experiment (translated from Norwegian): “The 2 nights before the lab-experiment you should sleep half your normal sleep length, i.e. ____ hours. We ask you to get up at 7:00 AM tomorrow and the day of the lab-experiment. You should therefore go to bed at ____ AM, both tonight and tomorrow night. We ask you not to sleep at other times.” For example, in the case of a subject having a habitual sleep length of 8 h, half the normal sleep length would be 4 h and the subject would be instructed to go to bed at 03:00, both tonight and tomorrow night.
Design
The study was a paired crossover design on which the protocol was repeated twice on the same subjects under two different conditions: after at least 2 nights with habitual sleep (HS) and after 2 nights with partial SR. On both test days the experiment started between 08:00 and 09:00. The order of the sleep conditions was counterbalanced (block-randomization) and there was at least 1 w between the first and second test. A pretest took place 2 d before the first test, in order to familiarize the subjects with the procedures.
Measurements of Sleepiness, Alertness, Sleep Latency, and Sleep Length
Time in bed (TIB) was calculated based on sleep diaries. Total sleep time (TST) was calculated based on actigraph measurements (ActiSleep+ monitor, ActiGraph, Pensacola, FL, USA). The actigraph was worn on the wrist 48 h prior to the experiment. TST was calculated based on the Cole-Kripke algorithm adjusted with In-bed and Out-bed times from the diary, as recommended by the manufacturer. At the start of each experiment, subjective sleepiness was measured by the Karolinska Sleepiness Scale (KSS)31 and behavioral alertness was measured by a computerized version of the 10-min Psychomotor Vigilance Test (PVT)32 (custom-written C++ program, National Institute of Occupational Health, Norway). Sleep latency was measured objectively by EEG + EOG at the end of the experiment (around 12:00). During the sleep latency measurement the room was darkened (< 1 lux), the subject's chair was reclined to a near horizontal position and the subject was left alone for 20 min and asked to try to fall asleep.
Electrical Pain Stimulation
High-density electrical stimulation was delivered through an electrode adapted from Klein et al.33 and Inui et al.30 The electrode consisted of a platinum electrode (diameter 0.2 mm) protruding 0.2 mm from the surface of a polyoxymethylene frame (custom made at the National Institute of Occupational Health, Oslo, Norway). The pin electrode served as cathode and was attached to the skin by double-adhesive tape at the volar forearm (approximately 10 mm medial to half the distance between the insertion point of the biceps brachii tendon and the distal end of ulna). The anode was a conductive Velcro-strap (Alpine Biomed ApS, Skovlunde, Denmark) soaked in an isotonic sodium chloride solution and placed on the ipsilateral upper arm, 5 cm proximal to the cubital fossa. The electrical stimuli were delivered by a constant current stimulator (DS7A and DG2A, Digitimer, Hertfordshire, England). Each electrical stimulation is delivered as a double pulse, where each pulse lasts 0.5 msec. The two pulses are separated by 10 msec and is perceived as one single pulse. In a reaction time experiment stimulating the distal and proximal arm, the conduction velocity was estimated to be compatible with the activation of Aδ-fibers (8 m/sec).
Each subject's pain threshold (PT) was determined by a ladder sequence consisting of three ascending series of stimuli. Each series started at 0 mA, increased by steps of 0.1 mA and stopped at the lowest mA-value that was rated painful by the subject. The PT was calculated as the mean of the last two mA-values.
Subjects rated the pain intensity of each electrical stimulus verbally on a numerical rating scale (NRS) with end points 0 (‘not painful’) and 10 (‘most intense pain imaginable’). Subjects were allowed to use decimals.
Pressure Pain Stimulation
The pressure pain threshold was measured by a handheld pressure algometer with probe size 1 cm2 (Wagner Force One model FDIX, Wagner Instruments, Greenwich, CT, USA). The test site was a point on the upper trapezius muscle (dominant side), one third of the distance on a straight line connecting the C6 spinal segment and the lateral acromion. The rate of pressure increase was 5 N/sec (50 kPa/sec). The pressure reading from the algometer was sampled continuously by a computer (custom-written C++ program, National Institute of Occupational Health, Norway). To assist the experimenter in increasing pressure with a steady rate, a guideline was displayed alongside with the applied pressure on the computer screen. The pressure continued until the subject reached a score of 5 cm on a 0–10 cm visual analog scale with end points ‘no pain’ and ‘worst pain imaginable’. The procedure was repeated three times.
EEG Registration
EEG registrations were made from 32 electrodes placed according to the international 10-20 system using a soft electrode cap matching the subjects head size (actiCAP, Brain Products GmbH, Gilching, Germany). The common reference electrode was placed at FCz. The continuous EEG signal was amplified, filtered (0.53–100 Hz) and sampled at 2 kHz (QuickAmp 40-channel amplifier and Brain Vision Recorder, Brain Products GmbH, Gilching, Germany). Impedance was kept below 20 kΩ. Ocular movements and eye blinks were monitored by two surface electrodes placed at the upper left (VEOG) and lower right (HEOG) side of the eye.
Experimental Procedure
After filling out the KSS scale and performing the PVT test at the start of the experiment, the EEG recording electrodes and the electrical stimulation electrode were mounted. Three blocks of 30 repeated noxious electrical stimuli were then applied. Stimuli were divided equally between intensity A (2 × PT), B (3 × PT) and C (4 × PT) within each block. The inter-stimulus interval varied between 10 and 15 sec. Each block lasted between 5 and 7 min and interblock interval was 2 min. Stimulus intensity was varied in pseudo-randomized order, i.e., for each block one of three predefined randomized sequences was followed. Subjects were instructed to keep their eyes open during the experiment and to focus on a sticker placed on the wall three meters away in order to minimize eye movements. Subjects were instructed to give their verbal pain intensity rating 3–4 sec after each stimulus. At the end of the experiment, sleep latency was measured before the EEG electrodes were unmounted.
EEG Preprocessing
Preprocessing was performed using Analyzer 2 software (BrainProducts GmbH, Gilching, Germany) and EEGlab Version 10.2.2.4b,34 an open source toolbox running under the Matlab environment (R2012 The Mathworks, Natick, MA, USA). Preprocessing in Analyzer included the following steps: downsampling to 512 Hz, band pass filtering at 1–100 Hz, notch filtering at 50 Hz, ocular correction with independent component analysis (ICA), re-referencing to linked mastoid (A1+A2) and segmentation relative to stimulus onset (−1 to 2 sec). The segments were then visually inspected and segments with artefacts (18.0 %) were discarded. EEG-data were extracted from electrodes Cz-A1A2, C3-A1A2, and C4-A1A2. Responses from C3/C4 were subsequently arranged as responses contralateral (Cc) to the stimulated side, since previous studies have shown that painful stimulation correlates with alpha and gamma activity at contralateral central electrodes.28,35–37
Time-Domain Analysis
For the time-domain analysis, across-trial average time courses were generated for each of the 18 experimental conditions (2 sleep × 3 block × 3 intensity). A semiautomatic search was performed in order to identify the maximum negative peak between 50 and 200 msec (N2) and the maximum positive peak between 150 and 500 msec (P2). If two P maxima were observed, the earlier was selected, in order to avoid P300. The N2P2 peak-to-peak amplitude was calculated as P2 peak amplitude minus N2 peak amplitude (Figure 1).
Figure 1.
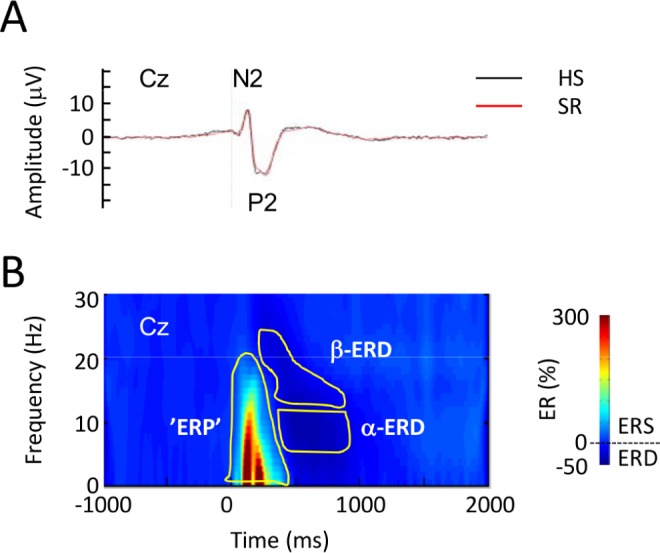
Group-level average event-related potentials elicited by high-density electrical stimulation. (A) Group-level average N2P2 potential after habitual sleep, (HS, black) and sleep restriction (SR, red) in the time domain. (B) Group-level average time-frequency representation of pain-elicited modulation of electroencephalographic oscillation magnitude (ER%). The color scale represents average increase or decrease of oscillation magnitude relative to a prestimulus reference interval (−0.9 to −0.1 sec) before the electrical stimulus. Statistically defined regions of interest (ROI) used in the time-frequency analysis are shown in yellow. Responses are from recording position Cz, referenced to linked mastoid (A1A2). ERD, event-related desynchronization; α-ERD, ERD in the α-frequency range; β-ERD, ERD in the β-frequency range; ‘ERP,’ event-related potential ROI; ERS, event-related synchronization.
Time-Frequency Domain Analysis
For the time-frequency analysis, segmented data were exported to Matlab format and analyzed by means of custom written Matlab scripts.36,38 The power spectral density of each epoch was calculated using the Windowed Fourier Transform (200 msec Hanning window), and averaged across trials to obtain the time-frequency representations for each subject and condition. To express the magnitude of event-related (ER) changes in oscillation amplitude, a percentage change in power for each TF-point after stimulus was calculated from a pre-stimulus reference interval (−900 msec to −100 msec) as follows:
where P(t,f) = |F(t,f)|2 is the power spectral density at each time-frequency point (t,f) and R(f) is the average power spectral density of the signal within the prestimulus reference interval36 for each subject and condition (2 sleep × 3 block × 3 intensity).
Defining Regions of Interest
To determine regions of interest (ROIs) within the TF-maps, we used a combination of bootstrapping and a paired t test.39 After bootstrapping 1,000 times the paired t test determined which TF-points in the 0–800 msec poststimulus interval that was different from the reference interval (−900 to −100 msec). The t test compared each ER% TF-point to the reference and provided statistical P values where TF-points with P < 0.01 (uncorrected) were retained. Clusters of significant P values were then identified visually. Only large clusters with a bandwidth of at least 10 Hz and 50 msec were considered. For each electrode position (Cz and Cc), the clusters were used to define regions of interest (ROIs) for the subsequent quantitative analysis of each condition (2 sleep × 3 block × 3 intensity) on changes in power magnitude (ER%). ER% was used as the dependent variable in separate statistical analyses assessing the effects of sleep and habituation. Time-frequency representations contained both phase-locked event-related potentials (ERPs) and non–phase-locked ERS and ERD.25 In addition, prior to baseline correction in the time-frequency domain, pre-stimulus α-power in the reference interval was quantified as the mean R(f) across the 8–13 Hz frequency range.
Time-frequency analysis revealed three clusters with significant changes in magnitude compared to the prestimulus reference interval. The three clusters were subsequently divided into four ROIs. The most significant response (ER%) was an early low frequency response corresponding to the N2P2 complex detected in the time domain in the 1–400 msec / 1–25 Hz range. In the following, this response in the time-frequency domain is denoted ‘ERP’. The response was seen at both electrodes (Figure 1 shows the response from Cz). Furthermore, a significant late ERD cluster was seen in the 200–800 msec range. This ROI was divided into α-ERD (8–13 Hz) and β-ERD (14–20 Hz), Figure 1. Finally, a significant response cluster was seen in the gamma frequency range (GBO) (33–60 Hz, 190–470 msec). The magnitudes of GBO and β-ERD did not vary with sleep as a main effect, neither with any interactions between sleep and block or intensity. These ROI therefore were not analyzed further.
Statistics
Paired comparisons of TIB, TST, response speed, sleepiness, and sleep latency were performed by Student's t-test or by the nonparametric Wilcoxon test, if data were nonnormally distributed.
Psychophysical and electrophysiological measurements were analyzed by linear mixed models (LMM) with maximum likelihood (ML) estimation. LMM have been shown to be advantageous relative to the more classic repeated-measures analysis of variance for analyzing ERPs.40 Dependent variables were subjective pain (NRS), electrophysiological data from the two electrode positions (Cz and Cc) analyzed in the time domain (N2P2 amplitude), in the time-frequency domain (‘ERP’ and α-ERD) and prestimulus α-power. One LMM analysis was performed for each electrode position. Independent fixed factors in the models were sleep, stimulus block (habituation) and stimulus intensity. Centering of the independent variables was not used.
To determine whether habituation to repeated blocks of painful stimulation varied between sleep conditions, a model with sleep, block, intensity, and sleep × block was tested. Similarly, to determine whether sleep condition affected painful stimulation differently at different intensities, a model with sleep, block, intensity, and sleep × intensity was tested. The interaction term was kept in the model only if P < 0.05. In this case a post hoc analysis was performed, in which only the effect of sleep was tested for each of the three levels of block or intensity. Bonferroni correction was then performed by multiplying the resulting P value by the number of comparisons. To determine whether the amplitudes differed between electrode positions, the datasets from electrode Cz and Cc were pooled and the dependent variables were tested with electrode (Cz, Cc) as the only independent fixed factor in the model. The number of repeated measures per subject was 18, yielding a total of 378 data points (21 subjects × 18 repeated measures).
From the painful pressure stimuli, the pressure pain threshold (PPT) was defined as the pressure corresponding to the first visual analog scale score above zero. The mean of the three PPT measurements was used in the statistical analysis.
The intercept was allowed to vary randomly in all models. Based on Akaike Information Criterion (AIC) random slope for sleep, intensity and/or block was added if it improved the model fit. No covariance structure was assumed.
Associations between selected continuous variables (NRS, N2P2, ‘ERP’, and α-ERD) were tested by separate LMM analyses in which one variable was the dependent variable, the other a covariate. Fixed factors (sleep, block, or intensity) were entered if it improved the model fit. Associations between electrical and pressure pain sensitivity were tested by Spear-man's nonparametric bivariate correlation test. NRS scores averaged across blocks were correlated against PPT, one analysis for each stimulus intensity and sleep condition. Delta scores (changes in electrical and pressure pain scores from HS to SR) were tested in the same manner.
Statistical analyses were performed with IBM SPSS version 21 (IBM, Chicago, IL, USA).
RESULTS
TIB, Sleep Time, Sleepiness, and Behavioral Alertness
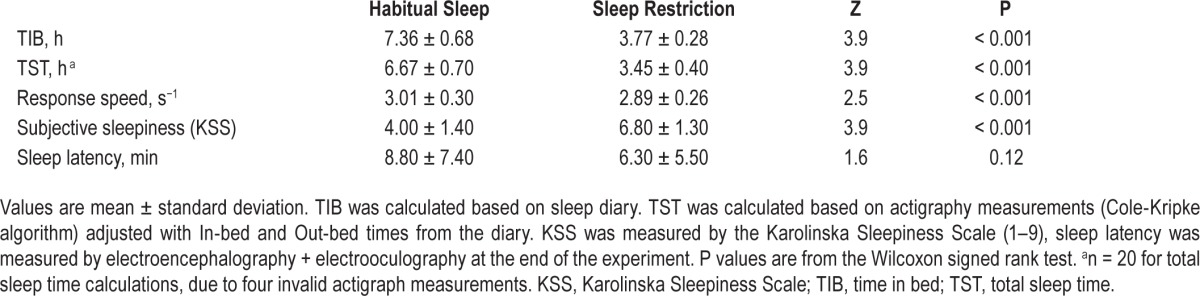
After SR, average TIB and TST was significantly shorter, PVT response speed was significantly slower, and subjects were significantly more sleepy (KSS), compared to after HS (Table 1). Sleep latency tended to be shorter after SR.
Table 1.
Sleepiness measures, time in bed, sleepiness, behavioral alertness.
Changes in Electrical Pain
Mean intensities of the high-density electrical stimulation were 2.4 ± 1.2 mA (intensity A), 3.7 ± 1.7 mA (intensity B), and 4.8 ± 2.3 mA (intensity C). This resulted in mean NRS scores of 2.6 ± 1.4, 3.5 ± 1.5, and 4.5 ± 1.6 on the 0–10 NRS scale, respectively.
Subjective pain ratings did not vary with sleep condition (P = 0.80), but increased with stimulus intensity (P < 0.001, Figure 2A) and decreased with stimulus block (P = 0.004, Figure 2B). There was a significant sleep × intensity interaction (P = 0.022). Post hoc analyses for each stimulus intensity showed a significant increase in pain ratings after SR for stimulus intensity C (P = 0.018, corrected): subjective pain ratings increased 8 %, from 4.4 ± 1.4 after HS to 4.7 ± 1.8 after SR (Figure 2A). Pain did not increase after SR for intensities A and B (P > 0.66, corrected). The decrease in pain ratings across stimulus blocks did not differ between sleep conditions, i.e. there was no sleep × block interaction (P = 0.99, Figure 2B). A statistical summary is shown in Table 2.
Figure 2.
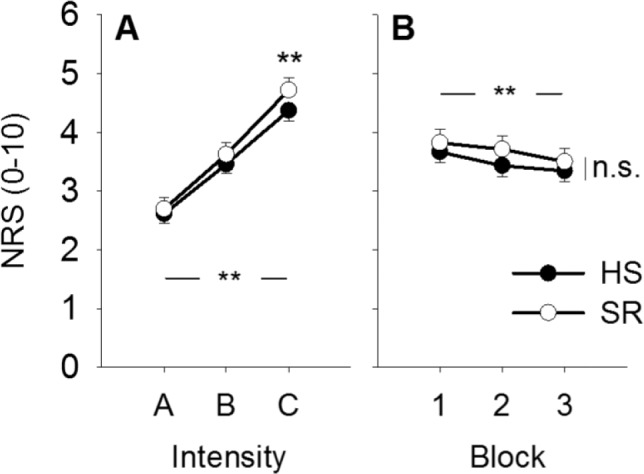
Changes in pain. (A) Mean pain intensity increased with stimulus intensity (**P < 0.001) and showed an interaction with sleep condition (P = 0.022). Post hoc analysis showed that pain ratings were higher after sleep restriction (SR) versus after habitual sleep (HS) for stimulus intensity C (**P = 0.018, Bonferroni corrected). (B) Mean pain intensity decreased with stimulus block (**P = 0.004), but the decrease did not change between sleep conditions (P = 0.99). Values are mean ± standard error of the mean. NRS, numerical rating scale; HS, habitual sleep; SR, sleep restriction.
Table 2.
Statistical summary of electrical pain after linear mixed-models analysis.
Changes in ERPs: Time-Domain Averaging
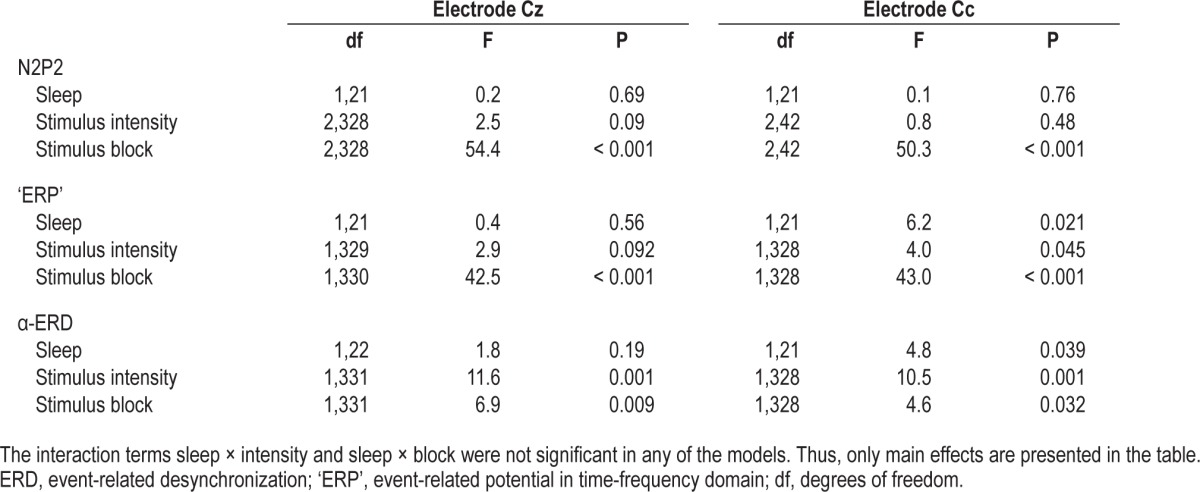
N2P2 amplitude was significantly larger (F(1,21) = 29.1, P < 0.001), and N2 peak latency was shorter (F(1,21) = 9.5, P = 0.006) at Cz, compared to Cc (Table 3). P2 peak latency did not differ between electrodes (F(1,714) = 0.1, P = 0.7).
Table 3.
Mean N2P2 amplitude, N2 peak latency, P2 peak latency, ‘event-related potential’, and α-event-related desynchronization.
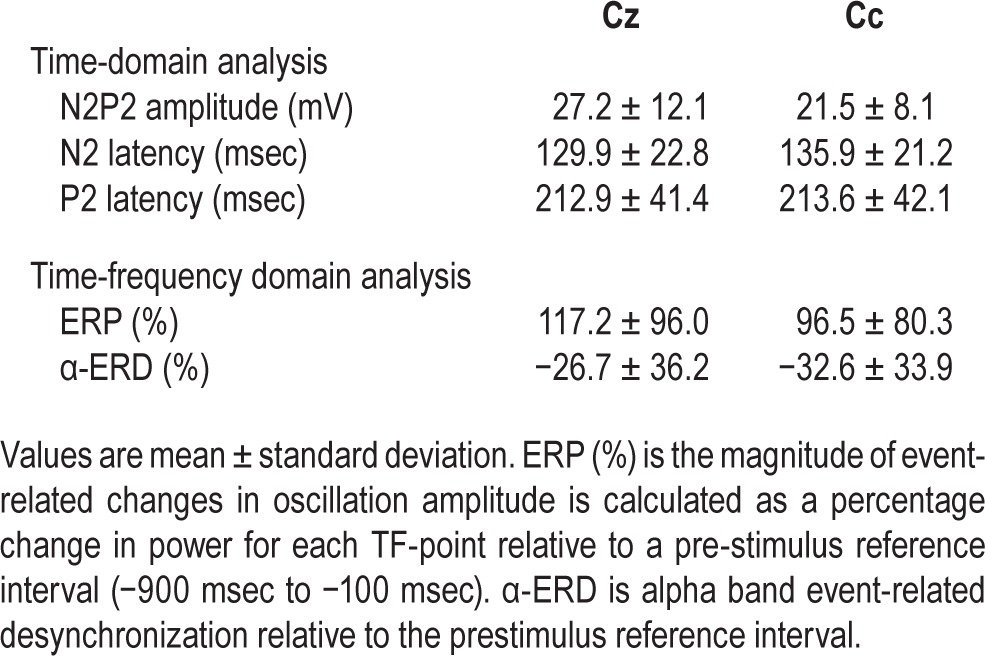
N2P2 amplitude did not vary with sleep condition at Cc (P = 0.76; Figure 3A), as well as at Cz (P = 0.69). At Cc, the amplitude of the N2P2 complex was 22.5 ± 8.5 mV after HS and 23.0 ± 7.5 mV after SR. At Cz, the N2P2 amplitude was 27.6 ± 11.7 mV after HS and 26.6 ± 12.2 mV after SR. There was a tendency toward an increased N2P2 amplitude with stimulus intensity at Cz (P = 0.09), but not at Cc (P = 0.48, Figure 3B). There was no sleep × intensity interaction for any of the electrodes (P > 0.63). N2P2 amplitude habituated across stimulus blocks at both electrodes (P < 0.001), but there was no sleep × block interaction for any of the electrodes (P > 0.14, Figure 3C). A statistical summary is shown in Table 4.
Figure 3.
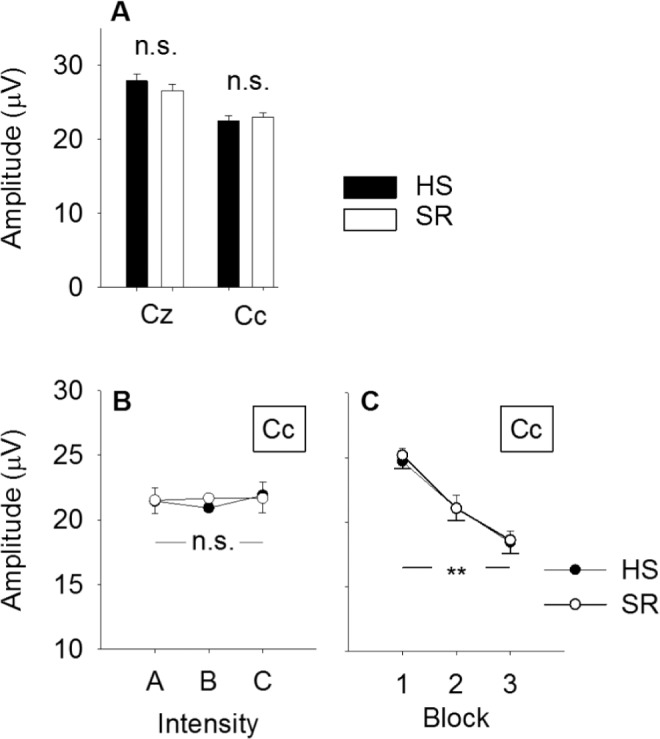
Changes in N2P2 amplitude. (A) Average N2P2 amplitude by sleep condition and electrode. There was no difference in amplitude after 2 nights of habitual sleep versus after 2 nights with 50 % sleep restriction at any of the recording electrodes (Cz, Cc; P > 0.69). (B) N2P2 amplitude did not change with stimulus intensity (P = 0.48, main effect) and there was no sleep × intensity interaction (P = 0.66). (C) N2P2 amplitude habituated across stimulus blocks (P < 0.001, main effect), but the degree of habituation was not different between sleep conditions (P = 0.63, sleep × block interaction). Values are mean ± standard error of the mean. HS, habitual sleep; SR, sleep restriction.
Table 4.
Statistical summary after linear mixed-models analysis of electrophysiological variables.
Changes in ERPs: Time-Frequency Domain Averaging
‘Event-Related Potential’
‘ERP’ was significantly larger in magnitude at Cz than at Cc (F(1,21) = 9.6, P < 0.005) (Table 3).
The ‘ERP’ response at electrode Cc was significantly larger in magnitude after SR (108.2 ± 88.9 %) versus after HS (84.8 ± 69.0 %, P = 0.021, Figure 4A). There was no difference in response magnitude at electrode Cz (P = 0.56).
Figure 4.
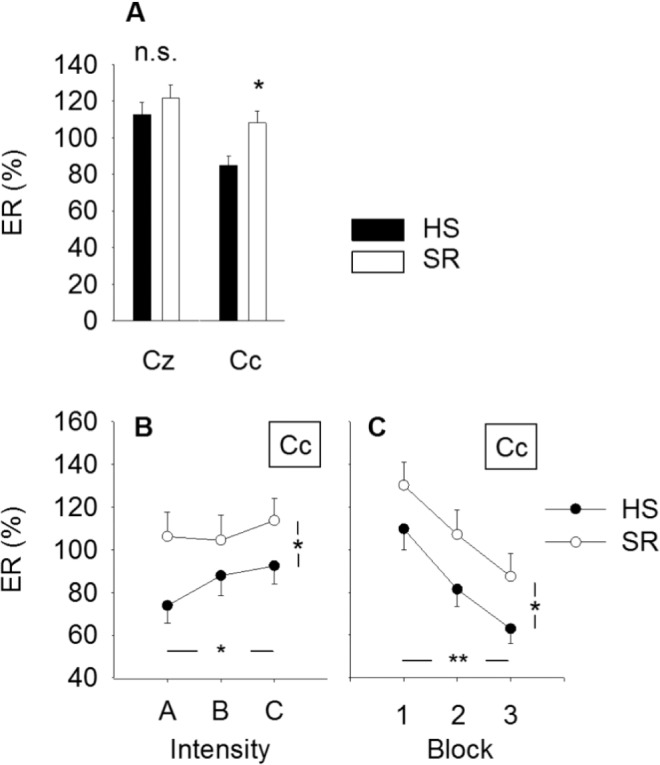
Changes in ‘ERP’ magnitude. (A) Average event-related synchronization (in % of the pre-stimulus reference interval) by sleep condition and electrode. At electrode Cc the magnitude was significantly larger after SR versus after habitual sleep, *P = 0.021. There was no sleep related differences at electrode Cz (P = 0.56). (B) ‘ERP’ magnitude increased with stimulus intensity (*P = 0.045), but there was no sleep × intensity interaction (P = 0.35). (C) The ‘ERP’ response habituated with stimulus block (**P < 0.001), but there was no sleep × block interaction (P = 0.68). Values are mean ± standard error of the mean. ‘ERP’, event-related potential in time-frequency domain; HS, habitual sleep; SR, sleep restriction.
The ‘ERP’ response habituated with stimulus block at Cc (P < 0.001, Figure 4C), as well as at Cz (P < 0.001). There was no sleep × block interaction at any of the electrodes (P > 0.33), i.e., habituation did not vary between sleep conditions. ‘ERP’ magnitude increased with stimulus intensity at electrode Cc (P = 0.045, Figure 4B) and showed a tendency at electrode Cz (P = 0.092). There was no sleep × intensity interaction at any of the electrodes (P > 0.21). A statistical summary is shown in Table 4.
α-ERD and Prestimulus α-power
α-ERD did not differ between electrodes (F(1,21) = 2.5, P < 0.13) (Table 3). α-ERD at electrode Cc was significantly weaker in magnitude after SR (−27.8 ± 38.0 %) versus after HS (−35.4 ± 28.9 %) (P = 0.039), indicating less ERD after SR versus after HS (Figure 5A). There was no effect of sleep on α-ERD at electrode position Cz (P = 0.19). α-ERD increased significantly in magnitude with stimulus intensity at both electrode positions (P < 0.001; Figure 5B). There was no sleep × intensity interaction at any of the electrodes (P > 0.78). α-ERD decreased significantly with stimulus block at Cc (P = 0.032; Figure 5C) and at Cz (P = 0.009), indicating habituation. There was no sleep × block interaction at any of the electrodes (P > 0.54). A statistical summary is shown in Table 4.
Figure 5.
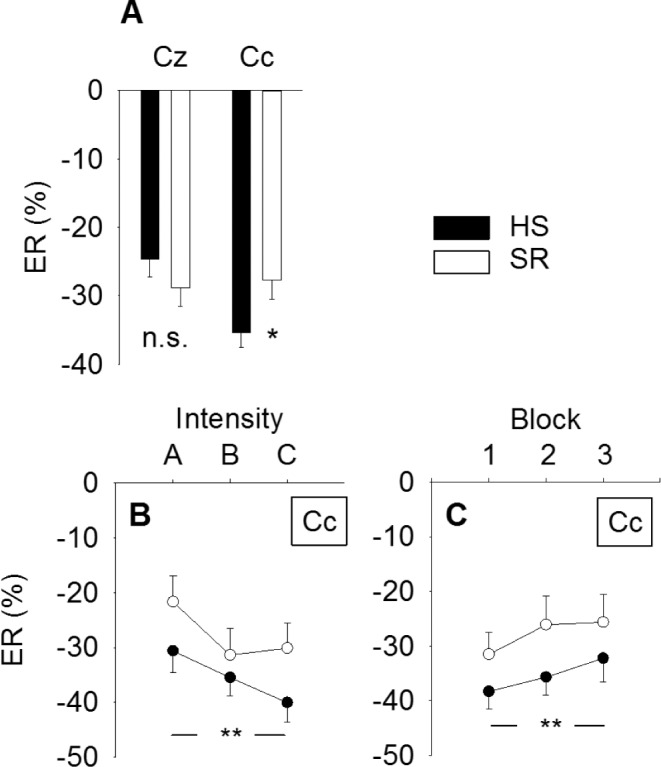
Changes in α-ERD magnitude. (A) Average event-related desynchronization (in % of the pre-stimulus reference interval) by sleep condition and electrode. α-ERD at electrode Cc was significantly weaker in magnitude after sleep restriction (*P = 0.039). (B) α-ERD magnitude increased with stimulus intensity (**P < 0.001), but no sleep × intensity interaction (P = 0.50). (C) α-ERD habituated with stimulus block (**P < 0.001), but no sleep × block interaction (P = 0.84). Values are mean ± standard error of the mean. ERD, event-related desynchronization; HS, habitual sleep; SR, sleep restriction.
α-ERD depends largely on prestimulus α-power,28 but there was no main effect of sleep on pre-stimulus α-power at electrode Cc (P = 0.46) or Cz (P = 0.45).
Changes in Pressure Pain Sensitivity
Mean PPT decreased from 28.6 ± 12.3 N after HS to 26.3 ± 9.2 N after SR, indicating an increased deep tissue pain sensitivity over the trapezius muscle (F = 6.4, P = 0.013).
Associations
In our post hoc analysis we found a significant positive association between ‘ERP’ and α-ERD both at Cc (F(1,374) = 163; P < 0.001) and Cz (F(1,363) = 118, P < 0.001), i.e., α-ERD is attenuated as ‘ERP’ is increased.
Electrical pain ratings (NRS) were significantly associated with the ‘ERP’ response at Cc (F(1,307) = 5.1, P = 0.025), an association that was not significant for the N2P2 amplitude (F(1,299) = 2.6, P = 0.11) nor for the α-ERD response (F(1,292) = 0.3, P = 0.58). At Cz there were no associations between pain and the ‘ERP’ (F(1,306) = 0.3, P = 0.61), N2P2 (F(1,314) = 1.5, P = 0.21), nor α-ERD (F(1,292) = 0.6, P = 0.45).
After SR, significant negative associations were found between electrical pain ratings at all intensities (NRS) and the PPT (rho < −0.51, P < 0.027). This indicates that subjects with the highest sensitivity to pressure pain also were most sensitive to electrical pain. After HS, no association was found between NRS and PPT (rho > −0.16, P > 0.5).
DISCUSSION
The main findings of the current study were that (1) subjective pain ratings to high-density electrical stimulation increased significantly after partial sleep restriction, but only for the highest stimulus intensity; (2) the magnitude of the pain-elicited EEG response analyzed in the time-frequency domain (‘ERP’) increased after partial SR, whereas the EEG response analyzed with the more conventional time-domain averaging (N2P2) did not, and (3) habituation across blocks did not differ between HS and SR. Finally, (4) pressure pain sensitivity of the trapezius muscle region increased after partial SR.
Effects on Sleepiness Measures
Subjects were actually more sleepy during the SR condition: both by the PVT32 and by the KSS. Shorter sleep latency after the experiment did not meet criteria for statistical significance. These measures confirm that our intervention had the intended effect on the participants.
Effects of SR on Pain
Experimental SR produced an increased pain sensation to the strongest electrical stimulus intensity (corresponding to NRS ≈ 4.5), but not to the weaker stimulus intensities (NRS ≈ 2.5–3.5). Neither was there an effect of SR on pain sensation averaged across all three stimulus intensities. This shows that 2 nights with partial SR is associated with hyperalgesia to electrically induced pain of moderate intensity. Weaker pain stimuli (lower third of the 0–10 NRS) were not affected by experimental SR. This differential effect on intensity confirms previous findings of SR-related hyperalgesia affecting only the highest mechanical pinprick intensity.21 To the authors' knowledge, no other studies have tested perception of painful electrical stimulation after SR. However, the electrical sensory threshold (generally nonpainful) was not found to be modulated following SR,41 indicating that low-intensity electrical stimuli may not be sensitive to reduced sleep length. Other studies have shown that reduced sleep is associated with hyperalgesic effects of both thermal and mechanical pain. Laser-induced heat-pain ratings was unchanged23 or increased by 30%14 following 2 nights of 4 h of sleep and increased by 57%22 after 2 nights of total sleep deprivation, respectively. Hypersensitivity to contact heat stimuli after SR has also been reported after 2 nights of total sleep deprivation,9 after 2 nights of 4 h of sleep23 and after 1 night of 4 h of sleep.12 Furthermore, Schuh-Hofer and coworkers,21 performing an extensive quantitative sensory testing protocol, reported that 1 night of total sleep deprivation led to lower pain thresholds to heat, cold, and mechanical pin-pricks, supporting SR-induced hyperalgesia.
Possible explanations for the weak increase in pain (≈ 8 %) after SR in the current study, compared to the 30% pain increase reported by Tiede and coworkers,14 could be related to differences between electrical and laser stimulation, as the former may stimulate some low-threshold Aβ-fibers whereas the latter is nociceptive specific.29,42 The difference in pain increase could also be related to different methods for rating pain: the subjects rated every single stimulus in the current study, whereas in the study by Tiede et al. subjects rated the stimulus intensity at the end of a block of 20 stimuli.14
The current study also found that experimental SR led to reduced pressure pain thresholds from the upper trapezius muscle. Reduced PPT after reduced sleep length corresponds with other experimental studies that investigated the effect of total sleep deprivation and insomnia on mechanical pain sensitivity of bone and muscular structures.16,21,43 Not only does SR seem to increase the pressure pain sensitivity within individuals, the current correlation between PPT and electrical pain ratings after SR indicate that the individuals who are most sensitive to pressure pain after SR also are the ones most sensitive to electrical pain stimuli.
Regarding the effects of SR on subjective responses to painful stimulation, the current study confirms knowledge from previous studies by showing SR-associated hyperalgesia to mechanical pain and extends knowledge to show that hyperalgesia exists also to electrically induced pain of moderate intensity. The overall hypothesis that insufficient sleep may contribute to the amplification of pain across several types of pain, involving both deep tissue afferents and more superficial afferents, is supported.
Effects of SR on N2P2 and ‘ERP’ Magnitude
The ‘ERP’ region of interest (based on time-frequency analysis) increased significantly in magnitude after SR at the contralateral Cc electrode, compared to HS, whereas the N2P2 amplitude (based on time-domain analysis) did not change. SR did not produce changes in response magnitude at the vertex Cz electrode, neither for ‘ERP’ nor for the N2P2 amplitude.
The current results differ from three similar studies that reported attenuated laser-evoked potential (LEP) amplitude at Cz after SR.14,22,23 Tiede and coworkers14 found that 2 nights of SR led to a 34% decrease in LEP amplitude, compared to HS. Decreased LEP amplitude was also found by Ødegård and coworkers,23 comparing LEP amplitude after 2 nights of 4 h of sleep versus 2 nights of 9 h of sleep. Azevedo and coworkers22 obtained LEP thresholds before and after 2 nights total sleep deprivation and observed a 23% increase in average thresholds. Furthermore, stimulating Aδ-fibers electrically led to reduced N120-P240 magnitude at Cz during sleep, compared to before sleep.44 These studies indicate that the amplitude of cortical responses recorded at the vertex after painful laser stimulation is attenuated by sleep restriction, as well as during actual sleep.
The discrepancy in SR-induced changes in N2P2 amplitude between the current study and the two aforementioned LEP-studies could be related to differences between painful test stimuli; electrical shocks vs. radiant heat induced by laser. Laser stimulation activates a combination of Aδ and C nociceptive fibers.42 Data from reaction time experiments in our lab show that the high-density electrode activates Aδ-fibers, although coactivation of Aβ-fibers cannot be ruled out. Although pain stimulation in general transmit information through the spinothalamic pathway to thalamus, primary and secondary somatosensory cortex, insular cortex, and anterior cingulate cortex, different types of pain produce different patterns of activations.45 Thus, given that laser and electrical stimuli probably produce different patterns of cortical activation per se, it may not be surprising if the pain-modulatory effect of reduced sleep also differ between laser and electrical stimuli.
N2 and P2 waves evoked by nociceptive stimuli reflect activations in the secondary somatosensory cortices, the insula, and the anterior cingulate gyrus.46,47 It is, however, worth noting that the N2P2 complex is similar to vertex potentials elicited by other sensory modalities (touch, auditory, visual).48 Thus, it has been suggested that the N2 and P2 waves are mainly determined by the ability of the external sensory stimulus to capture attention (i.e. stimulus saliency), rather than the ability to produce pain per se.49 Most of these findings, which are based on the studies of laser-evoked potentials, should be valid for other types of stimulation to activate small fibers, such as the current high-density electrical stimulation. Our findings of an increased ‘ERP’ magnitude for all intensities after SR, but increased pain scores only for the highest intensity, may thus indicate that the effects of SR on saliency is present for all intensities, but only for pain stimuli above a certain threshold.
The discrepancy between intensity and SR on N2P2 amplitude (unchanged) and ‘ERP’ magnitude (facilitation) could reflect that the latter methodology is more sensitive in picking up intensity-related changes of neural oscillations (see Figures 3B and 4B). Another explanation for the discrepancy between SR-induced effects on N2P2 amplitude and ‘ERP’ magnitude could be that in situations with large latency jitter the amplitude of across-trial averages in the time domain will be reduced.24 The time-frequency analysis overcome this by taking into account both the latency jitter that may occur between responses and account for phase-locked and non–phase-locked changes in amplitude. It should be noted that the P300 component of ERPs, which is generally generated by deviant (rare and unexpected) stimuli in oddball paradigms,50 could not be consistently evoked using our experimental design, and thus was not considered in the current study.
In summary, the current results extend previous findings by indicating that sleep restriction facilitates the EEG response analyzed in the time-frequency domain (‘ERP’), whereas the EEG response analyzed in the more conventional time-domain (N2P2) is unaffected.
Effect of SR on Habituation
Pain, N2P2, and ‘ERP’ habituated across the three stimulus blocks, but there was no interaction between sleep and habituation for any of the outcome variables. That habituation of N2P2 does not change with sleep restriction was also shown in a recent study of laser-evoked potentials.23 Because habituation of pain ratings after SR is unaltered relative to after HS, this indicates that the hyperalgesic effect of SR on subjective pain intensity cannot be explained by attenuation of habituation.
Because habituation of pain ratings did not change with sleep condition, the lower rates of habituation observed in chronic pain disorders such as migraine and fibromyalgia51,52 are probably not associated with relative sleep restriction.
Associations between Electrophysiological Outcomes
The painful stimulus induced α-ERD at both electrodes (Cz and Cc) in the 200–800 msec post-stimulus interval. This is an analog to results from previous experimental studies, which showed that painful events exert a global α-ERD.27,28,53 Our results also showed that α-ERD increased in magnitude with increasing stimulus intensity and pain, corresponding to a previous study.54 Increasing α-ERD may indicate increasing activation of cortical neuronal networks.55
α-ERD at electrode position Cc (overlying the primary sensory cortex) was reduced after SR, compared to after HS (Figure 5). Because α-ERD is considered to reflect cortical activation involved in processing of sensory or cognitive information, we interpret the reduction in α-ERD after SR as an indication of reduced cortical processing in the primary sensory cortex of the evoked nociceptive signal after SR.38 The SR-induced reduction in α-ERD magnitude was most prominent at the highest stimulus intensity, but was also evident at the lowest stimulus intensity (Figure 5B).
The magnitude of the ‘ERP’ response reflects the dipole generated as the sum of negative and positive postsynaptic potentials in the cortical cells underneath the nearby electrode. Contemporary cortical processing in the same cortical area may thus affect the magnitude of 'ERP' responses. One may speculate that the SR-induced reduction in α-ERD is related to the SR-induced increase in ‘ERP’. Our post hoc analysis of this association revealed that the α-ERD magnitude actually was significantly associated with the ‘ERP’ magnitude; i.e., α-ERD is attenuated as ‘ERP’ is increased. This may indicate that the increased ‘ERP’ after SR is related to reduced sensory-related processing of the response in the primary somatosensory cortex. Although speculative, one may suggest that the reduced α-ERD indicates that fewer resources are used for post-stimulus processing in this area of cortex after SR, i.e., that the stimuli are given less sensory-discriminative value. There was, however, no association between pain ratings and α-ERD. The lack of association between α-ERD and pain underlines the fact that the subjective evaluation of pain requires involvement of several brain structures. This may suggest differential SR- related modulation of brain structures involved in pain processing. Or, building on the aforementioned speculation, if the stimuli are given less sensory-discriminative value after SR, the SR-induced increase in pain intensity (for the highest stimulus intensity) may be driven by affective components of pain.
Finally, the relationship between pain, ‘ERP’magnitude, and N2P2 amplitude were investigated. It indicated a signifi-cant association between pain ratings and ‘ERP’ magnitude, but no significant association between pain ratings and N2P2 amplitude. Both findings correspond with previous studies on LEPs. A significant association between pain scores and LEP magnitude analyzed in the time-frequency domain was found by Zhang et al.36 In contrast, no association between pain and LEP amplitude was found when responses were analyzed in the time domain.48 Taken together, ERPs calculated in the time-frequency domain seem to be a better predictor of pain than ERPs calculated in the time domain. However, one must keep in mind that the ERP predominantly reflects sensory processing in primary somatosensory cortex. Indeed, it is not a measure of the global processing of pain.56
CONCLUSION
The current study extends previous findings by showing that experimental sleep restriction leads to increased perception of high-density electrically induced pain of moderate, but not low, intensity. Previous findings are confirmed and extended by showing muscular pressure pain hyperalgesia of the trapezius muscle region. The magnitude of the pain-elicited EEG response analyzed in the time-frequency domain (‘ERP’) increased after partial sleep restriction. Habituation to repeated blocks of painful stimulation was not affected by sleep restriction and cannot explain the increased pain perception or increased ‘ERP’. We hypothesize that the increased ‘ERP’ is related to reduced sensory-related processing of the response in the somatosensory cortex.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Jorid Thrane Stuenæs is acknowledged for collecting the data. All the subjects are acknowledged voluntarily abstaining from sleep for 2 half nights.
REFERENCES
- 1.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–16. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Uhlig BL, Sand T, Odegard SS, Hagen K. Prevalence and associated factors of DSM-V insomnia in Norway: the Nord-Trondelag Health Study (HUNT 3) Sleep Med. 2014;15:708–13. doi: 10.1016/j.sleep.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–52. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaila-Kangas L, Kivimaki M, Harma M, et al. Sleep disturbances as predictors of hospitalization for back disorders-a 28-year follow-up of industrial employees. Spine (Phila Pa 1976) 2006;31:51–6. doi: 10.1097/01.brs.0000193902.45315.e5. [DOI] [PubMed] [Google Scholar]
- 5.Siivola SM, Levoska S, Latvala K, Hoskio E, Vanharanta H, Keinanen-Kiukaanniemi S. Predictive factors for neck and shoulder pain: a longitudinal study in young adults. Spine (Phila Pa 1976) 2004;29:1662–9. doi: 10.1097/01.brs.0000133644.29390.43. [DOI] [PubMed] [Google Scholar]
- 6.Ødegård SS, Sand T, Engstrom M, Zwart JA, Hagen A. The impact of headache and chronic musculoskeletal complaints on the risk of insomnia: longitudinal data from the Nord-Trøndelag Health Study. J Headache Pain. 2013;14:24. doi: 10.1186/1129-2377-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Drewes AM, Rossel P, Arendt-Nielsen L, et al. Sleepiness does not modulate experimental joint pain in healthy volunteers. Scand J Rheumatol. 1997;26:399–400. doi: 10.3109/03009749709065709. [DOI] [PubMed] [Google Scholar]
- 9.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–7. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 10.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145:136–41. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 14.Tiede W, Magerl W, Baumgartner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 17.Arima T, Svensson P, Rasmussen C, Nielsen KD, Drewes AM, Arendt-Nielsen L. The relationship between selective sleep deprivation, nocturnal jaw-muscle activity and pain in healthy men. J Oral Rehabil. 2001;28:140–8. doi: 10.1046/j.1365-2842.2001.00687.x. [DOI] [PubMed] [Google Scholar]
- 18.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 19.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Older SA, Battafarano DF, Danning CL, et al. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–6. [PubMed] [Google Scholar]
- 21.Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154:1613–21. doi: 10.1016/j.pain.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Azevedo E, Manzano GM, Silva A, Martins R, Andersen ML, Tufik S. The effects of total and REM sleep deprivation on laser-evoked potential threshold and pain perception. Pain. 2011;152:2052–8. doi: 10.1016/j.pain.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Ødegård SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clin Neurophysiol. 2014 Dec 23; doi: 10.1016/j.clinph.2014.12.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26:1041–54. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 26.Schulz E, Tiemann L, Schuster T, Gross J, Ploner M. Neurophysiological coding of traits and states in the perception of pain. Cereb Cortex. 2011;21:2408–14. doi: 10.1093/cercor/bhr027. [DOI] [PubMed] [Google Scholar]
- 27.Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Oscillatory activity reflects the excitability of the human somatosensory system. NeuroImage. 2006;32:1231–6. doi: 10.1016/j.neuroimage.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Peng W, Valentini E, Zhang Z, Hu Y. Functional features of nociceptive-induced suppression of alpha band electroencephalographic oscillations. J Pain. 2013;14:89–99. doi: 10.1016/j.jpain.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner U, Greffrath W, Treede RD. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin. 2012;42:267–80. doi: 10.1016/j.neucli.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Adelta fibers by intra-epidermal needle electrode in humans. Pain. 2002;96:247–52. doi: 10.1016/S0304-3959(01)00453-5. [DOI] [PubMed] [Google Scholar]
- 31.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 32.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci. 2004;24:964–71. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–38. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci. 2007;27:9270–7. doi: 10.1523/JNEUROSCI.2283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu L, Xiao P, Zhang ZG, Mouraux A, Iannetti GD. Single-trial time-frequency analysis of electrocortical signals: baseline correction and beyond. Neuroimage. 2014;84:876–87. doi: 10.1016/j.neuroimage.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 39.Durka PJ, Zygierewicz J, Klekowicz H, Ginter J, Blinowska KJ. On the statistical significance of event-related EEG desynchronization and synchronization in the time-frequency plane. IEEE Trans Biomed Eng. 2004;51:1167–75. doi: 10.1109/TBME.2004.827341. [DOI] [PubMed] [Google Scholar]
- 40.Vossen H, Van Breukelen G, Hermens H, Van Os J, Lousberg R. More potential in statistical analyses of event-related potentials: a mixed regression approach. Int J Methods Psychiatr Res. 2011;20:e56–68. doi: 10.1002/mpr.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schestatsky P, Dall-Agnol L, Gheller L, et al. Pain-autonomic interaction after work-induced sleep restriction. Eur J Neurol. 2013;20:638–46. doi: 10.1111/ene.12011. [DOI] [PubMed] [Google Scholar]
- 42.Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- 43.Haack M, Scott-Sutherland J, Santangelo G, Simpson NS, Sethna N, Mullington JM. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16:522–33. doi: 10.1016/j.ejpain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Inui K, Qiu Y, Hoshiyama M, Tran TD, Kakigi R. Effects of sleep on pain-related somatosensory evoked potentials in humans. Neurosci Res. 2003;45:53–7. doi: 10.1016/s0168-0102(02)00198-0. [DOI] [PubMed] [Google Scholar]
- 45.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol. 1993;10:513–9. doi: 10.1097/00004691-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin. 2003;33:279–92. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–69. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 49.Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. 2008;100:815–28. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–8. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 51.Valeriani M, de Tommaso M, Restuccia D, et al. Reduced habituation to experimental pain in migraine patients: a CO(2) laser evoked potential study. Pain. 2003;105:57–64. doi: 10.1016/s0304-3959(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 52.de Tommaso M, Lo Sito L, Di Fruscolo O, et al. Lack of habituation of nociceptive evoked responses and pain sensitivity during migraine attack. Clinical Neurophysiol. 2005;116:1254–64. doi: 10.1016/j.clinph.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Pain suppresses spontaneous brain rhythms. Cereb Cortex. 2006;16:537–40. doi: 10.1093/cercor/bhj001. [DOI] [PubMed] [Google Scholar]
- 54.Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO(2) laser skin stimulations may reflect central interactions between A partial differential - and C-fibre afferent volleys. ClinNeurophysiol. 2003;114:710–22. doi: 10.1016/s1388-2457(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 55.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Clinical Neurophysiol. 2012;42:299–313. doi: 10.1016/j.neucli.2012.06.001. [DOI] [PubMed] [Google Scholar]


