Abstract
Study Objectives:
Following the 2009 pandemic, reports of an association between an AS03 adjuvanted H1N1 pandemic influenza vaccine and narcolepsy were published. Besides determining background incidence rates for narcolepsy in Germany this study aimed at investigating whether there was a change in incidence rates of narcolepsy between the pre-pandemic, pandemic, and the post-pandemic period on the population level.
Design:
Retrospective epidemiological study on the incidence of narcolepsy with additional capture-recapture analysis.
Setting:
German sleep centers.
Patients or Participants:
Eligible were patients with an initial diagnosis of narcolepsy (ICD10 Code G47.4) within the period from January 1, 2007 to December 31, 2011.
Interventions:
None; observational study.
Measurements and Results:
A total of 342 sleep centers were invited to participate in the study. Adequate and suitable data were provided by 233 sleep centers (68.1%). A total of 1,198 patients with an initial diagnosis of narcolepsy within the observed period were included, of whom 106 (8.8%) were children and adolescents under the age of 18 years and 1,092 (91.2%) were adults. In children and adolescents, the age-standardized adjusted incidence rate significantly increased from 0.14/100,000 person-years in the pre-pandemic period to 0.50/100,000 person-years in the post-pandemic period (incidence density ratio, IDR 3.57; 95% CI 1.94–7.00). In adults, no significant change was detectable. This increase started in spring 2009.
Conclusions:
For the years 2007–2011, valid estimates for the incidence of narcolepsy in Germany were provided. In individuals under 18, the incidence rates continuously increased from spring 2009.
Citation:
Oberle D, Drechsel-Bäuerle U, Schmidtmann I, Mayer G, Keller-Stanislawski B. Incidence of narcolepsy in Germany. SLEEP 2015;38(10):1619–1628.
Keywords: narcolepsy, incidence, epidemiology, Germany
INTRODUCTION
Narcolepsy is a rare sleep disorder with a prevalence of 26 to 50/100,000.1–3 Regarding incidence rates, prior to 2009, few data were available. Silber et al.4 found for Olmsted County, Minnesota, an incidence rate of 1.37/100,000 person-years (1.72 for men and 1.05 for women) and for narcolepsy with cataplexy 0.74/100,000 person-years for the period 1960 through 1989. After the 2009 H1N1 pandemic, in several European countries, a strong association between the pandemic influenza vaccination with a monovalent AS03-adjuvanted H1N1 influenza vaccine and narcolepsy was detected.5–11 A minor increase of the narcolepsy incidence in vaccinated compared to non-vaccinated individuals was observed in Quebec, Canada, where a different type of H1N1 vaccine was used.12 The underlying pathomechanism is unknown.
In the context of the increase in narcolepsy onset counts, especially in children and adolescents, incidence rates of narcolepsy were published for several European countries.
Partinen et al.13 based on register data for the years 2002– 2009, calculated an incidence rate for the whole Finnish population of 0.79/100,000 person-years (95% CI 0.62–0.96), for children and adolescents under 17 years of age 0.31/100,000 person-years (95% CI 0.12–0.51), for individuals aged 17 to 19 1.79 (95% CI 1.49–2.09), and for adults ≥ 20 years 0.87/100,000 person-years (95% CI 0.71–1.03). In 2010, the incidence in children and adolescents < 17 years was estimated to be 5.30/100,000 person-years corresponding to a 17-fold increase compared to previous years, and in 17- to 19-year-olds 5.46/100,000 person-years corresponding to a 3-fold increase. No increase was seen in individuals aged 20 years and above.
In Sweden, in the pre-pandemic period (January 1, 2000 to August 31, 2009), the incidence of narcolepsy in 2- to 17-year-old children and adolescents was 0.26/100,000 person-years (95% CI 0.12–0.50) and, in 2010, 6.6/100.000 person-years (95% CI 3.40–8.10) corresponding to a 25-fold increase.14
Furthermore, coordinated by the VAESCO (Vaccine Adverse Event Surveillance and Communication) group for the years 2000 through 2010 a dynamic retrospective cohort study was conducted based on data from 6 countries (Denmark, Finland, Italy, the Netherlands, Sweden, and United Kingdom).15 The pooled incidence rate was estimated to be 0.93/100,000 person-years (95% CI 0.90–0.97) in the whole population, 0.83/100,000 person-years (95% CI 0.75–0.91) in children and adolescents from 5 to 19 years, 1.06/100,000 person-years (95% CI 1.01–1.11) in adults from 20 to 59 years, and 0.88/100,000 person-years (95% CI 0.81–0.95) in adults over 60 years. In the age group of 5- to 19-year-olds, the country-specific incidence rates were increased after the mass vaccination campaign 2009/2010 in the Scandinavian countries as compared to the reference period (relative risk, RR 1.9 [95% CI 1.1–3.1]) in Denmark, 6.4 [95% CI 4.2–9.7] in Finland, and 7.5 [95% CI 5.2–10.7] in Sweden). In the other participating countries, a similar increase was not obvious.
In June 2013, the results of a register-based study conducted in Norway were published.16 This study investigated the potential change of the narcolepsy incidence in the years after the pandemic mass vaccination campaign in vaccinated and non-vaccinated children and adolescents at the age of 4 to 19 years. From October 2009 to January 2010 470,000 children and adolescents in Norway were vaccinated with Pandemrix. The coverage was 50%. The incidence rate of narcolepsy in vaccinees within 12 months after immunization was 10/100,000 person-years, with a clustering of cases within 6 months following immunization. The incidence rate decreased during the second year after the mass vaccination campaign to 1.1/100,000 person-years and thus reached the incidence rate estimated in non-vaccinated individuals (0.5–1.0/100,000 person-years); reference data from the pre-pandemic period were not available.
For Germany, hitherto only limited data regarding incidence rates were available. In 2002, the pediatrics working group of the German Society for Sleep Research and Sleep Medicine (Deutsche Gesellschaft für Schlafforschung und Schlafmedizin, DGSM) in collaboration with the Surveillance Unit for Rare Pediatric Diseases in Germany (Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland, ESPED) conducted a study on the incidence rate of narcolepsy in individuals under 18 years of age in Germany which delivered an estimate of 0.12/100,000 person-years.17,18 However, the completeness of case ascertainment was unclear. Regarding narcolepsy incidence in adults, prior to our study no data were available.
Our study aimed at providing background data for the incidence of narcolepsy in Germany in the years 2007 through 2011. In addition, we intended to investigate a potential change in the pandemic and post-pandemic period as compared to the pre-pandemic period on the population level.
METHODS
The Paul-Ehrlich-Institut as the responsible national competent authority for vaccines and biomedicines in collaboration with the German Society of Sleep Research and Sleep Medicine DGSM conducted a nationwide retrospective study on the incidence of narcolepsy from January 1, 2007 through December 31, 2011 in Germany.
Capture Investigation
All 342 DGSM accredited sleep centers were contacted and invited to participate. Centers which returned a completed and signed consent form were asked to report basic data with respect to the initial diagnoses of narcolepsy (International Classification of Diseases, version 10, ICD 10 code G47.4) within the observation period. For this purpose we used a written case report form (CRF) requesting for each eligible patient the following information: year of birth, gender, date of primary manifestation (symptom onset), date of initial diagnosis, and concomitant diseases. Sleep centers which refused to take part in the study were asked to give the reasons for non-participation on the reply form. Sleep centers which did not respond to the initial letter of invitation, received a written reminder. Hospitals which did not respond to the reminder were contacted by phone or email and asked to explain the reasons for non-responding/non-participation.
Recapture Investigation
An independent “recapture” investigation on the initial diagnoses of narcolepsy within the observation period (2007 to 2011) was carried out on-site in the sleep centers of the state (Bundesland) Rhineland-Palatinate. Patients with confirmed initial diagnosis of narcolepsy (ICD 10 code G47.4) from January 2007 through December 2011 were included.
All accredited sleep centers in Rhineland-Palatinate were contacted and asked to give consent to have a secondary investigation carried out on-site by an independent researcher. As soon as written agreement was received, a contract was concluded defining the modalities of study conduct, data protection, and rights and obligations of the contracting parties. If a hospital refused consent, it was requested to give the reasons. Hospitals which did not respond to the initial letter of invitation were contacted by email or phone until a definite confirmation or refusal was received.
Upon receipt of written consent, an appointment was made with the contact person of the sleep center. Initial diagnoses were identified based on paper or electronic patient records by an independent researcher. The written case report form (CRF) used in the capture investigation (see above) was also applied in the recapture investigation.
For both the capture and the recapture investigation, the same case definition was used (ICD 10 code G47.4 narcolepsy and cataplexy) to identify cases of narcolepsy in the hospitals' patient population. Only within the scope of the recapture investigation in Rhineland-Palatinate, an additional case validation was performed using the Brighton Collaboration criteria.
Data Linkage
Data linkage was performed manually on several variables including year of birth, gender, date of initial diagnosis, and concomitant diseases.
Observational Period
The observational period was divided into four sections: pre-pandemic period (January 1, 2007 to March 30, 2009), pandemic period prior to the mass vaccination campaign (April 1, 2009 to October 31, 2009), pandemic period during/ post mass vaccination campaign (November 1, 2009 to June 30, 2010), and post-pandemic period (July 1, 2010 to December 31, 2011). Durations of the pre-pandemic, pandemic prior to mass vaccination campaign, pandemic during/post mass vaccination campaign and post-pandemic periods comprised 27, 7, 8, and 18 months, respectively.
Statistics
The study population was divided into children and adolescents (0–17 years) and adults (≥ 18 years). For the incidence rate calculations, the study population under the age of 18 was additionally sub-divided into pre-pubertal (0–9 years) and pubertal (10–17 years) individuals, since, on average, girls enter puberty at ages 10–11 and boys at ages 11–12.19
Crude incidence rates for narcolepsy were calculated by dividing the number of patients initially diagnosed with narcolepsy by the total number of people in Germany in each age group during the same calendar year (2007, 2008, 2009, 2010, and 2011) or relevant period (January 2007 to March 2009, April 2009 to October 2009, November 2009 to June 2010, and July 2010 to December 2011). Demographics for 2007 through 2011 were provided by the Federal Statistical Office (https://www.destatis.de), Wiesbaden, Germany (delivery date: July 13, 2013). Under the assumption of a Poisson distribution, 95% confidence intervals (95% CI) of the crude incidence rates were estimated according to an algorithm described by Daly.20
By means of a capture-recapture analysis, the completeness of data capture was estimated. The capture-recapture analysis dates back to the original work initiated by Petersen.21,22 It is a valid method which is used to extrapolate from samples to the population. To do this, ≥ 2 independent data sources are needed. None of the 2 sources alone will capture all cases. From the number of cases which are captured in both sources (intersection), the total number of cases in the population can be estimated. The greater the intersection, the more complete is the data. In our setting, the capture units are to be understood as “analytic sources” since both investigations were based on the archived case reports.
Prerequisites of the capture–recapture analysis are23:
Both sources cover a common population.
The description of the individual cases in both sources enables a so-called record linkage.
The probability to be captured in a source is equal for all individuals.
The probability for each individual to be captured in one source is independent from the probability to be captured in the other source.
The probability to be captured in subpopulations, i.e., very young individuals, does not differ from the probability to be captured in the total population.
For the primary analysis, based on both independent investigations (sleep centers [capture], on-site investigation [recapture]) an estimate for “undercount” according to Chao24 was determined. Within the scope of sensitivity analyses, 3 further established capture-recapture estimates25–27 were used.
Based on the number of cases captured by source 1, the number of cases captured by source 2, and the estimate of undercount (i.e., for the number of cases not captured in both sources), a correction factor was calculated enabling extrapolation from Rhineland-Palatinate to the whole of Germany:
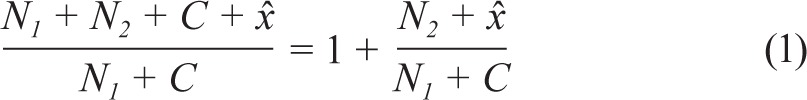 |
where N1 is the number of cases only captured by source 1 (investigation by sleep centers themselves), N2 is the number of cases only captured by source 2 (independent on-site investigation), C is the number of cases captured by both sources (intersection), and x̂ is the estimated number of cases captured neither by source 1 nor by source 2.
By multiplication of the crude numbers of cases with the correction factor, adjusted numbers of cases and, subsequently, adjusted incidence rates were calculated (method see above).
To compare the incidence rates of the pandemic period (prior to or during/post vaccination campaign) and the post-pandemic period with the reference period (pre-pandemic period), incidence density ratios (IDR)28 were calculated based on age-standardized (with the Standard European Population 201329 used as reference) adjusted incidence rates. Because of the exploratory character of the analysis, alpha-adjusting was not performed.
The statistical analyses were performed using the software package SAS, version 9.3 (SAS Institute Inc. Cary, NC, USA). With the aid of interrupted time series analyses30 using the AUTOREG procedure which provides a correction for autocorrelation, the temporal course of the incidence rates over 60 months was analyzed for changes in level and trend. An ITS analysis is a segmental linear regression analysis adjusting for temporal trends, i.e. for a potential preexisting change of the outcome variable over time without attributing it to a distinct event.
Ethics Statement
The study has been approved by the Ethics Committee of the State Chamber of Medicine in Hessen (ID No. FF 3/2011). According to the State Chamber of Medicine in Rhineland-Palatinate, no additional Ethics Committee approval for the on-site investigation in the state of Rhineland-Palatinate was required.
RESULTS
A total of 342 sleep centers were invited to participate of which 233 (68.1%) provided data on initial diagnoses of narcolepsy from January 2007 to December 2011. Among the non-participating hospitals were no major specialized centers for narcolepsy patients.
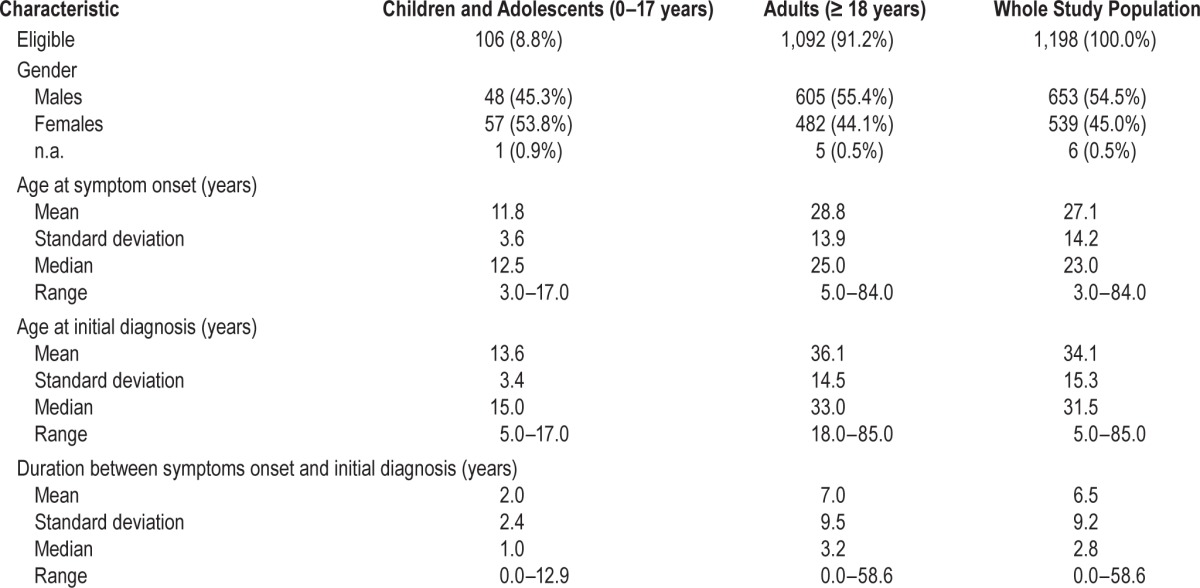
A total of 1,221 patients were included by the participating sleep centers, of whom 1,198 were eligible; 23 patients were excluded because they had initially been diagnosed with narcolepsy outside the observation period. Characteristics of the study population were shown in Table 1. The study population comprised 106 children and adolescents (8.8%) as well as 1,092 adults (91.2%). Slightly more female than male children and adolescents (53.3%) were included, whereas males outnumbered the females both in the adult and the whole study population. Median age at symptoms onset was 12.5 years in children and adolescents and 25.0 in adults; median age at initial diagnosis was 15.0 and 33.0 years, respectively. No child was younger than 5 years at the time of diagnosis. Median duration between symptoms onset and initial diagnosis was 1.0 year in children and adolescents and 3.2 years in adults.
Table 1.
Characteristics of the study population.
Regarding children and adolescents, the most frequently notified concomitant diseases were obesity (E66.xx, 4.7% based on 106 patients aged 0 to 17 years), sleepwalking (somnambulism; F51.3, 2.8%), sleep apnea (G47.3x, 2.8%), allergic rhinitis (J30.3, 2.8%), other somatoform disorders (F45.8, 1.9%), other non-organic sleep disorders (F51.8, 1.9%), other specified extrapyramidal and movement disorders (G25.8x, 1.9%), and mixed asthma (J45.8, 1.9%). With respect to adults, sleep apnea (G47.3x, 20.4% based on 1,092 patients aged 18 and above), other specified extrapyramidal and movement disorders (G25.8x, 10.0%), and obesity (E66.xx, 7.6%) were the most frequently recorded concomitant diseases.
In children and adolescents, the number of initially diagnosed cases of narcolepsy increased from 10 cases in 2007 to 41 cases in 2011 corresponding to an estimated crude incidence rate of 0.07/100.000 person-years in 2007 and 0.31/100,000 person-years in 2011 (Table 2A). In adults, the estimated crude incidence rate ranging from 0.29 to 0.35/100,000 person-years remained stable throughout the observation period. In prepubertal as well as in pubertal children and adolescents, the crude incidence rate continuously increased from 2007 to 2011 (Table 2B).
Table 2.
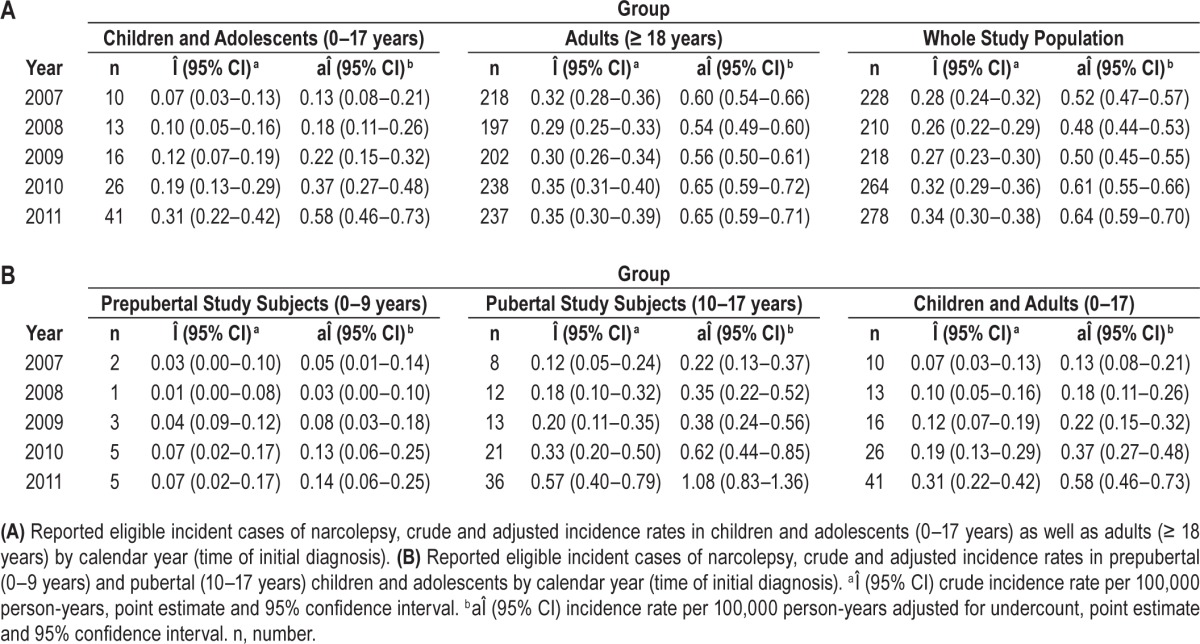
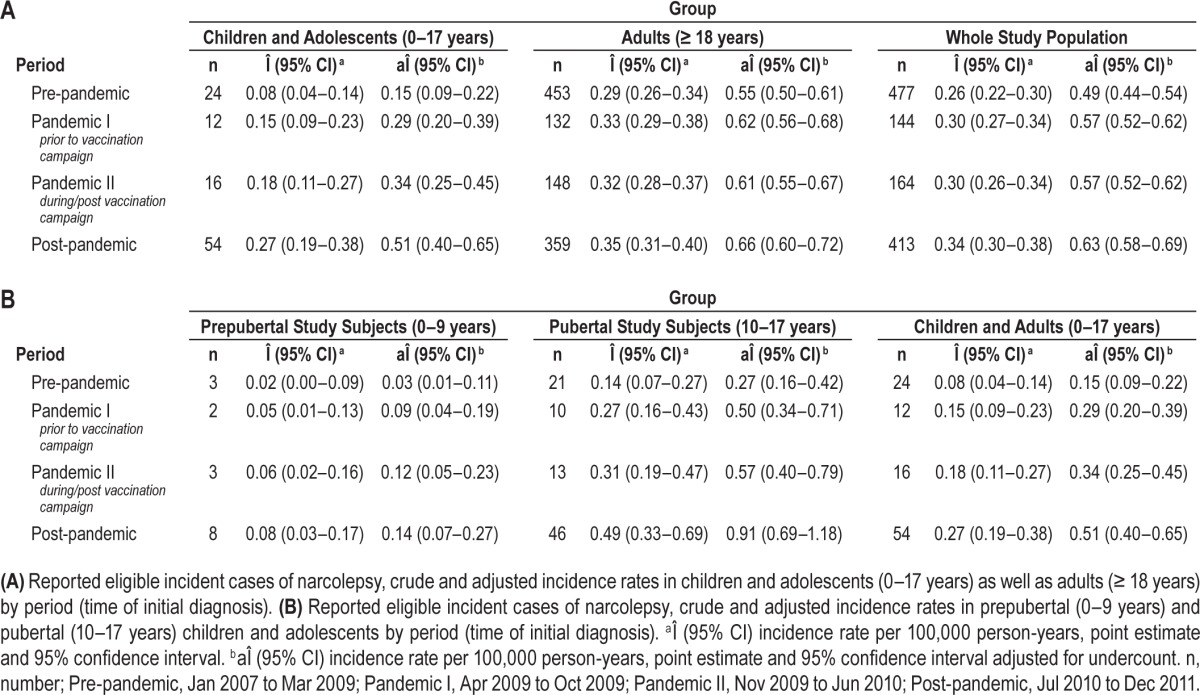
In the pre-pandemic period (January 2007 to March 2009), 24 children and adolescents had initially been diagnosed with narcolepsy corresponding to an estimated crude incidence rate of 0.08/100,000 person-years. In the post-pandemic period (July 2010 to December 2011), 54 new diagnoses were made in children and adolescents corresponding to an estimated crude incidence rate of 0.27/100,000 person-years (Table 3A). In adults, in the pre-pandemic period 453 patients and in the post-pandemic period 359 patients were initially diagnosed with narcolepsy corresponding to a slight increase of the estimated crude incidence rate from 0.29 to 0.35/100,000 person-years. An increase in incidence rates was observed in both prepubertal and pubertal children and adolescents (Table 3B).
Table 3.
From August 2013 to December 2013, a second investigation (recapture) was performed by an independent researcher in Rhineland-Palatinate. Thirteen of the 20 contacted sleep centers in Rhineland-Palatinate participated in the recapture investigation. The remaining 7 non-participating sleep centers did not diagnose narcolepsy and referred patients with suspected narcolepsy to specialized sleep centers. Of the 13 sleep centers participating in the recapture investigation 7 had participated in the capture investigation.
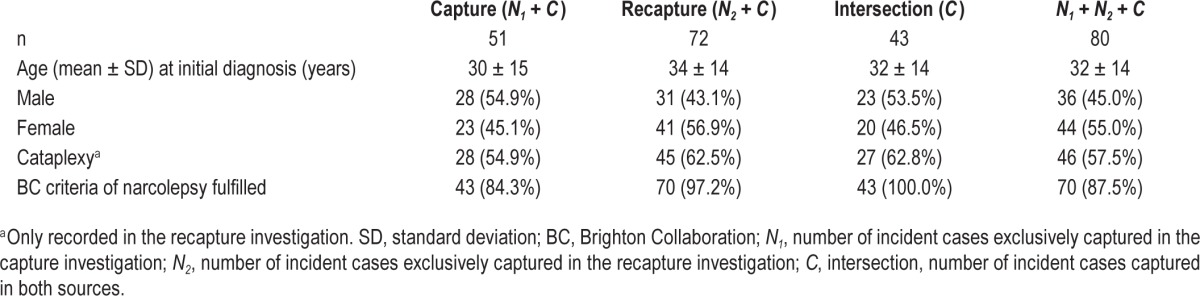
In Rhineland-Palatinate, a total of 80 initial diagnoses of narcolepsy were recorded (Table 4). In the capture investigation, 51 incident cases of narcolepsy were captured, 8 of which were exclusively within the scope of the capture investigation. In the recapture investigation, 72 incident cases were captured including 29 cases exclusively identified within the scope of the recapture investigation. A total of 43 incident cases were captured by both capture and recapture investigation. In Table 4 the study population in Rhineland-Palatinate is described in detail. Of the 51 cases included within the scope of the capture investigation 43 (84.3%) met the criteria of the BC case definition and of the 72 cases included within the scope of the recapture investigation 70 cases (97.2%) fulfilled the BC criteria. A total of 70 of the 80 cases (87.5%) included in Rhineland-Palatinate met the BC criteria, 2 cases did not, and 8 cases had not been identified within the scope of the recapture investigation.
Table 4.
Capture and recapture investigations in Rhineland-Palatinate.
According to the method described by Chao,24 x̂ = 15.92 incident cases had not been captured by both investigations. By inserting N1, N2, C, and x̂ into the mathematical equation (see equation 1) a correction factor of 1.88 for extrapolation to the whole of Germany was obtained.
By multiplying the observed numbers of incident cases with the correction factor, adjusted incidence rates by calendar year/ period were obtained (Table 2 and Table 3).
Besides the method according to Chao, 3 further established methods to calculate a capture–recapture estimate for under-count were used within the scope of sensitivity analyses.25–27 The adjusted incidence rates calculated in this way were only slightly different from those obtained by the method according to Chao (data not shown).
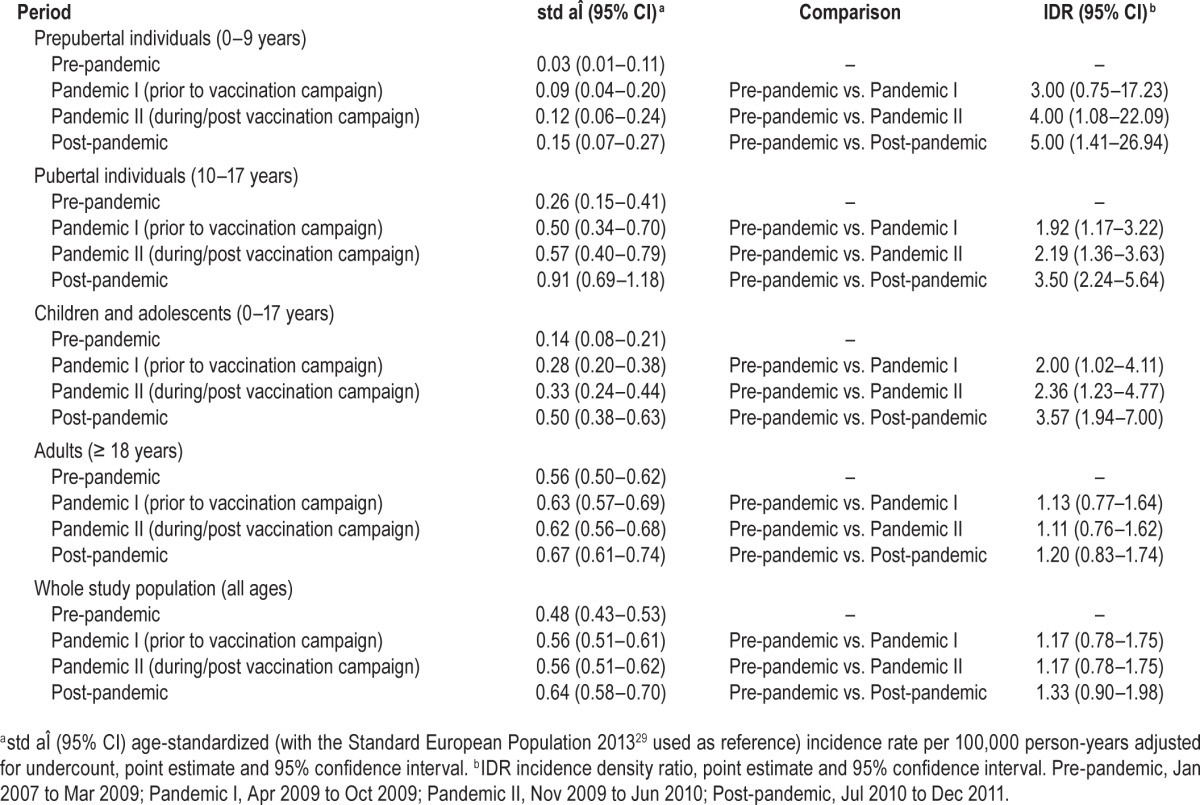
In children and adolescents, the age-standardized adjusted incidence rate significantly increased from 0.14/100,000 person-years (95% CI: 0.08–0.21) in the pre-pandemic period to 0.50/100,000 person-years (95% CI: 0.38–0.63) in the post-pandemic period (IDR 3.57; 95% CI 1.94–7.00) (Table 5). With respect to prepubertal individuals, the difference between the pre-pandemic period (0.03/100,000 person-years [95% CI: 0.01–0.11]) and the post-pandemic period (0.15/100,000 person-years [95% CI: 0.07–0.27]) was even more pronounced (IDR5.00; 95% CI: 1.41–26.94). In pubertal individuals, the age-standardized adjusted incidence rate significantly grew from 0.26/100,000 person-years (95% CI: 0.15–0.41) in the pre-pandemic period to 0.91/100,000 (95% CI: 0.69–1.18) in the post-pandemic period (IDR: 3.50; 95% CI: 2.24–5.64). In adults, no significant increase of the age-standardized adjusted incidence rate was detectable (0.56/100.000 person-years [95% CI: 0.50–0.62]) in the pre-pandemic period, 0.67/100,000 person-years [95% CI: 0.61–0.74] in the post-pandemic period; IDR 1.20; 95% CI 0.83–1.74). Regarding the whole study population, the age-standardized adjusted incidence rate was 0.48/100,000 person-years (95% CI: 0.43–0.53) in the pre-pandemic period and 0.64/100,000 person-years (95% CI: 0.58–0.70) in the post-pandemic period (IDR 1.33; 95% CI 0.90–1.98). In children and adolescents, but neither in adults nor in the whole study population, there were also significant differences between the pre-pandemic and the pandemic period (prior to and/or during/ post vaccination campaign; Table 5).
Table 5.
Comparison of age-standardized adjusted incidence rates between observational periods.
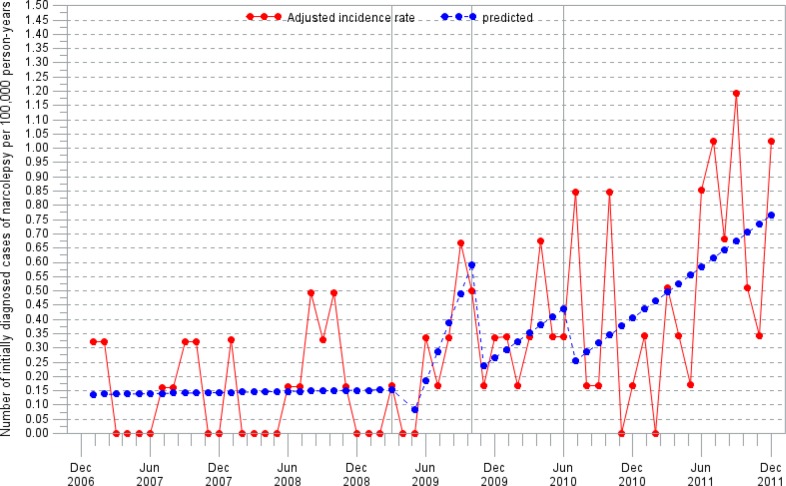
The ITS analysis revealed that regarding children and adolescents, the incidence rate started to increase significantly in spring 2009 (P = 0.0138); afterwards the trend remained stable during the remaining observation period (Figure 1).
Figure 1.
Adjusted incidence rate in children and adolescents (0–17 years old) by month. Segmental linear regression analysis.
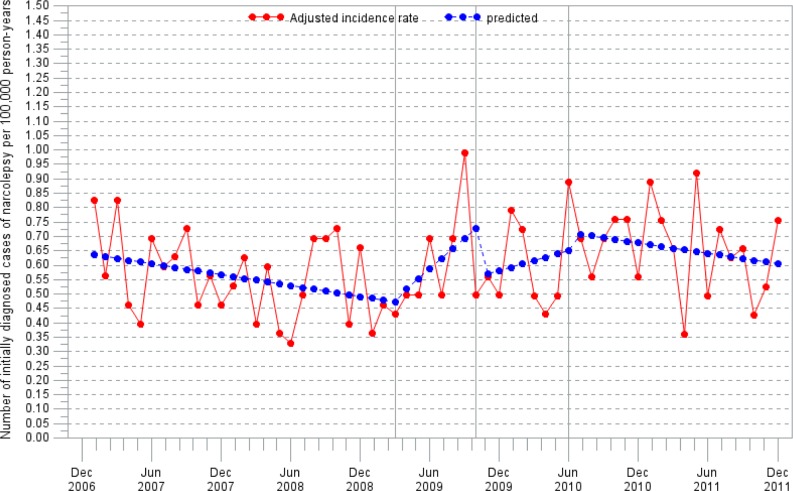
In adults, there were no significant changes in the incidence rate within the observation period (Figure 2).
Figure 2.
Adjusted incidence rate in adults (≥ 18 years old) by month. Segmental linear regression analysis.
DISCUSSION
This is the first nationwide investigation of the incidence of narcolepsy in all age groups and the biggest study conducted in one population using the same protocol and method, thus enabling a robust analysis of the incidence of narcolepsy in Germany.
The calculated estimates for adjusted incidence rates are in accordance with those obtained in other European countries in the pre-pandemic period13,14 and with those found within the scope of the ESPED study on the incidence rate of narcolepsy in children and adolescents in Germany conducted in 2002.17,18
As seen in several other countries,31 in Germany the incidence rate of narcolepsy in children and adolescents significantly increased between the pre-pandemic and the post-pandemic period on the population level. This increase (3.6-fold) was more pronounced than in Denmark (1.9-fold)15 and less marked than in Finland (17-fold)13 and Sweden (25-fold).14 In contrast, no increase of the incidence rate in children and adolescents was reported for the Netherlands, United Kingdom, and Italy (Tuscany and Emilia Romagna regions).15 For Beijing, China, Han et al. observed a 3-fold increase in narcolepsy onset counts (mostly children and adolescents) following the 2009 H1N1 pandemic irrespective of H1N1 immunization since only 5.6% of the patients reported a prior H1N1 vaccination (a non-adjuvanted H1N1 pandemic influenza vaccine was used).32 In contrast, no increase in the incidence rate for narcolepsy in the post-pandemic period was observed in South Korea,33 where a MF59-adjuvanted or a non-adjuvanted H1N1 pandemic influenza vaccine was used. Of note, in German children and adolescents, there was already a 2-fold incidence increase between the incidence rate of the pre-pandemic period and the pandemic period prior to the H1N1 mass vaccination campaign.
In Germany, no incidence increase was observed in adults on the population level. This is in line with findings from Finland, Sweden, the Netherlands, United Kingdom, and Italy.13,15 Only for Denmark, a 1.5-fold increased incidence rate in the post-pandemic period as compared to the pre-pandemic period in individuals aged 20 to 59 years was reported.15 Evidence of an increased risk of narcolepsy in H1N1 vaccinated adults, however, comes from recently published epidemiological studies: in Finland, a 3- to 5-fold increased risk of narcolepsy was reported for adults aged 20 to 64 years,34 and the French study found a 4.7-fold increased risk of narcolepsy in H1N1 vaccinated individuals aged 18 and over.9 In Swedish individuals aged 21 to 30 years, a two-fold increased risk was observed.10
According to the Robert Koch Institut (RKI), the Federal German Public Health Institute, the first lab-confirmed cases of influenza A/H1N1/v were registered from end of April 2009 in Germany. However, only from calendar week 42 a measurable influence on the morbidity of the population regarding acute airway diseases was observed.35 Considering that the mean time interval between symptom onset and initial diagnosis in children and adolescents between April 2009 and October 2009 was 1.4 years, it is not plausible that H1N1 influenza infections triggered the significant increase of initial narcolepsy diagnoses.
H1N1 vaccination coverage in Germany was rather low. The overall vaccination coverage was estimated to be 8.1% (95% CI: 7.4–8.8) in individuals > 14 years of age, 7.8% (95% CI: 6.1–10.0) in the age-group < 14 years, and 4.0% (95% CI: 2.6–6.4) in 14- to 17-year-old adolescents.36 Mass vaccination campaign was initiated on 26 October 2009. The last doses were administered at the beginning of March 2010. With very few exceptions, AS03 adjuvanted H1N1 vaccine was used. Considering the low vaccination coverage, the significant increase of the incidence rate observed in children and adolescents in the pandemic period during/post mass vaccination campaign and in the post-pandemic period as compared to the reference period (2.4-fold and 3.6-fold increase, respectively) may rather have other causes.
Compared to Sweden or Finland, the media paid little attention to the potential association between H1N1 vaccination and narcolepsy in Germany, probably due to the low vaccination coverage (about 8%). In August 2010, the Paul-Ehrlich-Institut, the Federal German Institute for Vaccines and Biomedicines, published on its homepage that the Swedish agency had received reports on narcolepsy in close temporal association with immunization against H1N1/A pandemic influenza in children and adolescents. In addition, a press release was issued. From late August until end of September 2010, the specialized press, e.g., Deutsches Ärzteblatt (German magazine for doctors) or Pharmazeutische Zeitung (pharmaceutical newspaper) addressed the topic in a few articles. In February 2011, media attention reached a first peak with several articles in large daily and weekly newspapers. Besides internet blogs, the subject was also taken up in radio and television broadcasts. A second peak occurred in July 2011 as a result of the European Medicines Agency's decision to restrict the use of AS03 adjuvanted pandemic H1N1 influenza vaccine in individuals younger than 20 years of age. Beyond July 2011, media attention eased after a new study from China32 put forward the thesis that the H1N1 pandemic itself may have triggered narcolepsy. The crucial point is that in Germany the incidence rates started to rise in spring 2009, i.e., at a time when media attention with respect to vaccinations and narcolepsy was literally nonexistent.
In 2002, the German Society for Neurology published guidelines for the diagnosis and management of narcolepsy which were updated in October 200837; the latest update dates back to 2012.38 The use of targeted diagnostics from 2008/2009 may have contributed to identify cases of narcolepsy earlier than before. An indication of this is a decreasing time interval between symptoms onset and initial diagnosis within the observation period. This decrease was already obvious between April 2009 and October 2009, i.e., prior to the mass vaccination campaign, as compared to the pre-pandemic period.
A limitation of the study is lacking completeness. A total of 233 (68.1%) of the 342 contacted sleep centers provided information regarding the frequency of initial diagnoses within the observation period. Further investigations regarding the technical thrust revealed that among the 112 hospitals which did not take part in our study were no sleep centers specialized on narcolepsy. Because of the limited completeness, an independent secondary investigation was conducted in one federal state of Germany, in Rhineland-Palatinate, to determine an estimate for undercount. Thirteen of the 20 contacted sleep centers in Rhineland-Palatinate participated in the recapture investigation. The remaining 7 nonparticipating sleep centers did not diagnose narcolepsy, but referred patients with suspected narcolepsy to specialized sleep centers. According to the method described by Chao, the number of missing cases in Rhineland-Palatinate was estimated. Subsequently, a correction factor was calculated to extrapolate to the whole of Germany. Sensitivity analyses using capture-recapture estimates according to Chapman, Sekar und Deming as well as Zelterman revealed a good concordance between the established methods. Because of the unequal catchability of the sleep centers, the method according to Chao was chosen for the primary analysis.
Ascertainment of narcolepsy cases in the capture investigation was based on registered diagnoses (ICD code G47.4). There was no opportunity in this study to independently validate these diagnoses by experts. Thus, we had to rely on the diagnosis made by the sleep centers. To face this limitation, within the recapture investigation it was investigated whether the cases included in Rhineland-Palatinate met the criteria of the BC case definition of narcolepsy and found that a remarkable 87% of the cases did.
This study did not aim at investigating a potential association between the pandemic influenza vaccination and narcolepsy, vaccine exposure data were not recorded within the scope of this investigation. The possible role of H1N1/A pandemic influenza vaccination and other vaccinations will be addressed within the scope of a second study, a multicenter matched case-control study on the risk factors of narcolepsy which is not subject of this publication.
To conclude, for the period 2007–2011, valid estimates for the incidence of narcolepsy in Germany were determined. In children and adolescents, a significant increase of the narcolepsy incidence rate between pre-pandemic and post-pandemic period was detected, with the incidence rate starting to rise in spring 2009. During the remaining observation period, this trend remained stable. This does not preclude a potential delayed effect of the mass vaccination campaign beyond the observation period. A similar increase was not observed in adults.
It is important to obtain country-specific incidence estimates to understand regional disease dynamics. The estimates that have been provided in this study will serve as starting point for further investigations.
DISCLOSURE STATEMENT
This was not an industry supported study. The project was sponsored by the German Ministry of Health (chapter 1501, title 54401). The authors have indicated no financial conflicts of interest. The work was performed at the Paul-Ehrlich-Institut, Federal Institute for Vaccines and Biomedicines, Langen, Germany.
ACKNOWLEDGMENTS
The authors thank all the sleep centers that participated in this study. We also express our thanks to Mrs. Barbara Sauer, DGSM, who was involved in the CRF handling, and to Mrs. Claudia Pönisch who performed the data management.
REFERENCES
- 1.Hublin C, Kaprio J, Partinen M, et al. The prevalence of narcolepsy: an epidemiological study of the Finnish Twin Cohort. Ann Neurol. 1994;35:709–16. doi: 10.1002/ana.410350612. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WTJ, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Priest RG, Caulet M, Guilleminault C. Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry. 1996;169:459–67. doi: 10.1192/bjp.169.4.459. [DOI] [PubMed] [Google Scholar]
- 4.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 5.MPA. Registerstudie med fokud pa neurologiska och immunrelaterade sjukdomar efter vaccination med Pandemrix. 2013. [Accessed March 2013]. http://www.lakemedelsverket.se/upload/nyheter/2013/PDX%20Rapport%20SV%20-%20registerstudie%207%20landsting%20regioner%202013-03-26.pdf.
- 6.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E, Andrews N, Stellitano L, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Control and Prevention. Narcolepsy in association with pandemic influenza vaccination - a multi-country European epidemiological investigation. [Accessed September 2012]. http://www.ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=959.2013.
- 9.Dauvilliers Y, Arnulf I, Lecendreux M, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136:2486–96. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 10.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population-and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275:172–90. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 11.O'Flanagan D, Barret AS, Foley M, et al. Investigation of an association between onset of narcolepsy and vaccination with pandemic influenza vaccine, Ireland April 2009-December 2010. Euro Surveill. 2014;19:15–25. [PubMed] [Google Scholar]
- 12.Montplaisir J, Petit D, Quinn M, et al. Risk of narcolepsy associated with inactivated adjuvanted (AS03) A/H1N1 (2009) pandemic influenza vaccine in Quebec. PLoS One. 2014;9:e108489. doi: 10.1371/journal.pone.0108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szakacs A, Darin N, Hallbook T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80:1315–21. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 15.Wijnans L, Lecomte C, de VC, et al. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2012;31:1246–54. doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Heier MS, Gautvik KM, Wannag E, et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14:867–71. doi: 10.1016/j.sleep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Berner R, Bialek R, Forster J. Erhebungseinheit für seltene pädiatrische Erkrankungen in Deutschland (ESPED) [Accessed January 2015];Monatsschr Kinderheilk. 2004 152:7779. Available at: http://www.esped.uni-duesseldorf.de/jabe2002_r.htm#narko. [Google Scholar]
- 18.Handwerker G. Narkolepsie im Kindes- und Jugendalter. Monatsschr Kinderheilk. Monatsschrift Kinderheilk. 2005;2:153. [Google Scholar]
- 19.Kail RV, Cavanaugh JC. Human development: a life-span view. 5th ed. Australia, Belmont, CA: Wadsworth Cengage Learning; 2010. [Google Scholar]
- 20.Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med. 1992;22:351–61. doi: 10.1016/0010-4825(92)90023-g. [DOI] [PubMed] [Google Scholar]
- 21.Hook EB, Regal RR. Capture-recapture methods in epidemiology: methods and limitations. Epidemiol Rev. 1995;17:243–64. doi: 10.1093/oxfordjournals.epirev.a036192. [DOI] [PubMed] [Google Scholar]
- 22.Petersen CG. The yearly immigration of young plaice into the Limfiord from the German Sea. Rep Dan Biol Stn. 1896;6:5–84. [Google Scholar]
- 23.Ruckinger S, Boneberger A. [Epidemiologic challenges in rare diseases]. Bundesgesundheitsblatt. Gesundheitsforschung. Gesundheitsschutz. 2008;51:483–90. doi: 10.1007/s00103-008-0533-6. [DOI] [PubMed] [Google Scholar]
- 24.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783–91. [PubMed] [Google Scholar]
- 25.Sekar CC, Deming WE. On a method of estimating birth and death rates and the extent of registration. J Am Stat Assn. 1949;44:102–5. [Google Scholar]
- 26.Chapman DG. Some properties of the hypergeometric distribution with applications to zoological sample censuses. University of California Publication in Statistics. 1951;1:1131–60. [Google Scholar]
- 27.Zelterman D. Robust estimation in truncated discrete distributions with application to capture-recapture experiments. J Stat Plan Inference. 1988;18:225–7. [Google Scholar]
- 28.Stokes M, Koch G. Up to speed with categorical data analysis. 2011. [Accessed February 2014]. Available at: https://support.sas.com/resources/papers/proceedings11/346-2011.
- 29.Eurostat European Commission. Revision of the European Standard Population. Report of Eurostat's Task Force. 2013. [Accessed February 2014]. http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-RA-13-028/EN/KS-RA-13-028-EN.pdf.
- 30.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19:613–23. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XZ, Penzel T, Han F. Increased incidence of narcolepsy following the 2009 H1N1 pandemic. Somnologie. 2013;17:90–3. [Google Scholar]
- 32.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–17. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 33.Choe YJ, Bae G, Lee D. No association between influenza A(H1N1) pdm09 vaccination and narcolepsy in South Korea: an ecological study. Vaccine. 2012;30:7439–42. doi: 10.1016/j.vaccine.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Nohynek H, et al. Increased risk of narcolepsy observed also among adults vaccinated with Pandemrix in Finland. 2013. [Accessed May 2013]. http://www.thl.fi/en_US/web/en/pressrelease?id=33516.
- 35.Buda S, Köpke K, Luchtenberg M, et al. Bericht zur Epidemiologie der Influenza Saison 2009/2010. Epidemiologisches Bulletin. 2010:21. [Google Scholar]
- 36.Walter D, Böhmer MM, an der Heiden M, Reiter S, Krause G, Wichmann O. Monitoring pandemic influenza A(H1N1) vaccination coverage in Germany 2009/10 - results from thirteen consecutive cross-sectional surveys. Vaccine. 2011;29:4008–12. doi: 10.1016/j.vaccine.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 37.Deutsche Gesellschaft für Neurologie. AWMF-Leitlinien-Register Nr. 030/056: “Narkolepsie.”. 2008. [Accessed February 2011]. http://www.awmf.org/uploads/tx_szleitlinien/030-056_S1_Narkolepsie_10-2008_10-2013.pdf.
- 38.Deutsche Gesellschaft für Neurologie. AWMF-Leitlinien-Register Nr. 030/056: “Narkolepsie.”. 2012. [Accessed February 2014]. http://www.awmf.org/leitlinien/detail/ll/030-056.html.