Abstract
Study Objectives:
Emerging evidence links obstructive sleep apnea (OSA) with increased cancer incidence and mortality. Invariant natural killer T (iNKT) cells play an important role in cancer immunity. We hypothesized that patients with OSA have low number of circulating invariant natural killer T (iNKT) cells, which may also be functionally impaired. This study aims to evaluate the frequency of circulating iNKT cells in OSA.
Design:
We evaluated the frequency of circulating iNKT cells by flow cytometry in 33 snorers being assessed for possible OSA. Using iNKT cell lines, we also evaluated the effect of exposure to hypoxia over 24 hours on apoptosis, cytotoxicity, and cytokine production.
Setting:
Teaching hospital based sleep unit and research laboratory.
Patients:
Thirty-three snorers were evaluated: 9 with no OSA (apnea-hypopnea frequency [AHI] < 5/h), 12 with mild-moderate OSA (AHI 5–30) and 12 with severe OSA (AHI > 30).
Measurements and Results:
Patients with severe OSA had considerably fewer iNKT cells (0.18%) compared to patients with mild-moderate (0.24%) or no OSA (0.35%), P = 0.0026. The frequency of iNKT cells correlated negatively with apnea-hypopnea index (r = −0.58, P = 0.001), oxygen desaturation index (r = −0.58, P = 0.0003), and SpO2% < 90% (r = −0.5407, P = 0.005). The frequency of iNKT cells increased following 12 months of nCPAP therapy (P = 0.015). Hypoxia resulted in increased apoptosis (P = 0.016) and impaired cytotoxicity (P = 0.035).
Conclusion:
Patients with obstructive sleep apnea (OSA) have significantly reduced levels of circulating invariant natural killer T (iNKT) cells and hypoxia leads to impaired iNKT cell function. These observations may partly explain the increased cancer risk reported in patients with OSA.
Citation:
Gaoatswe G, Kent BD, Corrigan MA, Nolan G, Hogan AE, McNicholas WT, O'Shea D. Invariant natural killer T cell deficiency and functional impairment in sleep apnea: links to cancer comorbidity. SLEEP 2015;38(10):1629–1634.
Keywords: obstructive sleep apnea, invariant natural killer T cells, cancer, cytotoxicity
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent disorder, affecting at least 4% of adult males and 2% of adult females, and is a well-established independent risk factor for cardiovascular disease and associated morbidity and mortality.1 Emerging evidence from both animal and human studies links OSA with increased cancer incidence and worse cancer outcomes.2–5 Epidemiological data from North America and Europe suggest malignancy is more likely to occur in subjects with severe sleep disordered breathing,3,4 while animal models suggest that intermittent hypoxia may promote tumor progression and metastasis.6,7 The mechanisms underlying these adverse outcomes in OSA are poorly understood, but chronic systemic inflammation, oxidative stress, and immune dysfunction have been postulated as potential contributory factors. However, to date there have been few if any clinical studies specifically evaluating potential pro-oncogenic factors in OSA cohorts.
Invariant natural killer T (iNKT) cells are a rare population of human innate T cells with an established role in cancer biology and other conditions such as autoimmune diseases.8 They express the invariant T cell receptor (TCR) Vα24Jα18 chain and NK-like surface molecules9 and are potent immunomodulatory cells capable of simultaneously secreting both TH1 and TH2 cytokines upon stimulation.10,11 Following stimulation, iNKT cells can also activate other cells of the innate and adaptive immune system.12 Studies of in vitro and animal models have demonstrated the ability of iNKT cells to direct anti-cancer responses in the prevention of tumors and in the eradication of existing tumors,13 while they have also been shown to directly lyse tumor cells.14 Furthermore, the number and function of circulating iNKT cells are reduced in cancer patients,8,15,16 and therapeutic strategies aimed at restoring iNKT cell number and function have shown significant promise in the context of cancer immunotherapy.17–21
To date there are no studies evaluating the relationship between OSA and iNKT cells, and in particular evaluating how OSA severity and burden of nocturnal hypoxemia may affect their number and function. In this study, we evaluate the frequency of circulating iNKT cells in participants with OSA, the effect of nocturnal continuous positive airway pressure (nCPAP) therapy on their number, and the effect of hypoxia on iNKT cell function.
METHODS
Participants
The study was approved by the ethics committee at St Vincent's University Hospital, Dublin, and was performed in accordance with the declaration of Helsinki. Male snorers referred to sleep clinic for assessment of suspected sleep disordered breathing, with no history of hypertension, dyslipidemia, or cardiovascular disease, and were non-smokers and taking no regular medications, were enrolled in the study after written informed consent. All subjects underwent inpatient polysomnography, performed and scored according to the 2007 AASM criteria,22 with the presence and severity of sleep disordered breathing classified according to the apnea-hypopnea index (AHI). An apnea was scored in the presence of ≥ 90% reduction in airflow of ≥ 10 sec duration. A hypopnea was scored when airflow was reduced by ≥ 30% in combination with 4% oxyhemoglobin desaturation from pre-event baseline, or when airflow was reduced by ≥ 50% in combination with an arousal from sleep. Subjects with moderate-severe OSA were commenced on nocturnal CPAP as clinically indicated, following an overnight, inpatient dose-titration study. Blood samples in the fasting state (at 08:00) were obtained for isolation of peripheral blood mononuclear cells (PBMCs).
Preparation of PBMCs
Ten mL of venous blood was collected in heparinized blood collection tubes and PBMCs were isolated by density centrifugation on Lymphoprep (Nycomed, Norway) at 400 g for 25 minutes. Cells were washed twice in Hank Buffered Salt Solution (HBSS), and the resulting cell pellet was suspended in 1 mL of complete RPMI 1640 medium for determination of cell count and viability by ethidium bromide/acridine orange staining. The frequency of circulating iNKT cells was evaluated using the combination of monoclonal antibodies (mAbs) 6b11 PE and CD3 APC by flow cytometry.
Generation of iNKT Cell Lines
PBMCs from healthy controls were used to generate iNKT cell lines by positive selection using anti-6b11 magnetic beads (Miltenyi Biotech, UK) as per the manufacturer's instructions. The purified iNKT cells were stained with 6b11 FITC and CD3 PE-Cy5 mAbs (BD Bioscience, UK) for 30 min and washed with phosphate buffered saline with azide (PBA). Double positive cells (CD3 and 6b11) were then sorted using fluorescence-activated cell sorting (FACS) Aria cell sorter (BD Biosciences). One thousand purified iNKT cells per well were plated in a round bottom 96-well plate with 150,000 irradiated PBMCs from 2 donors as feeders, 10 ng/mL of phytohemagglutinin (PHA) (Sigma Aldrich, UK) and 250 U/mL of interleukin-2 (IL-2) (Miltenyi Biotec). The PHA was diluted over 48 h using IL-2 supplemented complete RPMI 1640. The media was then replenished twice weekly and wells divided as required during growth. Mature iNKT cells were used for cytotoxicity and apoptosis experiments.
Flow Cytometric Staining and Analysis
PBMCs obtained from participants were stained with monoclonal antibodies (mAbs) against CD3, 6b11, CD8, CD4, CD56, and vα24. Stained cells were then acquired with FACS Calibur flow cytometer (BD Biosciences, NJ, USA) and analyzed with Cell Quest Pro (BD Biosciences). Invariant NKT cell numbers are expressed as percentage of lymphocytes.
Hypoxia Experiment
Invariant NKT cells (200,000 cells per well in triplicate) were incubated in a hypoxia chamber at 1% oxygen and 5% carbon dioxide, or incubated in normoxic conditions in an incubator containing 21% oxygen and 5% carbon dioxide at 37°C for 24 hours. The percentage of apoptotic cells compared to cells exposed to normoxia for 24 h was determined by flow cytometry using Annexin V apoptosis kit as per manufacturer's instructions (Milteny Biotech, Germany). Cell viability was checked by ethidium bromide/acridine orange staining, and equal numbers of viable cells were used for cytotoxicity studies. We assessed the effect of 24-h hypoxia on the ability of iNKT cells to lyse tumor cells by co-culturing hypoxic or normoxic iNKT cells with CD1d transfected C1R tumor cells at a ratio of 5:1 (effector to target ratio), and the proportion of lysed tumor cells expressed in arbitrary units were measured using the CytoTox-Fluor assay (Promega, UK). Cell supernatants harvested after culturing iNKT cells in hypoxia or normoxia with or without TCR stimulation (anti-CD3/28 stimulation beads (Miltenyi Biotech, Germany), were analyzed for cytokine levels (TNF-α and IL-4, classically produced simultaneously by iNKT cells) by Enzyme-linked immunosorbent assay (ELISA) (R&D Systems, USA) in triplicates.
Statistical Analysis
Statistical analysis was performed using Prism version 5b software (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± SEM and median ± interquartile range as appropriate. Groups were compared using ANOVA, student t-test or Mann-Whitney U test as appropriate. Correlation coefficients were generated to examine the relationship of iNKT cell numbers with indices of OSA severity and obesity. A P value < 0.05 was considered statistically significant.
RESULTS
A total of 33 patients were enrolled. Table 1 shows the baseline characteristics of the participants grouped according to OSA severity: no OSA (AHI < 5), mild-moderate OSA (AHI 5–30) and severe OSA (AHI > 30). Systolic blood pressure levels and subjective daytime sleepiness increased with increasing OSA severity, but no significant differences in demographic, anthropometric, or other clinico-metabolic variables were observed between the groups, except for LDL which was highest in the severe OSA group probably representing a cardiovascular risk marker in severe OSA.
Table 1.
Participant characteristics by OSA severity.
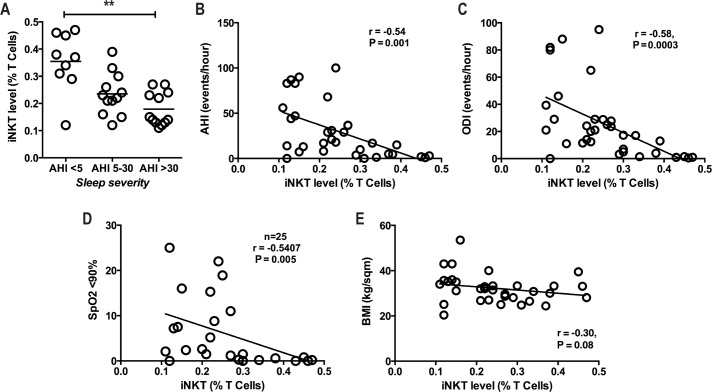
Participants with Severe OSA Have Low Frequency of Circulating iNKT Cells
The frequency of circulating iNKT cells was significantly lower in participants with AHI > 30 (mean 0.18% ± 0.06%) compared to patients with AHI 5–30 (mean 0.24% ± 0.08%) and < 5 (mean 0.35% ± 0.11%), P = 0.0026 (Figure 1A). There was no significant difference in the frequency of other lymphocyte subpopulations (Table 1). The frequency of iNKT cells correlated inversely with OSA severity as measured by apnea hypopnea index (AHI) (r = −0.54, P = 0.001; Figure 1B), oxygen desaturation index (ODI) (r = −0.58, P = 0.0003; Figure 1C), and percentage of sleep time with SpO2 < 90% (r = −0.54, P = 0.005, Figure 1D). There was no significant correlation with BMI (r = −0.31, P = 0.08, Figure 1E), and despite significant difference in LDL between the groups, there was no correlation between the frequency of iNKT cells and LDL (r = −0.033, P = 0.85).
Figure 1.
iNKT cell number and associations in OSA. (A) iNKT cell number according to sleep severity, comparisons made using Kruskal-Wallis test. (B-E) Correlation of iNKT cells with sleep severity (AHI), oxygen desaturation index (ODI), and % SpO2 < 90. **P < 0.01, comparisons made using spearman correlation coefficient. All analysis were performed in 33 patients apart from panel D.
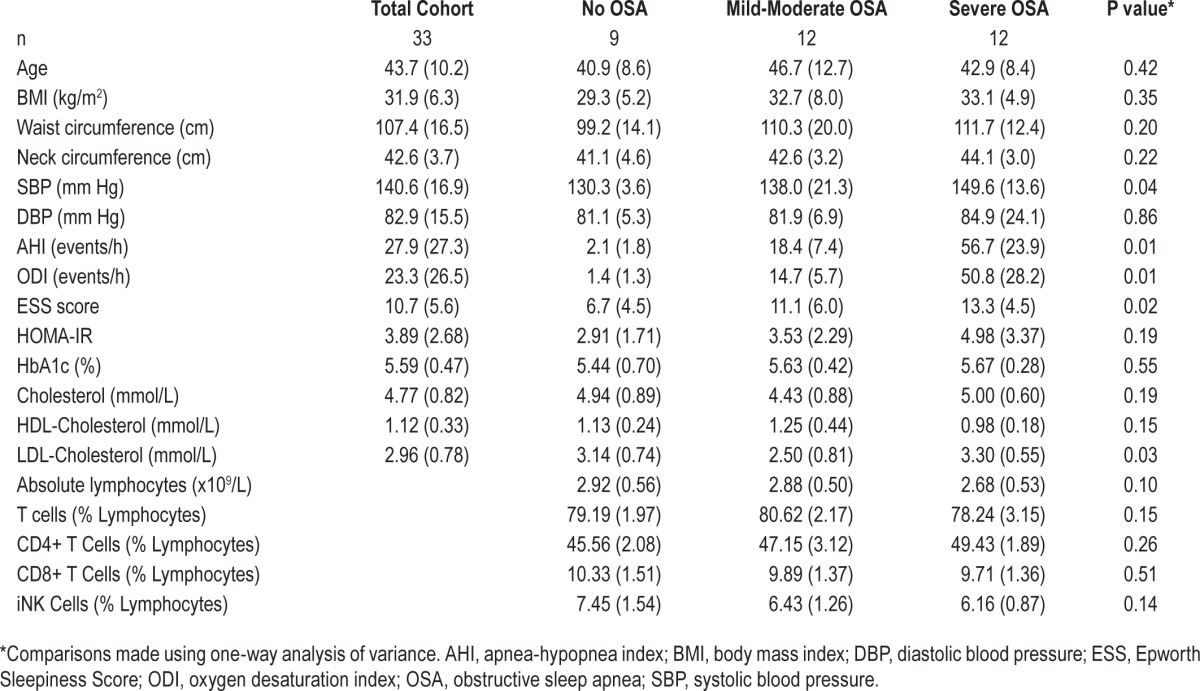
Circulating iNKT Cells Increase in Participants with Severe OSA after CPAP Therapy.
We prospectively followed up 10 participants with severe OSA who underwent treatment with nocturnal continuous positive airway pressure (nCPAP) and found a significant increase in the frequency of circulating iNKT cells following 12 months of therapy (mean 0.19% ± 0.06% vs 0.25% ± 0.10%, P = 0.015; Figure 2). When the change in iNKT cell number was assessed according to compliance, this did not reach statistical significance, P = 0.90, probably due to small numbers in each group.
Figure 2.
Effect of CPAP on iNKT frequency. iNKT cell frequency was assessed following 12 months of CPAP therapy and compared to baseline levels for each subject. *P < 0.05, comparisons made by paired t-test.
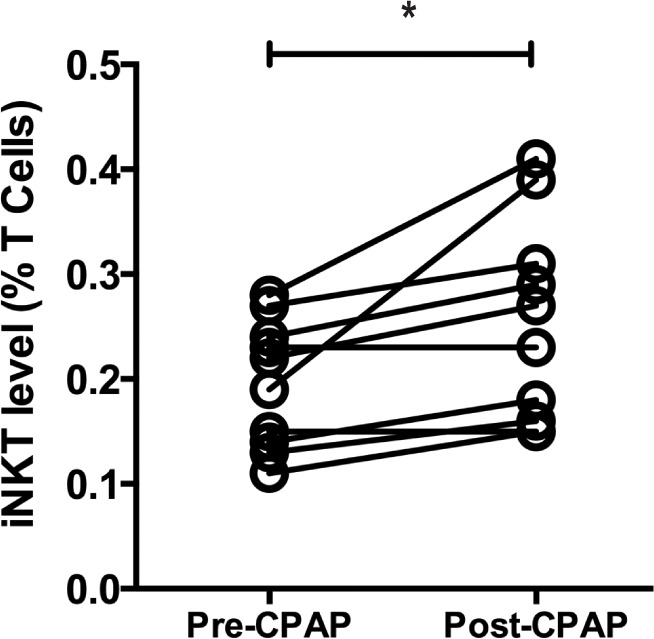
Exposure of iNKT Cells to Hypoxia Leads to Apoptosis and Impaired Cytotoxicity
Primary iNKT cell lines were used to study the in vitro effects of hypoxia on iNKT cell phenotype and function. Invariant NKT cells were exposed to 1% oxygen for 24 h and the percentage of apoptotic cells compared to cells exposed to normoxia for 24 hours. Significantly more iNKT cells exposed to hypoxia underwent apoptosis compared to cells exposed to normoxia (3.44% [1.90–4.16] vs 0.82% [0.75–1.69], P = 0.016, Figure 3A).
Figure 3.
Effects of hypoxia on iNKT cell function. (A) Exposure of iNKT cells to hypoxia leads to apoptosis; comparisons made by paired t test. (B) Exposure of iNKT cells to hypoxia leads to reduced cytotoxicity to CD1d cells; comparisons made by paired t test. (C,D) Hypoxia has no effect on TNF-α and IL-4 production by iNKT cells, comparisons between unstimulated iNKTs exposed to normoxia versus hypoxia and stimulated iNKTs exposed to normoxia versus hypoxia. *P < 0.05.
We also assessed the effect of 24-h hypoxia on the ability of iNKT cells to lyse CD1d transfected C1R tumor cells in a co-culture model. Invariant NKT cells exposed to hypoxia lysed fewer CD1d tumor cells compared to cells exposed to normoxia (3232 [2435–3590] vs 2757 [851–3159], P = 0.035, Figure 3B). Cell supernatants were interrogated for cytokine production by ELISA and there was no difference in cytokine levels (TNF-α and IL-4) between cells exposed to hypoxia and normoxia (Figures 3C, 3D).
DISCUSSION
The association between cancer and OSA is an emerging field, as yet unsupported by the wealth of evidence linking sleep disordered breathing with excess cardiometabolic morbidity and mortality.23 However, epidemiological data from Europe and North America are certainly suggestive of an important potential relationship between OSA and malignancy. Among 1,522 community-based subjects enrolled in the Wisconsin Sleep Cohort, severe sleep disordered breathing was associated with an almost five-fold risk of cancer death.3 Similarly, in a cohort of nearly 5,000 Spanish patients attending sleep clinics, severity of nocturnal hypoxemia predicted incident cancer even following rigorous adjustment for confounding variables.4 Data from animal models has shown that exposure to intermittent hypoxia may increase tumor progression, and appears to promote tumor metastasis.2,7
A number of potential contributory mechanistic factors have been suggested as mediators of this effect. A large body of literature has shown that OSA and associated intermittent hypoxemia lead to chronic systemic inflammation,24 a pro-oncogenic state.25 OSA is also now recognized as an oxidative stress disorder,26 with the generation of reactive oxygen species by sleep disordered breathing possibly increasing cancer risk. Meanwhile hypoxia, via stabilization of hypoxia-inducible factor, has an established role in promoting tumor angiogenesis, proliferation, and survival.27 However, to date there have been few data specifically exploring potential mechanisms in OSA patients.
Invariant NKT cells are of established importance in cancer biology. They constitute a rare subset of human innate T cells, and are potent drivers of innate and acquired immune responses, capable of driving anti-inflammatory Th2-type, regulatory T cell-type and pro-inflammatory Th1-type immune responses.11 Data from in vitro and rodent models have demonstrated the ability of iNKT cells to direct anti-tumor responses and to directly lyse tumor cells.13,28 In patients with a variety of advanced cancers, circulating iNKT cell numbers have been shown to be markedly reduced compared to healthy controls, and may also have impaired anti-tumor function.15,29 Moreover, a low frequency of circulating iNKT cells predicts a worse prognosis in subjects with head and neck squamous cell carcinoma.8,30 Our group has previously shown pro-oncogenic conditions such as smoking and obesity to be associated with depletion of iNKT cells.16,31 Meanwhile, a number of phase I/II clinical trials have suggested that augmentation of iNKT cell numbers and functionality may lead to improved outcomes in patients with advanced malignancy.20,32
In this first study of iNKT cells in subjects with OSA, we show that the frequency of circulating iNKT cells is reduced in patients with severe OSA, and that their numbers correlate inversely with AHI and the severity of nocturnal hypoxemia as measured by ODI and SpO2 < 90%. Furthermore, successful treatment with nCPAP appears to partially abrogate this reduction. A potential role for hypoxemia and associated tissue hypoxia in driving this observation is suggested by the increase in iNKT cell apoptosis following prolonged exposure to sustained hypoxia. Moreover, hypoxia appears to alter iNKT cell function, impairing their ability to lyse tumor cells, although cytokine production is not affected, which may be due to the differential effect of hypoxia on iNKT cells. Together these findings suggest that the presence of significant sleep disordered breathing may hinder an important innate anti-cancer defence mechanism. Indeed, previous work has shown that hypoxia impairs iNKT cell function with hypoxia leading to reduction of cytotoxicity, iNKT cell viability, and impaired interferon gamma secretion.33
Our study has a number of important limitations. First, while we observed an increase in iNKT cell numbers following successful treatment with nCPAP, we did not employ an nCPAP withdrawal model or include a sham nCPAP control group. However, the striking correlation between indices of OSA severity and frequency of circulating iNKT cells in parallel with a beneficial effect from appropriate therapy is suggestive of a potential causative effect. Second, our cohort was not necessarily typical of the standard sleep clinic population, being comprised of male nonsmokers without significant cardio-metabolic comorbidity. Finally, our in vitro model of sustained hypoxia does not mimic the cyclical intermittent hypoxemia seen in patients with sleep disordered breathing, and consequently does not replicate the environment of circulating iNKT cells in OSA patients. Additional experiments exposing iNKT cells to intermittent hypoxia were not feasible in our in vitro model, as the iNKT cell lines are maintained in suspension. However, only a small proportion of iNKT cells are located in the circulation, with the bulk being distributed throughout other tissues, most notably omental fat,16 and previous studies have shown that iNKT cells migrate to tumor microenvironment to interact with other immune cells.33 Recent data from rodent models suggest that—particularly in obese animals— exposure to intermittent hypoxia with consequent intermittent hypoxemia can translate into a profile of sustained hypoxia at a tissue level.34 Similarly, although our model utilized a comparatively severe degree of hypoxia, prior in vivo studies have measured oxygen tensions to be as low as 0.5% in murine lymphoid organs.35 While we are unaware of any comparable data from human studies, it can be speculated that oxygen tension might fall to these low levels in the lymphoid organs of OSA patients as they experience repeated cycles of apnea
In summary, we report a low frequency of circulating iNKT cells in patients with severe OSA, a relationship that is partially remediable with successful nCPAP therapy, while hypoxia appears to significantly inhibit the ability of iNKT cells to eradicate cancer cells. Further studies are required to longitudinally evaluate the contribution of low frequency of circulating iNKT cells to adverse cancer outcomes in OSA populations, and to further assess the impact of nCPAP therapy on their number and function.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Kent is supported by a grant from the Health Research Board, Ireland. Dr. Hogan is supported by a grant from the National Children's Research Centre, Ireland. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Drs. Hogan and O'Shea contributed equally to the study design, data interpretation, and manuscript preparation.
ABBREVIATIONS
- AHI
apnea-hypoapnea index
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence activated cell sorting
- HBSS
hank buffered salt solution
- IL-4
interleukin 4
- iNKT cells
invariant natural killer T cells
- mAbs
monoclonal antibodies
- nCPAP
nocturnal continuous positive airway pressure
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PBA
phosphate buffered saline with azide
- PBMCs
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- TCR
T cell receptor
- TNF-α
tumour necrosis factor-alpha
REFERENCES
- 1.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 2.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215–7. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 5.Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almendros I, Montserrat JM, Torres M, et al. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13:1254–60. doi: 10.1016/j.sleep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186:303–7. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Simoni Y, Diana J, Ghazarian L, Beaudoin L, Lehuen A. Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: are we close to reality? Clin Exp Immunol. 2013;171:8–19. doi: 10.1111/j.1365-2249.2012.04625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–9. [PubMed] [Google Scholar]
- 12.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–68. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawano T, Nakayama T, Kamada N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5. [PubMed] [Google Scholar]
- 15.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 16.Lynch L, O'Shea D, Winter DC, Geoghegan J, Doherty DG, O'Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 17.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- 18.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 19.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosylceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneiders FL, Scheper RJ, von Blomberg BM, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–41. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Hunn MK, Hermans IF. Exploiting invariant NKT cells to promote T-cell responses to cancer vaccines. Oncoimmunology. 2013;2:4. doi: 10.4161/onci.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israeli S, Chesson A, Quan S. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 23.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol. 2011;178:475–81. doi: 10.1016/j.resp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 25.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 26.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Takeda K, Yagita H, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–33. [PubMed] [Google Scholar]
- 29.Molling JW, Kolgen W, van der Vliet HJ, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 30.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 31.Hogan AE, Corrigan MA, O'Reilly V, et al. Cigarette smoke alters the invariant natural killer T cell function and may inhibit anti-tumor responses. Clin Immunol. 2011;140:229–35. doi: 10.1016/j.clim.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi M, Tashiro T, Dashtsoodol N, Hongo N, Watarai H. The specialized iNKT cell system recognizes glycolipid antigens and bridges the innate and acquired immune systems with potential applications for cancer therapy. Int Immunol. 2010;22:1–6. doi: 10.1093/intimm/dxp104. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Song L, Wei J, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–33. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–90. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caldwell CC, Kojima H, Lukashev D, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–9. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]