Abstract
Study Objective:
Preterm birth delays maturation of autonomic cardiovascular control, reflected in reduced heart rate variability (HRV) in preterm compared to term infants at term-equivalent age. It has been suggested that immature cardiovascular control contributes to the increased risk for the sudden infant death syndrome (SIDS) in preterm infants. However, the effects of prone sleeping, the major SIDS risk factor, and of gestational age (GA) at birth on HRV have not been assessed in preterm infants beyond term-equivalent age.
Subjects and Methods:
Very preterm (n = 21; mean GA 29.4 ± 0.3 weeks), preterm (n = 14; mean GA 33.5 ± 0.3 weeks), and term (n = 17; mean GA 40.1 ± 0.3 weeks) infants were recruited and underwent daytime polysomnography at 2–4 weeks, 2–3 months, and 5–6 months post-term corrected age (CA). Infants slept both supine and prone. HRV was assessed in the low frequency (LF) and high frequency (HF) ranges.
Results:
There was no effect of prone sleeping on HRV parameters in either preterm group. In term infants LF/HF was significantly elevated in the prone position in AS at 2–4 weeks (P < 0.05). HF HRV was significantly reduced (P < 0.05) and LF/HF increased (P < 0.05) in very preterm compared to both preterm and term infants at 2–3 months CA.
Conclusion:
Prone sleeping did not significantly impact on heart rate variability (HRV) in preterm infants. However, reduced maturation of high frequency HRV in very preterm infants resulted in significantly altered sympathovagal balance at 2–3 months corrected age, the age of peak sudden infant death syndrome (SIDS) risk. This may contribute to the increased risk of SIDS in infants born at earlier gestational age.
Citation:
Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. The effect of gestational age at birth on post-term maturation of heart rate variability. SLEEP 2015;38(10):1635–1644.
Keywords: preterm birth, sudden infant death syndrome, prone sleeping position, heart rate variability, autonomic nervous system, sleep
INTRODUCTION
Preterm birth is common and increasing in incidence, with rates ranging from 5% to 18% of live births worldwide.1 Prematurity is associated with a range of complications including an increased risk for the sudden infant death syndrome (SIDS),2 with infants born at earlier gestational ages (GA) at greater risk.2,3 SIDS occurs with a peak incidence at 2–3 months of age.4 One of the current theories to explain SIDS is impaired cardiovascular control leading to an uncompensated hypotensive episode and a failure to arouse from sleep.5 Despite successful reducing the risks campaigns alerting parents to the risks of prone sleeping, approximately 30% of infant deaths still occur in this position.3 The increased risk for SIDS with prone sleeping has been attributed to diminished cardiovascular control in this position, a finding reported for both term and preterm infants.6–9
Autonomic control of the cardiovascular system can be assessed noninvasively through spectral analysis of spontaneous beat-to-beat changes in heart rate (HR), known as heart rate variability (HRV).10 Spectral divisions of HRV reflect the relative contributions of the two branches of the autonomic nervous system: low frequency (LF; 0.04–0.15 Hz) reflects mixed sympathetic and parasympathetic activity, high frequency (HF; 0.4–1.5 Hz) reflects parasympathetic activity; while the ratio of LF to HF (LF/HF) reflects sympathovagal balance.11 Alterations in HRV have been reported in infants born pre-term12–17 and in adults with cardiovascular disease.18
Autonomic function matures across gestation before term-equivalent age.19–21 Preterm birth appears to delay maturation of HRV as preterm infants have lower HRV than term infants at term-equivalent age.12–14,17 Maturation of HF HRV, reflecting parasympathetic activity is primarily affected,13,15 resulting in alterations in sympathovagal balance in preterm infants at term-equivalent age. One study has shown that these alterations in HRV persist beyond term-equivalent age, with delayed maturation of both LF and HF HRV resulting in significant reductions in these parameters at 5–6 months post-term age compared to term infants in the supine position.22
Despite preterm infants born at earlier GA being at greater risk for SIDS, the effect of GA at birth on maturation of HRV beyond term-equivalent age in healthy preterm infants has not been assessed. Furthermore, it is unclear if prone sleeping has greater effects on HRV in infants born at earlier GAs. In this study we aimed to assess (1) the effect of GA at birth on HRV within the first six months after term-equivalent age and (2) the effect of prone sleeping on HRV in preterm infants. We hypothesized that HRV would be reduced in infants born at earlier GA and in preterm infants in the prone sleep position, particularly those infants born at younger GA.
METHODS
Ethical approval for this study was obtained from the Monash University and Monash Health human research ethics committees. Written parental consent was obtained prior to the commencement of each study.
Subjects
Thirty-five preterm infants (21M, 14F) born between 26–36 weeks of GA were recruited. Initial analysis identified an effect of GA at birth on HRV parameters; thus infants were subsequently divided into a very preterm group, born prior to 32 weeks GA (n = 21) and a preterm group, born between 32 and 36 weeks GA (n = 14). Data were compared to those of 17 term infants born between 38 and 42 weeks GA. Exclusion criteria for all infants included maternal smoking, family history of SIDS, and intrauterine growth restriction. For the preterm infants exclusion criteria also included significant intraventricular hemorrhage (Grade III or IV), clinically significant patent ductus arteriosus, major congenital abnormalities and chronic lung disease requiring respiratory stimulant medication or oxygen therapy at term-equivalent age. In the instance that twins were recruited, only one infant was studied. All infants routinely slept supine at home.
Infants underwent daytime polysomnography at three post-term ages; 2–4 weeks, 2–3 months, and 5–6 months (post-term corrected age [CA] for preterm infants). Fifteen very preterm and 9 preterm infants were studied at all 3 ages, 4 very preterm and 3 preterm infants were studied at only 2–4 weeks CA due to withdrawal from the study, and 2 very preterm and 2 pre-term infants were studied only at 2–3 months and 5–6 months CA as they were unable to attend the 2–4 weeks CA study due to illness or extenuating circumstances. Term infants were all studied at 3 ages.
Daytime Polysomnography
Polysomnography electrodes were applied during a morning feed and included electroencephalogram, electro-oculogram, submental electromyogram, electrocardiogram, and abdominal and thoracic respiratory belts (Resp-ez bands, EPM Systems, Midlothian, VA, USA). In addition to the standard polysomnography measures arterial oxygen saturation (SpO2) (Masimo, Frenchs Forest, NSW, Australia) and abdominal skin temperature (ADInstruments, Sydney, NSW, Australia) were also recorded. Blood pressure (BP) was recorded continuously using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) photoplethysmographic cuff placed around the infant's wrist using a technique previously validated by our group.23 Cerebral tissue oxygenation index (TOI) was also recorded using near-infrared spectroscopy (NIRO-200 spectrophotometer, Hamamatsu Photonics KK, Tokyo, Japan). BP and cerebral TOI data have been reported previously in the pre-term group24 and the term group25 and are not presented in this paper.
Daytime polysomnographic studies were performed in a quiet and dimly lit sleep laboratory kept at constant temperature (22–24°C). Sleep duration averaged 3.4 ± 0.2 h at 2–4 weeks, 3.0 ± 0.2 h at 2–3 months, and 2.3 ± 0.2 h at 5–6 months CA. Infants slept in both the prone and supine sleep position with the initial sleep position randomized between studies. Sleep position was usually changed following a midday feed. Data were recorded in both quiet sleep (QS) and active sleep (AS). In the supine position, the mean duration recorded for QS was 50 min at 2–4 weeks, 48 min at 2–3 months, and 54 min at 5–6 months CA; and for AS was 48 min at 2–4 weeks, 35 min at 2–3 months, and 34 min at 5–6 months CA. In the prone position, the mean sleep duration recorded for QS was 44 min at 2–4 weeks, 45 min at 2–3 months, and 34 min at 5–6 months CA; and for AS was 46 min at 2–4 weeks, 43 min at 2–3 months, and 22 min at 5–6 months CA.
Data were recorded in 1- to 2-min epochs during which the BP cuff was inflated with ≥ 2 min between cuff inflations to prevent venous pooling in the hand. At least 3 epochs were recorded in each sleep state and position in each infant.
All physiological variables, including ECG, were recorded with a sampling rate of 512 Hz using an E-series sleep system with Profusion software (Compumedics, Abbotsford, VIC, Australia).
Data Analysis
Data analysis was performed offline at the completion of each study. Our methods for analysis of HRV have been described previously.7,23,26,27 Briefly, artifact-free epochs of ECG data of 1–2 min in duration were selected from LabChart (ADInstruments, Sydney, NSW, Australia) and transferred to MATLAB (Mathworks, Natick, MA)7,27 for analysis. Heart period (HP) and respiratory rate (RR) were calculated using automated peak detection and beat-to-beat data were resampled at 200 Hz (cubic interpolation) to create an evenly spaced and continuous time series for further analysis.
Fast Fourier transformations were used to calculate the spectral power for HP. Each epoch was detrended and divided into 4 overlapping segments with 75% overlap. A Hamming window was used to reduce spectral leakage and the halving of measured power caused by windowing was corrected for. Power spectra were calculated for HRV within 2 frequency bands: low frequency ([LF] 0.04–0.15 Hz) reflecting slow oscillations attributed to the baroreflex which occur maximally at a frequency of approximately 0.1 Hz, and high frequency (HF) which was calculated for each infant based on respiratory frequency and is defined by the 10th and 90th centiles of the respiratory rate. Total power (TP) and the LF/HF ratio (LF/HF) were also calculated. The power values represent the square of the amplitude of an oscillatory signal in the identified frequency range.28,29
Statistical Analysis
Statistical analysis was performed using Sigmaplot 12.0 (Systat Software Inc, IL, USA). Data that failed normality were transformed using the logarithm function. The effects of GA were tested with linear regression, and as a signifi-cant effect on all HRV parameters was identified, all further analyses were performed comparing 2 groups of preterm infants, very preterm or preterm, and the term group. The effect of sleep state and position was determined using two way analysis of variance (ANOVA). The effect of GA at birth (very preterm, preterm, or term) and post-term CA was determined using two-way ANOVA within each sleep state and position. Where a significant difference was detected on ANOVA (P < 0.05), Student-Newman-Keuls post hoc analysis was used to determine where the difference occurred. Values are presented as mean ± standard error of the mean (SEM).
RESULTS
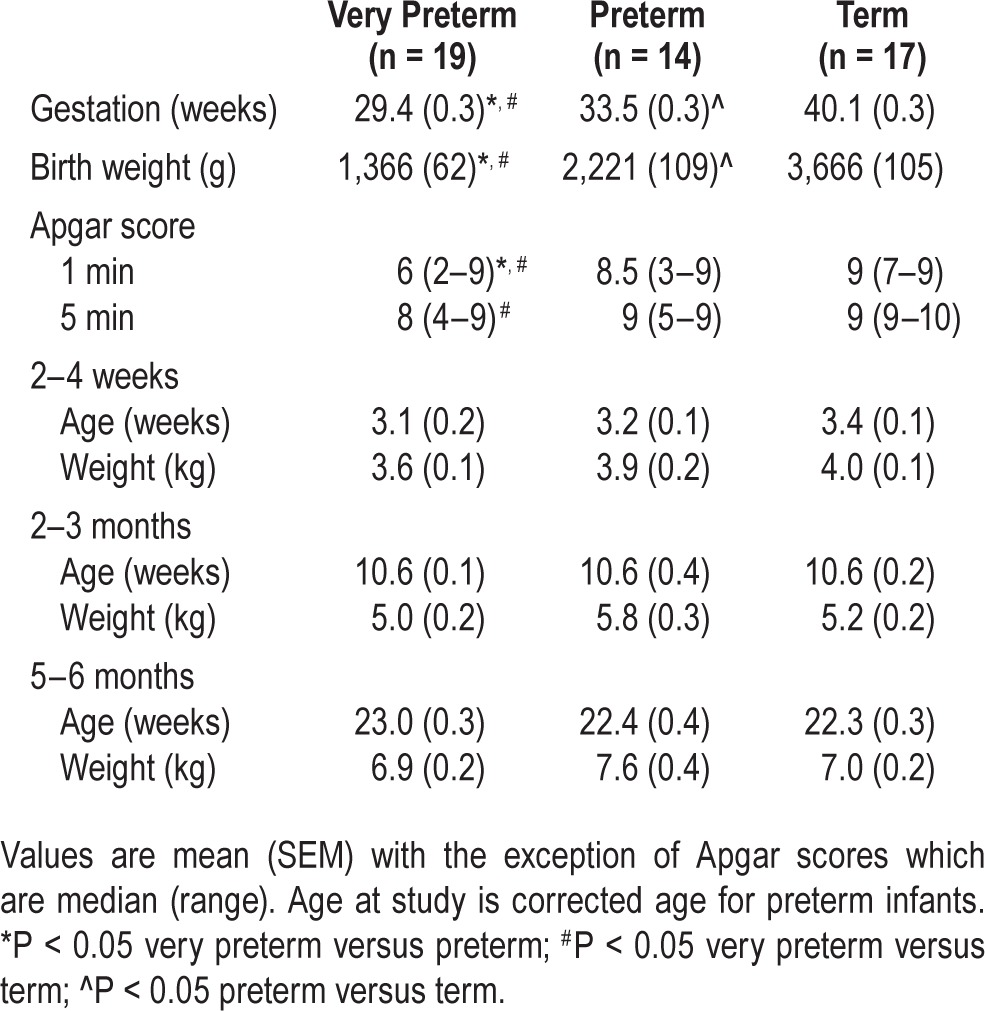
By design, very preterm and preterm infants had younger GA at birth and lower birth weight compared to term infants (very preterm < preterm < term; P < 0.05 for all). Very preterm infants had lower 1-min Apgar scores than preterm and term infants (P < 0.05) and lower 5-min Apgar scores than term infants (P < 0.05). There were no differences in age or weight between the 3 groups at the time of each study (Table 1).
Table 1.
Characteristics of very preterm, preterm and term infants.
Effect of Gestational Age
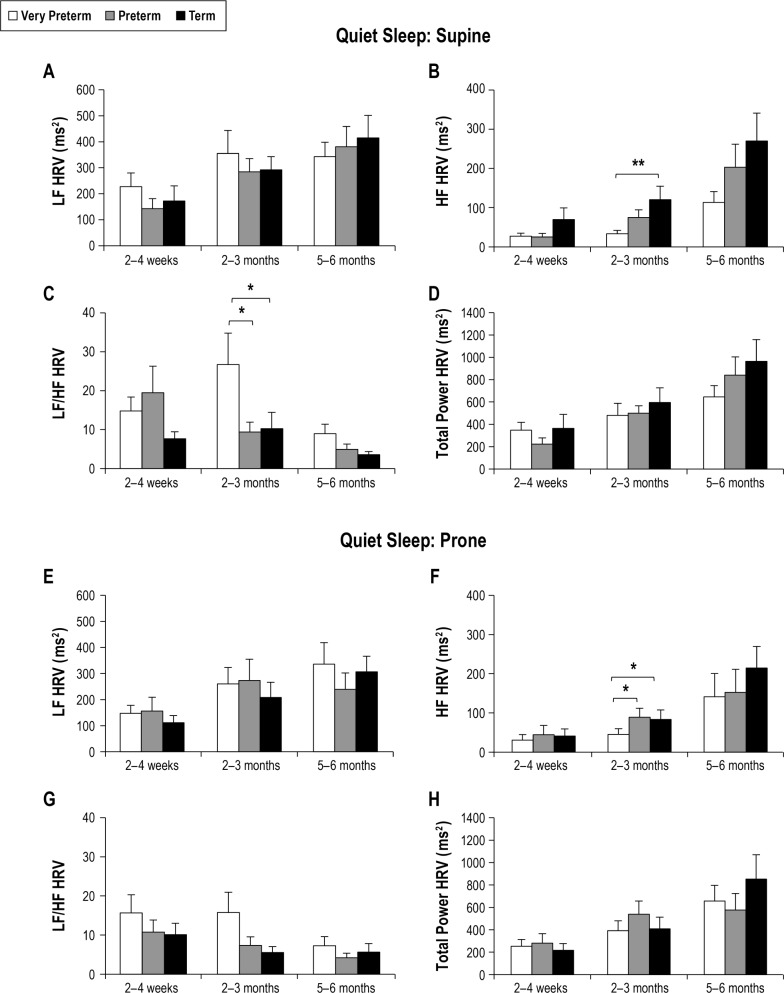
The effect of GA at birth on HRV in QS is shown in Figure 1. There was no significant effect of GA on LF HRV in either the supine or prone position. At 2–3 months CA, HF HRV was lower in very preterm than term infants (P < 0.01) in the supine position and in both the very preterm and preterm groups than the term group in the prone position (P < 0.05 for both). The LF/HF averaged higher in very preterm compared to both the preterm and term groups at 2–3 months CA, reaching statistical significance in the supine position (P < 0.05 for both). TP HRV was not different between groups in either the supine or prone position.
Figure 1.
Effect of gestational age on HRV parameters in quiet sleep in the supine (A–D) and prone (E–H) positions. Values are mean ± SEM. *P < 0.05, **P < 0.01. HF, high frequency; HRV, heart rate variability; LF, low frequency.
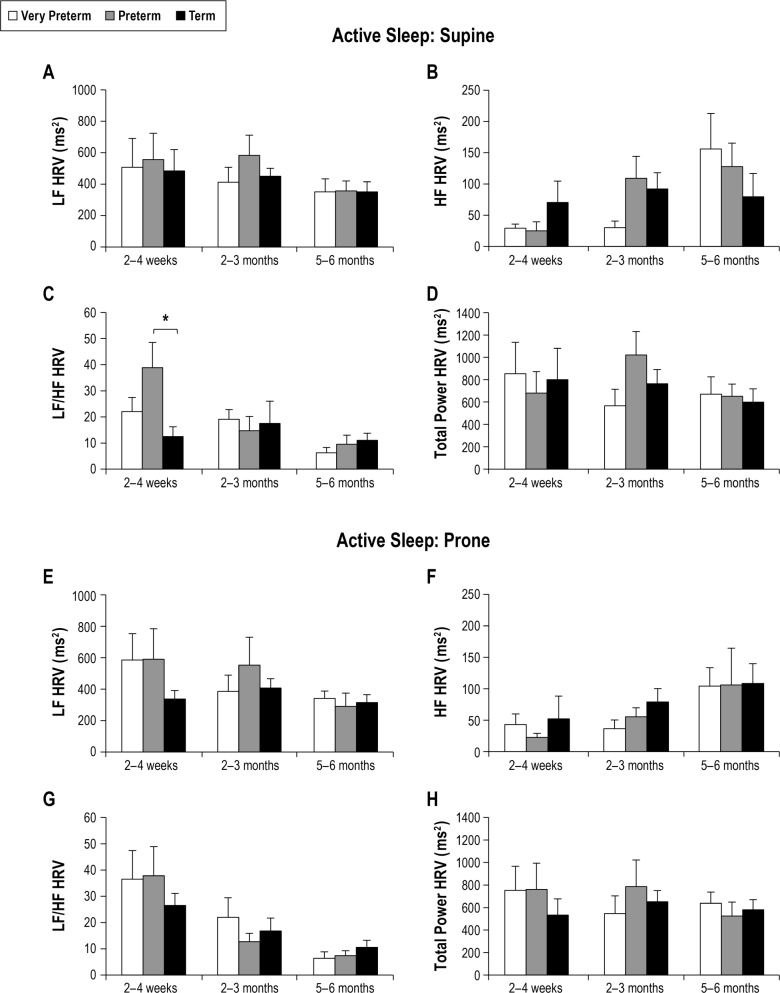
The effect of GA at birth on HRV in AS is shown in Figure 2. The LF/HF ratio was significantly higher in preterm than term infants at 2–4 weeks CA (P < 0.05). There were no other differences in HRV parameters between groups in either sleep position.
Figure 2.
Effect of gestational age on HRV parameters in active sleep in the supine (A–D) and prone (E–H) positions. Values are mean ± SEM. *P < 0.05. HF, high frequency; HRV, heart rate variability; LF, low frequency.
Maturation of HRV Parameters
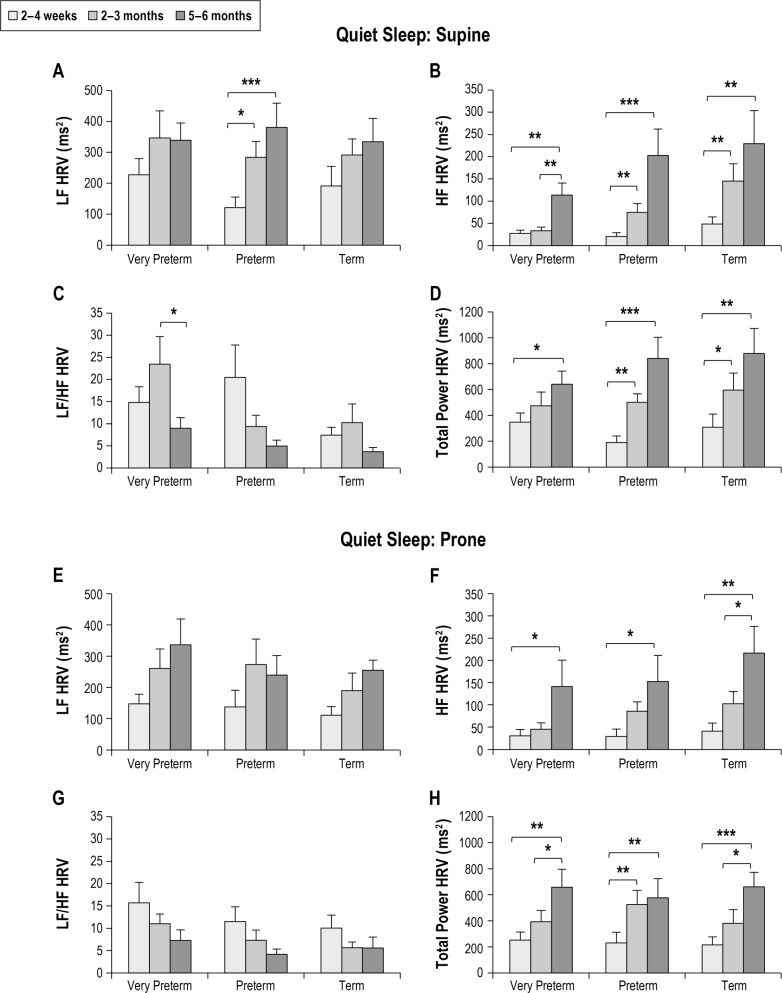
The effects of increasing post-term age on HRV indices during QS are presented in Figure 3. LF HRV increased in the supine position in preterm infants between 2–4 weeks and 2–3 months (P < 0.05) and between 2–4 weeks and 5–6 months (P < 0.001). In the prone position there was no effect of post-term age. HF HRV increased from 2–4 weeks to 2–3 months CA in all infant groups in both the supine (P < 0.01 for all) and prone positions (P < 0.05 for all). However, in very preterm infants this developmental increase was delayed, with the increase in HF HRV starting at a later age of 2–3 months CA. The LF/HF did not change with age in the preterm or term groups in the supine position. In contrast, there was a peak in the LF/HF at 2–3 months CA in very preterm infants, resulting in a sharp decrease between 2–3 months and 5–6 months CA. In the prone position, there was no effect of post-term age on LF/HF in any group. TP HRV increased with age in all infant groups, with a greater increase seen in the preterm and term groups in the supine position (2–4 weeks versus 5–6 months: very preterm P < 0.05; preterm P < 0.001; and term P < 0.01) and in the term group in the prone position (2–4 weeks versus 5–6 months: very preterm P < 0.01; preterm P < 0.01; term P < 0.001).
Figure 3.
Effect of post-term age on HRV parameters in quiet sleep in the supine (A–D) and prone (E–H) positions in infants born very preterm, preterm and term. Values are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. HF, high frequency; HRV, heart rate variability; LF, low frequency.
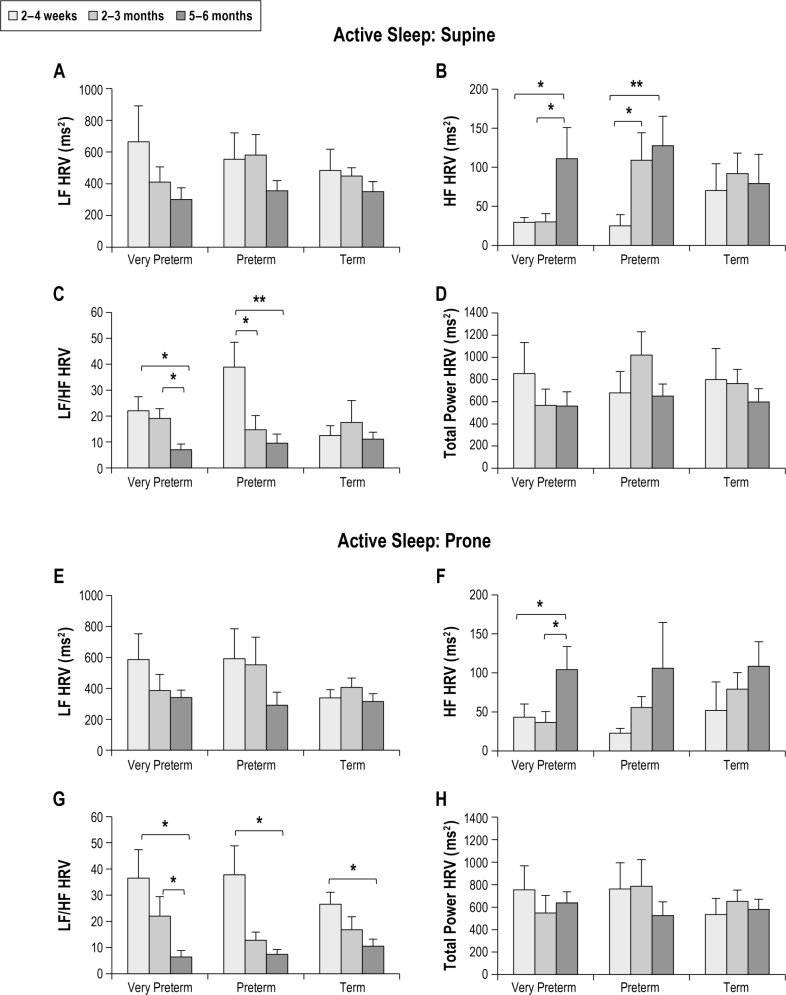
Figure 4 shows the effect of post-term age on HRV parameters in AS in the supine and prone positions. LF HRV was not altered by post-term age in either sleep position. HF HRV increased from 2–4 weeks to 5–6 months CA in both very pre-term (P < 0.05) and preterm infants (P < 0.05) in the supine position, and in very preterm infants (P < 0.05) in the prone position. LF/HF decreased from 2–4 weeks to 5–6 months CA in very preterm (P < 0.05) and preterm infants (P < 0.05) in both sleep positions. In term infants, post-term age had no effect on LF/HF in the supine position, but LF/HF decreased from 2–4 weeks to 5–6 months in the prone position (P < 0.05). There was no significant effect of post-term age on TP HRV in any group in either sleep position.
Figure 4.
Effect of post-term age on HRV parameters in active sleep in the supine (A–D) and prone (E–H) positions in infants born very preterm, preterm and term. Values are mean ± SEM. *P < 0.05, **P < 0.01. HF, high frequency; HRV, heart rate variability; LF, low frequency.
Effect of Sleeping Position
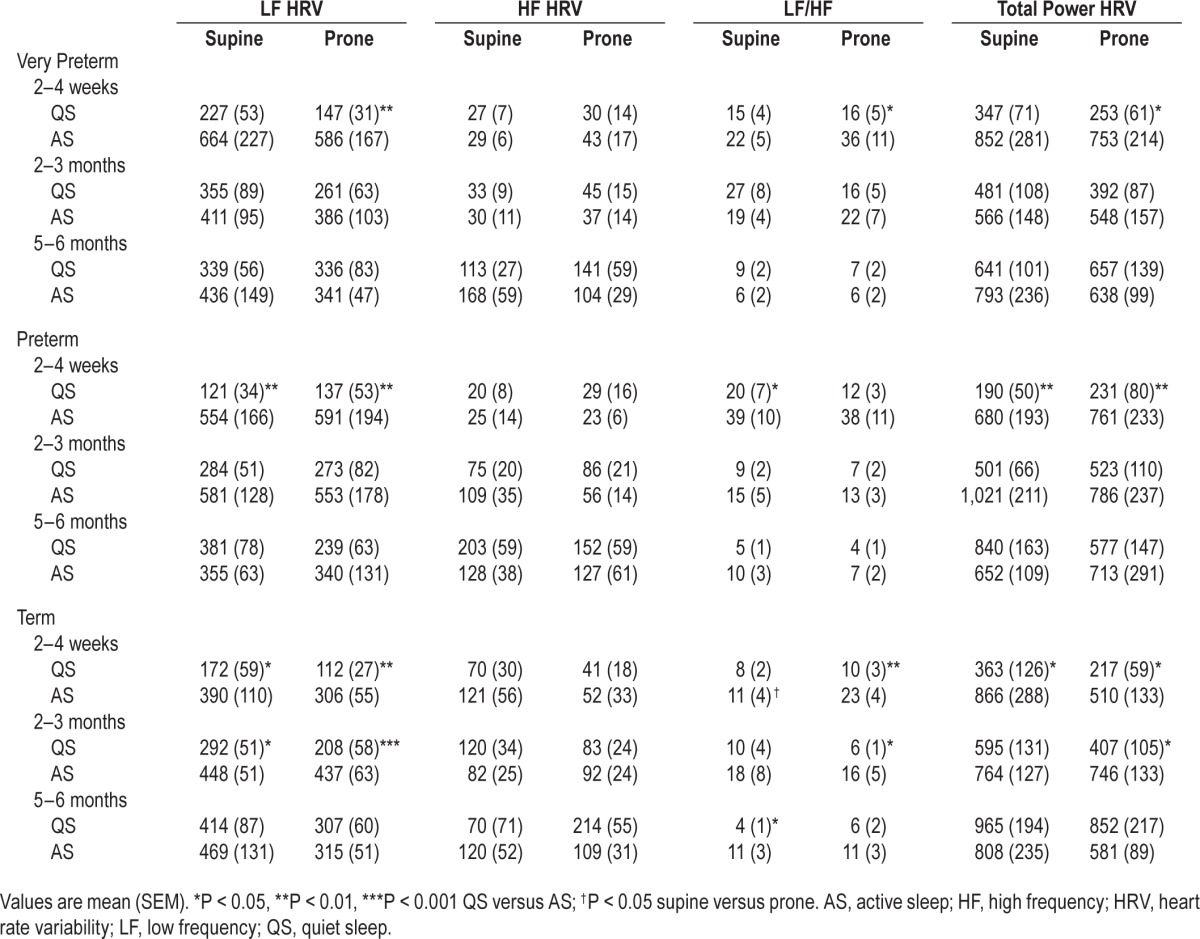
The effect of sleep position on HRV parameters is shown in Table 2. There was no significant effect of sleeping position on LF, HF, TP HRV or LF/HF in either the very preterm or preterm infants in either AS or QS at any age. In contrast, in term infants, LF/HF was higher in the prone position in AS at 2–4 weeks (P < 0.05). There was no significant effect of sleep position on LF, HF or TP HRV; however, LF HRV tended to be lower in the prone position in QS at 2–3 months (P = 0.09).
Table 2.
Effect of sleep state and sleep position on heart rate variability parameters in very preterm, preterm and term infants.
Effect of Sleep State
The effect of sleep state on HRV parameters is also shown in Table 2. In very preterm infants LF HRV, LF/HF, and TP HRV were higher in AS than QS in the prone position at 2–4 weeks CA (P < 0.05 for all). There was no effect of sleep state on HRV at 2–3 months or 5–6 months CA in either position.
In preterm infants LF HRV and TP HRV were higher in AS than QS in both sleep positions (P < 0.01), and LF/HF was higher in AS than QS in the supine position (P < 0.05) at 2–4 weeks CA. At 2–3 months, there was an overall effect of sleep state on LF HRV (P < 0.05); however, post hoc analysis was unable to identify where the difference occurred. At 5–6 months there was no effect of sleep state on HRV.
In term infants, LF HRV was higher in AS than QS at 2–4 weeks and 2–3 months in both the supine and prone positions (P < 0.05 for all). LF/HF was higher in AS than QS in the prone position at 2–4 weeks (P < 0.01) and 2–3 months (P < 0.05), and in the supine position at 5–6 months (P < 0.05). TP HRV was higher in AS than QS in both sleep positions at 2–4 weeks and in the prone position at 2–3 months (P < 0.05 for all). There was no effect of sleep state on HF HRV at any age or on LF or TP HRV at 5–6 months.
DISCUSSION
This study provides novel data on the effect of GA at birth and sleeping position on HRV parameters in preterm infants across the first six months following term-equivalent age. We have shown that HF HRV is lower and LF/HF higher at 2–3 months post-term in very preterm compared to preterm and term infants in QS. These findings suggest altered autonomic HR control in very preterm infants during the developmental period in which the risk of SIDS is maximal. Contrary to our hypothesis we found no effect of prone sleeping on HRV parameters in this cohort of preterm infants, even in those infants born at earlier GA.
Effect of Gestational Age at Birth
We have demonstrated that preterm birth has persistent effects on autonomic control of HR, particularly in those infants born very preterm. At 2–3 months CA, HF HRV, reflecting parasympathetic activity, was significantly reduced in very preterm compared to both preterm and term infants in QS. As a result, the LF/HF ratio was elevated in very preterm infants most prominently at 2–3 months CA, suggesting altered sympathovagal balance with a predominance of sympathetic activity.
Our findings of significantly reduced HF HRV and elevated LF/HF ratio at 2–3 months CA have significant implications for SIDS. Studies of infants who later succumbed to SIDS have found similar alterations in autonomic control of HR with reduced parasympathetic activity30 and elevated LF/HF ratio.31 Furthermore, our data suggest that the delay in maturation of HF HRV and subsequent alteration in sympathovagal balance in infants born very preterm only becomes significant at 2–3 months CA, coinciding with the period of peak risk for SIDS. This supports Filiano and Kinney's triple risk model, which suggests that SIDS deaths occur when three elements of risk interact; thus an underlying vulnerability may lie dormant until a critical developmental period is reached and an external stressor is present.32 We acknowledge that the period of peak SIDS risk in preterm infants occurs between 7–9 weeks post-term depending on GA at birth,2 slightly earlier than the peak seen in term infants. Therefore, it is possible that our study underestimates the effect of preterm birth on HRV and deficits in HF HRV in very preterm infants may be greater at a slightly earlier post-term age.
When examining the maturational trajectories of HRV across the first 6 months post-term we identified that delayed maturation of parasympathetic activity in very preterm infants is most prominent prior to 2–3 months CA. Between 2–3 months and 5–6 months CA, HF HRV follows a similar trajectory to that seen in preterm and term infants; however, although HF HRV activity increases to 5–6 months, it remains lower in very preterm infants than the other groups. This may be an indicator of early cardiovascular dysfunction, which could predispose infants born preterm to cardiovascular disease in adult life.33 In adults, reduced HRV has been reported in hypertensive patients18 and is associated with a significantly increased risk of experiencing a cardiac event.34
A number of previous studies in preterm infants conducted prior to term age have also found that maturation of parasympathetic activity is predominantly affected by preterm birth, while sympathetic maturation is relatively maintained.13,15,17 Our data build on these data and suggest that greater impairment in para-sympathetic maturation occurs in infants born very preterm (prior to 32 weeks of gestation). This is likely to be due to differences in fetal maturation of the branches of the autonomic nervous system. While sympathetic activity is predominant throughout gestation, parasympathetic activity undergoes a period of rapid maturation between 25 and 32 weeks of gestation.21,35,36 Therefore, very preterm birth, which occurs during this period, and the accompanying stresses of ex-utero life may significantly impact on normal parasympathetic maturation. We have demonstrated that this results in significantly reduced parasympathetic activity and altered sympathovagal balance in healthy infants born very preterm that persists until 2–3 months post-term age.
Finally, differences in HRV were observed only in QS, with no significant differences in HRV parameters seen between groups in AS. This is likely to be due to the predominance of sympathetic activity seen in AS and is consistent with previous studies in the supine position that have found no difference in HRV parameters between preterm and term infants in AS.22
Effect of Sleep Position and Sleep State
We found no significant effect of prone sleeping on HRV assessed in the frequency domain in either preterm or very preterm infants studied between 2–4 weeks and 5–6 months post-term CA. In term infants, LF/HF ratio was higher in the prone than the supine position at 2–4 weeks in AS. Prone sleeping is well established as a major risk factor for SIDS,4 and in term infants, prone sleeping has been associated with reduced blood pressure37 and alterations in cardiovascular control.7,38 However, the effect of sleep position on HRV in term infants is unclear. Gabai et al. found reduced short and long-term HRV in the prone compared to the supine position in term infants assessed within 3 days of birth overnight.39 Similarly, HRV parameters were found to be reduced prone compared to supine in term infants studied up to 4 months of age during the daytime.40,41 In term infants studied overnight at 2–3 months of age, Franco et al. found lower normalized power and higher bandwidth of HF HRV in the prone sleep position; but no effect of position on LF HRV or LF/HF ratio.6 In preterm infants assessed during daytime sleep prior to term-equivalent age, prone sleeping was associated with diminished HRV compared to the supine position in QS.42 Another study found prone sleeping particularly affected LF HRV, suggesting diminished sympathetic activity.8 When assessed at 1 and 3 months CA, only time-domain HRV parameters have been found to be lower in the prone position in QS with no effect of position on frequency-domain HRV parameters.9 In the current study, we also found no effect of prone sleeping on HRV assessed in the frequency domain in either preterm group. We suggest that the effect of prone sleeping on HRV parameters in preterm infants may be maximal prior to term age and diminishes with age; thus when studied at 2–4 weeks post-term age and beyond, we found no effect of sleep position. Similarly, in term infants the effect of prone sleeping was only present at 2–4 weeks. This may be due to maturational improvements in the cardiovascular response to prone sleeping.
Sleep state had a significant effect on HRV with elevated LF, LF/HF, and TP HRV in AS compared to QS at 2–4 weeks CA, with similar effects seen in both very preterm and preterm infants. This reflects the predominance of sympathetic activity in AS and may also be influenced by greater respiratory variability and more frequent body movements associated with AS.22 Consistent with previous studies in preterm infants,9 we found that the effect of sleep state on HRV was maximal at 2–4 weeks CA becoming less marked with increasing age. It is thought that this is due to increasing parasympathetic activity during this period resulting in a relative reduction in the influence of sympathetic activity on HR during sleep.43
CONCLUSIONS
We have shown that preterm birth results in impaired maturation of parasympathetic autonomic control of HR, most prominently in those infants born prior to 32 weeks GA. This delayed maturation results in significantly altered sympathovagal balance with a predominance of sympathetic activity in very preterm infants at 2–3 months post-term age. Altered autonomic cardiovascular control during the period of peak risk for SIDS may contribute to the increased risk of SIDS seen in infants born at earlier GAs; however, this does not appear to be exacerbated by prone sleeping.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by The National Health and Medical Research Council of Australia and the Victorian Government's Operational Infrastructure Support Program. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the parents and infants involved in this study and the staff at the Melbourne Children's Sleep Centre.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Malloy MH. Prematurity and sudden infant death syndrome: United States 2005-2007. J Perinatol. 2013;33:470–5. doi: 10.1038/jp.2012.158. [DOI] [PubMed] [Google Scholar]
- 3.Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of back-to-sleep campaign. Pediatrics. 2012;129:630–8. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030–9. doi: 10.1542/peds.2011-2284. [DOI] [PubMed] [Google Scholar]
- 5.Harper RM. Sudden infant death syndrome: a failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–2. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Franco P, Groswasser J, Sottiaux M, Broadfield E, Kahn A. Decreased cardiac responses to auditory stimulation during prone sleep. Pediatrics. 1996;97:174–8. [PubMed] [Google Scholar]
- 7.Yiallourou SR, Sands SA, Walker AM, Horne RS. Baroreflex sensitivity during sleep in infants: impact of sleeping position and sleep state. Sleep. 2011;34:725–32. doi: 10.5665/SLEEP.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean-Louis M, Anwar M, Rosen H, Craelius W, Hiatt M, Hegyi T. Power spectral analysis of heart rate in relation to sleep position. Biol Neonate. 2004;86:81–4. doi: 10.1159/000077782. [DOI] [PubMed] [Google Scholar]
- 9.Ariagno RL, Mirmiran M, Adams MM, Saporito AG, Dubin AM, Baldwin RB. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months' corrected age. Pediatrics. 2003;111:622–5. doi: 10.1542/peds.111.3.622. [DOI] [PubMed] [Google Scholar]
- 10.Task Force of the European Society the North American Society for Pacing Electrophysiology. Heart rate variability. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 11.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 12.Eiselt M, Curzi-Dascalova L, Clairambault J, Kauffmann F, Medigue C, Peirano P. Heart-rate variability in low-risk prematurely born infants reaching normal term: a comparison with full-term newborns. Early Hum Dev. 1993;32:183–95. doi: 10.1016/0378-3782(93)90011-i. [DOI] [PubMed] [Google Scholar]
- 13.Longin E, Gerstner T, Schaible T, et al. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat Med. 2006;34:303–8. doi: 10.1515/JPM.2006.058. [DOI] [PubMed] [Google Scholar]
- 14.De Rogalski Landrot I, Roche F, Pichot V, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Auton Neurosci. 2007;136:105–9. doi: 10.1016/j.autneu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Patural H, Barthelemy JC, Pichot V, et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res. 2004;14:391–5. doi: 10.1007/s10286-004-0216-9. [DOI] [PubMed] [Google Scholar]
- 16.Patural H, Pichot V, Jaziri F, et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum Dev. 2008;84:681–7. doi: 10.1016/j.earlhumdev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C. Heart rate variability in normal sleeping full-term and preterm neonates. Early Hum Dev. 1992;28:169–83. doi: 10.1016/0378-3782(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 18.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–7. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 19.Karin J, Hirsch M, Akselrod S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr Res. 1993;34:134–8. doi: 10.1203/00006450-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Okamura K, Yajima A. Spectral analysis of beat-to-beat intervals of the fetal heart obtained by doppler ultrasound. Gynecol Obstet Invest. 1996;41:5–9. doi: 10.1159/000292025. [DOI] [PubMed] [Google Scholar]
- 21.Tsukasa O, Kunihiro O, Yoshitaka K, et al. Alteration in the low-frequency domain in power spectral analysis of fetal heart beat fluctuations. Fetal Diagn Ther. 1999;14:92–7. doi: 10.1159/000020896. [DOI] [PubMed] [Google Scholar]
- 22.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89:145–52. doi: 10.1016/j.earlhumdev.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Yiallourou SR, Walker AM, Horne RS. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep. 2006;29:1083–8. doi: 10.1093/sleep/29.8.1083. [DOI] [PubMed] [Google Scholar]
- 24.Fyfe KL, Yiallourou SY, Wong FY, Odoi A, Walker AM, Horne RSC. Cerebral oxygenation in preterm infants. Pediatrics. 2014;134:435–45. doi: 10.1542/peds.2014-0773. [DOI] [PubMed] [Google Scholar]
- 25.Wong FY, Witcombe NB, Yiallourou SR, et al. Cerebral oxygenation is depressed during sleep in healthy term infants when they sleep prone. Pediatrics. 2011;127:e558–65. doi: 10.1542/peds.2010-2724. [DOI] [PubMed] [Google Scholar]
- 26.Witcombe NB, Yiallourou SR, Sands SA, Walker AM, Horne RS. Preterm birth alters the maturation of baroreflex sensitivity in sleeping infants. Pediatrics. 2012;129:e89–96. doi: 10.1542/peds.2011-1504. [DOI] [PubMed] [Google Scholar]
- 27.Yiallourou SR, Sands SA, Walker AM, Horne RS. Postnatal development of baroreflex sensitivity in infancy. J Physiol. 2010;588:2193–203. doi: 10.1113/jphysiol.2010.187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yiallourou SR, Sands SA, Walker AM, Horne RS. Maturation of heart rate and blood pressure variability during sleep in term-born infants. Sleep. 2012;35:177–86. doi: 10.5665/sleep.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andriessen P, Koolen AM, Berendsen RC, et al. Cardiovascular fluctuations and transfer function analysis in stable preterm infants. Pediatr Res. 2003;53:89–97. doi: 10.1203/00006450-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Kluge KA, Harper RM, Schechtman VL, Wilson AJ, Hoffman HJ, Southall DP. Spectral analysis assessment of respiratory sinus arrhythmia in normal infants and infants who subsequently died of sudden infant death syndrome. Pediatr Res. 1988;24:677–82. doi: 10.1203/00006450-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Franco P, Verheulpen D, Valente F, et al. Autonomic responses to sighs in healthy infants and in victims of sudden infant death. Sleep Med. 2003;4:569–77. doi: 10.1016/s1389-9457(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 32.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194–7. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 33.De Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust. N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 35.Schneider U, Schleussner E, Fiedler A, et al. Fetal heart rate variability reveals differential dynamics in the intrauterine development of the sympathetic and parasympathetic branches of the autonomic nervous system. Physiol Meas. 2009;30:215–26. doi: 10.1088/0967-3334/30/2/008. [DOI] [PubMed] [Google Scholar]
- 36.David M, Hirsch M, Karin J, Toledo E, Akselrod S. An estimate of fetal autonomic state by time-frequency analysis of fetal heart rate variability. J Appl Physiol. 2007;102:1057–64. doi: 10.1152/japplphysiol.00114.2006. [DOI] [PubMed] [Google Scholar]
- 37.Yiallourou SR, Walker AM, Horne RS. Effects of sleeping position on development of infant cardiovascular control. Arch Dis Child. 2008;93:868–72. doi: 10.1136/adc.2007.132860. [DOI] [PubMed] [Google Scholar]
- 38.Yiallourou SR, Walker AM, Horne RS. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep. 2008;31:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 39.Gabai N, Cohen A, Mahagney A, Bader D, Tirosh E. Arterial blood flow and autonomic function in full-term infants. Clin Physiol Funct Imaging. 2006;26:127–31. doi: 10.1111/j.1475-097X.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 40.Galland BC, Reeves G, Taylor BJ, Bolton DP. Sleep position, autonomic function, and arousal. Arch Dis Child Fetal Neonatal Ed. 1998;78:F189–94. doi: 10.1136/fn.78.3.f189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galland BC, Taylor BJ, Bolton DP, Sayers RM. Heart rate variability and cardiac reflexes in small for gestational age infants. J Appl Physiol. 2006;100:933–9. doi: 10.1152/japplphysiol.01275.2005. [DOI] [PubMed] [Google Scholar]
- 42.Goto K, Mirmiran M, Adams MM, et al. More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics. 1999;103:603–9. doi: 10.1542/peds.103.3.603. [DOI] [PubMed] [Google Scholar]
- 43.Harper RM, Hoppenbrouwers T, Sterman MB, McGinty DJ, Hodgman J. Polygraphic studies of normal infants during the first six months of life. I. Heart rate and variability as a function of state. Pediatr Res. 1976;10:945–8. doi: 10.1203/00006450-197611000-00008. [DOI] [PubMed] [Google Scholar]