Abstract
Study Objectives:
Evaluate whether levels of upsetting life events measured over a 9-y period prospectively predict subjective and objective sleep outcomes in midlife women.
Design:
Prospective cohort study.
Setting:
Four sites across the United States.
Participants:
330 women (46–57 y of age) enrolled in the Study of Women's Health Across the Nation (SWAN) Sleep Study.
Interventions:
N/A.
Measurements and Results:
Upsetting life events were assessed annually for up to 9 y. Trajectory analysis applied to life events data quantitatively identified three distinct chronic stress groups: low stress, moderate stress, and high stress. Sleep was assessed by self-report and in-home polysomnography (PSG) during the ninth year of the study. Multivariate analyses tested the prospective association between chronic stress group and sleep, adjusting for race, baseline sleep complaints, marital status, body mass index, symptoms of depression, and acute life events at the time of the Sleep Study. Women characterized by high chronic stress had lower subjective sleep quality, were more likely to report insomnia, and exhibited increased PSG-assessed wake after sleep onset (WASO) relative to women with low to moderate chronic stress profiles. The effect of chronic stress group on WASO persisted in the subsample of participants without baseline sleep complaints.
Conclusions:
Chronic stress is prospectively associated with sleep disturbance in midlife women, even after adjusting for acute stressors at the time of the sleep study and other factors known to disrupt sleep. These results are consistent with current models of stress that emphasize the cumulative effect of stressors on health over time.
Citation:
Hall MH, Casement MD, Troxel WM, Matthews KA, Bromberger JT, Kravitz HM, Krafty RT, Buysse DJ. Chronic stress is prospectively associated with sleep in midlife women: the SWAN Sleep Study. SLEEP 2015;38(10):1645–1654.
Keywords: EEG, midlife, sleep, stress, women
INTRODUCTION
Stress is commonly described as a key risk factor for sleep disturbances.1–21 However, the evidence linking stress with sleep is equivocal.2,22 Discrepant results in the published literature may be due, in part, to the varying approaches used to operationalize stress and sleep across studies.22 A majority of studies have examined a “snapshot” of stress measured at a single point in time (e.g., laboratory stressors, retrospective reports of traumatic stress) in relation to sleep, whereas current models of stress and disease emphasize the cumulative effect of stressors on health and disease outcomes over time.23,24 In a similar way, it is increasingly understood that sleep is best characterized across multiple dimensions including quality, duration, continuity, depth, and symptoms of common sleep disorders. However, studies of stress and sleep have focused primarily on a limited range of sleep outcomes.
Although disturbed sleep under situations of acute threat may be adaptive in the short term, chronic stress and stress-related sleep disturbances may impair recovery processes and alter the function of neural, cardiovascular, metabolic, and immune systems, with downstream consequences to health and functioning.25,26 A greater understanding of the influence of chronic stress on sleep is, in turn, critical to reducing stress-related morbidity and mortality. Although some longitudinal studies have evaluated links between acute life events and subjective sleep disturbances including insomnia,1,2 we are not aware of any studies that have prospectively modeled the effect of chronic stress on subsequent sleep outcomes, including comprehensive assessments of both self-report and objective indices of sleep.
In the current longitudinal analysis we evaluated associations among very upsetting life events measured prospectively over a 3- to 9-y period and subsequent indices of sleep in a community-based sample of midlife women. Trajectory analyses were applied to annual life events data to quantitatively identify distinct chronic stress groups based both on the average number and on the temporal profiles of life events over time. Our goal was to characterize participants' stress profiles over a nearly decade-long period of time. We focused on “gold standard” measures of sleep quality, reports of insomnia and polysomnography (PSG)-assessed sleep disturbances. Also assessed were daily diary measures homologous to PSG-assessed sleep duration and continuity outcomes in order to provide comparisons with studies focused on self-reported sleep outcomes. We hypothesized that participants whose lives were characterized by chronic, high levels of stress—relative to chronic, low or moderate stress—would experience poorer subjective sleep quality, increased prevalence of insomnia, and more PSG-assessed sleep disturbances, including increased sleep onset latency (SOL) and wakefulness after sleep onset (WASO), and decreased total sleep time (TST) and slow wave sleep (SWS), each of which has been associated with stressful life events in some, but not all, existing studies.22 Of these sleep outcomes, subjective sleep quality, symptoms of insomnia, and WASO have been most reliably associated with other indices of stress.22 Analyses adjusted for relevant sociodemographic and health characteristics that are also related to sleep.
METHODS
Participants
Participants were recruited from the Study of Women's Health Across the Nation (SWAN), a multisite, community-based, longitudinal study of women's health during the menopausal transition (n = 3,302). The SWAN study was conducted at seven clinical sites across the United States27 and baseline eligibility criteria were: age 42–52 y, intact uterus and at least one ovary, no use of exogenous hormones affecting ovarian function in the past 3 mo, and at least one menstrual period in the previous 3 mo. A total of 370 women elected to participate in the ancillary SWAN Sleep Study conducted at the Chicago, IL; Detroit area, MI; Oakland, CA; and Pittsburgh, PA study sites. Sleep study exclusion criteria were: postmenopausal status, current hormone replacement therapy, current chemotherapy or radiation, current oral corticosteroid use, regular night shift work, regular consumption of more than four alcoholic drinks/day, and noncompliance with Core SWAN procedures (i.e., missed > 50% of annual visits, refused annual visit blood draw). In the final year of the study, eligibility criteria were modified to allow inclusion of postmenopausal women.
Data relevant to the current analyses include assessments conducted in conjunction with participants' initial core SWAN assessment (baseline), annual core SWAN assessments (years 1–9), and the SWAN Sleep Study, a 35-day assessment of sleep during the menopausal transition (described below). A total of 330 participants were included in the current analyses due to missing data on baseline sleep complaints assessed at the initial Core SWAN assessment (n = 28) and model covariates (n = 12). Sleep outcomes did not differ in the full sample (n = 370) and the sample used here (n = 330).
Protocol
The SWAN Sleep Study was conducted over a full menstrual cycle or 35 days, whichever was shorter. The protocol began within 7 days of menstrual bleeding in women who still had regular menstrual cycles, and was scheduled by convenience for non-cycling women. Sleep was assessed by self-report, daily sleep diaries and unattended, in-home PSG at participants' habitual sleep-wake times during the first 3 nights of the protocol. Study staff met participants in their homes and applied and calibrated the equipment for PSG recording each evening. Participants turned the recorder off and removed the recording equipment upon rising in the morning. Data loss for PSG sleep studies was minimized through the use of a multi-night protocol and repeat studies, when possible. Indices of PSG-assessed sleep duration, continuity, and architecture were available for all participants (n = 330), whereas sleep disordered breathing data were missing for 15 participants (< 5% of the sample).
Upsetting life events for trajectory analyses were measured annually by self-report during baseline and at annual follow-up SWAN assessments. Participants had up to nine annual assessments of upsetting life events prior to the Sleep Study. Additional data relevant to the current report include sample characteristics measured in conjunction with the Core SWAN Study or the ancillary Sleep Study, as described in the next paragraphs. The institutional review board at each study site approved study procedures, and all participants provided written informed consent. Participants were compensated for participation in the Sleep Study.
Life Events
Upsetting life events were assessed using an 18-item version of the Psychiatric Epidemiology Research Interview Life Events Scale (PERI).28 The modified PERI assessed life events across eight domains: school, work, romantic relationships, children, family, criminal and legal matters, finances, and health. Participants rated whether an event occurred during the past year and how upsetting the event was (1 = not upsetting, 5 = very and still upsetting). Endorsed items scored as “very” and “still upsetting” were summed to yield an annual life events score with a range of 0 to 18. Each participant had between 3 and 9 y of annual life events data (mean = 8.67, standard deviation = 0.87, range = 3–9) measured prior to the Sleep Study. Our analytical approach to identifying chronic stress groups is described in the following paragraphs.
Sleep Outcomes
The SWAN Sleep Study assessment included two self-report questionnaires, a sleep diary, and in-home PSG. The Pittsburgh Sleep Quality Index (PSQI),29 an 18-item questionnaire, was administered to assess subjective sleep quality over the past month. Scores on the PSQI range from 0 to 21, with higher scores indicating more subjective sleep quality complaints. PSQI scores above 5 are associated with clinically significant sleep disorders.29 The Insomnia Symptom Questionnaire (ISQ),30 a 13-item measure, was administered to assess the presence/absence of insomnia symptoms. Because it was used as a diagnostic measure, insomnia was dichotomized as “present” or “absent.” Participants met criteria for insomnia if they endorsed difficulty falling asleep, difficulty staying asleep, or nonrestorative sleep at least three times per week for at least 4 w and reported insomnia-related functional impairment. Insomnia Symptom Questionnaires were available for 329 of 330 participants.
Polysomnographic sleep data were collected with Vitaport-3 (TEMEC VP3; Temec Instruments B.V., the Netherlands) ambulatory monitors over 3 consecutive nights. The PSG montage included bilateral central referential electroencephalogram (EEG) channels (C3 and C4, referenced to A1-A2), electro-oculogram (EOG), submentalis electromyogram (EMG), and electrocardiogram (EKG). Measures of sleep disordered breathing were collected on the first night of PSG using nasal pressure cannula, oronasal thermistor, respiratory inductance plethysmography, and fingertip oximetry. Quality assurance assessments, scoring, and processing of all Sleep Study data was performed at the University of Pittsburgh Neuroscience-Clinical and Translational Research Center (N-CTRC). At the time that data were collected, visual sleep stage scoring in 20-sec epochs was conducted by PSG technologists trained to reliability using standard scoring criteria31 and American Academy of Sleep Medicine (AASM) recommendations for sleep disordered breathing.32 Note that this study was conducted prior to the 2007 AASM scoring manual.
PSG-assessed sleep measures included indices of sleep duration, continuity, and sleep architecture. Although not theoretically linked to psychological stress, we also evaluated symptoms of sleep apnea, which is prevalent in midlife women.33 Sleep duration and continuity were defined as time in bed (PSG-TIB; minutes from getting into bed at night to getting out of bed in the morning), total sleep time (PSG-TST; minutes of sleep between sleep onset and the end of the sleep period), sleep onset latency (PSG-SOL; minutes from the lights out to the first 10 consecutive min of stage 2, 3, or 4 sleep interrupted by no more than 2 min of stage 1 sleep or wakefulness), wake after sleep onset (PSG-WASO; minutes of wakefulness between sleep onset and the end of the sleep period), sleep efficiency (PSG-SE; percent of time in bed spent asleep), and the number of awakenings of at least 11 sec in duration between sleep onset and the end of the sleep period (PSG-NAWK). Measures of sleep architecture included the percent of sleep time in non-rapid eye movement (NREM) stages 1, 2, and 3+4 sleep, as well as rapid eye movement (REM) sleep. Values for PSG-TIB, PSG-TST, PSG-SOL, PSG-WASO, PSG-SE, PSGNAWK, NREM sleep stages, and REM sleep were averaged across the second and third recording night to provide a reliable estimate of these outcomes.34 The apnea-hypopnea index (AHI) was used to quantify sleep disordered breathing on the first night of recording. Apnea data were missing in 15 participants due to equipment failure (AHI n = 315).
Participants completed the Pittsburgh Sleep Diary (PghSD)35 on each day of the protocol (mean = 29.95 ± 6.44 days). Included on the diary were questions about indices of sleep duration and continuity that are homologous to PSG-assessed time in bed (D-TIB), total sleep time (D-TST), sleep latency (D-SOL), wakefulness after sleep onset (D-WASO), sleep efficiency (D-SE), and number of awakenings (D-NAWK). Within-subject averages were computed for each diary-assessed sleep outcome. Sleep diary data were available for 322 of 330 participants.
Sample Characteristics
Sociodemographics, health characteristics, and health behaviors were assessed in conjunction with the Sleep Study or during the annual Core SWAN assessment preceding the Sleep Study.33 Race/ethnicity (Black, Chinese, White) and marital status (married/partnered, unmarried) assessed during the annual Core SWAN assessment preceding the Sleep Study were measured by self-report. Other sample characteristics assessed during the annual Core SWAN assessment included menopausal status, as determined by menstrual bleeding patterns36; self-perceived health status, dichotomized as “good” to “excellent” versus “fair” to “poor” based on the distribution of responses to the single-item general health rating of the Short-Form Health Survey (SF-36)37; and body mass index (BMI), based on measured height and weight. Medication use was assessed during the Sleep Study and coded according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification (http://www.whocc.no/atcddd). Medications that affect sleep (dichotomized as present or absent) were associated with the following ATC classification codes: N02A (opioids), N03A (antiepileptics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants), and R06A (antihistamines). Current smoking (any nicotine use) and regular exercise (exercising three or more times per week) were assessed by daily diary during the Sleep Study and coded as “present” or “absent.” Participants completed the 16-item Inventory of Depressive Symptomatology (IDS) on day 04, 14, and the last day of the Sleep Study.38 The IDS, minus sleep items, was averaged across administrations to yield a reliable estimate of self-reported symptoms of depression concurrent with the Sleep Study.
We also identified the number of acute life events identified by participants as “very upsetting” at the time of the Sleep Study. This variable was dichotomized as “none” or “1 or more” as 73% of participants indicated that no events were still “very upsetting” at the time of the Sleep Study. Finally, we identified the number of participants who endorsed subjective sleep complaints at baseline using a single item from the Women's Health Initiative.39 Participants were asked about their typical quality of sleep over the past 2 w; baseline sleep complaints was dichotomized for analyses (very sound/sound/average or restless/very restless).
Analytic Approach
Trajectory analysis was applied to annual PERI life events measures to categorize study participants into chronic stress groups based on the number of “very and still upsetting” life events endorsed at baseline and during annual Core SWAN assessments up to and including the assessment preceding the Sleep Study. We fit a hierarchical latent growth curve model to identify discrete clusters of individuals who share common pathways over time.40 Importantly, clusters were identified based on the entire dynamic pattern of the data, making the classification more robust to outlier values compared to simply averaging values over time, and clusters are formed quantitatively through maximum likelihood estimation rather than using arbitrary cutoffs. The resulting chronic stress trajectory groups, or clusters, reflect both longitudinally averaged values (i.e., mean number of events) and their temporal dynamics (i.e., profiles over time including increasing and decreasing slopes). The number of PERI assessments varied by participant depending on when they completed the Sleep Study in relation to ongoing annual Core SWAN assessments (range = 3–9). For analyses, we used the Statistical Analysis System Trajectory (SAS TRAJ; SAS Institute, Cary, NC) procedure41 to identify three trajectories that best characterized the samples' heterogeneity; descriptively, the three identified trajectory groups were “low chronic stress,” “moderate chronic stress,” and “high chronic stress” (Figure 1). The SAS TRAJ procedure calculated the probability of each participant belonging to each of the three trajectories and identified their group based on the largest probability value. Participants with missing PERI data were included, provided that a minimum of three observations were available.
Figure 1.
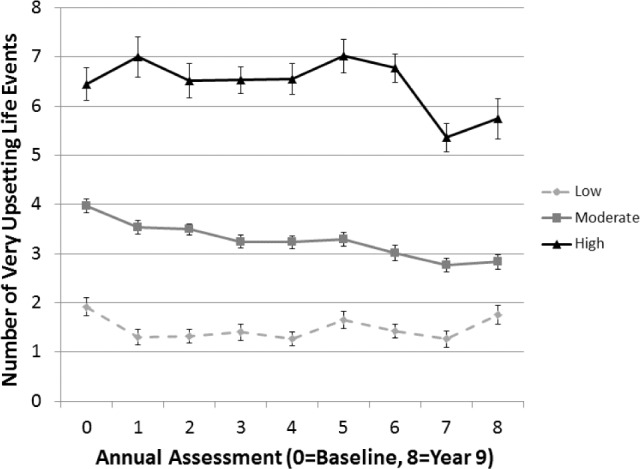
Chronic stress trajectory groups over the period of study (3 to 9 years of data, n = 330).
Sample characteristics and sleep outcomes were evaluated using descriptive statistics and skewed data were transformed prior to analysis. Univariate analyses examined group differences on study outcomes and covariates using analysis of variance (ANOVA), Pearson chi-square, and logistic regression. Tukey post hoc tests were applied to identify between-group differences in sleep outcomes.
Stepwise multivariate analyses were used to evaluate group differences on sleep outcomes. Step 1 covariates adjusted for self-reported sleep complaints at baseline and sample characteristics that differed among chronic stress groups including race, marital status, symptoms of depression, and BMI. Step 2 added “very upsetting acute life events” concurrent with the Sleep Study to model covariates. Low and moderate chronic stress groups referenced to the high chronic stress group were added to the model in Step 3. Menopausal status, perceived health, use of medications that affect sleep, exercise, and smoking were not included as covariates in multivariate analyses because they did not differ across chronic stress groups. Alpha level was set at P < 0.01 for all analyses. Multivariate sensitivity analyses were repeated in the 230 participants without sleep complaints at baseline (sleep rated as “very sound” to “average”).
RESULTS
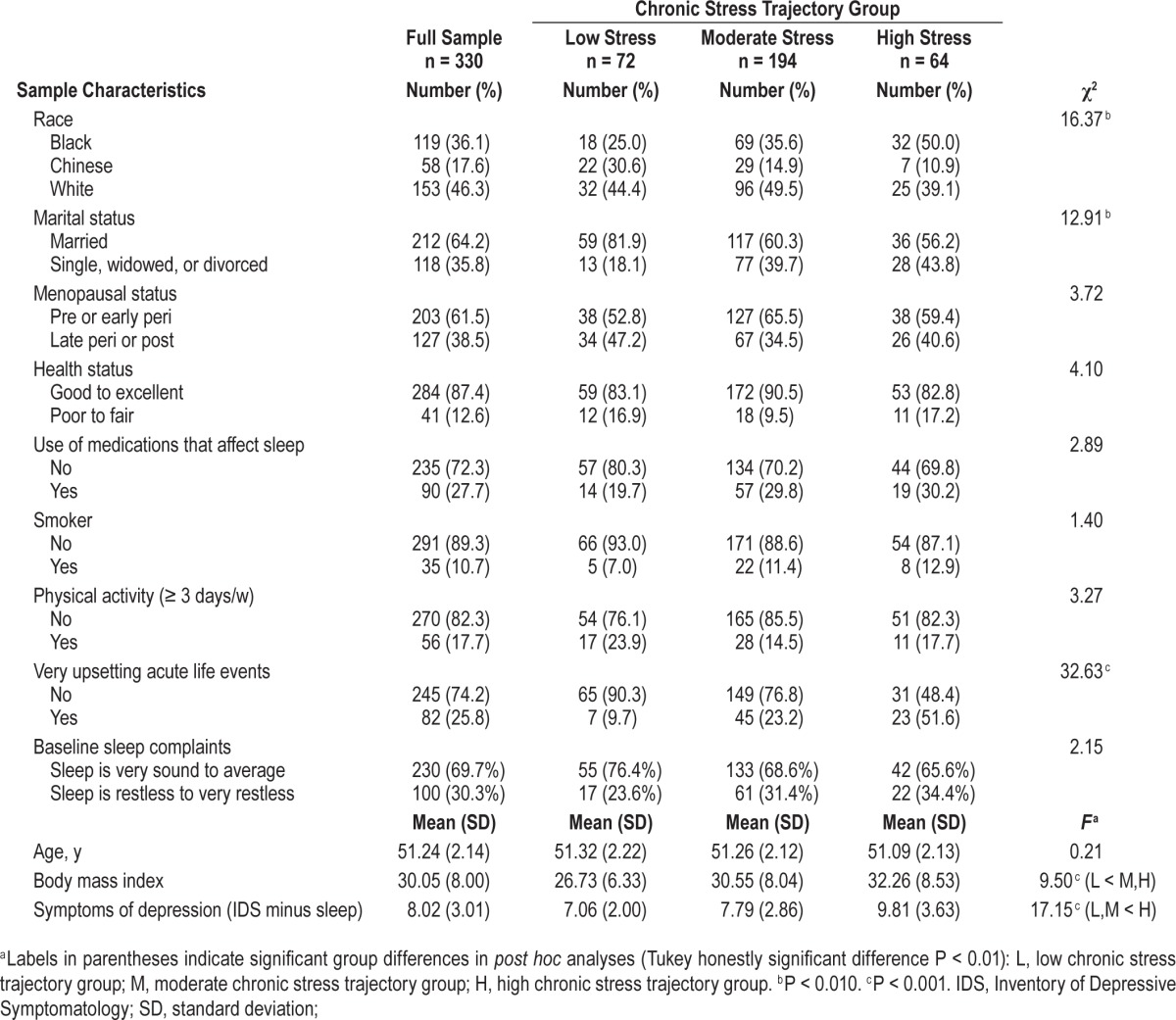
At the time of the Sleep Study, the average age of the sample was 51 y, half of the sample was comprised of minority racial/ ethnic groups, and 61.5% of participants were pre-menopausal or early perimenopausal. As shown in Table 1, most participants were married or living as married, perceived their health as “good” to “excellent,” were current nonsmokers, and did not use medications that affect sleep. However, average BMI was almost 30 and the majority of participants were sedentary. Univariate tests indicated that several sample characteristics differed by chronic stress group including race, marital status, “very upsetting acute life events,” BMI, and symptoms of depression (Table 1). For example, participants in the high and moderate chronic stress groups had higher BMIs and reported more symptoms of depression compared to participants in the low chronic stress group (P < 0.001). Participants in the high chronic stress group were also more likely to endorse “very upsetting acute life events” at the time of the sleep study (P < 0.001).
Table 1.
Sample characteristics according to chronic stress trajectory group.
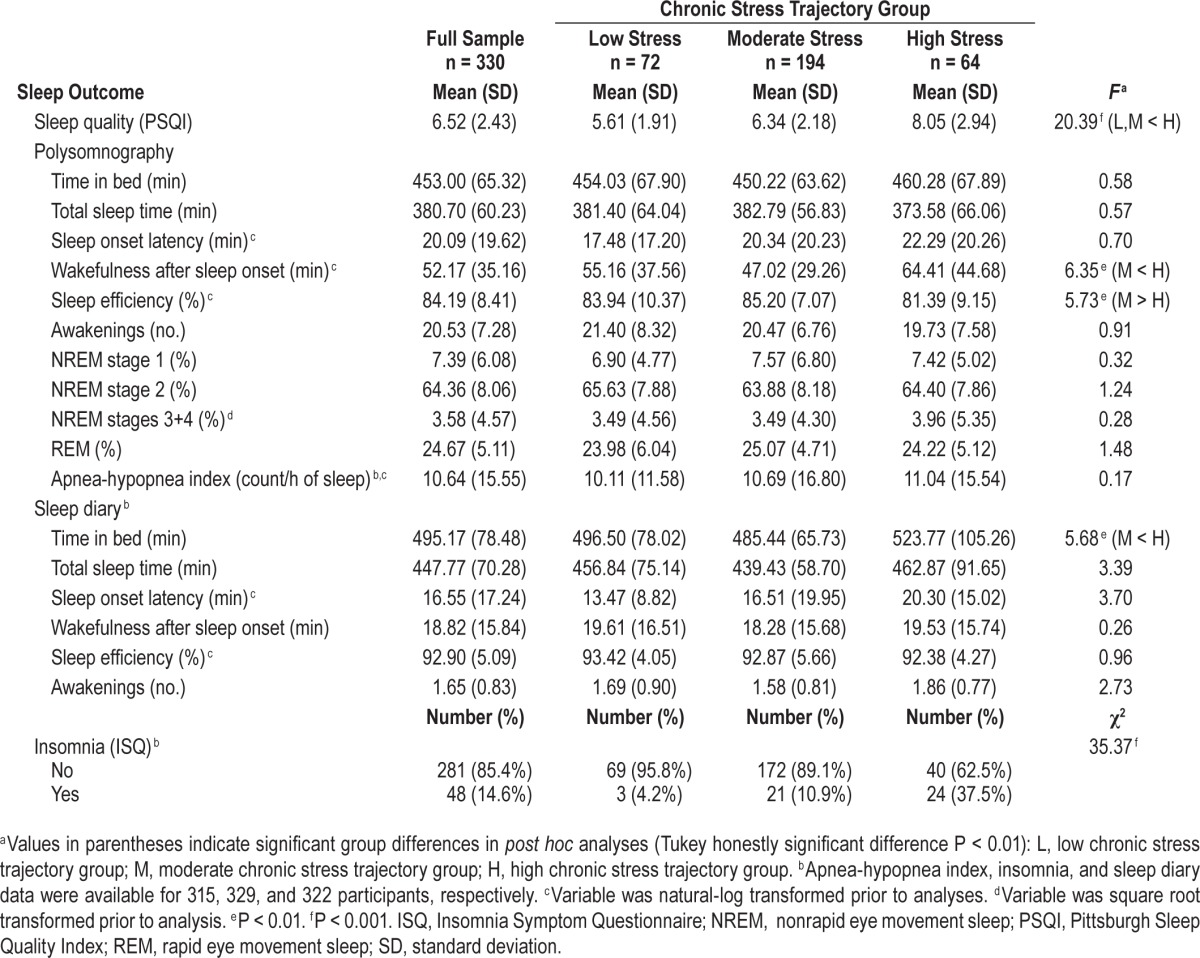
On average, the sample obtained less than 6.5 h of sleep, took 20 min to fall asleep, and had 52 min of WASO, as measured by PSG (Table 2). The average PSQI score for the sample was 6.52, falling within the “poor sleep quality” range,42 though most participants (85.4%) did not experience clinically significant insomnia, as assessed by the ISQ. Average diary-assessed sleep duration was 7.5 h and WASO was nearly 19 min. Univariate analyses showed significant chronic stress group differences for subjective sleep quality (P < 0.001), PSG-assessed WASO (P < 0.01) and sleep efficiency (P < 0.01), diary-assessed TIB (P < 0.01), and prevalence of insomnia (P < 0.001) (Table 2).
Table 2.
Univariate sleep outcomes according to chronic stress trajectory group.
Multivariate models evaluated the extent to which chronic stress group was prospectively associated with sleep outcomes after adjusting for race, baseline sleep complaints, marital status, symptoms of depression, BMI, and acute life events at the time of the Sleep Study. As shown in Tables 3 and 4, chronic stress group remained a significant predictor of subjective sleep quality, PSG-assessed WASO and reports of insomnia (Ps < 0.01) after adjusting for acute stress and other covariates. Participants in the high chronic stress group reported more subjective sleep complaints than did participants in the low and moderate chronic stress groups (standardized beta's = 0.19 and 0.18, respectively; Ps < 0.01). Participants in the high chronic stress group also exhibited greater PSG-assessed WASO than did participants in the moderate chronic stress group (standardized beta = 0.18, P < 0.01). The high chronic stress group also tended to have poorer PSG-assessed sleep efficiency and report more diary-assessed TIB compared to the moderate chronic stress group (standardized betas = −0.16 and 0.15, respectively; Ps < 0.05). In addition, insomnia was more prevalent in the high compared to the low and moderate chronic stress groups (Table 4). Sensitivity analyses in the 230 participants without baseline sleep complaints revealed a similar pattern of results for subjective sleep quality, PSG-assessed WASO and SE, diary-assessed TIB, and reports of insomnia (Tables 3 and 4). Finally, although acute stress was not a significant correlate of sleep in any of these models, participants who reported one or more “very upsetting” life events at the time of their sleep study tended to report greater subjective sleep complaints and more diary-assessed TIB and TST (see Step 2 multivariate model results for the full sample in Table 3).
Table 3.
Results for stepwise multivariate linear regression analyses predicting sleep outcomes in the full sample (n = 330) and in the subsample of participants without baseline sleep complaints (n = 230).
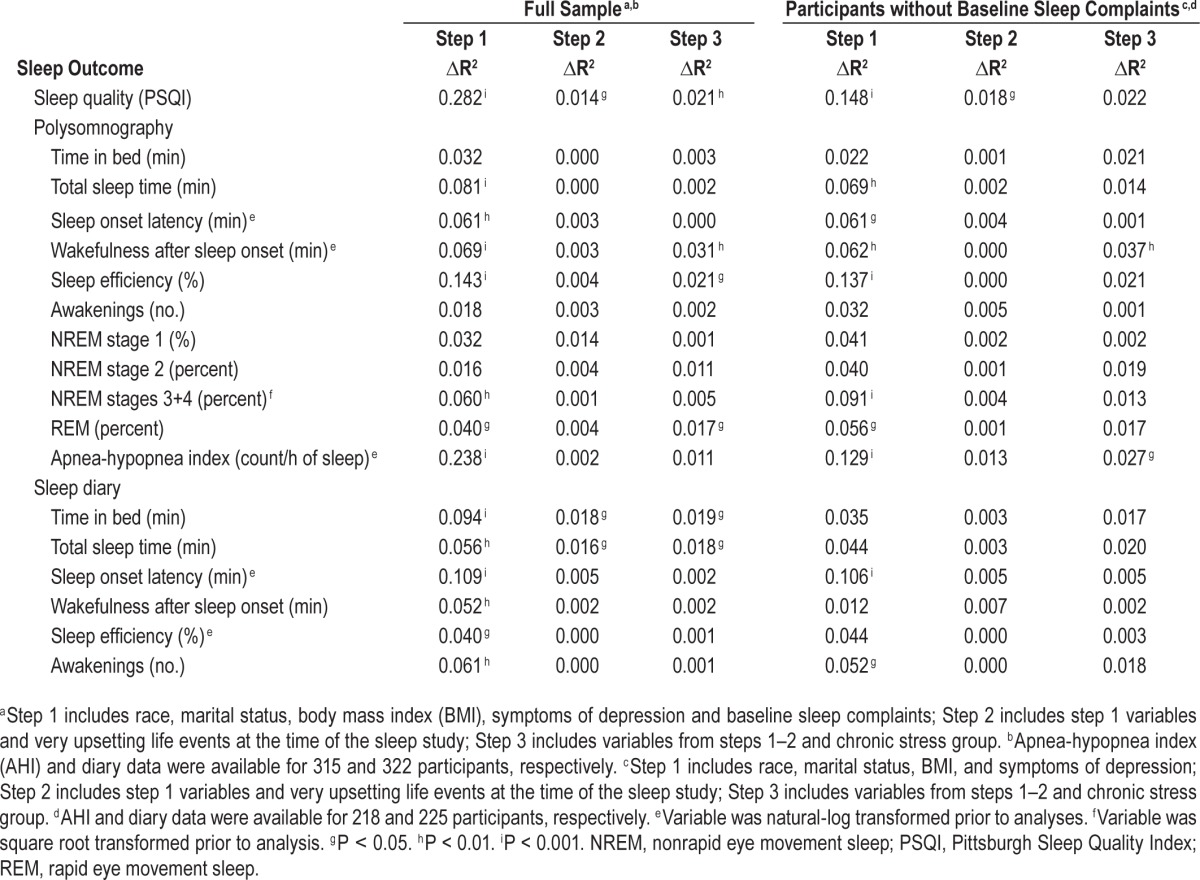
Table 4.
Results for multivariate logistic regression predicting odds of insomnia in the full sample (n = 329) and in the subsample of participants without baseline sleep complaints (n = 230).

DISCUSSION
This is the first study to evaluate the link between chronic psychological stress and sleep using prospective assessments of a range of life stressors over a nearly 10-y period and multidimensional assessment of subsequent sleep disturbance. Trajectory analyses were used to objectively identify orthogonal groups of participants with low, moderate, and high chronic stress profiles. Results indicate that there is a robust association between chronic stress burden, as defined by frequent and highly distressing events over a number of years, and disturbed sleep in midlife women. Women characterized by high chronic stress profiles had lower subjective sleep quality, were more likely to report insomnia, and exhibited greater PSG-assessed wakefulness after sleep onset relative to women with low to moderate chronic stress. Importantly, associations among chronic stress profiles and sleep were independent of acute life events at the time of the Sleep Study as well as other possible confounders.
The observed association between chronic stress profiles and self-reported poor sleep (sleep quality, reports of insomnia) is consistent with previous longitudinal studies of work stress and subjective sleep outcomes10–13,18 as well as a recent report linking stressful events and stress-related intrusive thoughts with insomnia over a 12-mo period.1 Studies linking psychological stress with objective indices of sleep have been more mixed,22 perhaps due to the narrow range of life events examined in previous prospective studies (e.g., bereavement, occupational stressors, traumatic events), differences in laboratory stressor characteristics (e.g., sleeping in a novel environment, expectation of a speech, overnight blood draws) and highly distressing life events,22 or due to the influence of negative affective bias on subjective reports of disturbed sleep.2,43,44 Our results indicate that the chronic experience of frequent and highly distressing events is prospectively associated with an important objective marker of sleep disruption, that is, wakefulness after sleep onset.
The current data provide a multidimensional picture of sleep continuity disturbances in relation to stressful life events in midlife women. First, significant effects were observed for chronic, not acute, stress. These data suggest that chronic stress histories may contribute to discrepancies across studies of acute stress and sleep. Second, high levels of chronic stress may precipitate sleep continuity disturbances. Sensitivity analyses in the subsample of participants without sleep complaints at baseline revealed robust associations among chronic stress group and PSG-assessed wakefulness after sleep onset. Third, on a day-to-day basis, midlife women may be unaware of the extent to which chronic stress interferes with their sleep. Unlike PSG and reports of sleep quality and insomnia, diary-assessed wakefulness after sleep onset was unrelated to chronic stress group. Finally, stress-related sleep continuity disturbances were uniquely associated with difficulties maintaining sleep, as measured by PSG-assessed wakefulness after sleep onset. Participants in the high chronic stress group did not spend more time in bed, take longer to fall asleep, or wake up more often during the night than did their counterparts in the low and moderate chronic stress groups. Although brief awakenings from sleep are routinely observed in healthy adults, difficulty falling back asleep during the night is characteristic of older adults and patients with insomnia.45 In effect, the sleep continuity profile observed in our sample of midlife women with a high chronic stress burden mirrors that of older adults and patients with insomnia.
The lack of hypothesized differences in TST and SWS may be related to the sleep profile observed in this sample. Given that participants obtained less than 6.5 h of sleep on average and SWS percent averaged less than 4% in the sample as a whole, the absence of group differences in TST and sleep depth may reflect a floor effect. Certainly, chronic stress has been linked to decreased SWS in younger samples.15 That chronic stress group was more strongly associated with subjective sleep quality and insomnia compared to sleep duration and SWS might suggest that these associations merely reflect a negative affective bias, yet our analyses adjusted for symptoms of depression and acute life events at the time of the Sleep Study. Moreover, diary-assessed indices of sleep duration were only modestly associated with chronic stress profiles, whereas no such associations were observed for diary-assessed indices of sleep continuity. It may also be that other indices of physiological arousal during sleep, including increased high-frequency EEG activity and decreased heart rate variability, may be more sensitive to stress than are traditional measures of visually scored sleep. In 1987, Davidson and colleagues reported a positive, linear association between stress-related intrusive thoughts and overnight cortisol levels in the wake of the Three Mile Island nuclear power plant leak.46 We have observed significant associations among symptoms of stress and increased high-frequency EEG activity during NREM sleep in several samples that did not exhibit differences in visually scored sleep.44,47,48 We also demonstrated that an experimental pre-sleep stressor blunted heart rate variability in a sample of healthy, young adults without altering visually scored parameters of sleep.49 The stress and sleep profile observed in the current sample of midlife women is quite similar to insomnia, which may be characterized by subjective sleep complaints and increased indices of physiological arousal during sleep, irrespective of decrements in PSG-assessed sleep duration, continuity, and depth.50
Several study characteristics limit the generalizability of the current findings. First, though our analyses adjusted for many sociodemographic and health characteristics that affect sleep, there may be other relevant predictors of sleep outcomes that we did not anticipate or statistically control for (e.g., genetics, coping style). Furthermore, given our desire to maximize study utility while minimizing participant burden, our assessment of upsetting life events covered a broad range of potential stressors (i.e., school, work, romantic relationships, children, family, criminal and legal matters, finances, and health) on a relatively brief 18-item questionnaire. This precluded evaluation of the differential effect of various types of stressors or stress characteristics. Our assessment of upsetting life events was also limited to a 9-y time span in midlife women. Early life events and/or longer stress chronicity may be stronger determinants of sleep outcomes than the stress trajectories that we assessed in the current study. Our results should not be generalized to other populations in whom stressors and sleep may be differentially related (e.g., men, older or younger age groups, other unrepresented racial/ethnic groups, shift workers). Although not measured in the current study, perceived control, cognitive arousal, avoidance behaviors, and coping style have been linked to disrupted sleep by our laboratory and others.1,43,44 Individual differences in vulnerability to stress-related sleep disturbances and stressor characteristics may be important to consider in future research on the mechanisms and prevention of stress-related sleep disturbances. This latter issue may be especially relevant to insomnia, which is exquisitely sensitive to individual differences in stress and sleep reactivity.1
In conclusion, the current study shows that high chronic stress burden is associated with increased subjective sleep quality complaints, reports of insomnia, and objectively assessed sleep continuity disturbances in midlife women. These associations were independent of preexisting sleep complaints, very upsetting acute life events, and symptoms of depression assessed at the time of the Sleep Study, as well as race, marital status, and BMI. The pattern of results observed in the current sample of midlife women raises the possibility that high levels of chronic stress may accelerate “sleep aging,” or a sleep profile that would be expected in older women. More research is needed to determine whether coping strategies that reduce both the number and the subjective effect of stressful life events lessens the effect of life's “slings and arrows” on sleep and their more downstream effects on health and functioning.
DISCLOSURE STATEMENT
This was not an industry supported study. The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). In addition, support for Drs M. Hall and R. Krafty was provided by HL104607 and GM113243, support for Dr. Casement was provided by MH093605 and MH013511, support for Dr. Troxel was provided by HL093220. Sleep data were processed with the support of UL1TR000005. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Aging, National Institute of Nursing Research, Office of Research on Women's Health or the National Institutes of Health. Dr. Buysse has served as a paid consultant on scientific advisory boards for the following companies: Eisai, Merck, Otsuka, Medscape, and CME Outfitters. Total fees from each of these sources were less than $10,000 per year. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the dedicated study staff at each site and all the women who participated in SWAN Sleep Ancillary Study.
Clinical Centers
University of Michigan, Ann Arbor – Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999– present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office
National Institute on Aging, Bethesda, MD – Winifred Rossi 2012–present; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Coordinating Center
University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001.
Steering Committee
Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- ATC Code
Anatomical Therapeutic Chemical Code
- BMI
body mass index
- D-NAWK
diary-assessed number of awakenings
- D-SE
diary-assessed sleep efficiency
- D-SOL
diary-assessed sleep onset latency
- D-TIB
diary-assessed time in bed
- D-TST
diary-assessed total sleep time
- D-WASO
diary-assessed wake after sleep onset
- EEG
electroencephalogram
- EKG
electrocardiogram
- EMG
electromyogram
- EOG
electro-oculogram
- ISQ
Insomnia Symptom Questionnaire
- N-CTRC
University of Pittsburgh Neuroscience - Clinical and Translational Research Center
- NREM
non-rapid eye movement
- PERI
Psychiatric Epidemiology Research Interview Life Events Scale
- PSG
polysomnography
- PSG-NAWK
PSG-assessed number of awakenings
- PSG-SE
PSG-assessed sleep efficiency
- PSG-SOL
PSG-assessed sleep onset latency
- PSG-TIB
PSG-assessed time in bed
- PSG-TST
PSG-assessed total sleep time
- PSG-WASO
PSG-assessed wake after sleep onset
- PSQI
Pittsburgh Sleep Quality Index
- PSS
Perceived Stress Scale
- REM
rapid eye movement
- SAS TRAJ
Statistical Analysis System Trajectory
- SF-36
Short-form Health Survey
- SWAN
Study of Women's Health Across the Nation
- SWS
slow wave sleep.
REFERENCES
- 1.Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37:1295–304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 3.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–22. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53:741–8. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen HK, Ducharme LJ, Roman PM. Job stress and poor sleep quality: data from an American sample of full-time workers. Soc Sci Med. 2007;64:1997–2007. doi: 10.1016/j.socscimed.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lallukka T, Rahkonen O, Lahelma E, Arber S. Sleep complaints in middle-aged women and men: the contribution of working conditions and work-family conflicts. J Sleep Res. 2010;19:466–77. doi: 10.1111/j.1365-2869.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai K, Nakata A, Ikeda T, Otsuka Y, Kawahito J. Employment type, workplace interpersonal conflict, and insomnia: a cross-sectional study of 37,646 employees in Japan. Arch Environ Occup Health. 2014;69:23–32. doi: 10.1080/19338244.2012.713040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailshire JA, Burgard SA. Family relationships and troubled sleep among U.S. adults: examining the influences of contact frequency and relationship quality. J Health Soc Behav. 2012;53:248–62. doi: 10.1177/0022146512446642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribet C, Derriennic F. Age, working conditions, and sleep disorders: a longitudinal analysis in the French cohort E.S.T.E.V. Sleep. 1999;22:491–504. [PubMed] [Google Scholar]
- 11.Hanson LL, Akerstedt T, Naswall K, Leineweber C, Theorell T, Westerlund H. Cross-lagged relationships between workplace demands, control, support, and sleep problems. Sleep. 2011;34:1403–10. doi: 10.5665/SLEEP.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgard SA, Ailshire JA. Putting work to bed: stressful experiences on the job and sleep quality. J Health Soc Behav. 2009;50:476–92. doi: 10.1177/002214650905000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lange AH, Kompier MA, Taris TW, et al. A hard day's night: a longitudinal study on the relationships among job demands and job control, sleep quality and fatigue. J Sleep Res. 2009;18:374–83. doi: 10.1111/j.1365-2869.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- 14.Akerstedt T, Orsini N, Petersen H, Axelsson J, Lekander M, Kecklund G. Predicting sleep quality from stress and prior sleep--a study of dayto-day covariation across six weeks. Sleep Med. 2012;13:674–9. doi: 10.1016/j.sleep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effects of a major stressful event and its resolution. Psychiatry Res. 1991;39:199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- 16.Kecklund G, Akerstedt T. Apprehension of the subsequent working day is associated with a low amount of slow wave sleep. Biol Psychol. 2004;66:169–76. doi: 10.1016/j.biopsycho.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Nakata A, Haratani T, Takahashi M, et al. Job stress, social support, and prevalence of insomnia in a population of Japanese daytime workers. Soc Sci Med. 2004;59:1719–30. doi: 10.1016/j.socscimed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Linton SJ. Does work stress predict insomnia? A prospective study. Br J Health Psychol. 2004;9:127–36. doi: 10.1348/135910704773891005. [DOI] [PubMed] [Google Scholar]
- 19.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Bastien CH, Vallieres A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2:50–62. doi: 10.1207/s15402010bsm0201_5. [DOI] [PubMed] [Google Scholar]
- 21.Friedman L, Brooks JD, Bliwise DL, Yesavage JA, Wicks DS. Perceptions of life stress and chronic insomnia in older adults. Psychol Aging. 1995;10:352–7. doi: 10.1037//0882-7974.10.3.352. [DOI] [PubMed] [Google Scholar]
- 22.Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med. 2007;5:256–78. doi: 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEwen BS, Stellar E. Stress and the individual. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- 24.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. Oxford, England: John Wiley & Sons; 1988. pp. 629–49. [Google Scholar]
- 25.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal translition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: biology and pathology. London: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 28.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the Peri Life Events Scale. J Health Soc Behav. 1978;19:205–29. [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 32.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 33.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 34.Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. Sleep. 2012;35:1285–91. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–20. [PubMed] [Google Scholar]
- 36.World Health Organization Scientific Group. Geneva, Switzerland: World Health Organization; 1996. Research on the Menopause in the 1990s. [PubMed] [Google Scholar]
- 37.Ware JE, Sherbourne CD. The MOS-36-Item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 38.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 39.Matthews KA, Shumaker SA, Bowen DJ, et al. Women's health initiative. Why now? What is it? What's new? Am Psychol. 1997;52:101–16. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- 40.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4:139–57. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 41.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Soc Meth Res. 2001;29:375–93. [Google Scholar]
- 42.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychol. 2004;23:542–5. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- 44.Hall M, Buysse DJ, Dew MA, Prigerson HG, Kupfer DJ, Reynolds CF. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depress Anxiety. 1997;6:106–12. [PubMed] [Google Scholar]
- 45.Ohayon MM, Lemoine P. [Sleep and insomnia markers in the general population] Encephale. 2004;30:135–40. doi: 10.1016/s0013-7006(04)95423-1. [DOI] [PubMed] [Google Scholar]
- 46.Davidson LM, Fleming R, Baum A. Chronic stress, catecholamines, and sleep disturbance at Three Mile Island. J Human Stress. 1987 Summer;:75–83. doi: 10.1080/0097840X.1987.9936798. [DOI] [PubMed] [Google Scholar]
- 47.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 49.Hall M, Vasko R, Buysse DJ, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]


