Abstract
Study Objectives:
Mitochondrial DNA (mtDNA) copy number is an important component of mitochondrial function and varies with age, disease, and environmental factors. We aimed to determine whether mtDNA copy number varies with habitual differences in sleep duration within pairs of monozygotic twins.
Setting:
Academic clinical research center.
Participants:
15 sleep duration discordant monozygotic twin pairs (30 twins, 80% female; mean age 42.1 years [SD 15.0]).
Design:
Sleep duration was phenotyped with wrist actigraphy. Each twin pair included a “normal” (7–9 h/24) and “short” (< 7 h/24) sleeping twin. Fasting peripheral blood leukocyte DNA was assessed for mtDNA copy number via the n-fold difference between qPCR measured mtDNA and nuclear DNA creating an mtDNA measure without absolute units. We used generalized estimating equation linear regression models accounting for the correlated data structure to assess within-pair effects of sleep duration on mtDNA copy number.
Measurements and Results:
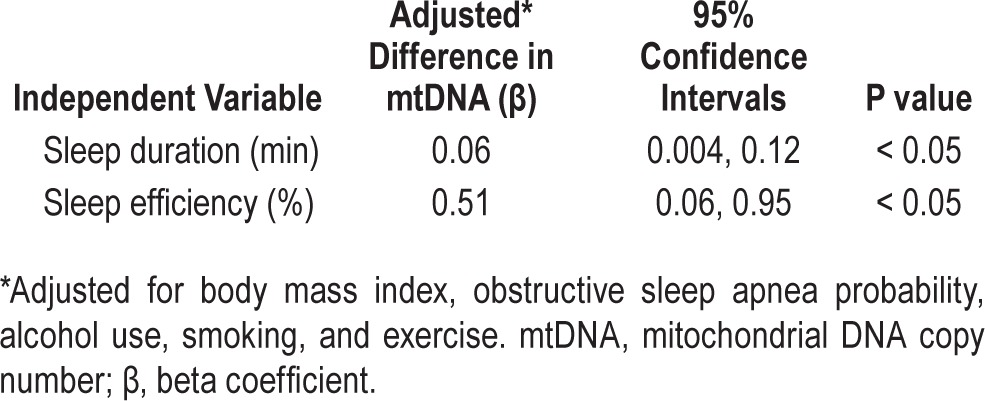
Mean within-pair sleep duration difference per 24 hours was 94.3 minutes (SD 62.6 min). We found reduced sleep duration (β = 0.06; 95% CI 0.004, 0.12; P < 0.05) and sleep efficiency (β = 0.51; 95% CI 0.06, 0.95; P < 0.05) were significantly associated with reduced mtDNA copy number within twin pairs. Thus every 1-minute decrease in actigraphy-defined sleep duration was associated with a decrease in mtDNA copy number of 0.06. Likewise, a 1% decrease in actigraphy-defined sleep efficiency was associated with a decrease in mtDNA copy number of 0.51.
Conclusions:
Reduced sleep duration and sleep efficiency were associated with reduced mitochondrial DNA copy number in sleep duration discordant monozygotic twins offering a potential mechanism whereby short sleep impairs health and longevity through mitochondrial stress.
Citation:
Wrede JE, Mengel-From J, Buchwald D, Vitiello MV, Bamshad M, Noonan C, Christiansen L, Christensen K, Watson NF. Mitochondrial DNA copy number in sleep duration discordant monozygotic twins. SLEEP 2015;38(10):1655–1658.
Keywords: mitochondrial DNA, copy number, twins, sleep discordance, sleep duration
INTRODUCTION
Mitochondrial DNA (mtDNA) is integral to mitochondrial function and vital to cellular energy production. Properly functioning mitochondria are crucial to healthy human physiology.1–3 Unlike nuclear DNA, double-stranded circular mtDNA molecules are present in multiple copies per mitochondrion and can respond to insult by increasing mtDNA copy number to support normal mitochondrial functioning.4–6 Lack of protective histones and limited repair capacity make mtDNA fragile and susceptible to insults that can overwhelm its replicative machinery or stimulate mitochondrial fusion causing mtDNA levels to vary. Whether mtDNA copy number increases or decreases following an insult depends upon the nature of the damaging process involved including multiple forms of cancer, toxin exposures, aging, and oxidative stress.7–13
Sleep curtailment is a wide-spread problem in Western societies with myriad untoward effects on health including increased risk of coronary heart disease, ischemic stroke, hypertension, insulin resistance, obesity, and cognitive impairment.14–21 Short sleep induces oxidative stress in animal models22 suggesting that sleep duration could influence mtDNA copy number, but no studies have examined this relationship. Because sleep duration and mtDNA copy number are heritable,23,24 a co-twin study design method that accounts for familial factors (e.g., genetics and shared environment) can substantially increase study power to detect subtle environmental effects, such as the impact of short sleep on mtDNA copy number. The aim of this study was to compare mtDNA copy number between sleep duration discordant monozygotic co-twins with one twin reporting a normal amount of sleep (7–9 h/24) and the other a short sleeper (< 7 h/24). Demonstrating a significant association between copy number and sleep duration would be a first step toward elucidating the mechanisms by which short sleep contributes to untoward health outcomes.
METHODS
Participant Selection
Participants were monozygotic twins identified through the University of Washington Twin Registry.25 All twins enrolled in the Registry complete a health questionnaire which includes questions about usual sleep duration. Based on that questionnaire and a study-specific phone interview, we identified twin pairs with self-reported average sleep duration discordance ≥ 1 hour per night. We excluded twins with a history of sleep illness, a high risk for sleep apnea on the Multivariable Apnea Prediction Index,26 and shift workers. Participating twins provided a fasting blood sample at the completion of a 14-day actigraphy monitoring period. Collected DNA was purified and frozen within 24 hours. A total of 15 twin pairs (30 individuals) ≥ 45 min discordant in sleep duration per 24 h, with one twin sleeping 7–9 h and the other sleeping < 7 h/24 h, underwent the final mtDNA copy number analysis.
Sleep Duration Phenotyping
All twins were monitored concurrently for 14 days with the Actiwatch-2 actigraph (Philips Respironics, Murrysville, PA, USA) worn on the non-dominant wrist, starting at 17:00 of the first day of the research protocol. To facilitate actigraph record scoring, each twin completed a sleep diary concurrently for the entire 14 days. Actigraphy differentiates sleep from wake with good agreement to polysomnography.27–29 Actigram analysis was performed using Respironics Actiware 5.57 software (Philips, Respironics, Murrysville, PA, USA) and previously described protocols.30 All actigraphy records were scored by the same individual. The senior investigator (NFW) assessed 10% of the records to ensure actigraphy scoring consistency. We utilized the patient's corresponding sleep diary to assess bed times, wake times, and nap times. We also used event marker button data and photo-optic light data to determine wake and sleep. Total sleep time included all sleep through the 24-h day, including naps. Sleep onset was defined by marked reduction in activity counts within 15 min of sleep diary defined bedtime. This determination was further informed by drop in lux levels and event marker data. Sleep efficiency included sleep latency as per standard protocol. Wake time was defined by a marked increase in activity counts within 15 min of sleep diary defined wake time, which was also informed by increasing lux levels and event marker data.
Quantification of Mitochondrial DNA Copy Number
DNA was extracted from peripheral blood leukocytes (PBL) in whole blood using Qiagen Puregene standard protocols. An assay based on real-time polymerase chain reaction and SYBR Green technology was adapted as a measure of the amount of mitochondrial DNA compared to nuclear DNA. Each DNA sample was assayed in triplicate in an identical procedure to that described by Mengel-From et al.11 (see supplemental materials for additional details).
Statistical Methods
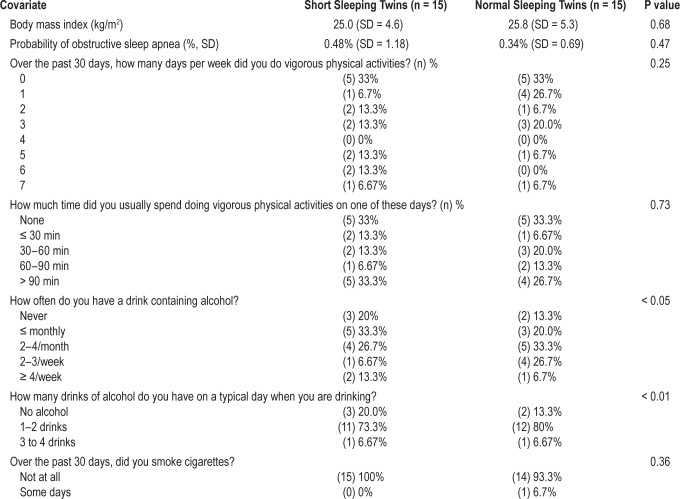
Descriptive statistics were calculated using mean and standard deviation for continuous variables and percentages for categorical variables. Comparison of covariates between sleep duration groups utilized generalized estimating equation (GEE) linear regression for continuous, ordinal, and dichotomous variables using robust standard error estimates. We explicitly modeled within-pair effects of sleep duration on mtDNA copy number; the within-pair effect is of particular interest since this controls for genetic and common environmental influences shared by twins within a pair. For this analysis, we generated a within pair mean sleep duration and then subtracted this value from the measured sleep duration for each twin, thus generating the individual difference from the within-pair mean for each twin, which became the within-pair GEE linear regression independent variable.31
For multivariate within-pair GEE regression models the independent variables were actigraphy defined sleep duration and sleep efficiency. The dependent variable remained mtDNA copy number. Results are presented as regression generated β coefficients and 95% confidence intervals. All analyses were adjusted for body mass index, sleep apnea probability, alcohol use, smoking, and physical exercise levels (Table S1, supplemental material).
Table S1.
Analysis covariates by sleep duration group.
RESULTS
Study Population
An initial pool of 1,283 monozygotic twin pairs was identified from the Registry based on the enrollment questionnaire. These twins were sent an invitation letter if their subjective sleep duration was discordant by at least 1 hour. One-hundred fifteen twin pairs replied to this invitation and were interviewed by phone to confirm ongoing sleep discordance. Of those, 52 twin pairs met criteria, were enrolled, and underwent actigraphy for 2 weeks, followed by blood draw and DNA extraction. After actigraphy results were analyzed, 30 twins (15 pairs) were included who met criteria for 1 short sleeping twin (< 7 h/24) and 1 normal sleeping (7–9 h/24) twin in each pair.
This final study group included 80% female participants. The mean age was 42.1 years (standard deviation [SD] 15.0, range 20–71). Participants were predominantly of European ancestry based on self-report (90%). For educational level, 70% had a college degree or higher, 20% had an Associate's degree or some college, and 10% were high school graduates.
Actigraphy
Sleep-related characteristics as determined by actigraphy included a mean sleep duration among all participants of 418.9 min (SD 63.7, range 190.5–499.4). The mean within-pair sleep duration difference was 94.3 min (SD 62.6, range 45.9–300.3). The mean sleep efficiency among all participants was 87.4% (SD 9.8, range 39.7%–95.5%). The mean within-pair sleep efficiency difference was 6.5% (SD 11.8, range 0.12%–48.3%).
mtDNA Analysis
The mean PBL mtDNA copy number was 63.8 (SD 17.7, range 24.7–110.3). We found a significant overall effect of sleep duration on mtDNA copy number (Table 1), such that every 1-minute decrease in actigraphy-defined sleep duration was associated with a decrease in mtDNA copy number of 0.06. Likewise, a 1% decrease in actigraphy-defined sleep efficiency was associated with a decrease in mtDNA copy number of 0.51. Prior to adjustment for covariates, the beta coefficient was in the same direction of effect, but did not reach statistical significance.
Table 1.
Sleep duration and sleep efficiency are positively associated with peripheral blood leukocyte mitochondrial DNA copy number.
DISCUSSION
We demonstrate that reduced sleep duration and sleep efficiency are associated with decreased mtDNA copy number within monozygotic twin pairs. Many factors, including oxidative stress, have been shown to reduce mitochondrial fission, increase mitochondrial fusion, or otherwise decrease mtDNA copy number. In rodent models, sleep deprivation produces oxidative stress22 and is associated with mitochondrial dysfunction,32 suggesting that chronic sleep curtailment in human subjects might be associated with changes in mtDNA copy number. While further investigation is required to more fully define this relationship, this finding provides a potential molecular pathway by which short sleep results in poor health.
Peripheral blood mtDNA copy number declines with age and decreased number correlates with poorer health outcomes in the elderly.10,11 Short sleep is also associated with increased morbidity and mortality.33 Could decreased mtDNA copy number induced by short sleep duration be a mechanism that explains the association between short sleep and increased mortality? mtDNA copy number has been proposed as a potentially useful clinical marker because it can predict severity of neoplastic processes including gastric cancer34 and forecast lung cancer risk.35 This raises questions as to whether changes in mtDNA copy number predict other health consequences of sleep curtailment such as cardiovascular disease or diabetes. Could mtDNA copy number be a biomarker of sleep curtailment?
In our study, reduced sleep efficiency was also associated with reduced mtDNA copy number. This could reflect short sleep duration, since low sleep efficiency is typically associated with reduced sleep duration. To further explore this relationship we assessed a multivariate model that included both sleep duration and sleep efficiency. However, collinearity artificially inflated the standard errors mitigating effects and obviating conclusions (data not shown). Another possibility is that reduced sleep efficiency represents overall sleep disruption. If so, this raises the possibility that other conditions that disrupt sleep—obstructive sleep apnea, insomnia, narcolepsy—may also be associated with reduced mtDNA copy number. Future studies should assess mitochondrial health and functioning in these sleep illnesses.
Our study examined habitual patterns of sleep in the twins' home setting and, therefore, offers insights that differ from those garnered by acute changes in sleep patterns obtained in controlled laboratory conditions. This implies strong ecological validity because the mitochondrial physiology of our twins likely had time to compensate for the chronic impact of short sleep duration. Another strength of our study was the use of monozygotic twins which controlled for genetics and early life shared family environment. Our sample was derived from the community and not from a clinical population seeking healthcare, which increases the generalizability of the results. However, a notable limitation is that our participants were predominantly younger adult women of European ancestry, and therefore our results should be applied to the general population with caution. Another limitation is that this was an exploratory pilot study with small sample size, and results warrant confirmation by further research.
Our results did not reach statistical significance without adjustment for covariates. Covariates were determined a priori, as established factors which either influence mtDNA copy number directly, or through known associations with oxidative stress. A recent study by Kim et al.36 showed an association between sleep apnea and decreased mtDNA copy number. Exercise is well known to increase mtDNA copy number as observed in recent studies of mouse muscle37 and peripheral blood of older women.38 A component of cigarette smoke, poly-cyclic aromatic hydrocarbons, is associated with increased PBL mtDNA copy number,39 and cigarette smoke exposure increases mtDNA copy number in esophageal tissues.40 Similarly, alcohol has been shown to increase mtDNA mutagenesis in blood.41 Higher BMI is associated with decreased mtDNA copy number.42 Many of these same factors (especially BMI, exercise, smoking, and alcohol consumption) were adjusted as confounding variables in these studies. Given that these factors are all known to influence mtDNA copy number, we feel adjustment increases the validity of our association between sleep duration and mtDNA copy number.
It is important to note that mtDNA numbers are currently not directly comparable between studies using different protocols for mtDNA extraction and data handling, including the use of ratios or transformed data versus untransformed data. In our study, we utilized a procedure identical to that of Mengel-From et al.11 using untransformed data, in which similar levels of mtDNA copy number were measured in individuals with similar ages when compared to our data.
In conclusion, we found that short sleep and reduced sleep efficiency were associated with reduced mtDNA copy number within sleep duration-discordant monozygotic twin pairs. Considering the importance of mitochondria to human physiology, and the association of short sleep with numerous untoward health outcomes, our findings offer intriguing evidence of a potential mechanism by which sleep curtailment is associated with poor health.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grants K23HL083350, P30NR011400, RC2HL103416, a Northwest Institute of Genetic Medicine Resource Access Grant, a University of Washington General Clinical Research Center Pilot Grant, and grants from the Oda and Hans Svenningsens Foundation and Dagmar Marshalls Foundation. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the twins for their participation and the staff of the University of Washington Twin Registry, in particular Ally Avery and Linda Floyd.
SUPPLEMENTAL MATERIAL
METHODS
Quantification of Mitochondrial DNA Copy Number
An assay based on real-time polymerase chain reaction (qPCR) and SYBR Green technology was adapted as a measure of the amount of mitochondrial DNA compared to nuclear DNA. One 153bp PCR was targeted to the mitochondrial NADH dehydrogenase (ND1) gene using the primer sequences 5'-AACATACCCATGGCCAACCT-3' for the forward primer and 5'-AGCGAAGGGTTGTAGTAGCCC-3' for the reverse primer. To quantify the amount of mtDNA another 268bp PCR targeted to the nuclear Beta-globin (BG) the primer sequences were for the forward primer 5'-GAAGAGCCAAGGACAGGTAC-3' and the reverse primer 5'-CAACTTCATCCACGTTCACC-3'. All primer designs were originally published by Liu CS and co-workers43 and applied in the later study.44 The assay was adapted to a high through-put 96-plate format using a StepOne instrument and SYBR Green technology (Applied Biosystems). The reactions were performed in a total volume of 10 μl including 1x Fast SYBR Green Master Mix, 5μM of each of the primers and 2 ng of DNA. The amplification was preheated at 95°C at 30 sec followed by a 40 cycle program of 0.3 sec at 95°C, 15 sec at 58°C and 30 sec at 72°C. The assay was calibrated using a serial dilution of 10 ng, 5 ng, 2.5 ng, 1.25 ng, and 0.625 ng DNA and a straight linear correlation was observed with an R2 of 0.996 and was used to set the threshold cycle number (Ct) of both the nuclear and the mitochondrial genes. Each DNA sample was assayed in triplicate using either the ND1 primers or BG primers in parallel reactions. For each 96-plate, a DNA control sample from the same individual and a “no template control” was added in triplicate. All samples were above our quality criteria of low or no amplification (CT > 31) in any of the 6 amplifications. The six amplifications were 3 real-time PCR amplifications for each sample with the primers for ND1 and BG amplification, respectively. The median of the values were used to reduce variance from potential outliers. The mtDNA copy number was calculated as the relative number of mtDNA to the nucleus BG gene by the formula 2(CtBG median − CtND median) as described elsewhere44 except we did not conduct log transformation, thus measuring the n-fold difference in relation to a nuclear diploid gene.
REFERENCES
- 1.Leem J, Koh EH. Interaction between mitochondria and the endoplasmic reticulum: implications for the pathogenesis of type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:242984. doi: 10.1155/2012/242984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang JM, Xu HY, Zhang XJ, Li X, Zhang HB, Ge PF. Role of mitochondrial function in the protective effects of ischaemic postconditioning on ischaemia/reperfusion cerebral damage. J Int Med Res. 2013;41:618–27. doi: 10.1177/0300060513476587. [DOI] [PubMed] [Google Scholar]
- 3.Raimundo N. Mitochondrial pathology: stress signals from the energy factory. Trends Mol Med. 2014;20:282–92. doi: 10.1016/j.molmed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Moraes CT. What regulates mitochondrial DNA copy number in animal cells? Trends Genet. 2001;17:199–205. doi: 10.1016/s0168-9525(01)02238-7. [DOI] [PubMed] [Google Scholar]
- 5.Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36:125–31. doi: 10.1016/S1673-8527(08)60099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babbar M, Sheikh MS. Metabolic stress and disorders related to alterations in mitochondrial fission or fusion. Mol Cell Pharmacol. 2013;5:109–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Yu M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011;89:65–71. doi: 10.1016/j.lfs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JN, Leung MC, et al. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma DR, Sunkaria A, Wani WY, et al. Aluminium induced oxidative stress results in decreased mitochondrial biogenesis via modulation of pgc-1alpha expression. Toxicol Appl Pharmacol. 2013;273:365–80. doi: 10.1016/j.taap.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Chu HT, Hsiao WW, Tsao TT, et al. Quantitative assessment of mitochondrial DNA copies from whole genome sequencing. BMC Genomics. 2012;13(Suppl 7):S5. doi: 10.1186/1471-2164-13-S7-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–59. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Kafaji G, Golbahar J. High glucose-induced oxidative stress increases the copy number of mitochondrial DNA in human mesangial cells. Biomed Res Int. 2013;2013:754946. doi: 10.1155/2013/754946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012;13:909–15. doi: 10.1038/embor.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 15.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the united states. Soc Sci Med. 2010;71:1027–36. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–92. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first national health and nutrition examination survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun DA. Sleep and hypertension. Chest. 2010;138:434. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 21.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57:649–55. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–90. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6:11–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Xing J, Chen M, Wood CG, et al. Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst. 2008;100:1104–12. doi: 10.1093/jnci/djn213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strachan E, Hunt C, Afari N, et al. University of Washington twin registry: poised for the next generation of twin research. Twin Res Hum Genet. 2013;16:455–62. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 27.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/ wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 28.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 29.Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep. 1982;5:389–99. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- 30.Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. J Clin Sleep Med. 2013;9:217–25. doi: 10.5664/jcsm.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591–602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 32.Yang RH, Hu SJ, Wang Y, Zhang WB, Luo WJ, Chen JY. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res. 2008;1230:224–32. doi: 10.1016/j.brainres.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Luyster FS, Strollo PJ, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Qu Y, Dang S, Yang Q, Shi B, Hou P. Variable copy number of mitochondrial DNA (mtdna) predicts worse prognosis in advanced gastric cancer patients. Diagn Pathol. 2013;8:173. doi: 10.1186/1746-1596-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosgood HD, 3rd, Liu CS, Rothman N, et al. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis. 2010;31:847–9. doi: 10.1093/carcin/bgq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YS, Kwak JW, Lee KE, et al. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid Redox Signal. 2014;21:1285–8. doi: 10.1089/ars.2014.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao X, Zhao ZW, Zhou HY, Chen GQ, Yang HJ. Effects of exercise intensity on copy number and mutations of mitochondrial DNA in gastrocnemus muscles in mice. Mol Med Rep. 2012;6:426–8. doi: 10.3892/mmr.2012.913. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Ko JH, Lee DC, Lim I, Bang H. Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause. 2012;19:1109–15. doi: 10.1097/gme.0b013e3182503e97. [DOI] [PubMed] [Google Scholar]
- 39.Pavanello S, Dioni L, Hoxha M, Fedeli U, Mielzynska-Svach D, Baccarelli A. Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 22:1722–9. doi: 10.1158/1055-9965.EPI-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin C, Wang L, Chou T, et al. Cigarette smoking and hogg1 ser326cys polymorphism are associated with 8-ohdg accumulation on mitochondrial DNA in thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2013;20(Suppl 23):S379–88. doi: 10.1245/s10434-012-2576-z. [DOI] [PubMed] [Google Scholar]
- 41.von Wurmb-Schwark N, Ringleb A, Schwark T, et al. The effect of chronic alcohol consumption on mitochondrial DNA mutagenesis in human blood. Mutat Res. 637:73–9. doi: 10.1016/j.mrfmmm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Lee DC, Im JA, Lee JW. Mitochondrial DNA copy number in peripheral blood is independently associated with visceral fat accumulation in healthy young adults. Int J Endocrinol. 2014;2014:586017. doi: 10.1155/2014/586017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CS, Tsai CS, Kuo CL, et al. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37:1307–17. doi: 10.1080/10715760310001621342. [DOI] [PubMed] [Google Scholar]
- 44.Lee JW, Park KD, Im JA, Kim MY, Lee DC. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta. 2010;411:592–6. doi: 10.1016/j.cca.2010.01.024. [DOI] [PubMed] [Google Scholar]