Abstract
Background
Aminoglycosides (AG) are a common cause of acute kidney injury (AKI) in CF patients. This study aimed to identify factors associated with AKI during intravenous AG courses in this population.
Methods
Matched case-control study utilizing two independent cohorts of hospitalized CF patients receiving ≥3 days of intravenous AG at Cincinnati Children's and Children's of Alabama. All admissions with AKI (cases, N=82) were matched to 2 randomly selected admissions without AKI (controls, N=164) by center, gender, and age +/-3 years of case. AKI was defined as an increase in serum creatinine (SCr) 1.5 times baseline or by 0.3 mg/dL within 48h. Admissions with AKI before day 4 or without at least weekly SCr monitoring were excluded. Simple and multiple conditional logistic regression compared factors among cases and controls.
Results
Multivariable analysis identified receipt of an AG within 90 days prior to admission, longer AG duration, low serum albumin, and receipt of TMP/SMX as independent risk factors for developing AKI. Infection with Staphylococcus aureus diminished the odds of developing AKI.
Conclusions
This study identifies risk factors contributing to aminoglycoside-associated AKI in CF patients. These findings can be used to anticipate high-risk scenarios and limit AKI in CF clinical care.
Keywords: acute renal failure, adverse drug events, antibiotics, drug-induced nephrotoxicity, pediatrics
INTRODUCTION
Acute kidney injury (AKI) is a known side effect of aminoglycoside (AG) antibiotics and has been shown to occur from direct effects on the renal proximal tubule epithelial cells by the medication.[1] Aminoglycosides have good activity against many Gram-negative bacteria, including Pseudomonas aeruginosa, and are frequently used in the management of pulmonary exacerbations in cystic fibrosis (CF). AKI develops during an AG course in 24-30% of non-critically ill children receiving ≥ 5 consecutive days of the medication and is associated with increased hospital costs and prolonged length of stay.[2] Importantly, children and adults who sustain AKI are at increased risk for long-term morbidity, mortality and the development of chronic kidney disease.[3-8]
Previous studies have examined the relationship between AG use and nephrotoxicity in primarily adult and critically-ill patients.[9-20] Risk factors, such as hypoalbuminemia, increased AG exposure (duration and doses), and concomitant receipt of other nephrotoxic medications, have been described in these non-CF populations. However, these factors have not been consistently reported from one patient population to another and may not apply to younger CF patients. While AG therapy has been implicated as a primary risk factor for AKI in patients with CF,[21-23] no study has sought specifically to identify other factors that may contribute to the risk of AG-associated AKI in pediatric CF.
Repeated courses of intravenous (IV) AG in CF patients may be associated with long-term kidney damage.[24, 25] With a paucity of suitable alternative antibiotic choices, it is imperative to 1) understand the factors which contribute to an increased risk of AKI from AG use in patients with CF and 2) develop effective methods to decrease injury associated with each individual exposure. We conducted a matched case-control analysis to identify risk factors for AG-associated AKI in hospitalized pediatric CF patients in order to define high risk scenarios that might benefit from reno-protective strategies.
METHODS
Study design, setting, and participants
This retrospective two-center matched case-control study was conducted at Cincinnati Children's Hospital Medical Center (CCHMC, Cincinnati, Ohio) and Children's of Alabama (COA, Birmingham, Alabama). All individual admissions were eligible for inclusion if the CF patient was admitted to a noncritical care unit of the hospital for a pulmonary exacerbation and received at least 3 consecutive days of an IV AG antibiotic (gentamicin, tobramycin, or amikacin). Recurrent hospitalizations could be included if the repeat admission occurred least 7 days following completion of the previous antibiotic course. Individuals with a history of lung transplantation or hospitalizations with insufficient renal monitoring (less than weekly serum creatinine (SCr) measurement) were excluded from study. Hospitalizations complicated by early-onset AKI (within 72 hours of the start of AG therapy) were also excluded, as this was felt to possibly reflect a separate group in which different factors precedent to hospitalization and unrelated to AG receipt may have contributed to early renal injury.
Cases were defined as any subject admission during which AKI developed according to Kidney Disease – Improving Global Outcomes (KDIGO) criteria[26] on or after day 4 of AG therapy (see Definitions). All cases were included in the study. Individual admissions in which AKI was not detected during AG therapy were eligible to be controls and were matched to each case according to gender, age (+/- 3 years), and center at which care was provided (CCHMC or COA). Two controls were randomly selected per case. A specific subject could contribute separate admissions as cases or controls depending on whether AKI developed, but could not provide both case and control admissions within the same matched group. This study was approved by the Committees for the Protection of Human Subjects with a waiver of informed consent at both institutions.
Data collection
Data were collected from two independent CF cohorts receiving IV AG therapy at CCHMC and at COA using a common data abstraction form. The CCHMC study population included subjects admitted from January 10, 2010 through June 30, 2012, while the COA study population included subjects admitted from August 3, 2009 through April 28, 2011. The medical records of all patients receiving an IV AG in the hospital for >48 hours were reviewed to determine eligibility and identify those who met case and control definitions. Each subject's hospital medical record was reviewed for information pertaining to demographics, past medical history, laboratory assessments, and hospital management. Two sets of respiratory cultures were reviewed (on admission and most recent prior to admission). Spirometry performed within the 6 months prior to and at the time of hospital admission was reviewed. Data related to administration of the aminoglycoside (drug, dosing regimen, duration), concurrent antibiotics and nephrotoxic medications were collected.
Study definitions
Acute kidney injury was defined based on changes in SCr, according KDIGO criteria:[26] a rise in SCr by ≥0.3 mg/dL within 48 hours or an increase in SCr ≥1.5 times baseline. Baseline SCr was defined as the lowest value obtained within 3 months prior to and following admission. In order to avoid situations in which a very small SCr rise was adjudicated as AKI (for example, a change from 0.3 to 0.5 mg/dL) the SCr value had to be at least 0.6 mg/dL to be considered AKI. AKI occurring on/after day 4 of an AG course was defined as AG-associated AKI (case definition). KDIGO urine output criteria for AKI were not used since urine volume measurements are not routinely obtained during cystic fibrosis patient admissions and drug-induced AKI is usually non-oliguric in nature.
A history of underlying kidney disease included a history of any of the following conditions: diabetic nephropathy, kidney stones, or documented diagnosis of chronic kidney disease. Prior AKI was identified via review of all available SCr data for included subjects. Recent AG receipt was defined as IV AG administration within 90 days of hospital admission. Hypoalbuminemia was considered an albumin level below 3.5 g/dL[27, 28] obtained within 48 hours of admission. Anemia was defined according to World Health Organization criteria for age and gender.[29] Underweight was categorized as a body mass index (BMI) below the 5th percentile for age and gender for children <20 years of age according to the Center for Disease Control and Prevention growth curves[30] or less than 18.5 for adults. Vancomycin, amphotericin, penicillins, cephalosporins, antivirals, non-steroidal anti-inflammatory medications, calcineurin inhibitors, loop diuretics, angiotensin converting enzyme inhibitors, and computed tomography contrast were classified as nephrotoxins.[7, 31] Concomitant nephrotoxic medication use was defined to be present if given ≥24 hours of the AG treatment course.
Baseline forced expiratory volume in one second (FEV1) was defined as the highest value within the 6 months prior to admission. The change in FEV1 from baseline to admission was calculated as the absolute difference between these two values. Baseline estimated glomerular filtration rate (eGFR) was calculated using established formulae for children (<18 years of age) and adults. Subject height at start of AG therapy and baseline SCr were used to calculate eGFR among children using the Schwartz equation,[32] while eGFR was derived using baseline SCr, age, gender, and race among adults according to the Modification of Diet in Renal Disease (MDRD) equation.[33] Due to the matched nature of the study, eGFR was calculated using the same equation for cases and matched controls regardless of age of the control.
Statistical analysis
Data were collected and entered independently at the two centers into a single electronic database for data management and analysis using REDCap electronic data capture tools hosted at CCHMC.[34] Statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, TX).
Categorical variables were described using frequencies and percentages while continuous variables were described using means with standard deviations or medians with interquartile ranges, as appropriate. Unadjusted analyses using simple conditional logistic regression compared variables among cases and matched controls. Antibiotics were analyzed individually and by antibiotic class if more than one agent among a class had been administered among the entire study population. Antibiotics with activity against methicillin-resistant Staphylococcus aureus were grouped as vancomycin, trimethoprim-sulfamethoxazole (TMP-SMX), and other (clindamycin, linezolid, doxycycline) due to the different nephrotoxic potential of these medications. Inhaled antibiotics with potential nephrotoxic effects (tobramycin and colistin) were grouped together for analysis; use of inhaled aztreonam was not assessed.
A stepwise, multiple conditional logistic regression model was constructed to determine the independent effects of clinical variables on the development of AG-associated AKI. Variables with a p-value <0.2 on univariate analysis were considered for inclusion in the final multivariable model; factors with an adjusted p-value <0.05 remained in the final model. The change in FEV1 from baseline to admission was not assessed in multivariable analyses because complete data were available for only 80% of cases and missing data was not assumed to be random (sicker patients were less likely to have spirometry performed on admission).
To examine whether repeated measurements (admissions) among individual subjects affected the results, we performed several additional analyses. First, to investigate whether admissions were statistically independent, a mixed effects logistic regression model tested the significance of subject level clustering in the final multivariate model. Next, a subset of cases and controls was derived in which one admission per subject was randomly selected from each group. Subject-specific risk factors (those listed below in Table 1, as well as culture results) were compared among the single admission subset and total study population using a two-sample test of proportions, t-test or Mann-Whitney U test for categorical, normally- and non-normally distributed continuous variables, respectively. The inclusion of multiple admissions was considered to have a significant impact on a variable if: a) there was a significant (p<0.05) change in the proportion or distribution of the variable among cases or controls, or b) the p-value derived from univariate analysis of cases and controls was significant (p<0.05) among the single admission subset but not among the entire study population.
Table 1.
Unadjusted analyses of potential subject-related risk factors for aminoglycoside-associated acute kidney injury.
| Variable | Cases (n=82) | Controls (n=164) | Odds ratio (95% CI)a | p-value |
|---|---|---|---|---|
| Demographics and medical history | ||||
| Medical center, n (%) | ||||
| Children's of Alabama | n=52 (63.4%) | 104 (63.4) | N/A | N/A |
| CCHMC | 30 (36.6) | 60 (36.6) | ||
| Age in years, median (IQR) | 17.4 (14.9-19.6) | 17.7 (15.0-19.6) | 1.06 (0.87-1.28) | .56 |
| African-American race | 5 (6.1) | 2 (1.2) | 5.00 (0.97-25.8) | .05 |
| CF-related diabetes | 55 (67.1) | 105 (64.0) | 1.19 (0.63-2.25) | .59 |
| F508del mutation | 77 (93.9) | 148 (90.2) | 1.67 (0.59-4.77) | .34 |
| Number of admissions in previous 5 years, median (IQR) | 15 (7-22) | 12 (4-20) | 1.02 (0.99-1.04) | .24 |
| Prior AKI | 64 (78.1) | 103 (62.8) | 2.06 (1.11-3.80) | .02 |
| Body mass index, median (IQR) | 18.8 (17.4-21.0) | 19.2 (17.6-20.7) | 0.98 (0.88-1.08) | .64 |
| Underweightb | 19 (23.2) | 25 (15.2) | 1.74 (0.87-3.49) | .12 |
| AG therapy within 90 days of AG start | 52 (63.4) | 76 (46.3) | 2.44 (1.28-4.65) | .007 |
| Receipt of inhaled antibiotics within 30 days of AG start | 47 (57.3) | 90 (54.9) | 1.11 (0.64-1.91) | .71 |
| Receipt of azithromycin within 30 days of AG start | 68 (82.9) | 131 (79.9) | 1.27 (0.60-2.67) | .53 |
| Receipt of IV antibiotics within 30 days of AG start | 19 (23.2) | 31 (18.9) | 1.32 (0.67-2.61) | .42 |
| Receipt of ≥1 nephrotoxic medication within 30 days of AG start | 24 (29.3) | 35 (21.3) | 1.56 (0.83-2.92) | .16 |
| Baseline SCr in mg/dL, mean (SD) | 0.55 (0.13) | 0.59 (0.15) | 0.79 (0.65-0.96)c | .02 |
| Baseline eGFR in mL/min/1.73 m2 | ||||
| Case age < 18 years, median (IQR)d | 114 (107-152) | 112 (98-133) | 1.08 (0.98-1.18) | .10 |
| Case age ≥ 18 years, median (IQR)d | 160 (132-189) | 143 (114-161) | 1.11 (1.01-1.22) | .02 |
| Labs at initiation of AG therapy | ||||
| Albumin <3.5 g/dL | 19 (23.2) | 16 (9.8) | 2.63 (1.29-5.38) | .008 |
| Abnormal hepatic profilee | 36 (43.9) | 61 (37.2) | 1.36 (0.77-2.41) | .29 |
| Anemia for age | 29 (35.4) | 40 (24.4) | 1.75 (0.96-3.19) | .07 |
| Spirometry testingf | ||||
| Baseline FEV1, median (IQR) | 73 (55-89) | 74 (48-89) | 1.01 (0.89-1.15) | .85 |
| Admission FEV1, median (IQR) | 55 (46-69) | 60 (39-74) | 1.03 (0.88-1.22) | .84 |
| Change in FEV1 from baseline to admission, median (IQR) | 16 (7-27) | 11 (5-20) | 1.29 (1.01-1.65) | .05 |
Odds ratios and p-values derived by simple conditional logistic regression. Odds ratio >1 signifies increased odds of AKI during admission, <1 signifies decreased odds of AKI.
Underweight defined as a body mass index (BMI) below the 5th percentile for age and gender for subjects <20 years of age or less than 18.5 if ≥20 years.
Odds ratio of AKI for every 0.1 mg/dL increase in baseline SCr.
eGFR was calculated using same equation (Schwartz or Modification of Diet in Renal Disease) for matched controls as case regardless of age of control subject. Odds ratios presented per 10 mL/min/1.73 m2 increase in eGFR.
Defined as AST, ALT, or total bilirubin above the upper normal limits for age.
Baseline FEV1 compared among 78 cases and 154 matched controls. Admission FEV1 compared among 65 cases and 114 matched controls. Change in FEV1 compared among 63 cases and 109 matched controls. Odds ratios presented per 10% change in FEV1.
RESULTS
During the stated time periods, we identified 593 pediatric hospital admissions in which IV aminoglycosides were used for treatment of a CF exacerbation (n = 199 at CCHMC, n = 394 at COA). AKI occurred in 131 (22%) of these, of which 49 occurred within 72 hours of admission (deemed early-onset AKI and excluded from further study). Figure 1 displays a flow chart of the remaining 82 admissions that met the study definition of AG-associated AKI. Fifty-four unique subjects sustained AKI during the study, of which 35 (75%) had a single AKI episode, 12 (22%) had 2 AKI episodes, 5 (9%) had 3 episodes and 2 (4%) had 4. Ninety-one unique patients contributed hospital admissions which comprised the 164 matched controls for this study. Among subjects contributing admissions as controls (n = 91), 53 contributed a single control admission, 18 contributed two, and 20 contributed 3 or more. Overall, the 246 hospital admissions were derived from 110 unique subjects: 19 subjects contributed admissions as cases only, 56 as controls only, and 35 as both cases and controls.
Figure 1.
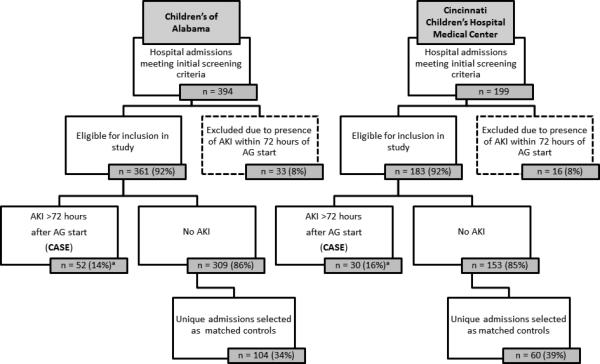
Flow chart of intravenous aminoglycoside courses administered among hospitalized cystic fibrosis patients.
a The proportion of eligible hospital admissions meeting the case definition at the two centers were similar (two-sample test of proportions: p = 0.54).
Table 1 presents the unadjusted associations of clinical factors with AG-associated AKI. Fifty-one percent of both cases and controls were male. Cases more often had recent AG exposure (< 90 days) and prior AKI. Cases were more likely to have low albumin and anemia on admission. Baseline SCr was higher among controls. Complete baseline and admission spirometry data were available for 66 cases (80%) and 114 of their matched controls (70% of all controls): the decline in FEV1 from baseline to admission was greater in cases than controls (Cases: median change 16% [IQR 7-27], Controls: median 11% [IQR 5-20]; p < 0.05). No differences in the prevalence underlying kidney disease were present between groups (Cases: 20%, Controls: 20%; p = 0.91). Receipt of oral (Cases: 28%, Controls: 33%; p = 0.40), IV (Cases: 23%, Controls 19%; p = 0.37), and inhaled (Cases: 57%, Controls: 55%; p = 0.75) antibiotics within the preceding 30 days was similar. The number of admissions in the preceding 5 years was similar among the two groups (Cases: 15, Controls: 12; p = 0.24).
The most commonly identified pathogens on respiratory cultures were Pseudomonas species (71%), Staphylococcus aureus (58%), Achromobacter xylosoxidans (10%), and Stenotrophomonas maltophilia (8%). Among subjects with MIC data available for Pseudomonas species (n = 173), the tobramycin MIC was similar between cases and controls (p > 0.05 for both highest and lowest MIC available). Staphylococcus aureus was cultured less often from cases than controls (Cases: 49%, Controls: 62%; p = 0.04), as was methicillin-resistant S. aureus (Cases: 28%, Controls: 38%; p = 0.13). No differences were detected in the prevalence of other pathogens.
Subjects with S. aureus (n = 142) less often had prior AKI (60% vs 79%, p = 0.002) or recent AG receipt (44% vs 63%, p = 0.005), were less often underweight (11% vs 28%, p < 0.001), and had fewer hospital admissions in the prior 5 years (median: 12 vs 14, p = 0.01). Subjects with and without S. aureus were similar in gender, age, baseline SCr, and duration of AG receipt. Among subjects with complete spirometry data, those with and without S. aureus had similar changes in FEV1: 13% vs 11%, respectively (rank sum p = 0.12).
Results related to antibiotic and medication administration are displayed in Table 2. Tobramycin was the AG agent administered to the majority of subjects: 96% of Cases vs. 93% Controls (p = 0.33). The mean daily AG dose was similar among cases and controls. Cases received longer AG courses and were more likely to receive TMP/SMX, while they were less likely to receive other anti-staphylococcal antibiotics, most notably linezolid (Case: 5%, Controls: 13%, p = 0.05). Receipt of 2 or more nephrotoxins during therapy occurred in 16% of both cases and controls. There were no differences in the proportion of cases and controls receiving AG dose reductions (Case: 15%, Control: 14%, p = 0.90) or a prolongation of the AG dosing interval (4% vs 9%, p = 0.18).
Table 2.
Unadjusted analyses of antibiotic- and nephrotoxin-related risk factors for aminoglycoside-associated acute kidney injury.
| Variable | Cases (n=82) | Controls (n=164) | Odds ratioa (95% CI) | p-value |
|---|---|---|---|---|
| Aminoglycoside administration | ||||
| Days of AG therapy, median (IQR) | 13 (10-14) | 11 (9-14) | 1.10 (1.02-1.18) | .01 |
| Gentamicin or amikacin administration, n (%) | 3 (3.7) | 11 (6.7) | 0.52 (0.14-1.95) | .33 |
| Tobramycin administered once daily on all AG days | 70 (85.4) | 132 (80.5) | 1.45 (0.68-3.06) | .33 |
| AG dose in mg/kg/dayb, median (IQR) | 9.8 (7.5-10.6) | 9.7 (7.9-10.5) | 0.97 (0.86-1.10) | .63 |
| Concomitant receipt of other antimicrobialsc | ||||
| Cephalosporins | 27 (32.9) | 66 (40.2) | 0.71 (0.40-1.27) | .25 |
| Ceftazidime | 25 (30.5) | 60 (29.4) | 0.74 (0.41-1.34) | .32 |
| Penicillins | 26 (31.7) | 46 (28.1) | 1.21 (0.66-2.26) | .53 |
| Piperacillin/tazobactam | 21 (25.6) | 34 (20.7) | 1.34 (0.70-2.67) | .37 |
| Ticarcillin/clavulanate | 5 (6.1) | 11 (6.7) | 0.90 (0.29-2.77) | .85 |
| Meropenem | 28 (34.2) | 57 (34.8) | 0.97 (0.56-1.69) | .93 |
| Fluoroquinolones | 16 (19.5) | 20 (12.2) | 1.77 (0.85-3.66) | .13 |
| Trimethoprim/sulfamethoxazole | 42 (51.2) | 38 (23.2) | 3.07 (1.77-5.32) | <.001 |
| Vancomycin | 11 (13.4) | 35 (21.3) | 0.57 (0.27-1.19) | .14 |
| Other anti-staphylococcal antibioticsd | 9 (11.0) | 34 (20.7) | 0.40 (0.17-0.98) | .04 |
| Triazole antifungals | 13 (15.9) | 15 (9.2) | 1.87 (0.84-4.17) | .13 |
| Inhaled colistin or tobramycin | 12 (14.6) | 19 (11.6) | 1.31 (0.60-2.86) | .50 |
| Colistin | 9 (11.0) | 14 (8.5) | 1.32 (0.55-3.18) | .54 |
| Tobramycin | 3 (3.7) | 5 (3.1) | 1.20 (0.29-5.02) | .80 |
| Concomitant receipt of non-antibiotic nephrotoxins | ||||
| Non-steroidal anti-inflammatory drugs | 6 (7.3) | 10 (6.1) | 1.22 (0.43-3.47) | .72 |
| Contrast for computed tomography | 4 (4.9) | 4 (2.4) | 2.00 (0.50-8.00) | .33 |
| Receipt of 2+ concomitant nephrotoxins | 13 (15.9) | 26 (15.9) | 1.00 (0.48-2.09) | 1.00 |
Odds ratios and p-values derived by simple conditional logistic regression. Odds ratio >1 signifies increased odds of AKI during admission, <1 signifies decreased odds of AKI.
Averaged over entire AG course. Subjects receiving amikacin therapy were excluded for this analysis.
Data not displayed for individual agents administered in <5% of the study population.
Included clindamycin, doxycycline, and linezolid.
Table 3 shows the results of our multivariable model. After controlling for other significant variables, receipt of an IV AG within 90 days of admission, low serum albumin (<3.5 g/dL) at AG initiation, a longer duration of AG therapy and concomitant receipt of TMP/SMX, were independently associated with increased odds of AG-associated AKI. The presence of Staphylococcus aureus on recent respiratory culture was associated with decreased odds of AKI. No specific nephrotoxins were associated with AG-associated AKI in this study even when added to our final model (data not shown). We examined possible interaction between the presence of Staphylococcus aureus and receipt of TMP/SMX and found that the interaction term was not significant (p = 0.45).
Table 3.
Multivariable analysis for acute kidney injury during aminoglycoside therapy.
| Variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| Factors related to subject medical history | ||
| Serum albumin <3.5 g/dL at initiation of AG | 3.45 (1.41-8.45) | .007 |
| Receipt of an intravenous aminoglycoside within 90 days prior to admission | 2.28 (1.10-4.72) | .03 |
| Infection with Staphylococcus aureus | 0.47 (0.25-0.90) | .02 |
| Factors related to AG course | ||
| Concomitant receipt of trimethoprim/sulfamethoxazole | 3.27 (1.75-6.10) | <.001 |
| Days of aminoglycoside therapy | 1.10 (1.02-1.20) | .01 |
When variables in the final multivariate model were incorporated into a generalized linear mixed effects logistic regression model fit with maximum likelihood estimation using subject as a random effect, subject-level clustering was not significant: the 95% confidence interval for the variance of the regression coefficient attached to subject crossed zero. When the random effect was removed, there was no difference in the model outputs with or without the random effects. Therefore, repeated visits from individual subjects were deemed independent.
Univariate analyses of risk factors among the subset which included only a single admission per subject demonstrated few differences compared to analyses of all hospitalizations. The only variable significantly impacted by inclusion of multiple admissions for a single subject was the number of admissions in the previous 5 years. Among the single admission subset, the median number of previous admissions was higher among cases (13.5 vs 8, two-sample Mann-Whitney U test p = 0.007).
DISCUSSION
Our study identifies several important predictors for AG-associated AKI in children and young adults with cystic fibrosis including recent receipt of an AG, duration of AG therapy, low albumin, and concomitant receipt of TMP/SMX. These findings will help clinicians manage children and young adults with CF and assist investigators in the design of future studies on AKI in CF patients. Recognition of clinical- and patient-related factors which increase the risk for AKI may allow providers to implement strategies to mitigate AKI in this high-risk population.
Aminoglycoside nephrotoxicity results from drug accumulation within proximal tubule cells. Thus, longer courses of AG lead to increased drug exposure and opportunities for toxicity.[12-17] The threshold for development of AKI likely varies between individuals and a recent receipt of an AG, for example, could lead to an impaired ability to handle the drug safely. Therefore, development of nephrotoxicity in individuals with recent AG exposure may be more likely. There are no clear guidelines as to the optimal duration of antibiotic courses in CF, but our findings suggest that efforts to identify the duration of AG exposure which maximizes efficacy and minimizes toxicity are needed.
We found an independent association between low albumin levels and AKI. Previous studies in other populations have also reported low albumin as a risk factor for AG nephrotoxicity.[9, 11] Albumin and AG are transported via the same endocytic receptor, megalin, in the proximal tubule.[35] While low albumin levels could result from poor nutrition, deficiency may instead reflect decreased renal reserve and signal an increased sensitivity to AG drug accumulation. In vitro studies[36] have also demonstrated that megalin substrates can prevent renal uptake of gentamicin and mitigate toxicity; it is possible that normal albumin levels could be protective. Consideration of nutritional (and pre-hospitalization hydration) status may therefore mitigate the risk of toxicity from AG exposure. We chose a cut-off of 3.5 mg/dL to define hypoalbuminemia based on normative values at our centers, as well as those reported for children and adults,[27, 28] but this association between low albumin and AKI was also present when analyzed as a continuous measure (p=0.003) or when dichotomized at more profound levels of deficiency (<3.0 g/dL, p= 0.007).
Our study also identified several unexpected associations. First, we found a strong association between TMP/SMX use and AKI. Trimethoprim can elevate serum creatinine through inhibition of tubular secretion of creatinine without a true change in GFR.[37] However, cases of AKI attributable to TMP/SMX have been reported[38] as TMP/SMX may potentiate the nephrotoxic effects of other nephrotoxins.[39] Therefore, it is challenging to know whether the positive association seen in our study is real or, in part, artifact. History of prior AKI, baseline SCr, and duration of AG receipt did not differ among subjects based on TMP/SMX use and the association between TMP/SMX and AKI was significant both among subjects with and without S. aureus-positive cultures. We suspect that the association of TMP/SMX and AKI is not the result of the presence of other confounders. Given the frequent use of TMP/SMX in the CF population, we would suggest that close monitoring of SCr is necessary when this drug is co-administered with an AG. Other formal measures of glomerular filtration rate are not routinely performed in CF patients, but utilization of these could ascertain the effect of TMP/SMX on renal function. Alternatively, use of urinary biomarkers may better identify kidney injury during AG receipt. With the currently available data, we would not identify TMP/SMX as a nephron-sparing antibiotic (e.g., when considering TMP/SMX or vancomycin for MRSA coverage) for CF exacerbations.
When controlling for other variables, the presence of Staphylococcus aureus on recent respiratory culture was associated with a decreased risk of AKI in our study. Subjects with S. aureus less often had prior AKI or recent AG exposure, were less often underweight, and had fewer hospital admissions in the prior 5 years despite having a similar age, gender, and degree of respiratory impairment as subjects without S. aureus. This leads us to believe that this subset of CF patients with Staphylococcus aureus co-infections may have different underlying risk for AKI. Even though all subjects in our study received an AG, it is possible that those with recent S. aureus had decreased cumulative lifetime nephrotoxin exposure.
Finally, it was unexpected that receipt of other nephrotoxic agents was not associated with AKI in our study. Nephrotoxins may have been avoided in subjects deemed to be at higher risk for AKI. Another possibility is that the specific nephrotoxic agents administered in our study population, which were mostly antibiotics (cephalosporins, penicillins, and vancomycin), do not add to the significant toxicity of AG in CF. Contrary to studies in critically ill and adult populations,[17, 18, 20] utilization of vancomycin also did not carry an increased risk of AKI during CF hospitalization. Since the majority of nephrotoxins administered in patients with CF are antibiotics, the lack of detected impact of nephrotoxins may have resulted from receipt of similar regimens among cases and controls. Patients frequently receive the same antimicrobial medications on each admission and the specific antibiotic regimen used may contribute minimally to the nephrotoxicity of AGs in this population.
The strengths of this study include utilizing data from independent cohorts of patients at two urban, free-standing children's hospitals. Importantly, the rate of AG-associated AKI at the two institutions was similar (~15%). Both centers had an additional 8% incidence of early AKI (<72 hours) which we excluded for the purposes of this analysis. Combined data from these two large pediatric institutions represent standardized inpatient CF care and offer a sufficient population to identify risk factors for AG-associated nephrotoxicity. Inpatient management of patients at these hospitals is expected to be similar to the care provided at other pediatric institutions. Thus, risk factors identified by this study are expected to be generalizable to pediatric CF inpatients at other facilities. Other strengths include a case-control design, which allowed us to match for important potential confounders (gender, age and center), and use of a contemporary AKI definition.
Despite these strengths, we acknowledge several important limitations. Differences in therapeutic drug management between the two institutions limited direct investigation of the impact of serum AG concentrations and AKI development. However, we confirmed no differences in the proportion of cases and controls requiring a reduction of the AG dose or an extension of the dosing interval, suggesting detection of unstable serum drug concentrations did not contribute to AKI risk.
Second, we defined cases and controls based on admissions rather than individual subjects. Since episodes of AKI occur sporadically rather than consistently for any subject, we evaluated each admission as an independent event. In order to ascertain the potential bias of our approach, we tested for statistical independence of repeated admissions among individual subjects using a mixed effects model and found no significant effects of subject. Defining cases and controls according to admissions allowed us to focus on potentially modifiable risk factors which could be identified at the time of AG administration.
Lastly, the study's retrospective design also conferred limitations. For instance, SCr continues to be the most commonly used and widely accepted measure of renal function in the clinical setting, necessitating its use in this study. We acknowledge the limitation in using a SCr-based demarcation of kidney injury and recommend incorporation of more sensitive markers of kidney injury (e.g., urinary biomarkers) to better evaluate the impact of AG exposure on renal function. Additionally, complete lung function data were available in only 80% of cases. Spirometry was less often performed in sicker patients (lower baseline FEV1 among subjects without admission FEV1), preventing reliable inclusion of respiratory data in the multivariable model. The impact of acuity of pulmonary decline on the development of AKI should be explored in future prospective studies.
In conclusion, we have identified a number of potential risk factors for AG-associated acute kidney injury in children and young adults with cystic fibrosis. Patients with CF are frequently exposed to numerous courses of antibiotics over a lifetime, including intravenous AG. Identification of those at highest risk for toxicity may allow selection of alternative medications or more rigorous therapeutic drug management to minimize untoward effects of these important drugs. Future prospective studies are needed to further explore the associations described in our study and improve the safety of recurrent aminoglycoside use in this population.
Acknowledgments
Sources of Funding: There was no direct funding for this study. Dr. Downes was supported by the National Institute of Child Health and Human Development of the National Institutes of Health under award number 5T32HD069054, Cincinnati Training Program in Pediatric Clinical and Developmental Pharmacology. Dr. Harris receives funding from the Cystic Fibrosis Foundation (HARRIS12Q0). Dr. Clancy receives grant support from the NIH (NHLBI-FOA-HL 12–035) and the Cystic Fibrosis Foundation (R457-CR11, AMIN09YO). Dr. Goldstein is supported in part by the Agency for Healthcare Research and Quality Center for Education and Research on Therapeutics grant (AHRQ CERT 1U19HS021114). Dr. Askenazi receives funding from the Pediatric and Infant Center for Acute Nephrology (PICAN) which is sponsored by Children's of Alabama and the University of Alabama at Birmingham's School of Medicine, Department of Pediatrics and Center for Clinical and Translational Science (CCTS). Funding for REDCap at CCHMC is provided by Center for Clinical and Translational Science and Training grant support (UL1-RR026314). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AG
aminoglycoside
- AKI
acute kidney injury
- CCHMC
Cincinnati Children's Hospital Medical Center
- CF
cystic fibrosis
- COA
Children's of Alabama
- FEV1
forced expiratory volume in 1 second
- IQR
inter-quartile range
- IV
intravenous
- KDIGO
Kidney Disease – Improving Global Outcomes
- mg/kg/day
milligrams per kilogram per day
- SCr
serum creatinine
- SD
standard deviation
- TMP/SMX
trimethoprim/sulfamethoxazole
Footnotes
Conflict of Interest: There are no conflicts of interest to disclose. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
REFERENCES
- 1.Rougier F, Claude D, Maurin M, Maire P. Aminoglycoside nephrotoxicity. Current drug targets Infectious disorders. 2004;4:153–162. doi: 10.2174/1568005043340858. [DOI] [PubMed] [Google Scholar]
- 2.Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in noncritically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26:144–150. doi: 10.1093/ndt/gfq375. [DOI] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 4.Abitbol CL, Bauer CR, Montane B, Chandar J, Duara S, Zilleruelo G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol. 2003;18:887–893. doi: 10.1007/s00467-003-1186-1. [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehbe E, Brock R, Budev M, Xu M, Demirjian S, Schreiber MJ, Stephany B. Short-term and long-term outcomes of acute kidney injury after lung transplantation. J Heart Lung Transplant. 2012;31:244–251. doi: 10.1016/j.healun.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxicmedication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6:856–863. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165:522–527. e522. doi: 10.1016/j.jpeds.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Contreras AM, Ramirez M, Cueva L, Alvarez S, de Loza R, Gamba G. Low serum albumin and the increased risk of amikacin nephrotoxicity. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 1994;46:37–43. [PubMed] [Google Scholar]
- 10.Cortes J, Gamba G, Contreras A, Pena JC. Amikacin nephrotoxicity in patients with chronic liver disease. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 1990;42:93–98. [PubMed] [Google Scholar]
- 11.Gamba G, Contreras AM, Cortes J, Nares F, Santiago Y, Espinosa A, Bobadilla J, Jimenez Sanchez G, Lopez G, Valadez A, et al. Hypoalbuminemia as a risk factor for amikacin nephrotoxicity. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 1990;42:204–209. [PubMed] [Google Scholar]
- 12.Prins JM, Buller HR, Kuijper EJ, Tange RA, Speelman P. Once versus thrice daily gentamicin in patients with serious infections. Lancet. 1993;341:335–339. doi: 10.1016/0140-6736(93)90137-6. [DOI] [PubMed] [Google Scholar]
- 13.Rougier F, Ducher M, Maurin M, Corvaisier S, Claude D, Jelliffe R, Maire P. Aminoglycoside dosages and nephrotoxicity: quantitative relationships. Clinical pharmacokinetics. 2003;42:493–500. doi: 10.2165/00003088-200342050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Prins JM, Weverling GJ, de Blok K, van Ketel RJ, Speelman P. Validation and nephrotoxicity of a simplified once-daily aminoglycoside dosing schedule and guidelines for monitoring therapy. Antimicrobial agents and chemotherapy. 1996;40:2494–2499. doi: 10.1128/aac.40.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leehey DJ, Braun BI, Tholl DA, Chung LS, Gross CA, Roback JA, Lentino JR. Can pharmacokinetic dosing decrease nephrotoxicity associated with aminoglycoside therapy. J Am Soc Nephrol. 1993;4:81–90. doi: 10.1681/ASN.V4181. [DOI] [PubMed] [Google Scholar]
- 16.Bertino JS, Jr., Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis. 1993;167:173–179. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 17.Pauly DJ, Musa DM, Lestico MR, Lindstrom MJ, Hetsko CM. Risk of nephrotoxicity with combination vancomycin-aminoglycoside antibiotic therapy. Pharmacotherapy. 1990;10:378–382. [PubMed] [Google Scholar]
- 18.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrobial agents and chemotherapy. 1999;43:1549–1555. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrobial agents and chemotherapy. 2009;53:2887–2891. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlach AT, Stawicki SP, Cook CH, Murphy C. Risk factors for aminoglycoside-associated nephrotoxicity in surgical intensive care unit patients. International journal of critical illness and injury science. 2011;1:17–21. doi: 10.4103/2229-5151.79277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Aloul M, Miller H, Stockton P, Ledson MJ, Walshaw MJ. Acute renal failure in CF patients chronically infected by the Liverpool epidemic Pseudomonas aeruginosa strain (LES). J Cyst Fibros. 2005;4:197–201. doi: 10.1016/j.jcf.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Bertenshaw C, Watson AR, Lewis S, Smyth A. Survey of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2007;62:541–545. doi: 10.1136/thx.2006.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth A, Lewis S, Bertenshaw C, Choonara I, McGaw J, Watson A. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax. 2008;63:532–535. doi: 10.1136/thx.2007.088757. [DOI] [PubMed] [Google Scholar]
- 24.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatric pulmonology. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 25.Hmiel SP, Beck AM, de la Morena MT, Sweet S. Progressive chronic kidney disease after pediatric lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:1739–1747. doi: 10.1111/j.1600-6143.2005.00930.x. [DOI] [PubMed] [Google Scholar]
- 26.Kellum JA. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012;2:19–36. [Google Scholar]
- 27.Elin RJ. Reference Intervals and Laboratory Values. Goldman's Cecil Medicine. 2011:2558–2569. [Google Scholar]
- 28.Arcara KM. Blood Chemistries and Body Fluids. The Harriet Lane Handbook. Mosby, Inc.; 2012. pp. 639–650. [Google Scholar]
- 29.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public health nutrition. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:2000, 1–190. [PubMed] [Google Scholar]
- 31.Drug Information Handbook. Lexi-Comp, Inc.; Hudson, Ohio: 2009. [Google Scholar]
- 32.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaseda R, Hosojima M, Sato H, Saito A. Role of megalin and cubilin in the metabolism of vitamin D(3). Ther Apher Dial 15 Suppl. 2011;1:14–17. doi: 10.1111/j.1744-9987.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe A, Nagai J, Adachi Y, Katsube T, Kitahara Y, Murakami T, Takano M. Targeted prevention of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists. J Control Release. 2004;95:423–433. doi: 10.1016/j.jconrel.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Maki DG, Fox BC, Kuntz J, Sollinger HW, Belzer FO. A prospective, randomized, double-blind study of trimethoprim-sulfamethoxazole for prophylaxis of infection in renal transplantation. Side effects of trimethoprimsulfamethoxazole, interaction with cyclosporine. J Lab Clin Med. 1992;119:11–24. [PubMed] [Google Scholar]
- 38.Fraser TN, Avellaneda AA, Graviss EA, Musher DM. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother. 2012;67:1271–1277. doi: 10.1093/jac/dks030. [DOI] [PubMed] [Google Scholar]
- 39.de Araujo M, Seguro AC. Trimethoprim-sulfamethoxazole (TMP/SMX) potentiates indinavir nephrotoxicity. Antivir Ther. 2002;7:181–184. [PubMed] [Google Scholar]


